Key Points
Question
What are the characteristics of the design of clinical efficacy trials of biosimilars for treating cancer, and what evidence do trials of biosimilars provide?
Findings
This meta-analysis and systematic review of 31 cancer biosimilar studies of 3 reference products involving 12 310 patients found that, compared with pivotal trials of reference drugs, studies of 3 cancer drugs with biosimilars available at the time of the analysis were, on average, larger, more often a randomized clinical trial, and more often double blinded. Meta-analyses showed biosimilars to be as effective as their reference products in all disease settings.
Meaning
This study suggests that studies of biosimilars for treating cancer have design elements of rigorous trials and show equivalent effectiveness to their reference products.
Abstract
Importance
Biologics account for almost half of US drug spending but may be subject to competitive pricing pressures by US Food and Drug Administration–approved biosimilars. The extent of the preapproval clinical testing that is needed and how these biosimilars compare with the originator biologic products remain critical issues in establishing a vibrant biosimilar market.
Objectives
To analyze the design of cancer biosimilar efficacy studies compared with the reference drug pivotal trials and provide summary risk ratio estimates for each cancer type drug subgroup.
Data Sources
A systematic search was performed of articles and abstracts published using Embase, PubMed/MEDLINE, and ClinicalTrials.gov, last updated April 18, 2021.
Study Selection
All studies or abstracts in English comparing a disease-modifying cancer biologic and its biosimilar and reporting efficacy or surrogate efficacy results were included.
Data Extraction and Synthesis
Outcome estimates and study characteristics were extracted from each study. Among biosimilar efficacy studies, random-effects meta-analyses were performed for each cancer type molecule outcome subgroup, calculating pooled relative estimates and 95% CIs.
Main Outcomes and Measures
Study characteristics, such as population size, blinding, and randomization, were compared between biosimilar trials and those of reference drugs. Risk ratio estimates for relative change to surrogate measures (eg, progression-free survival) were collected for biosimilars and their reference products.
Results
A total of 31 cancer biosimilar studies of 3 reference products involving 12 310 patients were included. In all 7 subgroups, the biosimilars analyzed were indistinguishable from their reference drug on surrogate efficacy. Six reference drug trials were included, involving 1811 patients. On average, biosimilar studies involved more patients than reference drug trials (mean number of patients, 397 vs 302), were more likely to be randomized clinical trials rather than single-group or observational studies (100% [31 of 31] vs 50% [3 of 6]), and were more likely to be double blind rather than open label (84% [26 of 31] vs 17% [1 of 6]).
Conclusions and Relevance
This systematic review and meta-analysis found that the biosimilars for the cancer drugs in this sample were subjected to rigorous clinical evaluations, and the results were statistically indistinguishable from those of original products across drugs, cancer types, and outcome measures.
This systematic review and meta-analysis examines the design of cancer biosimilar efficacy studies compared with the reference drug pivotal trials and provides summary risk ratio estimates for each cancer type drug subgroup.
Introduction
Biologic drugs, which are manufactured via genetically modified bacteria, can be transformative in caring for patients with cancer, eye diseases, and rheumatologic conditions. Although biologics are used by fewer than 2% of individuals in the US, they contribute a large and increasing share of US prescription drug costs.1 In 2019, biologic manufacturers earned $211 billion in revenue, or 43% of national drug sales.2 To address the steep prices of biologics, the Biologics Price Competition and Innovation Act of 2009 created an abbreviated regulatory pathway for biosimilars—products that are chemically and clinically comparable to approved biologic therapies but made by different manufacturers.3 Facilitating competition from biosimilars was expected to lower prices and overall spending on biologic drugs.4
The limited availability of biosimilars in the US has been due to several factors. Although small molecules usually involve few chemical synthetic steps, biologics require more chemically complex manufacturing processes that can shift over time and lead to variations in the product. The US Food and Drug Administration (FDA) has generally required biosimilars to undertake substantial testing before approval, including human trials to assess pharmacologic similarity between the biosimilar and the reference drug and to detect adverse immune system reactions.3,5 The FDA also prefers a comparative efficacy trial between the biosimilar and the original product.6 The clinical development of biosimilars can thus take almost as long as the trials used to secure FDA approval of the originator drugs.7
After approval, most biosimilars have not been deemed interchangeable with the original product, and some physicians remain skeptical about their relative effectiveness.8 For example, biosimilars for the treatment of cancer are on pace to take half or more of the market share in their first 2 years since approval,9 which is less than the 75% to 90% market share that generic drugs typically take in the first year.2 Policy makers have proposed various strategies to increase the number of biosimilars competing per brand-name biologic to increase uptake and lower prices.9,10 Focusing on biologics intended to treat cancer, we sought to understand the extent of preapproval efficacy testing of biosimilars and to compare the effectiveness of biosimilars with that of originator biologic products at the drug-disease stage-outcome level.
