Abstract
Objective:
The objective of this meta-analysis was to summarize evidence on the therapeutic effects of non-invasive brain stimulation (NIBS) on core symptoms of multiple sclerosis (MS). Specifically, findings from studies deploying transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS) protocols were summarized in this review.
Methods:
We systematically searched articles published in four databases, until 31 May 2021, which compared the effects of active tDCS or rTMS with sham intervention in MS patients. We used a random-effects model for this meta-analysis. Meta-regression and subgroup meta-analysis were used to examine the effects of stimulation dose and different stimulation protocols, respectively.
Results:
Twenty-five randomized controlled trials (RCTs) were included in this review, consisting of 19 tDCS and 6 rTMS studies. tDCS led to a significant and immediate reduction of fatigue with a large effect size (Hedges’s g = −0.870, 95% confidence intervals (CI) = [−1.225 to −0.458], number needed to treat (NNT) = 2). Particularly, a subgroup analysis showed that applying tDCS over the left DLPFC and bilateral S1 led to fatigue reductions compared to sham stimulation. Furthermore, tDCS had favorable effects on fatigue in MS patients with low physical disability but not those with high physical disability, and additionally improved cognitive function. Finally, whereas rTMS was observed to reduce muscle spasticity, these NIBS protocols showed no further effect on MS-associated pain and mood symptoms.
Conclusion:
tDCS in MS alleviates fatigue and improves cognitive function whereas rTMS reduces muscle spasticity. More high-quality studies are needed to substantiate the therapeutic effects of different NIBS protocols in MS.
Keywords: multiple sclerosis, repetitive transcranial magnetic stimulation, transcranial direct current stimulation, fatigue, meta-analysis
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory disease of the central nervous system (CNS). 1 The location, number, and size of lesions within the CNS determine the neurological symptoms and the burden of MS. The course of this disease is highly unpredictable and variable between patients. Characteristically, neurological deficits are initially reversible, followed by progressive neurological deterioration over time. 2 Symptoms include fatigue, vision problems, motor deficits, sensory disturbances, pain, spasticity, cognitive deficits, depression, bladder, and bowel dysfunction, all of which can occur in various combinations. 1 Furthermore, symptoms exacerbate each other, leading to accelerated deterioration. In one such case, spasmodic muscle contraction can cause secondary pain. 3 In another case, pain and fatigue can aggravate spasticity, movement problems, and cognitive problems. 3 Concerns about falling due to spasticity, paralysis, or postural instability often result in loss of activity. A decline of mobility function, in turn, deteriorates fitness, gait function, and endurance. 4
Multidisciplinary approaches are recommended to relieve symptoms and decrease the extent of MS exacerbation. Interventions include pharmacotherapy, exercise, and alternative or complementary approaches.5,6 Whereas a milder course for MS has been realized following the 25 years since disease-modifying therapies (MDTs) became available, the incidence of MS has increased. 7 Despite recent and ongoing therapeutic pharmacologic advances in disease-modifying therapies (DMTs), MS remains a progressive disease in most cases, leading to disability and high socioeconomic costs. 8 Consequently, symptomatic therapies become necessary, especially in the later course of the disease. However, depending on the symptom, they considerably differ in their response to therapeutic interventions. A few symptoms like spasticity have a long and successful pharmaceutical history, starting with oral muscle-relaxants, intrathecal baclofen in severe cases, and, more recently, cannabinoids and botulinumtoxin Type-A. Other conditions including fatigue, muscle weakness, and postural instability are more challenging to treat pharmaceutically – instead, physical exercise programs and neuro-rehabilitation techniques are used to address these symptoms. Presupposing patient access, insurance coverage or the financial means to physical exercise programs, the therapeutic success highly varies between patients, depending on many variables such as regularity and patient’s adherence. Therefore, auxiliary methods add therapeutic value. A potential set of methods are non-invasive brain stimulation (NIBS) techniques that include transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS). NIBS recommended as effective treatments for many neurological and chronic diseases include neuropathic pain, Parkinson disease, and fibromyalgia.9,10 These techniques can be applied either as a single therapy or, potentially more promising, in combination with pharmaceutical intervention or physical exercise.
Lefaucheur and colleagues summarized the therapeutic effects of rTMS and tDCS on MS symptoms in their recent guidelines.9,10 Based on three randomized controlled trials (RCTs), a probable efficacy (Level-B evidence) was concluded for intermittent theta-burst stimulation (iTBS), an rTMS protocol, to the leg motor cortex to treat lower-limb spasticity. However, no recommendations were made in the guideline for tDCS on MS due to the considerable variability in protocols and methodological heterogeneity. One recent meta-analysis demonstrated the favorable effects of tDCS on cognitive processing speed, pain, fatigue, and mood. 11 However, the effects of different NIBS protocols on MS need more attention as individual responses are disparate. Investigations into the differential effects and subgroup analyses (i.e. targets and types) of NIBS on MS are currently missing. Hence, the current review and meta-analysis aims to summarize the latest evidence on the therapeutic effects of NIBS on core symptoms of MS and compare the outcomes of different stimulation protocols. The NIBS in this review mainly focuses on tDCS and rTMS due to the limited use of other NIBS to treat and investigate MS. To elaborate, we included RCTs investigating the effects of tDCS and rTMS on symptoms of MS, including fatigue, pain, spasticity, mood, motor function, and cognitive deficits.
Material and methods
Data source and literature search
This review followed Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA). 12 We systematically searched four English bibliographic databases including PubMed, Medline, EMBASE, and Web of Science for articles published until 31 May 2021. The search was performed using the keywords (Multiple sclerosis OR Disseminated sclerosis OR Sclerosis) AND (non-invasive brain stimulation OR NIBS OR Transcranial direct current stimulation OR tDCS OR TMS OR transcranial magnetic stimulation) AND (randomized controlled trial OR randomly OR RCT OR randomized). In addition, reference lists of related published reviews and meta-analyses were screened for additional relevant studies. Three authors (GXJX, KTS, and RLDK) independently identified potential studies by reading study titles and abstracts, with disagreements settled through discussion with a fourth author (GSK).
Inclusion and exclusion criteria
Inclusion criteria were (1) studies defined MS diagnoses according to the standard McDonald’s criteria; (2) studies used a form of tDCS or rTMS intervention; (3) studies needed to include a sham stimulation control group; and (4) studies needed to be classified as an RCT and published in English. Exclusion criteria were studies published as conference abstracts, book chapters and dissertations, or if the stimulation target site was outside the brain.
Quality assessment and data extraction
Three authors (GXJX, KTS, and RLDK) extracted the relevant information and assessed the quality of each study independently after identifying eligible studies. Any disagreement between these authors was resolved by discussion. The Physiotherapy Evidence Database (PEDro) scale was used to assess the quality of included RCTs. A customized form was used for data extraction. Extracted information included the study design, sample size, characteristics of participants, stimulation protocols, time points of assessments, the measurement of outcomes, adverse effects, findings and whether the trial had been pre-registered. Studies were further classified into Class I–III studies as done by Lefaucheur et al. 13 A Class-I study is defined as an RCT with masked outcome assessments in an adequate population (enrolled population’s size consistent with proper sample size estimate). Whereas a Class-II study is an RCT performed with a smaller sample size or an RCT that lacks at least one term listed in Class I, and a Class-III study included all other controlled trials (for details, see Lefaucheur et al. 13 ).
Statistical analysis
Comprehensive meta-analysis version 3.0 for Windows was used for the statistical analyses. For studies with incomplete data, corresponding authors were contacted by email. Standard errors of the mean (SEM) were converted to standard deviations (SD) for studies reporting only SEM using the formula
where n equals sample size. The formula,
was used to combine SD from subscales. Means and standard deviations were estimated according to Luo et al. 14 and Wan et al. 15 for studies that only provided median and interquartile ranges. GetData Graph digitizer 2.26 was used to extract data that were reported only as a graph. 16
Individual study effect estimates
In our review, we define an immediate effect of stimulation treatment as the effect directly after the last stimulation treatment, that is, calculated as the change from baseline to the end of the last treatment. We define short-term durability as the effect observed at a follow-up visit (1–4 weeks after the last stimulation treatment), that is, calculated as the change from baseline to the follow-up visit. Individual effect sizes for immediate effects and short-term durability were estimated using absolute change scores (post-minus pre-stimulation scores) to correct for baseline differences between groups. The standardized mean difference, or Hedges’s g (a variation of Cohen’s d, but accounting for sample size 17 ), and 95% confidence interval (CI) were computed for each trial by comparing patients undergoing active versus sham tDCS or rTMS.
