Abstract
Background:
Infection caused by Actinomyces species is a rare cause of head and neck infection in children. This chronic cervicofacial infection can present with localized swelling, abscess formation, sinus drainage and can be complicated by osteomyelitis.
Methods:
Presented are 2 pediatric cases of secondary actinomycosis in the context of congenital lesions: 1 patient with a previously excised preauricular sinus and another with a persistent sublingual mass. A comprehensive literature search was conducted for reported cases of pediatric actinomycosis in the cervicofacial region.
Results:
Both cases presented were successfully treated with a combination of complete surgical excision of the lesions and prolonged antibiotic therapy. Thirty-four pediatric cases of cervicofacial actinomycosis are reviewed, 2 presented herein, and 32 from the published literature. There was equal gender distribution and the median age was 7.5 years. The most common site for infection was the submandibular area. Four (12%) of cases arose in pre-existing congenital lesions. Most patients were treated with penicillin-based antibiotics for a median duration of 6 months following surgical excision or debridement.
Conclusions:
Actinomycosis is a rare infection of the cervicofacial region; secondary infections arising from congenital lesions of the head and neck are even more rare. A previously excised pre-auricular sinus and a sublingual dermoid cyst are not previously reported sites of infection. Actinomycosis should be suspected in chronically draining sinuses of the head and neck region and confirmed through anaerobic culture. Osteomyelitis is a potential complication and magnetic resonance (MR) imaging is warranted. Long-term antibiotic therapy with a penicillin-based antibiotic and surgical excision should be considered.
Keywords: infection, anaerobic bacteria, children, intraoral excision, common childhood external ear problems
Introduction
Cervicofacial actinomycosis is a rare cause of head and neck abscesses in healthy children. It is a rare, slowly progressive, subacute, or chronic infection caused by the Actinomyces species of bacteria. 1 Actinomyces species are gram positive filamentous anaerobic bacteria that can present with characteristic sulfur granules. These bacteria commonly colonize the human oral cavity, gastrointestinal, and genitourinary tract. 2 Mucosal injury, poor oral hygiene, and immunosuppression have been implicated in pathogenesis of this disease. Actinomyces infection is commonly polymicrobial and requires prolonged anerobic culture for up to 15 days to grow. 3 The most common species involved in human infection is A. israeli but there are at least 25 other species less commonly described in the literature identified by 16S ribosomal RNA sequencing. 4
Infection is classically described as a chronic submandibular swelling but has been described in multiple regions in the head and neck. 5 Actinomycosis can present with pain, fever, and weight loss and may be misdiagnosed as other granulomatous diseases or malignancy. 6 Infections may be complicated by draining sinuses, osteomyelitis, and bacteremia.5,7 Its tendency to persist, spread to adjacent tissue, and cause significant morbidity if not treated for extended periods with a penicillin-based therapy makes it important to confirm the diagnosis and initiate appropriate management. 8 Anerobic cultures are required for diagnosis and should be obtained in an atypical pediatric head or neck mass. 9 Appropriate treatment often includes both medical management with penicillin-based antibiotics to treat a potentially polymicrobial infection, and surgery for extensive or recurrent disease. 10 We present 2 cases of children with secondary cervicofacial actinomycosis in sites not previously described and review the literature on this rare infection in children. The first of these cases is the first report of A. turicensis cervicofacial infection in a child.
Cases
Case 1: A previously healthy, immunized, 10-year-old female presented with a 1-year history of recurrently infected left pre-auricular sinus. She was referred to the pediatric otolaryngology service after previous surgical debridement. However, despite excision with dissection down to the helical cartilage, she subsequently developed a chronic infection post-operatively with poor response to short courses of multiple oral antibiotics including cephalexin, amoxicillin, and trimethoprim-sulfamethoxazole (TMP/SMX) for presumed cellulitis including possible MRSA. All wound swabs were negative, including MRSA screening. Anaerobic cultures had not been done.
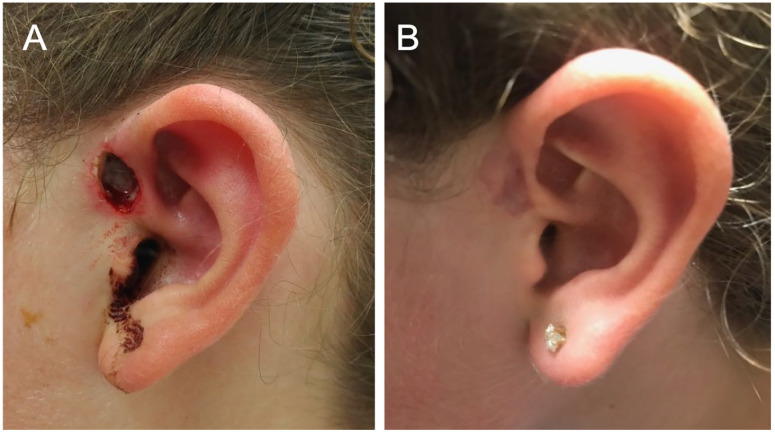
Physical examination at presentation revealed erythema and inflammation surrounding the previous surgical site. There was purulent drainage from this site which was sent for aerobic culture which showed gram positive cocci but no growth, likely due to TMP/SMX treatment at the time of the swab. Subsequently, the patient was consented for re-exploration and re-excision. In the operating room, a pre-auricular sinus was identified, which tracked down to the cartilaginous helix. A lacrimal probe was placed in the sinus to guide the dissection. Purulence was readily expressed, collected, and swabs were sent for both aerobic and anerobic culture. The sinus tract was circumferentially dissected out and resected en bloc at the root of the helix. The wound was copiously irrigated followed by a layered closure (Figure 1A). Pathology showed dermal granulation tissue with acute and chronic inflammation in keeping with secondary infection after the initial resection.
