Abstract
This study uses serologic testing to characterize natural immunity and the long-term durability of SARS-CoV-2 antibodies among unvaccinated US adults by history of COVID-19 infection.
As of December 28, 2021, approximately 27% of the US population was unvaccinated against SARS-CoV-2,1 yet the prevalence of natural immunity remains unknown. Blood donor studies may have selection bias and lack clinical information.2 Previous COVID-19 infection is a possible surrogate for natural immunity, but 1 study suggested that 36% of COVID-recovered individuals are serologic nonresponders.3 Even among individuals who develop antibodies, durability of this response beyond 6 months remains unknown. We characterized natural immunity and long-term durability among unvaccinated individuals using anti-spike antibodies, the first line of defense against SARS-CoV-2.
Methods
Healthy adults who reported no SARS-CoV-2 vaccination were recruited via 1 public Twitter post and 1 public Facebook advertisement between September 11, 2021, and October 8, 2021. Participants completed an online questionnaire about demographics, COVID-19 status, and mask use. Using weighted random sampling (relative weights based on the estimated unvaccinated US population by age, race and ethnicity, and education1), we created 3 equally sized sample groups among those who reported a test-confirmed COVID-19 infection (“COVID-confirmed”), who believed they had COVID-19 but were never tested (“COVID-unconfirmed”), and who did not believe they ever had COVID-19 and never tested positive (“no-COVID”). These groups were invited to undergo antibody testing at LabCorp facilities nationwide.
Qualitative detection of antibodies against the SARS-CoV-2 antinucleocapsid (N) protein (positive cutoff index ≥1.0) and semiquantitative detection of antibodies against the SARS-CoV-2 spike protein receptor-binding domain (RBD) (positive cutoff ≥0.8 U/mL) were performed (Elecsys; Roche Diagnostics International Ltd). Various cutoffs are reported (≥250 U/mL, ≥500 U/mL, ≥1000 U/mL) based on reported associations with neutralization.4
Population characteristics were compared using χ2 test for categorical (Fisher exact test for rare outcomes) and Wilcoxon rank sum test for continuous variables. We used linear regression to analyze the association between time after infection and log antibody titer. The threshold for statistical significance was P < .05 (2-sided). All analyses were performed using Stata 17.0/SE (StataCorp). The study was approved by the Johns Hopkins institutional review board. Participants provided electronic informed consent.
Results
Of 1580 individuals invited to undergo serologic testing, 816 (52%) did so between September 24, 2021, and November 5, 2021. Participants had a mean age of 48.0 years, 421 (52%) were women, and 669 (82%) were White (Table). Fourteen percent reported routine mask use in public. Anti-RBD and anti-N antibody presence/absence were correlated (95%; Cohen κ=0.908).
Table. Population Characteristics and Antibody Result Stratified by COVID-19 Diagnosis, Confirmed or Suspected.
| Total, No. (%) | No. (%) | P valuea | |||
|---|---|---|---|---|---|
| COVID-19 infection | Believes never had COVID-19 | ||||
| Confirmed | Unconfirmed | ||||
| No. | 816 | 295 | 275 | 246 | |
| Age, median (IQR), y | 48 (37-59) | 47 (37-59) | 48 (37-58) | 49 (38-62) | .49 |
| Men | 395 (48) | 140 (47) | 132 (48) | 123 (50) | .83 |
| Women | 421 (52) | 155 (53) | 143 (52) | 123 (50) | |
| Raceb | |||||
| African American/Black | 12 (2) | 4 (1) | 7 (3) | 1 (0.4) | .01 |
| Asian | 35 (4) | 16 (5) | 12 (4) | 7 (3) | |
| White | 669 (82) | 228 (77) | 221 (80) | 220 (89) | |
| Other | 100 (12) | 47 (16) | 35 (13) | 18 (7) | |
| Hispanic ethnicityb | 106 (13) | 43 (15) | 39 (14) | 24 (10) | .40 |
| Attended college | 518 (64) | 179 (61) | 162 (59) | 177 (72) | .004 |
| Mask use | |||||
| Routinely | 114 (14) | 53 (18) | 28 (10) | 33 (13) | <.001 |
| Sometimes | 214 (30) | 103 (35) | 76 (28) | 68 (28) | |
| Rarely | 355 (44) | 117 (40) | 122 (44) | 116 (47) | |
| Never | 100 (12) | 22 (8) | 49 (18) | 29 (12) | |
| Nucleocapsid-positivec | 440 (54) | 280 (95) | 138 (50) | 22 (9) | <.001 |
| Anti-RBD–positive | 471 (58) | 293 (99) | 152 (55) | 26 (11) | <.001 |
| Antinucleocapsid/anti-RBD agreement | 779 (95) | 248 (96) | 219 (92) | 215 (98) | <.001 |
| Anti-RBD, U/mLc | |||||
| Median (IQR) | 158 (52-499) | 205 (61-535) | 131 (35-402) | 82 (19-172) | .005 |
| ≥250 | 185 (23) | 129 (44) | 50 (18) | 6 (2) | <.001 |
| ≥500 | 117 (14) | 79 (27) | 33 (12) | 5 (2) | <.001 |
| ≥1000 | 63 (8) | 43 (15) | 16 (6) | 4 (2) | <.001 |
| Days since COVID-19 diagnosis, median (IQR)c | 261 (56-387) | ||||
Abbreviation: RBD, receptor-binding domain.