Summary effect estimates
Random-effects meta-analysis was used to account for the clinical and methodological diversity among included trials, both for immediate effects and for short-term durability. Heterogeneity among the included studies was assessed by using Higgins’ I2 statistic.18,19 Meta-regression was used to test the relationship between dose and effect size. Subgroup analysis was used to explore the effects of different tDCS and rTMS protocols (i.e. intensity and sites) on symptom reductions. Sensitivity analysis was performed using the leave-one-out method in cases where results were significant. Publication bias was assessed by visual inspection (of a funnel plot) and Egger’s test in cases where there were more than 10 articles.20,21 For significant meta-analytic results, we calculated the number needed to treat (NNT) using the following formula in MATLAB:
where d equals Hedges’s g. The statistical threshold was set at p < 0.05 and p < 0.1 (two-tailed) for the main tests and the Egger’s test, respectively. 20
Results
Characteristics of included studies
Study selection
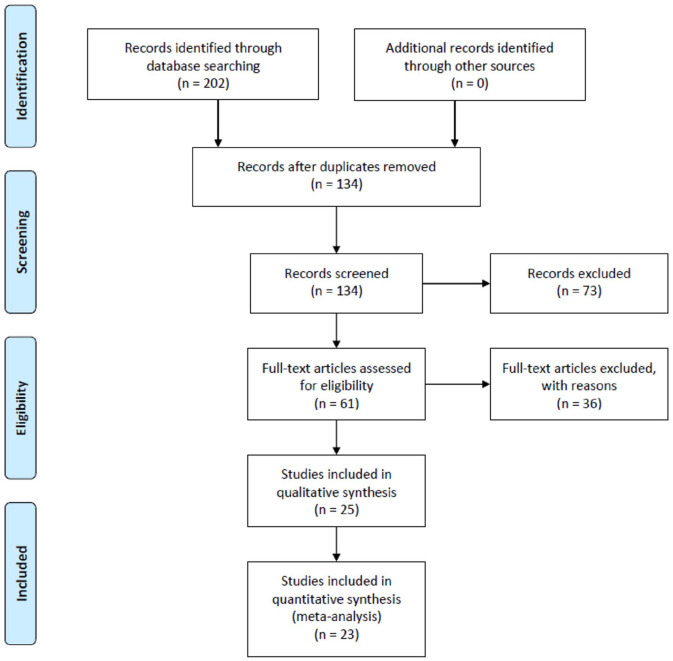
Twenty-five RCTs were identified as suitable for inclusion into the present review, and 23 RCTs were included in the meta-analysis (see Figure 1).22–46 For details, see Supplementary Materials.
Figure 1.
Flow diagram of the search strategy.
Participants
The demographic characteristics of the 25 RCTs included are presented in Table 1. These studies comprised a total of 491 patients. Patients’ diagnoses covered three types of MS including relapsing-remitting MS (RRMS), secondary-progressive MS (SPMS), and primary-progressive MS (PPMS), with six studies including patients exclusively in the relapsing-remitting stage.22,30,31,33,34,45 The mean score of the Expanded Disability Status Scale (EDSS) of included patients ranged from 2.3 to 6.5. Patients in three studies received no pharmacological treatment except for interferon-beta,26,30,40 while patients in five other studies were on stable pharmacological treatment.23,24,37,39,42 The remaining 17 studies did not provide information on pharmacological treatment.
Table 1.
Demographic characteristics (n = 25 studies).
| Author and year | Study design | Intervention | Sample size (M/F) | Age (years) | Participants condition | Clinicaltrials.gov or others (yes/no) | Disease type (RR/PP/SP) | Disease severity (EDSS) | Disease duration (years) |
|---|---|---|---|---|---|---|---|---|---|
| Mori et al. 33 | RCT | tDCS | 19 (8/11) | 44.8 ± 27.5 | RRMS patients | – | 19/0/0 | – | – |
| Ferrucci et al. 26 | RCT, crossover | tDCS | 23 (7/16) | 44.54 ± 6.6 | MS patients, MFIS > 45 EDSS (0, 6.5) |
– | 19/0/4 | 3.28 ± 0.24 | 13.23 ± 3.16 |
| Meesen et al. 29 | Pseudo-RCT, crossover | tDCS | 31 (9/22) | 48.16 ± 10.13 | MS patients, mild to moderate | – | 20/2/9 | 3.15 ± 1.22 | – |
| Saiote et al. 30 | Pseudo-RCT, crossover | tDCS | 13 (3/10) | 46.9 ± 6.8 | MS patients, FSS ⩾ 4 | – | 13/0/0 | 3.5 ± 4.0 | 9.0 ± 5.4 |
| Tecchio et al. 32 | Crossover | tDCS | 10 (3/7) | 45.8 ± 7.6 | MS patients, mild | – | 7/2/1 | 1.5 (0–3.5) | 7.1 ± 8.2 |
| Iodice et al. 45 | RCT | tDCS | 20 (5/15) | 41.8 ± 6.213 | RRMS patients | – | 20/0/0 | 3.7 ± 0.88 | 7.4 ± 2.54 |
| Tecchio et al. 31 | RCT, crossover | tDCS | E1:13 (4/9) E2: 8 (2/6) |
E1:45.8 ± 7.6 E2:38.1 ± 9.8 |
E1: MS patients, MFIS > 15 E2: MS patients, MFIS > 16 |
– | E1: 13/0/0 E2: 8/0/0 |
E1: 1.5 (0–3.5) E2: 2 (1–2.5) |
E1: 7.6 ± 8.2 E2: 13.5 ± 4.2 |
| Mattioli et al. 44 | RCT | tDCS | 20 (4/16) | / | MS patients | – | / | / | / |
| Ayache et al. 39 | RCT, crossover | tDCS | 16 (3/13) | 48.9 ± 10.0 | MS with neuropathic pain | yes | 11/1/4 | 4.25 ± 1.4 | 11.8 ± 9.4 |
| Hanken et al. 28 | RCT | tDCS | 40 (15/25) | 49.25 ± 9.43 | MS with cognitive fatigue | – | 15/0/25 | 4.18 ± 1.55 | 12.19 ± 8.39 |
| Cancelli et al. 22 | RCT, crossover | tDCS | 10 (2/8) | 43.2 ± 13.1 | MS with MFIS > 35 BDI ⩽ 19 |
– | 10/0/0 | 0.9 (0–3.5) | 6.6 ± 3.7 |
| Chalah et al. 24 | RCT, crossover | tDCS | 10 (6/4) | 40.50 ± 11.18 | MS with FSS > 5 BDI ⩽ 19 |
– | 9/0/1 | 2.3 ± 2.5 | 14 ± 9.9 |
| Charvet et al. 25 | RCT | tDCS | 27 (11/16) | 44.18 ± 15.90 | MS patients | – | 13/14 | E:6.0 (0.0–7.0) C:3.5 (0.0–8.5) |
14.69 ± 10.16 |
| Fiene et al. 40 | RCT, crossover | tDCS | 15 (7/8) | 43.20 ± 14.97 | MS with WEIMuS ⩾ 9 BDI ⩽ 19 |
– | 14/0/1 | 3.54 ± 1.94 | 9.63 ± 8.57 |
| Oveisgharan et al. 43 | RCT | tDCS | 13 (3/10) | 38.9 ± 12.3 | MS patients | – | 3/10/0 | 3.8 ± 1.2 | 11.8 ± 8.9 |
| Pilloni et al. 41 | RCT | tDCS | 15 (4/11) | 52.7 ± 11.3 | MS patients, 1.0 < EDSS < 6.5 | yes | 5/0/10 | 4.98 ± 1.37 | – |
| Young et al. 42 | RCT | tDCS | 30 (15/15) | 50.54 ± 11.07 | MS patients | – | 16/3/11 | – | – |
| Mortezanejad et al. 38 | RCT | tDCS | 36 (6/30) | – | MS patients, FSS > 4 EDSS < 4 |
yes | – | – | – |
| Chalah et al. 23 | RCT, crossover | tDCS | 11 (3/8) | 43.91 ± 9.69 | MS patients | – | 10/0/1 | <6.5 | 6.30 ± 3.83 |
| Mori et al. 34 | RCT | rTMS | 20 (13/7) | 38.4 ± 11.24 | RRMS patients | – | 20/0/0 | 3.7 ± 1.38 | – |
| Boutiere et al. 35 | RCT | iTBS | 17 (9/8) | 51.59 ± 10.57 | MS patients, 4 ⩽ EDSS ⩽ 7 | – | 4/0/13 | – | 15.26 ± 9.89 |
| Gaede et al. 27 | RCT | dTMS | 28 (6/22) | – | MS patients, FSS > 4 0 < EDSS < 6 |
yes | 26/0/2 | – | – |
| Korzhova et al. 36 | RCT | E1: rTMS E2: iTBS |
34 (14/20) | – | MS patients | – | 0/0/34 | 6.5 | 4–20 |
| Şan et al. 37 | RCT | rTMS | 16 (8/8) | 49.93 ± 12.27 | MS patients | – | – | – | 16.5 ± 9.01 |
| Tramontano et al. 46 | RCT | iTBS | 16 (11/5) | 51.75 ± 7.9 | MS patients, 4.5 < EDSS < 6.5 | – | 0/7/9 | 5.75 ± 0.89 | 18.44 ± 8.66 |
BDI, Beck Depression Inventory; EDSS, Expanded Disability Status Scale; FSS, Fatigue Severity Scale; MFIS, Modified Fatigue Impact Scale; MS, multiple sclerosis; RCT, randomized controlled trials; RRMS, relapsing-remitting multiple sclerosis; WEIMuS, Würzburger Fatigue Inventory for MS.