Figure 1.
Post-operative image of preauricular sinus and healing of the sinus tract following therapy. (A) Recurrent preauricular sinus after wide local excision. Healing by secondary intention. (B) Healed pre-auricular sinus following a 6-month course of antibiotics post-operatively.
Gram-positive bacilli and gram-positive cocci were seen on microscopy, although only Actinomyces turicensis subsequently grew. Given polymicrobial findings on microscopy, the infectious diseases team recommended switching antibiotic coverage from amoxicillin to amoxicillin-clavulanate to cover both Actinomyces spp. and Staphylococcus spp. Subsequent magnetic resonance (MR) imaging confirmed that there was no evidence of osteomyelitis.
Surgical re-excision was completed 1 month later because of intermittent drainage from the operative site. The area was re-excised down to the cartilage. Soft tissue and perichondrium were scraped from the underlying cartilage. The area was packed and subsequently healed by secondary intention after 2 weeks. Gram stains of intraoperative specimens again identified gram positive bacilli and cocci and cultures subsequently grew only A. turicensis. The patient completed a 6-month course of amoxicillin-clavulanate post-operatively and has had no recurrence. When seen at the 6-month follow-up after completing antimicrobial therapy, there was no contour defect or evidence of ongoing infection (Figure 1B).
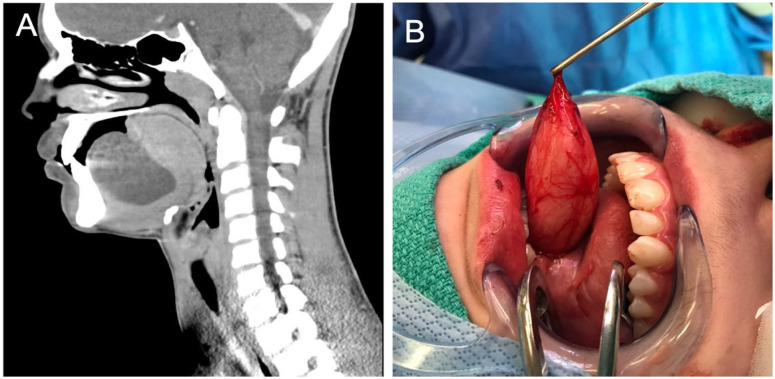
Case 2: A 14-year-old, immunized, male presented with a history of a sublingual mass initially thought to represent a plunging ranula. Contrast enhanced CT neck showed a 5 cm cystic mass displacing the tongue superiorly (Figure 2A). The mass was first managed with decompression and marsupialization under local anesthetic. Aspirates sent for aerobic culture were negative. No anerobic cultures were requested. Clindamycin and chlorhexidine mouthwash were given for antimicrobial prophylaxis following the procedure. There were no signs of recurrence at the 1-week follow-up.
Figure 2.
CT image and intraoperative image of the sublingual mass. (A) Sagittal CT view showing a ~5 cm × 5 cm sublingual mass. (B) Case 2: Intra-operative image of sublingual mass excision. Dermoid cyst measured ~5 cm × 5 cm.
The patient presented to the emergency department 2 months later with concerns the lesion was increasing in size following trauma to the face and jaw. Muffling of the voice was noted. The mass was decompressed and 80 mL of yellow cloudy fluid was collected and sent for cytology, culture, and sensitivity. A 7-day course of amoxicillin was prescribed empirically for oral infection secondary to a congenital mass. There was no recurrence at the 3-week follow-up. At 5 months there was recurrence of sublingual swelling and the area was again aspirated and sent for both aerobic and anerobic cultures. This aspirate grew only Actinomyces spp. which was treated with 6-months of amoxicillin therapy. MRI confirmed that there was no bony involvement suggesting osteomyelitis. Another aspiration was needed 4 months later, this grew Veillonella spp. in addition to Actinomyces spp. Antibiotic coverage was broadened to high-dose amoxicillin-clavulanate. Due to the persistent infection and recurrence of swelling surgical excision was done. The pathology-confirmed dermoid cyst (Figure 2B) was completely excised and high-dose amoxicillin-clavulanate was extended for 3 months post-operatively. Total duration of antibiotic therapy was 9 months. There were no signs of recurrence 2 months post-operatively.