A χ2 test was used for categorical variables (Fisher exact test for rare outcomes) and a Wilcoxon rank sum test for continuous variables.
Race and ethnicity data were collected to perform weighted random sampling among the 3 groups for antibody testing. Participants could select from predefined categories of African American/Black, Asian, White, or other. Ethnicity was self-reported. Participants could select among predefined categories of Hispanic/Latino yes/no.
Among participants with positive titers.
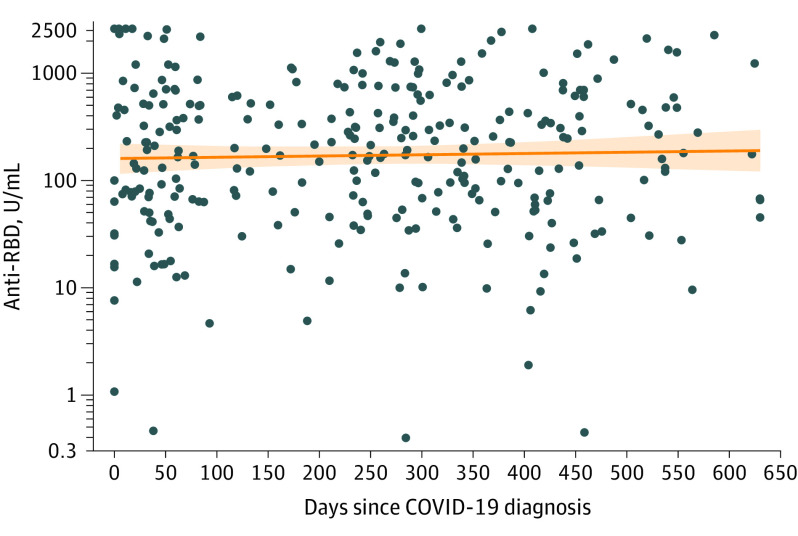
Among 295 reported COVID-confirmed participants, 293 (99%) tested positive for anti-RBD antibodies (≥250 U/mL, 44%; ≥500 U/mL, 27%; ≥1000 U/mL, 15%). A median of 8.7 (IQR, 1.9-12.9; range, 0-20) months passed since reported COVID-19 diagnosis. The median anti-RBD level among those who tested positive was 205 (IQR, 61-535) U/mL. There was no evidence of association between time after infection and antibody titer (0.8% increase [95% CI, –2.4% to 4.2%] per month; P = .62) (Figure).
Figure. Anti-Spike RBD Levels by Time Since COVID-19 Diagnosis.
Anti–receptor-binding domain (RBD) levels did not differ by months since COVID-19 diagnosis (0.8% increase [95% CI, –2.4% to 4.2%] per month; P = .62). Data markers indicate individual anti-RBD titers; solid orange curve with shaded area, linear regression with 95% confidence range.
Among 275 reported COVID-unconfirmed participants, 152 (55%) tested positive for anti-RBD antibodies (≥250 U/mL, 18%; ≥500 U/mL, 12%; ≥1000 U/mL, 6%). The median level among those who tested positive was 131 (IQR, 35-402) U/mL.
Among 246 reported no-COVID participants, 11% tested positive for anti-RBD antibodies (≥250 U/mL, 2%; ≥500 U/mL, 2%; ≥1000 U/mL, 2%). The median level among those who tested positive was 82 (IQR, 19-172) U/mL.
Discussion
In this cross-sectional study of unvaccinated US adults, antibodies were detected in 99% of individuals who reported a positive COVID-19 test result, in 55% who believed they had COVID-19 but were never tested, and in 11% who believed they had never had COVID-19 infection. Anti-RBD levels were observed after a positive COVID-19 test result for up to 20 months, extending previous 6-month durability data.5
Study limitations include lack of direct neutralization assays, the fact that antibody levels alone do not directly equate to immunity,4,6 the cross-sectional study design, a convenience sample with an unknown degree of selection bias due to public recruitment, self-reported COVID-19 test results, the study population being largely White and healthy, and lack of information on breakthrough infections. Participants were given only 1 month to complete antibody testing, which may have contributed to the 52% rate among those invited to test.
Although evidence of natural immunity in unvaccinated healthy US adults up to 20 months after confirmed COVID-19 infection is encouraging, it is unclear how these antibody levels correlate with protection against future SARS-CoV-2 infections, particularly with emerging variants. The public health implications and long-term understanding of these findings merit further consideration.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Associate Editor.
References
- 1.USAFacts . Secondary US coronavirus vaccine tracker 2021. Accessed December 28, 2021. https://usafacts.org/issues/coronavirus/
- 2.Jones JM, Stone M, Sulaeman H, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA. 2021;326(14):1400-1409. doi: 10.1001/jama.2021.15161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu W, Russell RM, Bibollet-Ruche F, et al. Predictors of nonseroconversion after SARS-CoV-2 infection. Emerg Infect Dis. 2021;27(9):2454-2458. doi: 10.3201/eid2709.211042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert PB, Montefiori DC, McDermott AB, et al. ; Immune Assays Team; Moderna Inc Team; Coronavirus Vaccine Prevention Network (CoVPN)/Coronavirus Efficacy (COVE) Team; US Government (USG)/CoVPN Biostatistics Team . Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43-50. doi: 10.1126/science.abm3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Israel A, Shenhar Y, Green I, et al. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines (Basel). 2021;10(1):64. doi: 10.3390/vaccines10010064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999-1009. doi: 10.1016/S2213-2600(21)00220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]