Stimulation parameters
Table 2 depicts the stimulation parameters of included studies regarding fatigue symptoms. For studies that focused on other symptoms, please see Supplemental Table S1. All 19 RCTs deploying tDCS used excitatory (anodal) stimulation with eight studies targeting the left dorsolateral prefrontal cortex (DLPFC),23–25,30,38–40,44 three studies targeting bilateral primary somatosensory cortex (S1)22,31,32 and four studies targeting primary motor cortex (M1).26,29,38,41 The cathode was placed supraorbitally or over the contralateral hemisphere on the homologous brain region in most studies, except for two studies that used a monocephalic montage and placed the cathode on the contralateral shoulder and under the chin, respectively.31,40 For studies that used anodal stimulation of bilateral S1, the cathode was positioned over the occipital cortex on electrode position Oz. The duration of each session ranged from 15 to 30 minutes, while the number of sessions ranged from 1 to 20, and the intensity of stimulation ranged from 1.0 to 2.5 mA. Table 3 depicts the stimulation characteristics of the seven RCTs utilizing rTMS. All RCTs used excitatory stimulation (frequency > 5 Hz). Treatment intensity ranged from 80% to 110% MT, and the number of intervention sessions ranged from 10 to 18. In nine studies, subjects received additional exercise or cognitive training in the experimental and control group.29,34–37,40,41,44,46
Table 2.
Treatment parameters of tDCS interventions for fatigue symptoms (n = 14).
| Author and year | Intervention site | Intensity (mA) | Duration | Intervention | Control | Outcome measure | Assessment timepoint | Side affect | Findings |
|---|---|---|---|---|---|---|---|---|---|
| Ferrucci et al. 26 | C3, C4 | 1.5 | 15 min/session, 5 sessions |
Active tDCS | Sham tDCS | FIS | Before, after, 1w after, 3w after | Two had skin reaction | A-tDCS applied at M1 improves fatigue when compared to sham stimulation |
| Meesen et al. 29 | C3, C4 | 1 | 20 min/session, 1 session |
Active tDCS + motor training | Sham tDCS + motor training | VAS-fatigue | Before, during, 30 minutes after | / | A single session a-tDCS does not relieve fatigue when compared to sham stimulation |
| Saiote et al. 30 | Left DLPFC | 1 | 20 min/session, 5 sessions |
Active tDCS | Sham tDCS | FSS, MSFSS, MFIS | Day 0, day 5, 8, 10, 15, 30 | / | A-tDCS of the DLPFC does not relieve fatigue when compared to sham stimulation |
| Tecchio et al. 32 | Bilateral S1 | 1.5 | 5 sessions | Active tDCS | Sham tDCS | MFIS | Before, after, 4w after, 8w after | / | A-tDCS of bilateral somatosensory areas improves fatigue when compared to sham stimulation |
| Tecchio et al. 31 | E1: Bilateral S1 E2: SM1 |
1.5 | 5 sessions | Active tDCS | Sham tDCS | MFIS | Before, after | No | A-tDCS of bilateral somatosensory areas improves fatigue when compared to sham stimulation |
| Ayache et al. 39 | Left DLPFC | 2 | 3 sessions | Active tDCS | Sham tDCS | MFIS, BPI, VAS | Before, after | tDCS group: 3 headaches. sham group: 1 headache, 1 Phosphenes |
A-tDCS significantly relieves pain; no effects on mood, fatigue, or attention when compared to sham stimulation. |
| Hanken et al. 28 | Right PC | 1.5 | 20 min/session, 1 session |
ActivetDCS | Sham tDCS | VAS-fatigue | Before, after | / | A-tDCS of the right parietal cortex shows positive effects on fatigue-associated performance but does not relieve fatigue when compared to sham stimulation |
| Chalah et al. 24 | E1: Left DLPFC E2: Right PPC |
2 | 20 min/session, 5 sessions |
Active tDCS | Sham tDCS | FSS, MFIS VAS-fatigue |
Before, after | Active right PPC: Three insomnia, 2 headaches |
A-tDCS of left DLPFC stimulation significantly ameliorates fatigue when compared to sham stimulation; a-tDCS of the right PPC improves anxiety and depression when compared to sham stimulation; no effect of any intervention on attention |
| Charvet et al. 25 | left DLPFC | 2 | 20 min/session, 20 sessions |
Active tDCS | Sham tDCS | PROMIS-FS | Before, after | Two pain patients | A-tDCS significantly reduces fatigue when compared to sham stimulation, longer treatment periods and higher stimulation intensity are of greater benefit |
| Cancelli et al. 22 | Bilateral S1 | 1.5 | 15 min/session, 5 sessions |
Active tDCS | Sham tDCS | MFIS | Before, after | Weariness, tingling | A-tDCS leads to a significant reduction in fatigue when compared to sham stimulation |
| Fiene et al. 40 | left DLPFC | 1.5 | 27.29 ± 1.15 30 min/session, one session |
Active tDCS + reaction time task | Sham tDCS + reaction time task | VAS-fatigue | Before, after | / | A-tDCS of the left DLPFC reduces fatigue when compared to sham stimulation |
| Chalah et al. 23 | left DLPFC | 2 | 20 min/session, 5 sessions |
Active tDCS | Sham tDCS | FSS, MFIS | Before, after, 1w after | no | A-tDCS shows effects on fatigue and anxiety but not depression when compared to sham stimulation |
| Mortezanejad et al. 38 | E1: DLPFC E2: M1 |
1.5 | 20 min/session, 6 sessions | Active tDCS | Sham tDCS | FSS | Before, after, 1 month after | Tingling, itching | Both M1 and DLPFC a-tDCS show effects on fatigue when compared to sham stimulation and effects of DLPFC a-tDCS. last up to four weeks. |
| Pilloni et al. 41 | C3 | 2.5 | 20 min/session, 10 sessions | Active tDCS + physical training | Sham tDCS + physical training | MFIS-21 | Before, after, 1 month after | / | A-tDCS has immediate and long-lasting effects on walking and endurance when compared to sham stimulation |
BPI, Brief Pain Inventory; DLPFC, dorsolateral prefrontal cortex; FIS, Fatigue Impact Scale; FSS, Fatigue Severity Scale; MFIS, Modified Fatigue Impact Scale; MSFSS, the MS-specific FSS; PC, parietal cortex; PPC, posterior parietal cortex; PROMIS-FS, the Patient-Reported Outcomes Measurement Information System-fatigue short form; SM1, hand sensorimotor areas; VAS, Visual Analog Scale.
Table 3.