Methods
A search of the published literature up to March 28, 2020 was conducted using the online search databases PubMed, EMBASE, and Medline. The search used MeSH terms or free text phrases that combined infection-specific terms (Actinomyces AND actinomycosis) with condition-specific terms (head and neck OR head OR skull, neck OR neck muscles; OR cervicofacial OR facial OR face OR cervical; OR mandibular, sublingual OR oral) and age-specific terms (child, neonate, pediatric, pediatric, boy, girl). Cochrane and Web of Science were also searched but no relevant, non-duplicate articles were identified. Articles selected for review were written in English and included confirmed cases of actinomycosis restricted to the head and neck in patients under 18 years of age. Abstracts and full articles were reviewed by 2 authors independently (KG and BVW). References of included articles were reviewed to identify additional reports that were not included in the original search.
Articles were excluded if they were not specific to the management of pediatric head and neck actinomycosis, if they reported on immunocompromised hosts, or if they specifically addressed actinomycosis of the tonsils, thyroid, intracranial, or dental actinomycosis as these have a different presentation than cervicofacial actinomyces infection. Demographic, clinical, microbiological, management, and outcome data were extracted using a standardized spreadsheet document.
Results
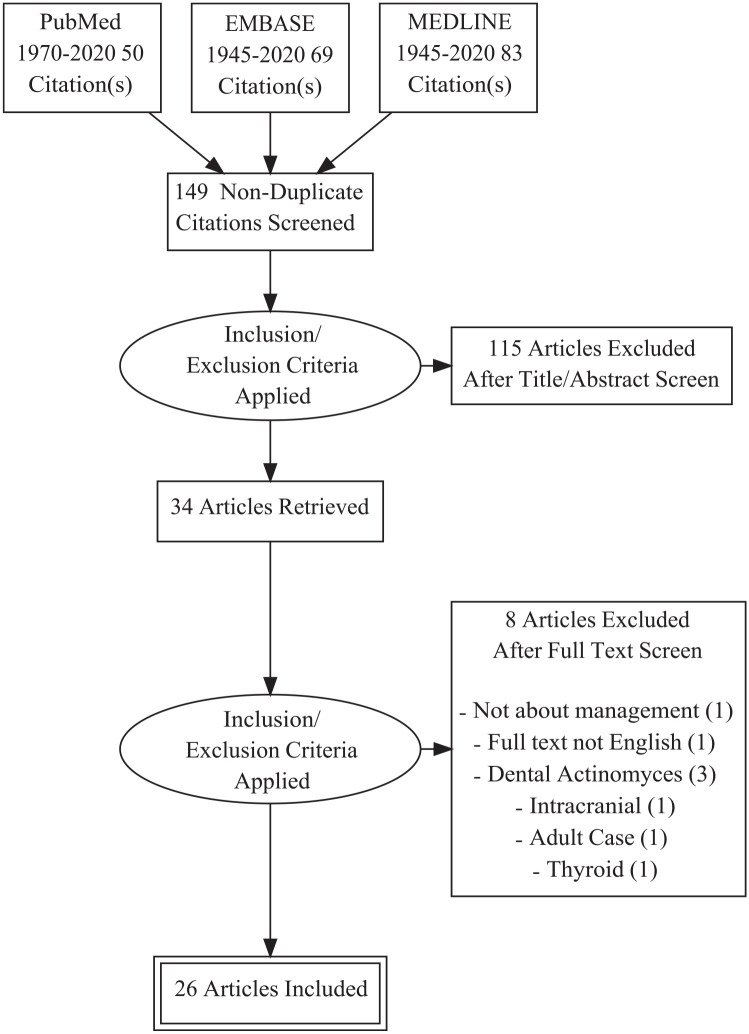
With our pre-defined search criteria, we identified a total of 149 English language articles. The authors excluded 115 articles during title and abstract screening; 34 articles received full text review and 26 were included in the final review (Figure 3). The 8 articles that did not fulfill study criteria after receiving full text review were excluded for the following reasons: site not qualifying (N = 6), non-English language (N = 1), and management not reported (N = 1).
Figure 3.
Flow diagram showing the literature review process for this literature review. The diagram shows the process by which studies were incorporated into the results of the literature search.
From the 26 articles, 32 patient cases were reported, 16 (50%) male, 15 (48%) female, and 1 article (2%) where gender was not specified. The median age was 7.5 years, with a range of 20 months to 17 years. The most common site of infection was the mandibular region accounting for 15 cases (48%), 8 patients (25%) had infections in the cervical region, and 2 patients (6%) had an infra-auricular infection. Other sites of infection included the labial mucosa, temporal/temporo-facial, mastoid, parotid, temporal bone, and middle ear, which accounted for 7 cases (22%) combined. Literature review showed 2 (6%) cases of actinomycosis in the setting of congenital lesions, first in the setting of a dermoid cyst and the other in a pyriform sinus fistula. Along with our cases, actinomycosis arising in congenital head and neck lesions accounted for 4 (12%) of head and neck infections. Actinomycosis most commonly presented with a cervicofacial mass in 29 cases (91%). Of the 23 cases that included information about patient symptoms, 15 (65%) had pain at the site. Sinus tracts developed in 6 cases (19%) and 13 cases (41%) reported features consistent with osteomyelitis. Otalgia or otorrhea were reported in 3 cases, but only presented in middle ear infections. The most common organisms were A. israelii; reported in 8 cases (28%) and A. odontolyticus; reported in 4 cases (13%). A. neuii, A. naeslundii and were reported in 1 case each. The organism was unspecified in 18 cases (56%).
Penicillin-based therapy was given in 30 cases (94%), this included penicillin G/V, amoxicillin, and ampicillin. Two children (6%) received no penicillin-based antibiotics during treatment. Antibiotics used in these cases were clindamycin or cefuroxime and metronidazole. Seven of the 30 children (23%) who received penicillin-based therapy also received non-penicillin-based antibiotics for some or most of their antimicrobial therapy. Non-penicillin antibiotics in these cases included: meropenem, clindamycin, doxycycline, pristinamycine cefazolin, clarithromycin, and azithromycin. Surgery or aspiration was performed in 24 cases (75%) and no procedures were performed in 8 cases (25%). Antibiotic therapy was continued for a median of 6 months with a range of 1 week to 25 months, with some patients receiving antibiotic therapy intermittently over years due to recurrence. All included case reports reported either cure of the infection or loss to follow-up. This data is summarized in Table 1.
Table 1.
Data from 34 Cases of Pediatric Cervicofacial Actinomycosis.
| Paper | Age/gender | Site of infection | Presentation | Actinomyces species (spp) | Antibiotic (duration) | Surgery | Primary or secondary infection |
|---|---|---|---|---|---|---|---|
| Present study Case 1, 2019 | 10/F | Pre-auricular sinus | Inflammation Sinus tract |
Actinomyces turicensis | Amoxicillin/clavulanate (6 mo) | Excision | Secondary to congenital lesion |
| Present study Case 2, 2019 | 15/M | Sublingual | Painless mass | Not stated (NS) | Amoxicillin/clavulanate (9 mo) | Decompression Marsupialization | Secondary to congenital lesion |
| Drake and Holt, 1970 24 | 3/F | Cervical | Mass | A. israelii | Cloxacillin (0.25 mo) Penicillin (3.5 mo) |
Drainage | Primary |
| 5/F | Cervical | Mass | A. israelii | Penicillin (3.3 mo) | Aspiration Resection | Primary | |
| Roveda, 1973 25 | 17/M | Parotid | Painful mass | NS | Penicillin (0.5 mo) | Drainage | Primary |
| Walker et al, 1981 26 | 7/F | Mandibular | Mass Fever Osteomyelitis |
A. israelii | Penicillin (3 mo) | Curette | Primary |
| Badgett and Adams, 1987 27 | 5/F | Submandibular | Painful mass Osteomyelitis |
NS | Clindamycin (10 mo) | Drainage | Primary |
| Feder, 1990 28 | 6/M | Mandibular | Painful mass Fever Osteomyelitis |
A. israelii | Penicillin (3.5 mo) | Drainage | Primary |
| 13/M | Mandibular | Painful mass | A. israelii | Penicillin (4 mo) | Drainage | Primary | |
| Carrau et al, 1993 29 | 6/M | Temporofacial | Painless mass | NS | Penicillin (9 mo) | Excision | Primary |
| Foster et al, 1993 30 | 11/M | Submental | Mass | NS | Penicillin (7 mo) | Curette | Primary |
| Altundal et al, 2000 31 | 10/M | Submandibular | Painful mass Sinus tract Osteomyelitis |
NS | Penicillin (19.25 mo) | — | Primary |
| Schwartz and Wilson, 2001 32 | 17/M | Mandibular | Painful mass | NS | Amoxicillin (6 mo) | Surgical Incision | Primary |
| Hong et al, 2004 33 | 9/M | Posterior neck | Mass | NS | Penicillin (0.25 mo) | — | Primary |
| Sobol et al, 2004 34 | 12/F | Temporal bone | Otalgia, otorrhea Hearing loss Osteomyelitis |
NS | Penicillin (6 months) | Excision | Primary |
| Robinson et al 22 | 7/F | Mandibular | Painful mass Osteomyelitis | NS (2 species) | Penicillin (7.5 mo) Clindamycin (0.5 mo) Doxycycline (3 mo) Clindamycin (8 mo) |
Debridement | Primary |
| 4/F | Mandibular | Mass Osteomyelitis |
A. israelii | Penicillin, Clindamycin, Clarithromycin, and Metronidazole Azithromycin (~4 y)* |
Debridement | Primary | |
| 3/M | Mandibular | Painful mass Osteomyelitis Sinus tract |
A. naeslundii | Cefazolin Penicillin Clindamycin Amoxicillin/clavulanate Metronidazole (22 mo)* |
Biopsies Debridement Sequestrectomy | Primary | |
| 4/F | Mandibular | Painful mass Osteomyelitis |
A. israelii | Amoxicillin (6 mo) Clindamycin (7 mo) Amoxicillin/clavulanate (12 mo) |
Sequestrectomy Mandibulectomy Debridement | Primary | |
| Mehta et al, 2007 35 | 11/M | Temporal bone | Otorrhea, otalgia Mass Osteomyelitis |