Treatment parameters of rTMS interventions on fatigue symptoms (n = 4).
| Author and year | Brain target | Type of coil | Intensity (RMT) | Frequency (HZ) | Total pulses per session | Number of sessions | Experiment group | Control group | Assessment of time points | Outcome measures | Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mori et al. 34 | Leg area of M1 | / | 80% of AMT | 5/50 | / | 10 | Active iTBS + exercise | Sham iTBS + exercise | Before, after | MAS, MSSS-88, FSS |
iTBS significantly reduces spasticity and fatigue compared to sham stimulation |
| Korzhova et al. 36 | Both M1 | Figure of eight | 80% | E1: 20 E2: 5-35 |
E1: 1600 E2: 1200 |
10 | E1: active HF-rTMS + physical therapy E2: active iTBS |
E1: sham HF-rTMS + physical therapy E2: sham iTBS |
Before, after, 2w after, 12w after | MAS, NAS, SESS; MFIS; |
Both HF-rTMS and iTBS reduce spasticity, but only iTBS has long-term effects on spasticity compared to sham stimulation HF-rTMS also relieves pain and fatigue. compared to sham stimulation |
| Gaede et al. 27 | E1: LPFC E2: M1 |
E1: H6 E2: H10 |
E1: 120% of RMT E2: 90% of RMT |
E1: 18 E2: 5 |
E1: 1800 E2: 800 |
18 | Active rTMS | sham rTMS | Week1-6, 8, 10, 12 | FSS | rTMS of M1 significantly reduces fatigue compared to sham stimulation |
| Tramontano et al. 46 | Active: left cerebellum Sham: right cerebellum | Figure of eight | / | / | / | 10 | Active iTBS + vestibular rehabilitation | Sham iTBS + vestibular rehabilitation | Before, after | FSS | C-iTBS shows significant improvement of lower limb function compared to sham stimulation |
2MWT, Two-minute walking test; 5STS, Five repetition sit to stand test; AP, average power; A-tDCS, anodal tDCS; AMT, active motor threshold; BBS, Berg Balance Scale; BI, the Barthel Index; BPI, brief Pain Inventory; C-iTBS, cerebellar-iTBS; C3/C4, motor cortex; FDI, First dorsal interosseous; FSS, Fatigue Severity Scale; iTBS, intermittent transcranial magnetic theta burst stimulation; LPFC, lateral prefrontal cortex; MAS, modified Ashworth scale; MFIS, Modified Fatigue Impact Scale; MSSS-88, the 88 items Multiple Sclerosis Spasticity Score questionnaire; MSWS-12, Multiple sclerosis walking scale-12; NAS, numerical analog scale; NPS, Neuropathic Pain Scale; PLAS, pain level associated with spasticity; PT, peak torque; PT/BW, peak torque/ body weight; RDSS, the Rekand disability and spasticity score; RMT, rest motor threshold; S1, whole body somatosensory areas; SDMT, Symbol digit modalities test; SESS, the Subjective Evaluating Spasticity Scale; SPART, Spatial recall test; SRT, Select reminding test; T25-FW, Timed 25-foot walk; TBG, Tinetti Balance and Gait Scale; TPT, time to peak torque; TW, total work; WCST, Wisconsin card test; .
Quality assessment of included studies
The results of the quality assessment (PEDro) for the 25 RCTs can be found in the Supplementary Materials (Table S2). In short, four studies had a score of 9 on the PEDro scale, 11 studies had a score of 8, 8 studies had a score of 7, and 2 studies had a score of 6. Studies were designed as double-blind, except for four studies, for which patients were blinded but assessors were aware of the stimulation group.25,37,40,42 With regards to Lefaucheur et al’.s criteria, 13 only one study could be classified as Class I, 36 whereas 24 studies were classified as Class II, and no study was classified as Class III.
Meta-analysis results for fatigue symptoms
Immediate effects
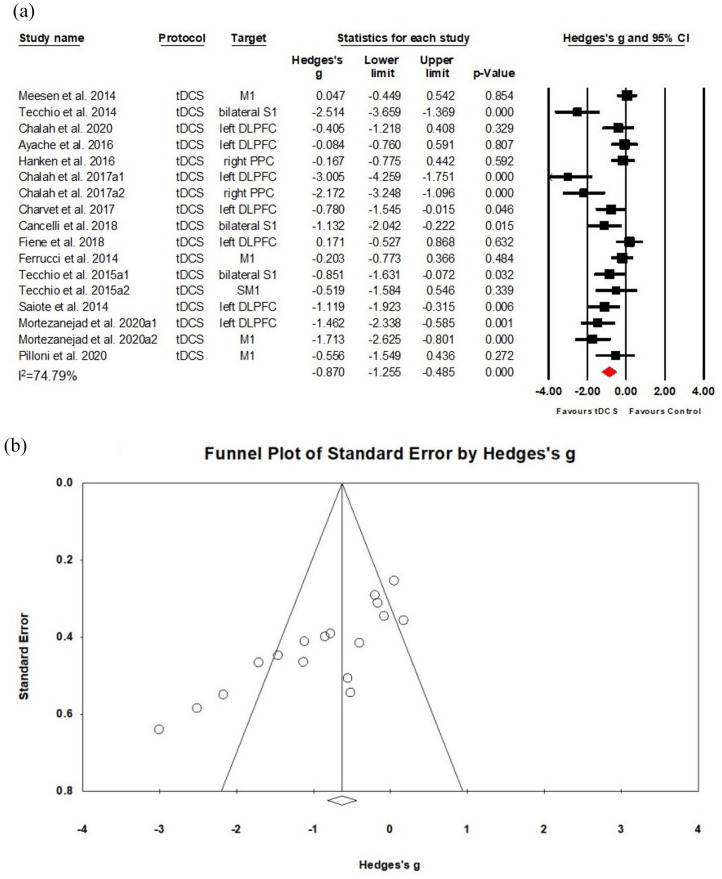
A total of 18 studies were included in this meta-analysis, with four RCTs involving two separate subgroups.24,31,36,38 Thus, 22 data sets, including 17 tDCS data sets (Class II) and 5 rTMS data sets (Class I–II) with a total of 376 patients were subjected to meta-analysis. The analysis of 17 tDCS studies (278 patients) revealed a positive effect on fatigue with a large effect size (Hedges’s g = −0.870, (95%CI: −1.255, −0.485), NNT = 2), despite substantial heterogeneity between individual study estimates (I2 = 74.79% for tDCS; see Figure 2(a)). Although this result was robust to leave-one-out sensitivity analysis, the Funnel plot indicated the possibility of a publication bias (see Figure 2(b)). Moreover, meta-regression indicated no significant effect of stimulation dose as defined tapped by the number of sessions. Performing the meta-analysis on the five rTMS data sets (98 patients) revealed no significant difference between active and sham stimulation (Hedges’s g = −0.336, (95% CI: −0.720, 0.047), p = 0.086); see Supplementary Figure S.1a).
Figure 2.
Effects of tDCS on fatigue in MS: (a) a forest plot showing studies that compare anodal tDCS with sham stimulation for fatigue symptoms on MS. (b) Examination of bias. The figure depicts an asymmetric funnel plot (p < 0.001). Possible sources of asymmetry are publication bias, poor methodological quality, true heterogeneity, and chance.
Subgroup analysis
Subgroup analysis of tDCS on fatigue aimed at the differential effects of stimulation targets (bilateral S1, left DLPFC, M1), stimulation intensities (1.5 mA, 2 mA), and MS subtypes. In addition, in order to investigate the differential therapeutic efficacies of NIBS on different stages of MS, we divided the included studies into low physical disability (L-PD) and high physical disability (H-PD) groups based on the baseline EDSS score. The cut-off value was 3.5, with subgroups defined as L-PD (EDSS ⩽ 3.5) and H-PD (EDSS > 3.5). 47 Moreover, the short-term durability of tDCS on fatigue in MS was also investigated (please refer to Supplementary 06 and Supplementary Table S.1b).
Targets
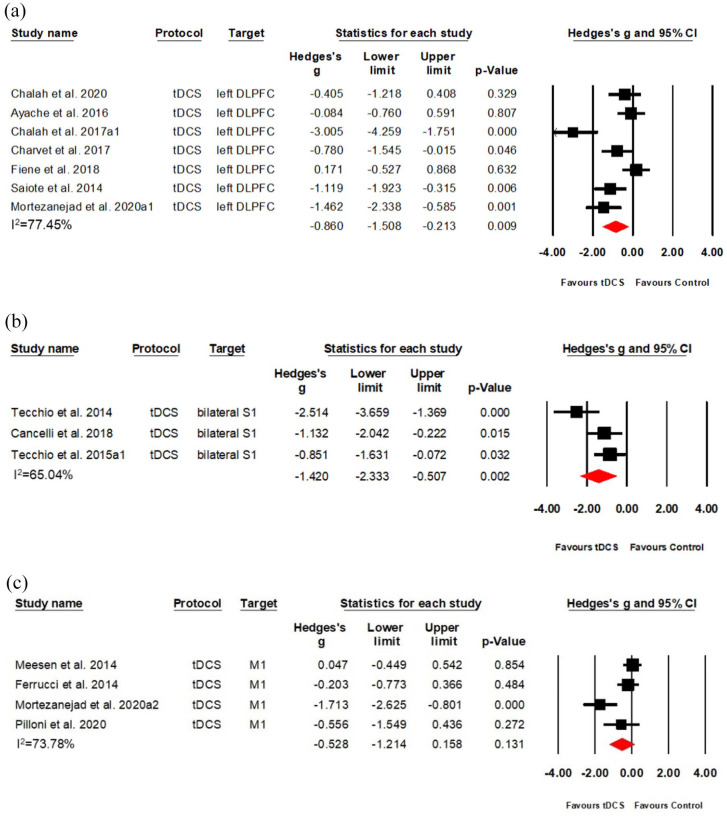
Among 17 tDCS studies (20 data sets), seven RCTs (Class II) targeted the left DLPFC,23–25,30,38–40 whereas four RCTs (Class II) targeted M126,29,38,41 and three RCTs (Class II) targeted bilateral S1.22,31,32 Left DLPFC stimulation produced a large effect size (Hedges’s g = −0.860, (95%CI: −1.508, −0.213), NNT = 2) but also substantial heterogeneity among the included studies (I2 = 77.45%, see Figure 3(a)). Similarly, bilateral S1 stimulation showed a large effect size (Hedges’s g = −1.420, (95% CI: −2.333, −0.507), NNT = 1) and substantial heterogeneity (I2 = 65.039%, see Figure 3(b)), whereas M1 stimulation indicated no significant effects (Hedges’s g = −0.528, (95% CI: −1.214, 0.158), heterogeneity: I2 = 73.78%) (see Figure 3(c)).
Figure 3.
Effects of tDCS on fatigue in MS, separated for different stimulation targets: (a) A forest plot showing studies comparing anodal tDCS of the left DLPFC with sham stimulation. (b) A forest plot showing studies comparing anodal tDCS of bilateral S1 with sham stimulation. (c) A forest plot showing studies comparing anodal tDCS of M1 with sham stimulation.
Intensity
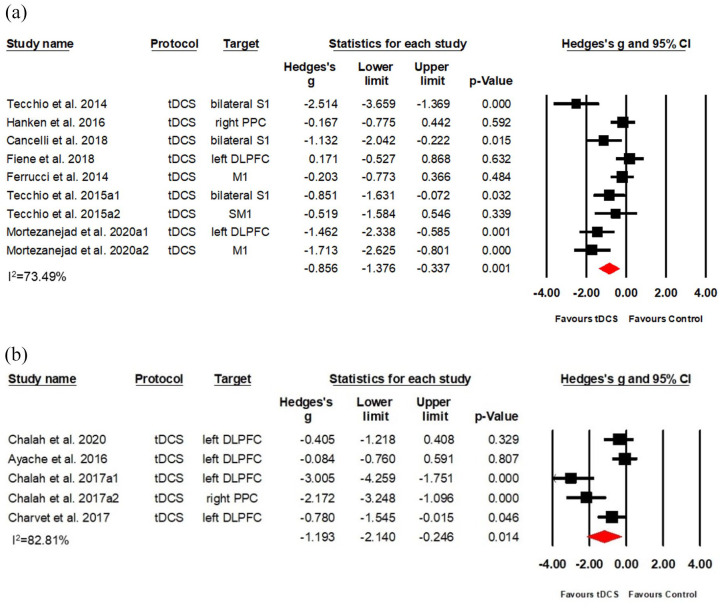
A total of nine data sets among seven RCTs (Class II) applied a current of 1.5 mA.22,26,28,31,32,38,40 Results indicated a large effect size (Hedges’s g = −0.856, (95%CI: −1.376, −0.337), NNT = 2) despite substantial heterogeneity (I2 = 70.49%; see Figure 4(a)). Five data sets among four RCTs (Class II) applied a current of 2 mA23–25,39 and revealed a large effect size (Hedges’s g = −1.193, (95%CI: −2.140, −0.246), NNT = 2) despite substantial heterogeneity (I2 = 82.81%; see Figure 4(b)).
Figure 4.
Effects of different intensities of anodal tDCS on MS-associated fatigue: (a) a forest plot showing studies comparing 1.5 mA anodal tDCS with sham stimulation and (b) a forest plot showing studies comparing 2.0 mA anodal tDCS with sham stimulation.
RR-MS patients
Four data sets (three RCTs, Class II) only included RRMS subtype patients.30,37,46 Other studies included all MS subtypes or did not mention the MS type of their participants. When only including studies with the RR subtype in the meta-analysis, which was the most frequently diagnosed subtype of MS, we found a large effect size (Hedges’s g = −0.938, (95%CI: −1.373, −0.503), NNT = 2) without heterogeneity (I2 = 0%) (see Supplementary Figure S.1c). Results were robust to leave-one-out sensitivity analysis.
Baseline EDSS score
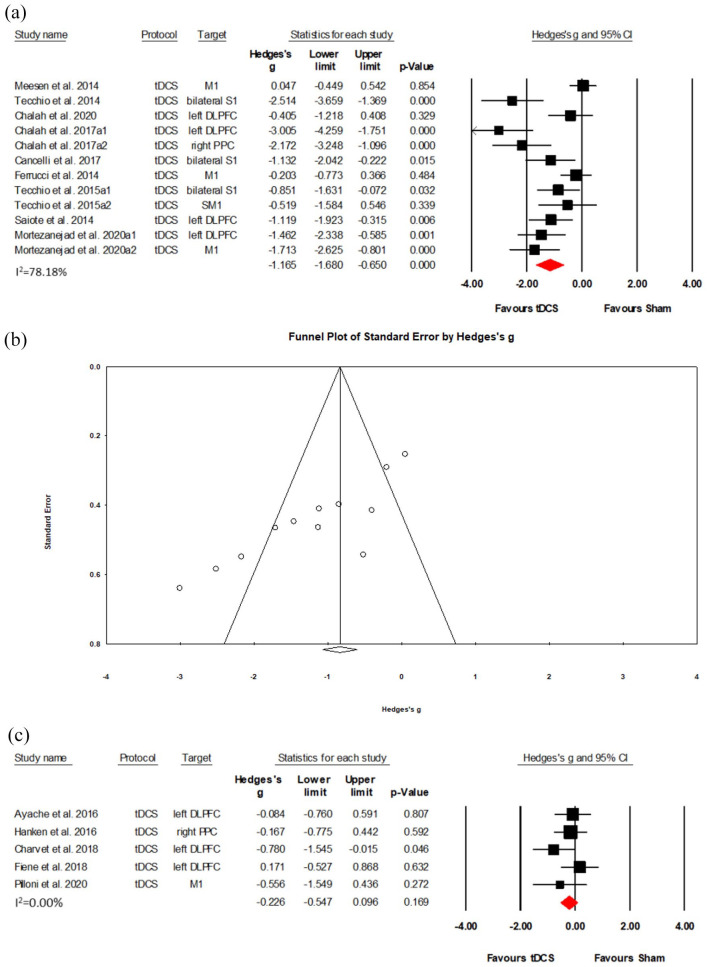
Nine RCTs (12 data sets) in total with 165 participants were in the L-PD group.22–24,26,29–32,38 Results show a large effect size (Hedges’s g = −1.165, (95% CI: −1.680, −0.650), NNT = 2) despite substantial heterogeneity (I2 = 78.18%; see Figure 5(a)). This result was robust to leave-one-out sensitivity analysis, although the Funnel plot indicated the possibility of a publication bias (see Figure 5(b)). Meta-analysis of five RCTs that were in the H-PD group with 113 participants25,28,39–41 revealed no significant results (Hedges’s g = −0.226, (95%CI: −0.547, 0.096), p = 0.169) (see Figure 5(c)).
Figure 5.
Effects of tDCS on fatigue in low physical disability and high physical disability MS: (a) a forest plot showing studies that compare anodal tDCS with sham stimulation for fatigue symptoms on low physical disability MS. (b) Examination of bias. The figure depicts an asymmetric funnel plot (p < 0.001). Possible sources of asymmetry are publication bias, poor methodological quality, true heterogeneity and chance. (c) A forest plot showing studies that compare anodal tDCS with sham stimulation for fatigue symptoms on high physical disability MS.