NS | Ampicillin/Sulbactam (2.25 mo) | Labyrinthectomy Debridement | Primary |
| Ciftologan et al, 2009 36 | 9/M | Submandibular | Painless mass | NS | Amoxicillin (3 mo) | Excision | Primary |
| Gazzano et al, 2010 37 | 8** | Middle ear | Otorrhea | NS | Amoxicillin (5.75M) Amoxicillin + Pristinamycine (2 mo) |
Excision | Primary |
| Hung et al, 2014 38 | 7/F | Posterior cervical region | Painful mass Neck pain |
NS | Penicillin (12 mo) | Partial excision | Primary |
| Lezcano et al, 2014 39 | 10/M | Mastoid | Painful Mass Fever |
NS | Meropenem (1.5 mo) Amoxicillin/clavulanate (6 mo) |
Mastoidectomy | Primary |
| Thacker and Mary Healy, 2014 40 | 16/F | Submandibular | Painless mass | NS | Penicillin (7 mo) | — | Primary |
| Verma et al, 2014 41 | 6/F | Labial mucosa | Mass Sinus tracts |
A. israelii | Amoxicillin/clavulanate (1 mo) | — | Secondary to congenital lesion |
| Walther et al, 2014 42 | 1.67/F | Cervical region | Painless mass Sinus tract |
A. neuii | Amoxicillin/clavulanate (6 mo) | Excision | Primary |
| Chatterjee et al, 2015 43 | 13/M | Infra-auricular | Painful mass Facial swelling Osteomyelitis |
NS | Cefuroxime/metronidazole | Incisional Biopsy Resection | Primary |
| Prajapati et al, 2016 44 | 8/M | Cervical spine | Painful ulcer Osteomyelitis |
NS | Ampicillin/sulbactam Penicillin (1 mo) |
— | Primary |
| Sama et al 8 | 11/F | Infra-auricular | Painless mass Sinus tracts |
NS Others |
Penicillin | Debulking drainage | Primary |
| Yanagisawa et al, 2017 45 | 2/M | Cervical | Painless Mass Sinus tract |
A. odontolyticus | Ampicillin/sulbactam (0.5M) Amoxicillin/clavulanate (6 mo) |
Biopsy | Secondary to congenital lesion |
| Glass et al, 2019 46 | 5/M | Submandibular | Painless Mass Odontalgia Osteomyelitis |
A. odontolyticus | Amoxicillin/clavulanate (7.5 mo) | Debridement | Primary |
| 5/F | Submandibular | Odontalgia Mass Osteomyelitis |
A. odontolyticus | Amoxicillin/clavulanate (6 mo) | Drainage | Primary | |
| Savoca et al, 2019 47 | 10/F | Cervical | Painful mass | A. odontolyticus | Clindamycin*
Amoxicillin (2 mo) Penicillin (7.5 mo) |
Biopsy | Primary |
Abbreviations: F, female; M, male.
Not included in average duration of treatment calculation as the exact duration of treatment was not reported.
Gender not specified.
Discussion
We present 2 cases of pediatric cervicofacial actinomycosis in the setting of congenital lesions representing the first report of actinomycosis at each respective site, although not the first report of infections arising from congenital lesions. Actinomyces species are a part of the normal oral flora and infection typically ensues when the host’s mucosal barriers or immune responses are compromised, which is likely the source of the sublingual infection reported. We postulate that the pre-auricular sinus became infected from the drooling of oral secretions during sleep. It is possible that in the case of the sublingual cyst actinomycosis could have developed spontaneously from previous aspiration or a microscopic breach in its mucosal surface that may have happened with the rapid growth of the cyst and possible thinning of its wall. Alternatively, the cyst could have become secondarily infected at the time of the initial surgery. Both children had polymicrobial infection, which is commonly present in actinomycosis and can make diagnosis more challanging. 11
Actinomycosis is rare in childhood but more common in middle aged individuals. It can mimic a wide variety of diseases, including malignancy and tuberculosis and presents with an array of symptoms making diagnosis challenging. 12 Cervicofacial presentations include localized swelling often in the mandibular area with or without abscess formation, fibrosis, and sinus drainage.3,13 A. israelli is the most common species known to cause infection in humans and was identified as the leading species causing pediatric cervicofacial actinomycosis in this review. Treatment is often comprised of surgical debridement followed by long-term penicillin-based therapy.