Meta-analysis results for pain symptoms
Immediate effects
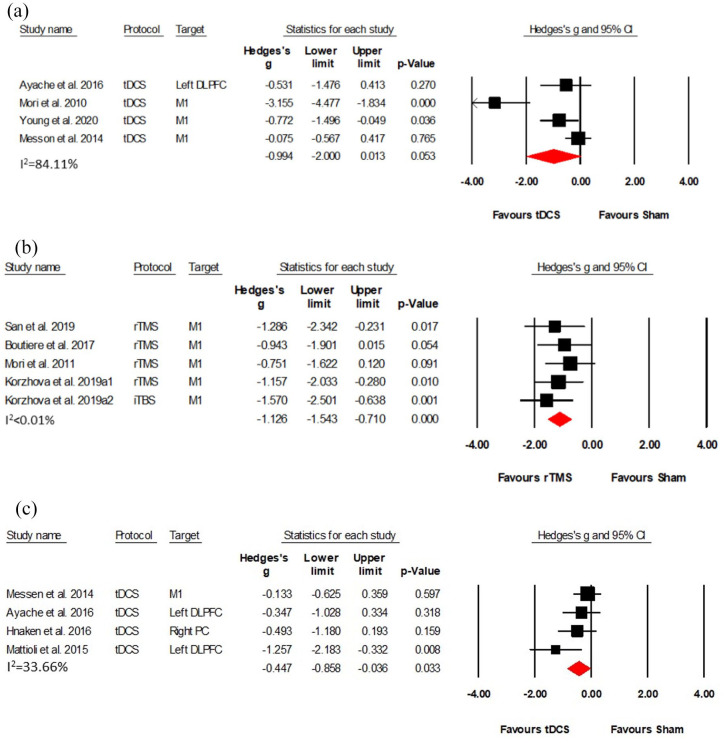
Five RCTs (Class I–II, four tDCS studies, one rTMS study) with 130 patients investigated the effects of NIBS on pain symptoms.29,33,36,39,42 Results of the four tDCS (Class II) studies with 96 patients showed no significant effect, albeit a clear trend (Hedges’s g = −0.994, (95%CI: −2.000, 0.013), p = 0.053) (see Figure 6(a)). The only rTMS study (Class I), with 34 patients, indicated that high-frequency rTMS significantly improved MS pain, with effects lasting over 2 weeks posttreatment. 36 However, no significant effect was found in the iTBS group. 36
Figure 6.
Effects of NIBS on pain, spasticity and cognitive function in MS: (a) a forest plot showing studies comparing effects of NIBS with sham stimulation on pain in MS, (b) a forest plot showing studies comparing NIBS with sham NIBS stimulation for spasticity on MS, and (c) a forest plot showing studies comparing NIBS with sham NIBS stimulation for cognitive function on MS.
Meta-analysis results for spasticity symptoms
Immediate effects
Five studies (Class I–II) assessed spasticity in MS patients, with one using tDCS as intervention and four using rTMS.34–37,45 Performing the meta-analysis on the five rTMS (Class I–II) data sets with a total of 87 patients revealed a large effect size (Hedges’s g = −1.126, (95% CI: −1.543, −0.710), NNT = 2); (see Figure 6(b)). However, the result of the tDCS study showed that five sessions of stimulation did not improve the lower spasticity limb in MS. 45 Only one of these studies (Class I) investigated the short-term durability of NIBS effects on spasticity, revealing that both HF-rTMS and iTBS significantly reduced spasticity. Notably, these effects lasted 2 weeks after HF-rTMS, while effects of iTBS lasted for 12 weeks. 36
Review results for motor function
Immediate effects
Six studies investigated the effects of NIBS on motor function, including lower limb function (i.e. walking; 3 tDCS and 1 rTMS)41,43,45,46 and fine hand motor function (2 tDCS studies).29,31 No meta-analysis was conducted on these studies due to the low count and variety of stimulation protocols and outcomes. In terms of the effects of tDCS on lower-limb function, patient walking speed increased significantly when the target site was M1,41,43 but no significant change on the MS Walking Scale (MSWS-12) was observed.43,45 Only one rTMS study showed a positive effect on lower limb function after stimulation was applied to the cerebullaem. 46 As for two studies that investigated the effects of tDCS on fine hand motor function, the first study observed no significant differences between active and sham tDCS when the target site was M1, 43 whereas the second study found positive effects of tDCS on the fine motor function when bilateral S1 was stimulated. 31 Only one study investigated the short-term durability of effects, revealing improved of lower limb function that lasted for 4 weeks after active tDCS stimulation. 41
Review and meta-analysis results for depression and anxiety
Immediate effects
Five Class II studies investigated depression and anxiety symptoms. Meta-analysis including four tDCS studies (five data sets totaling 75 patients)24,33,39,42 showed no effects of active tDCS on depression when compared to sham stimulation (Hedges’s g = −0.814, (95% CI: −1.737, 0.109)); (see Supplementary Figure S.2a).24,33,39,42 Three of these studies (four data sets including 56 patients) also investigated anxiety symptoms.24,39,42 As with the depression set of studies, meta-analysis indicated no significant effects of active tDCS when compared with sham stimulation (Hedges’s g = −0.954, (95% CI: −2.030, 0.122)); (see Supplementary Figure S2b). One study without valid data for quantitative analysis was not subjected to meta-analysis, 23 but suggested an anxiolytic effect of tDCS 1 week poststimulation and no immediate effect. However, the study did not report a comparison between active and sham stimulation groups.
Meta-analysis results for cognitive function
Immediate effects
Four Class II studies with 107 patients investigated the effects of tDCS on attention (three studies) or executive function (one study).28,29,39,44 Meta-analysis revealed a positive effect of active tDCS on attention and executive function with a medium effect size compared to sham stimulation (Hedges’s g = −0.447, (95% CI: −0.858, −0.036), NNT = 4); (see Figure 6(c)).
Discussion
Patients with MS show multiple neurological dysfunctions or disease-related complications due to chronic inflammatory demyelinating of the CNS and focal or diffuse neurodegeneration. Both rTMS and tDCS are presumed to modify neural and cortical activity, and may further elicit changes to non-neuronal tissues (i.e. glial cells) in the brain as all tissues and cells in the CNS are sensitive to electromagnetic fields.9,10 As such, these NIBS devices are promising therapeutic options for MS in regards to the change in neuronal excitability and influence on inflammation.
In this review with 25 RCTs including 491 patients, we investigated the effects of NIBS on core symptoms of MS. First, we found that anodal tDCS but not rTMS can significantly relieve fatigue compared to sham stimulation. The effect was observed immediately after the end of treatment and remained significant during a follow-up visit 1–4 weeks posttreatment in a subset of studies. Second, our analysis further revealed a spasticity relieving effect of rTMS and cognitive improvement upon tDCS. Third, studies indicated no effect of tDCS on pain and mood. Finally, no conclusion could be made about the effects of NIBS on motor function because of the variety of the relevant studies.
With regards to the stimulation parameters and type, significantly positive effects of anodal tDCS on fatigue were found when bilateral S1 and left DLPFC were stimulated but not M1. Notably, both 1.5 mA and 2 mA tDCS current intensities produced improvements. Patients with RRMS seemed to benefit from tDCS treatment, whereas no conclusions could be made about tDCS effects on other subtypes of MS due to a lack of data. tDCS showed favorable effects on fatigue for low compared to high physical disability patients.
When interpreting these results, it is important to note that fatigue in MS patients may be the result of multiple factors related to structural or functional brain network impairments. For instance, impaired functional connectivity between the striatum and sensorimotor cortex has been linked to fatigue in MS, which may be restored by NIBS.48,49 Another study has shown that anodal tDCS can enhance axonal conduction through a subthreshold polarizing effect, mitigating MS fatigue. 49 In addition, while fatigue symptoms of MS have been associated with inflammatory cytokines and synaptic changes, tDCS has been observed to promote beneficial synaptic changes and counteract the harmful effects of MS on neurotransmission, resulting in reduced fatigue. 49 However, to what extent tDCS can relieve the motor or cognitive components of fatigue remains to be investigated.
Anodal tDCS targeted at bilateral S1 and left DLPFC showed a large effect size in our review, whereas stimulation of M1 produced no effects. Previous studies indicated that increased connectivity between DLPFC and sensory cortical regions may contribute to the pathophysiology of MS-related fatigue. 48 Hence, the large positive effect of anodal tDCS over bilateral S1 and left DLPFC observed in our analysis may be associated with normalization of S1 and DLPFC connectivity. In addition, Jaegar et al. 48 suggested a bi-directional relationship between fatigue and mood characteristics, with several lines of evidence indicating that tDCS of the left DLPFC has positive effects on depression and anxiety.50–52 Hence, we hypothesize that changes in fatigue after stimulation are related to the positive effects of left DLPFC stimulation on mood symptoms. However, our meta-analysis indicated no effects of NIBS on mood and anxiety symptoms in MS, which speaks against our view.
As for the intensity of tDCS, our review suggested that both 2 mA (Hedges’s g = 1.193) and 1.5 mA (Hedges’s g = 0.856) significantly improved fatigue symptoms. There are inconsistencies among electrophysiological studies, such as one study finding stronger cortical excitability with higher current intensities, 53 while another study did not find such a relationship. 54 We conclude that a dose-response relationship between tDCS intensity and treatment efficacy remains an open question, with preliminary evidence indicating both 1.5 mA and 2.0 mA intensities show promising effects on fatigue relief.
Our preliminary analysis on tDCS in RRMS (four data sets) indicated a similar effect size compared to the overall effect size that included all studies, yet, no conclusions can be drawn for other types of MS given a lack of data and future studies need to further explore a possible differential effect of tDCS on different MS types.
One study showed that both cortical demyelinating lesions and inflammation were frequent in early-stage MS, 55 which may theoretically be modulated by tDCS. 9 The progression of MS in this review is determined by baseline EDSS scores, and our study indicated that applying tDCS to MS in early stages lead to promising results on fatigue.
Our meta-analysis revealed that tDCS did not ameliorate chronic neuropathic pain, contradicting a recent review’s claim that tDCS in MS may have positive effects on pain 56 – though it should be noted that their conclusion was based on one study. In healthy subjects, tDCS applied over M1 or DLPFC alleviated pain and increased pain thresholds.57,58 Incidentally, rTMS has also been shown to have positive effects on chronic pain. 59 More high-quality studies are necessary to reach a conclusion.
Our meta-analysis further revealed that rTMS can significantly improve MS-associated spasticity. Our proposed mechanism for this antispastic effect is that MS spasticity may be due to excessive excitation of the neural stretch reflex caused by lesions of the corticospinal tract, so the application of rTMS may improve corticospinal tract excitability and thus reduce the activation of the stretch reflex.60,61 Indeed, the antispastic effect of rTMS has also been shown in patients with spinal cord injuries, cerebral palsy, and stroke.62,63 However, the longevity of the antispastic effect of rTMS observed in our meta-analysis after treatment is unknown and subject to future research.
No conclusion was reached on the effects of NIBS on motor function. Studies indicated that multiple sessions of anodal tDCS stimulation of M1 had a cumulative effect on motor function,29,41 but further investigation is needed.
Finally, our meta-analysis indicated a positive short-term effect of tDCS on cognitive function. But more well-powered studies are needed to substantiate the therapeutic effects of NIBS on cognitive function in MS.
Limitations
Our meta-analysis has multiple limitations: (1) several included studies had a cross-over design but did not mention details about the length of the washout period, so potential carry-over effects in these studies may affect the overall result of our meta-analysis; (2) the meta-analysis results were based on self- and observer-rating outcome measures, with self-rating scales being especially susceptible to subjective factors that may bias these results; (3) results indicated a potential publication bias and so should be interpreted with caution; (4) medication effects likely confound NIBS effects; (5) most patients in the included studies belonged to the RRMS subtype, so our findings may not generalize to other forms of MS; (6) our review and meta-analysis include several low-quality studies with small sample sizes and heterogeneous study designs, therefore, future reviews should include higher-quality studies with sufficient sample sizes to substantiate the therapeutic effects of NIBS in MS; (7) finally, our study was not registered on PROSPERO and a protocol for this study was not prepared.
Conclusion
In conclusion, this systematic review and meta-analysis showed that anodal tDCS of bilateral S1 or left DLPFC can significantly reduce fatigue in patients with MS. Importantly, tDCS relieves fatigue in low physical disability but not high physical disability MS. Furthermore, rTMS relieves MS-associated spasticity and tDCS improves MS-associated cognitive function, but tDCS has no effects on MS-associated mood and pain. No conclusion can be made regarding motor function effects of NIBS due to the variety of protocols. Open questions pertain to the longevity of effects, the differential outcomes following different stimulation intensities and whether specific subtypes of MS benefit more from stimulation treatments than others.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223211069198 for Effects of non-invasive brain stimulation in multiple sclerosis: systematic review and meta-analysis by Rebecca L.D. Kan, Grace X.J. Xu, Kate T. Shu, Frank H.Y. Lai, Gottfried Kranz and Georg S. Kranz in Therapeutic Advances in Chronic Disease
Footnotes
Author contributions: Rebecca L. D. Kan: Conceptualization; Methodology; Software; Writing-original draft; Writing-review & editing.
Grace X. J. Xu: Conceptualization; Methodology; Software; Writing-original draft.
Kate T. Shu: Conceptualization; Methodology; Software; Writing-original draft.
Frank H. Y. Lai: Resources; Writing-review & editing.
Gottfried Kranz: Resources; Writing-review & editing.
Georg S. Kranz: Conceptualization; Formal analysis; Methodology; Project administration; Supervision; Funding acquisition; Writing-review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Hong Kong Research Grants Council (project no. 25100219 and 15100120) to Georg S. Kranz.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Georg S. Kranz  https://orcid.org/0000-0002-3892-1804
https://orcid.org/0000-0002-3892-1804
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Rebecca L.D. Kan, Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, SAR, China
Grace X.J. Xu, Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, SAR, China
Kate T. Shu, Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, SAR, China Department of Rehabilitation, Third Military Medical University Southwest Hospital, Chongqing, China.
Frank H.Y. Lai, Faculty of Health and Life Sciences, The Northumbria University Newcastle, Newcastle upon Tyne, UK
Gottfried Kranz, Neurological Rehabilitation Center Rosenhügel, Vienna, Austria.
Georg S. Kranz, Department of Rehabilitation Sciences, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, SAR, 999077, China; Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria; The State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Pokfulam, Hong Kong, SAR, China.
References
- 1. Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med 2000; 343: 938–952. [DOI] [PubMed] [Google Scholar]
- 2. Goldenberg MM. Multiple sclerosis review. P T 2012; 37: 175–184. [PMC free article] [PubMed] [Google Scholar]
- 3. Fernández Ó, Costa-Frossard L, Martínez-Ginés M, et al. The broad concept of ‘Spasticity-Plus Syndrome’ in multiple sclerosis: a possible new concept in the management of multiple sclerosis symptoms. Front Neurol 2020; 11: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsuda PN, Shumway-Cook A, Ciol MA, et al. Understanding falls in multiple sclerosis: association of mobility status, concerns about falling, and accumulated impairments. Phys Ther 2012; 92: 407–415. [DOI] [PubMed] [Google Scholar]
- 5. Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: disease-modifying therapies for adults with multiple sclerosis: report of the guideline development, dissemination, and implementation Subcommittee of the American Academy of Neurology. Neurology 2018; 90: 789–800. [DOI] [PubMed] [Google Scholar]
- 6. Beer S, Khan F, Kesselring J. Rehabilitation interventions in multiple sclerosis: an overview. J Neurol 2012; 259: 1994–2008. [DOI] [PubMed] [Google Scholar]
- 7. Koch-Henriksen N, Magyari M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat Rev Neurol 2021; 17: 676–688. [DOI] [PubMed] [Google Scholar]
- 8. Berger T, Kobelt G, Berg J, et al. New insights into the burden and costs of multiple sclerosis in Europe: results for Austria. Mult Scler 2017; 23: 17–28. [DOI] [PubMed] [Google Scholar]
- 9. Lefaucheur J-P, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017; 128: 56–92. [DOI] [PubMed] [Google Scholar]
- 10. Lefaucheur J-P, Aleman A, Baeken C, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol 2020; 131: 474–528. [DOI] [PubMed] [Google Scholar]
- 11. Hsu W-Y, Cheng C-H, Zanto TP, et al. Effects of transcranial direct current stimulation on cognition, mood, pain, and fatigue in multiple sclerosis: a systematic review and meta-analysis. Front Neurol 2021; 12: 626113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lefaucheur J-P, André-Obadia N, Antal A, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS) 2014; 125: 2150–2206. [DOI] [PubMed] [Google Scholar]
- 14. Luo D, Wan X, Liu J, et al. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 2018; 27: 1785–1805. [DOI] [PubMed] [Google Scholar]
- 15. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. GetData Graph digitizer 2.26, http://getdata-graph-digitizer.com/download.php
- 17. Higgins JP, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons, 2019. [Google Scholar]
- 18. Borenstein M, Higgins JP, Hedges LV, et al. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods 2017; 8: 5–18. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 22. Cancelli A, Cottone C, Giordani A, et al. Personalized, bilateral whole-body somatosensory cortex stimulation to relieve fatigue in multiple sclerosis. Mult Scler J 2017; 24: 1366–1374. [DOI] [PubMed] [Google Scholar]
- 23. Chalah MA, Grigorescu C, Padberg F, et al. Bifrontal transcranial direct current stimulation modulates fatigue in multiple sclerosis: a randomized sham-controlled study. J Neural Transm 2020; 127: 953–961. [DOI] [PubMed] [Google Scholar]