Pre-auricular sinuses are common congenital abnormalities that are thought to be the consequence of incomplete fusion of the 6 auditory Hillocks of His. 14 These indentations near the anterior margin of the helix may become secondarily infected, most commonly with Staphylococcus aureus. 15 As Actinomyces also causes sinus tract formation, it is possible that the infection may have created a de novo tract following successful and complete excision of the original preauricular sinus tract. Despite its rarity, Actinomyces spp should be considered in chronically draining sinuses, which show poor response to short courses of anti-Staphylococcal antibiotic therapy. Early diagnosis and treatment are important as long-term antibiotic therapy is important and serious complications, such as osteomyelitis, may ensue. In such cases, sending purulent secretions, rather than swabs, for cultures, especially anerobic cultures, maximizes detection of the organism. This allows for targeted antimicrobial therapy with a better chance of clearing the infection. Complete surgical excision of the pre-auricular pit along with the sinus tract is important to definitively manage recurrently infected sinuses. 15 Wounds are closed primarily in most cases of surgical excision of pre-auricular sinuses. However, for actinomycoses with draining sinuses, the wounds should be packed for optimal healing by secondary intention. 16
Dermoid cysts are benign lesions that can occur in the head and neck region. 17 They are midline lesions resulting from sequestration of ectodermal tissue along embryonic fusion planes. 18 This is the first report of a sublingual dermoid cyst infected with Actinomyces spp. As the earliest sampling taken from this cyst was not sent under anerobic conditions, the failure to grow actinomyces from that sample does not exclude its presence. We cannot determine if this lesion was spontaneously infected. The lesion was decompressed and marsupialized in clinic but failed to resolve and subsequently needed multiple aspirations. The failure of this cyst to resolve following initial surgical management and need for multiple aspirations raised concerns for secondary infection. Given the location of the dermoid cyst, there were also concerns for difficulties with speech, swallowing, mastication, and airway obstruction.19,20 The growth of Actinomyces spp directed targeted amoxicillin therapy. Despite antibiotic treatment and multiple aspirations, the lesion continued to enlarge. As a result, complete surgical excision of the cyst was necessary (Figure 2). An additional 3 months of antibiotic therapy was prescribed post-operatively, after which time there was complete resolution of the infection.
Cervicofacial actinomycoses commonly presents with a painful mass or swelling, with up to 50% arising in the mandibular region. 3 However, the absence of pain should not be used to exclude the diagnosis, as up to one-third of cases may not present with pain. Osteomyelitis rarely complicates head and neck infections caused by S.aureus and other common acute pyogenic organisms. In contrast, osteomyelitis is a recognized complication of cervicofacial actinomycosis occurring in 38% of pediatric cases reported in the literature ; 67% of these bony infections involved the mandible. 21 Infections complicated by bony infection may be challenging to clear and require extended antimicrobial therapy. Given the frequency of this complication and clinically silent pattern among reported cases, MRI should be done to exclude bony involvement. Although sinus tract formation is an important characteristic feature, we found that it only occurred in 16% pediatric reports and emphasizes that most pediatric cases do not demonstrate this distinguishing feature. It therefore emphasizes the importance of cultures in maximizing detection if pathognomonic features are not present. Actinomyces can form abscesses and tracts, which made it challenging in our first case to determine whether the tract was from previous incomplete excision of a congenital preauricular sinus tract or novel from the unsuspected actinomyces, however, symptoms only resolved after complete surgical excision and prolonged antibiotics. 3
Treatment with a penicillin-based regimen for up to 6 months is recommended therapy in uncomplicated disease. Treatment may need to be longer if complications, including osteomyelitis, occur. Most cases of actinomycosis in the literature were treated with a penicillin-based therapy, including penicillin G, penicillin V, and amoxicillin. Co-infection with other organisms from skin such as S. aureus as well as oral flora may occur. In such cases, the cultures should guide the need for combination therapy. Both of our cases required beta-lactamase inhibitor combinations to address coinfections.