- 24. Chalah MA, Riachi N, Ahdab R, et al. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J Neurol Sci 2017; 372: 131–137. [DOI] [PubMed] [Google Scholar]
- 25. Charvet LE, Dobbs B, Shaw MT, et al. Remotely supervised transcranial direct current stimulation for the treatment of fatigue in multiple sclerosis: results from a randomized, sham-controlled trial. Mult Scler 2018; 24: 1760–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrucci R, Vergari M, Cogiamanian F, et al. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. Neurorehabilitation 2014; 34: 121–127. [DOI] [PubMed] [Google Scholar]
- 27. Gaede G, Tiede M, Lorenz I, et al. Safety and preliminary efficacy of deep transcranial magnetic stimulation in MS-related fatigue. Neurol Neuroimmunol Neuroinflamm 2018; 5: e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hanken K, Bosse M, Möhrke K, et al. Counteracting fatigue in MS with right parietal anodal tDCS -preliminary results of a doubleblind placebo-controlled study. Neurologie Und Rehabilitation 2016; 22: 69–70. [Google Scholar]
- 29. Meesen RL, Thijs H, Leenus DJ, et al. A single session of 1 mA anodal tDCS-supported motor training does not improve motor performance in patients with multiple sclerosis. Restor Neurol Neurosci 2014; 32: 293–300. [DOI] [PubMed] [Google Scholar]
- 30. Saiote C, Goldschmidt T, Timaus C, et al. Impact of transcranial direct current stimulation on fatigue in multiple sclerosis. Restor Neurol Neurosci 2014; 32: 423–436. [DOI] [PubMed] [Google Scholar]
- 31. Tecchio F, Cancelli A, Cottone C, et al. Brain plasticity effects of neuromodulation against multiple sclerosis fatigue. Front Neurol 2015; 6: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tecchio F, Cancelli A, Cottone C, et al. Multiple sclerosis fatigue relief by bilateral somatosensory cortex neuromodulation. J Neurol 2014; 261: 1552–1558. [DOI] [PubMed] [Google Scholar]
- 33. Mori F, Codecà C, Kusayanagi H, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J Pain 2010; 11: 436–442. [DOI] [PubMed] [Google Scholar]
- 34. Mori F, Ljoka C, Magni E, et al. Transcranial magnetic stimulation primes the effects of exercise therapy in multiple sclerosis. J Neurol 2011; 258: 1281–1287. [DOI] [PubMed] [Google Scholar]
- 35. Boutière C, Rey C, Zaaraoui W, et al. Improvement of spasticity following intermittent theta burst stimulation in multiple sclerosis is associated with modulation of resting-state functional connectivity of the primary motor cortices. Mult Scler 2017; 23: 855–863. [DOI] [PubMed] [Google Scholar]
- 36. Korzhova J, Bakulin I, Sinitsyn D, et al. High-frequency repetitive transcranial magnetic stimulation and intermittent theta-burst stimulation for spasticity management in secondary progressive multiple sclerosis. Eur J Neurol 2019; 26: 680–e44. [DOI] [PubMed] [Google Scholar]
- 37. Şan AU, Yılmaz B, Kesikburun S. The effect of repetitive transcranial magnetic stimulation on spasticity in patients with multiple sclerosis. J Clin Neurol 2019; 15: 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mortezanejad M, Ehsani F, Masoudian N, et al. Comparing the effects of multi-session anodal trans-cranial direct current stimulation of primary motor and dorsolateral prefrontal cortices on fatigue and quality of life in patients with multiple sclerosis: a double-blind, randomized, sham-controlled trial. Clin Rehabil 2020; 34: 1103–1111. [DOI] [PubMed] [Google Scholar]
- 39. Ayache SS, Palm U, Chalah MA, et al. Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front Neurosci 2016; 10: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fiene M, Rufener KS, Kuehne M, et al. Electrophysiological and behavioral effects of frontal transcranial direct current stimulation on cognitive fatigue in multiple sclerosis. J Neurol 2018; 265: 607–617. [DOI] [PubMed] [Google Scholar]
- 41. Pilloni G, Choi C, Shaw MT, et al. Walking in multiple sclerosis improves with tDCS: a randomized, double-blind, sham-controlled study. Ann Clin Transl Neurol 2020; 7: 2310–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Young J, Zoghi M, Khan F, et al. The effect of transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis: randomized controlled trial. Pain Med 2020; 21: 3451–3457. [DOI] [PubMed] [Google Scholar]
- 43. Oveisgharan S, Karimi Z, Abdi S, et al. The use of brain stimulation in the rehabilitation of walking disability in patients with multiple sclerosis: a randomized double-blind clinical trial study. Iran J Neurol 2019; 18: 57–63. [PMC free article] [PubMed] [Google Scholar]
- 44. Mattioli F, Bellomi F, Stampatori C, et al. Neuroenhancement through cognitive training and anodal tDCS in multiple sclerosis. Mult Scler 2016; 22: 222–230. [DOI] [PubMed] [Google Scholar]
- 45. Iodice R, Dubbioso R, Ruggiero L, et al. Anodal transcranial direct current stimulation of motor cortex does not ameliorate spasticity in multiple sclerosis. Restor Neurol Neurosci 2015; 33: 487–492. [DOI] [PubMed] [Google Scholar]
- 46. Tramontano M, Grasso MG, Soldi S, et al. Cerebellar intermittent theta-burst stimulation combined with vestibular rehabilitation improves gait and balance in patients with multiple sclerosis: a preliminary double-blind randomized controlled trial. Cerebellum 2020; 19: 897–901. [DOI] [PubMed] [Google Scholar]
- 47. Goodkin D, Cookfair D, Wende K, et al. Inter and intrarater scoring agreement using grades 1.0 to 3.5 of the Kurtzke Expanded Disability Status Scale (EDSS). Neurology 1992; 42: 859–863. [DOI] [PubMed] [Google Scholar]
- 48. Jaeger S, Paul F, Scheel M, et al. Multiple sclerosis–related fatigue: altered resting-state functional connectivity of the ventral striatum and dorsolateral prefrontal cortex. Mult Scler 2019; 25: 554–564. [DOI] [PubMed] [Google Scholar]
- 49. Chalah MA, Riachi N, Ahdab R, et al. Fatigue in multiple sclerosis: neural correlates and the role of non-invasive brain stimulation. Front Cell Neurosci 2015; 9: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chalah MA, Kauv P, Créange A, et al. Neurophysiological, radiological and neuropsychological evaluation of fatigue in multiple sclerosis. Mult Scler Relat Disord 2019; 28: 145–152. [DOI] [PubMed] [Google Scholar]
- 51. Boggio PS, Rigonatti SP, Ribeiro RB, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol 2008; 11: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Lima AL, Braga FMA, da Costa RMM, et al. Transcranial direct current stimulation for the treatment of generalized anxiety disorder: a randomized clinical trial. J Affect Disord 2019; 259: 31–37. [DOI] [PubMed] [Google Scholar]
- 53. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000; 527: 633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jamil A, Batsikadze G, Kuo HI, et al. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J Physiol 2017; 595: 1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lucchinetti CF, Popescu BF, Bunyan RF, et al. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med 2011; 365: 2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Palm U, Ayache SS, Padberg F, et al. Non-invasive brain stimulation therapy in multiple sclerosis: a review of tDCS, rTMS and ECT results. Brain Stimul 2014; 7: 849–854. [DOI] [PubMed] [Google Scholar]
- 57. Boggio PS, Zaghi S, Lopes M, et al. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol 2008; 15: 1124–1130. [DOI] [PubMed] [Google Scholar]
- 58. Vaseghi B, Zoghi M, Jaberzadeh S. Does anodal transcranial direct current stimulation modulate sensory perception and pain? A meta-analysis study. Clin Neurophysiol 2014; 125: 1847–1858. [DOI] [PubMed] [Google Scholar]
- 59. Goudra B, Shah D, Balu G, et al. Repetitive transcranial magnetic stimulation in chronic pain: a meta-analysis. Anesth Essays Res 2017; 11: 751–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Centonze D. Advances in the management of multiple sclerosis spasticity: multiple sclerosis spasticity nervous pathways. Eur Neurol 2014; 72: 6–8. [DOI] [PubMed] [Google Scholar]
- 61. Mori F, Koch G, Foti C, et al. The use of repetitive transcranial magnetic stimulation (rTMS) for the treatment of spasticity. Prog Brain Res 2009; 175: 429–439. [DOI] [PubMed] [Google Scholar]
- 62. Kumru H, Murillo N, Samso JV, et al. Reduction of spasticity with repetitive transcranial magnetic stimulation in patients with spinal cord injury. Neurorehabil Neural Repair 2010; 24: 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gunduz A, Kumru H, Pascual-Leone A. Outcomes in spasticity after repetitive transcranial magnetic and transcranial direct current stimulations. Neural Regen Res 2014; 9: 712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223211069198 for Effects of non-invasive brain stimulation in multiple sclerosis: systematic review and meta-analysis by Rebecca L.D. Kan, Grace X.J. Xu, Kate T. Shu, Frank H.Y. Lai, Gottfried Kranz and Georg S. Kranz in Therapeutic Advances in Chronic Disease