Surgical debridement, in conjunction with prolonged antibiotic therapy (6-12 months), was the most common management approach used in the literature and is recommended for congenital lesions that have become secondarily infected. Therapy for osteomyelitis was typically longer in duration than for uncomplicated infection. 22 Tetracycline, erythromycin, clindamycin, or third generation cephalosporins can also be used, especially in cases where the patient has a penicillin allergy. 23
Conclusions
Cervicofacial actinomyces is a rare but important infection in children and most often arises in the mandibular region. Anaerobic cultures of exudate facilitate early diagnosis. Given this organism’s propensity to spread to bone and to form sinuses, MR imaging is recommended to ensure that there is no bony involvement. Microbiologic confirmation of this infection allows long-term, targeted, penicillin-based therapy for extended periods up to 6 months or longer if complications occur. These chronic infections are best managed with a combination of penicillin-based antibiotics and surgery.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Karan Gandhi  https://orcid.org/0000-0003-4159-8670
https://orcid.org/0000-0003-4159-8670
M. Elise Graham  https://orcid.org/0000-0001-7159-2953
https://orcid.org/0000-0001-7159-2953
References
- 1. Wong VK, Turmezei TD, Weston VC. Actinomycosis. BMJ Case Rep. 2011;343:d6099-d6099. [DOI] [PubMed] [Google Scholar]
- 2. Kononen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valour F, Senechal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014;7:183-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schaal KP, Lee HJ. Actinomycete infections in humans–a review. Gene. 1992;115(1-2):201-211. [DOI] [PubMed] [Google Scholar]
- 5. Boyanova L, Kolarov R, Mateva L, Markovska R, Mitov I. Actinomycosis: a frequently forgotten disease. Future microbiol. 2015;10(4):613-628. [DOI] [PubMed] [Google Scholar]
- 6. Varghese BT, Sebastian P, Ramachandran K, Pandey M. Actinomycosis of the parotid masquerading as malignant neoplasm. BMC Cancer. 2004;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McHugh KE, Sturgis CD, Procop GW, Rhoads DD. The cytopathology of Actinomyces, Nocardia, and their mimickers. Diagn Cytopathol. 2017;45(12):1105-1115. [DOI] [PubMed] [Google Scholar]
- 8. Sama CB, Mbarga NF, Oben CE, Mbarga JA, Nfor EK, Angwafo FF. Massive paediatric cervicofacial actinomycoses masquerading as an ulcerative malignancy. BMC Infect Dis. 2016;16(1):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sharma S, Hashmi MF, Valentino ID. Actinomycosis. In: StatPearls. StatPearls Publishing; 2020. [Google Scholar]
- 10. Gelada K, Halli R, Mograwala H, Sethi S. Actinomycosis which impersonates malignancy. Ann Maxillofac Surg. 2018;8(2):358-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmed S, Ali M, Adegbite N, Vaidhyanath R, Avery C. Actinomycosis of tongue: rare presentation mimicking malignancy with literature review and imaging features. Radiol Case Rep. 2019;14(2):190-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Acevedo F, Baudrand R, Letelier LM, Gaete P. Actinomycosis: a great pretender. Case reports of unusual presentations and a review of the literature. Int J Infect Dis. 2008;12(4):358-362. [DOI] [PubMed] [Google Scholar]
- 13. Pulverer G, Schutt-Gerowitt H, Schaal KP. Human cervicofacial actinomycoses: microbiological data for 1997 cases. Clin Infect Dis. 2003;37(4):490-497. [DOI] [PubMed] [Google Scholar]
- 14. Kumar Chowdary KVS, Sateesh Chandra N, Karthik Madesh R. Preauricular sinus: a novel approach. Indian J Otolaryngol Head Neck Surg. 2013;65(3):234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scheinfeld NS, Silverberg NB, Weinberg JM, Nozad V. The preauricular sinus: a review of its clinical presentation, treatment, and associations. Pediatr Dermatol. 2004;21:191-196. [DOI] [PubMed] [Google Scholar]
- 16. Dinah F, Adhikari A. Gauze packing of open surgical wounds: empirical or evidence-based practice? Ann R Coll Surg Engl. 2006;88(1):33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kandogan T, Koc M, Vardar E, Selek E, Sezgin O. Sublingual epidermoid cyst: a case report. J Med Case Rep. 2007;1:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dutta M, Saha J, Biswas G, Chattopadhyay S, Sen I, Sinha R. Epidermoid cysts in head and neck: our experiences, with review of literature. Indian J Otolaryngol Head Neck Surg. 2013;65(suppl 1):14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nguyen BN, Malone BN, Sidman JD, Barnett Roby B. Excision of sublingual gland as treatment for ranulas in pediatric patients. Int J Pediatr Otorhinolaryngol. 2017;97:154-156. [DOI] [PubMed] [Google Scholar]
- 20. George MM, Mirza O, Solanki K, Goswamy J, Rothera MP. Serious neonatal airway obstruction with massive congenital sublingual ranula and contralateral occurrence. Ann Med Surg (Lond). 2015;4(2):136-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sezer B, Akdeniz BG, Gunbay S, Hilmioglu-Polat S, Basdemir G. Actinomycosis osteomyelitis of the jaws: report of four cases and a review of the literature. J Dent Sci. 2017;12(3):301-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robinson JL, Vaudry WL, Dobrovolsky W. Actinomycosis presenting as osteomyelitis in the pediatric population. Pediatr Infect Dis J. 2005;24(4):365-369. [DOI] [PubMed] [Google Scholar]
- 23. Lerner PI. Susceptibility of pathogenic actinomycetes to antimicrobial compounds. Antimicrob Agents Chemother. 1974;5(3):302-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drake DP, Holt RJ. Childhood actinomycosis. Report of 3 recent cases. Arch Dis Child. 1976;51(12):979-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roveda SIL. Cervicofacial actinomycosis. Report of two cases involving major salivary glands. Aust Dent J. 1973;18(1):7-9. [DOI] [PubMed] [Google Scholar]
- 26. Walker S, Middelkamp JN, Sclaroff A. Mandibular osteomyelitis caused by Actinomyces israelii. Oral Surg Oral Med Oral Pathol. 1981;51(3):243-244. [DOI] [PubMed] [Google Scholar]
- 27. Badgett JT, Adams G. Mandibular actinomycosis treated with oral clindamycin. Pediatr Infect Dis J. 1987;6:221–3. [PubMed] [Google Scholar]
- 28. Feder HM. Actinomycosis Manifesting as an Acute Painless Lump of the Jaw. Pediatr. 1990;85(5). [PubMed] [Google Scholar]
- 29. Carrau RL, Greenwall LCK, Canaan LCRE, Moore JC. Actinomycosis of the infratemporal fossa. Am J Otolaryngol. 1993;14(1):1-4. [DOI] [PubMed] [Google Scholar]
- 30. Foster SV, Demmler GJ, Hawkins EP, Tillman JP. Pediatric cervicofacial actinomycosis. South Med J. 1993;86(10):1147-1150. [DOI] [PubMed] [Google Scholar]
- 31. Altundal H, Gursoy B, Salih I, Olgaç V. Pediatric cervicofacial actinomycosis: Acase report. J Dent Child (Chicago, Ill). 2000;71(1):87-90. [PubMed] [Google Scholar]
- 32. Schwartz HC, Wilson MC. Cervicofacial actinomycosis following orthognathic surgery: Report of 2 cases. J Oral Maxillofac Surg. 2001;59(4):447-449. [DOI] [PubMed] [Google Scholar]
- 33. Hong IS, Mezghebe HM, Gaiter TE, Lofton J. Actinomycosis of the neck: diagnosis by fine-needle aspiration biopsy. J Natl Med Assoc. 1993;85(2):145-146. [PMC free article] [PubMed] [Google Scholar]
- 34. Sobol SE, Samadi DS, Wetmore RF. Actinomycosis of the temporal bone: A report of a case. Ear, Nose Throat J. 2004;83(5):327-329. [PubMed] [Google Scholar]
- 35. Mehta D, Statham M, Choo D. Actinomycosis of the temporal bone with labyrinthine and facial nerve involvement. Laryngoscope. 2007;117(11):1999-2001. [DOI] [PubMed] [Google Scholar]
- 36. Ciftologan DY, Bayram N, Akalin T, Vardar F. Actinomycosis in differential diagnosis of cervicofacial mass: A case report. Cocuk Enfeksiyon Derg. 2009;3(1):28-30. [Google Scholar]
- 37. Gazzano E, Chanteret C, Duvillard C, Folia M, Romanet P. A case of actinomycosis of the middle ear and a review of the literature. Int J Pediatr Otorhinolaryngol. 2010;5(2):70-73. [Google Scholar]
- 38. Hung PC, Wang HS, Chiu CH, Wong AMC. Cervical spinal cord compression in a child with cervicofacial actinomycosis. Brain Dev. 2014;36(7):634-636. [DOI] [PubMed] [Google Scholar]
- 39. Lezcano C, Simons JP, Colman KL, Cohen MS, Lin PL, Reyes-Mugica M. Actinomycotic mastoiditis complicated by sigmoid sinus thrombosis and labyrinthine fistula. Pediatr and Devel Pathol. 2014;17(6):478-481. [DOI] [PubMed] [Google Scholar]
- 40. Thacker SA, Mary Healy C. Pediatric cervicofacial actinomycosis: An unusual cause of head and neck masses. J Pediatric Infect Dis Soc. 2014;3(2): e15-9. [DOI] [PubMed] [Google Scholar]
- 41. Verma S, Verma GK, Shanker V, et al. Pediatric cervicofacial actinomycosis disclosing an underlying congenital dermoid cyst. J Dent Res. 2014;11(2):281-283. [PMC free article] [PubMed] [Google Scholar]
- 42. Walther K, Bruder E, Goldenberger D, Mayr J, Schaad UB, Ritz N. Actinomyces neuii isolated from a 20-month-old girl with cervical lymphadenitis. J Pediatric Infect Dis Soc. 2015;4(3):e32-e37. [DOI] [PubMed] [Google Scholar]
- 43. Chatterjee RP, Shah N, Kundu S, Mahmud SA, Bhandari S. Cervicofacial actinomycosis mimicking osseous neoplasm: A rare case. Journal of clinical and diagnostic research: JCDR. 2015;9(7):ZD29-ZD31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prajapati S, Yoon DJ, Benitez CL, Buyuk A. Cervical vertebral actinomycosis mimicking malignancy in a paediatric patient. BMJ Case Rep. 2016;2016:2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yanagisawa R, Minami K, Kubota N, Iwade T, Ogiso Y. Asymptomatic subcutaneous cervical mass due to Actinomyces odontolyticus infection in a pyriform sinus fistula. Pediatr Int. 2017;59(8):941-942. [DOI] [PubMed] [Google Scholar]
- 46. Glass GE, Staruch RMT, Bradshaw K, Charles AK, Stotland MA. Pediatric cervicofacial actinomycosis: lessons From a craniofacial unit. J Craniofac Surg. 2019;30(8):2432-2438. [DOI] [PubMed] [Google Scholar]
- 47. Savoca E, Mehra S, Waldman EH. A case of pediatric cervicofacial actinomyces masquerading as malignancy: Case report and review of the literature. Int J Pediatr Otorhinolaryngol. 2019;116:204-208. [DOI] [PubMed] [Google Scholar]