Methods
Data Sources
We performed a systematic search of published articles and abstracts using Embase, PubMed/MEDLINE, and ClinicalTrials.gov (last updated April 18, 2021). We used a text string designed to identify trials of brand-name biologics indicated as disease-modifying therapies for cancer that have available biosimilars on the US market (eMethods in the Supplement). Three biologic drugs met our criteria: rituximab, trastuzumab, and bevacizumab. The search string was then modified for ClinicalTrials.gov while retaining an approach common to other databases. We also looked for any missing studies in the bibliographies of systematic reviews and meta-analyses that we found in our original search. To find pivotal trials supporting FDA approval of brand-name cancer biologics, we relied on references from the original US drug labeling and FDA medical reviews available at Drugs@FDA.11 We then compared study design characteristics of the retrieved pivotal trials with those of the included biosimilar trials (eg, sample size, trial control such as placebo, active comparison or no control, blinding, and outcome). This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Study Selection
We included biosimilar studies if they reported a trial comparing a biosimilar for cancer treatment and its brand-name reference product. The comparison had to include at least 1 efficacy outcome, even if it was a surrogate measure (eg, progression-free survival). Studies that reported only pharmacokinetic comparisons (eg, area under the serum concentration-time curve or maximum observed serum concentration) were excluded, as were case reports.
When there were multiple publications reporting from a single trial, we included the earliest peer-reviewed publication with at least 1 reported clinical efficacy comparison in the full study cohort. If a publication lacked data in a field, we supplemented our data collection with information posted on ClinicalTrials.gov or India’s Clinical Trials Registry.
Data Extraction, Quality Assessment, and Data Synthesis
Three of us (D.B., E.D., and S.N.) extracted data on trial design and efficacy outcomes and 1 of us (D.B.) extracted data on study funding and authorship. For trial design and efficacy, we extracted data on blinding, duration of therapy and median follow-up, number of patients in each study group, patient characteristics, and efficacy results. We also extracted information about study funding, including the nature and name of the trial sponsor, the number of authors employed by or receiving funds from the sponsor, and the participation of a medical writer. In coding studies for noninferiority, we relied on each study’s own prespecified noninferiority or equivalence margins, if provided. We coded studies that did not provide prespecified noninferiority margins as failing to show noninferiority.
Two of us (E.D. and S.N.) independently evaluated the methodological quality of each trial on randomization (generation of allocation sequences and concealment of allocation), blinding, adequacy of analyses (including dropouts and withdrawals), and selective reporting of outcomes using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials.12,13 To account for heterogeneity, we stratified the analyses based on brand-name reference product, type of cancer, and main clinical outcome recorded by the trial. Using Stata, version 15 (StataCorp), we performed random-effects analyses for each subgroup, calculating pooled relative estimates (ie, risk ratio or hazard ratio) and 95% CIs. If the relative estimates and 95% CIs were not reported in the trials, we calculated them based on the available information (number of cases and noncases by treatment group). When estimating 95% CIs was not possible, we extracted and pooled the alternative CIs (ie, 90% CIs) reported homogenously across the trials of the same subgroup. Between-study heterogeneity within subgroups was assessed using the I2 statistic.14,15 To investigate further sources of heterogeneity, we performed sensitivity analyses by excluding trials that did not examine the studied clinical outcome as the primary outcome.15 A funnel plot, the Begg test, and the Egger test were used to estimate potential publication biases.16 Statistical tests were 2-sided and used a significance threshold of P < .05.
Results
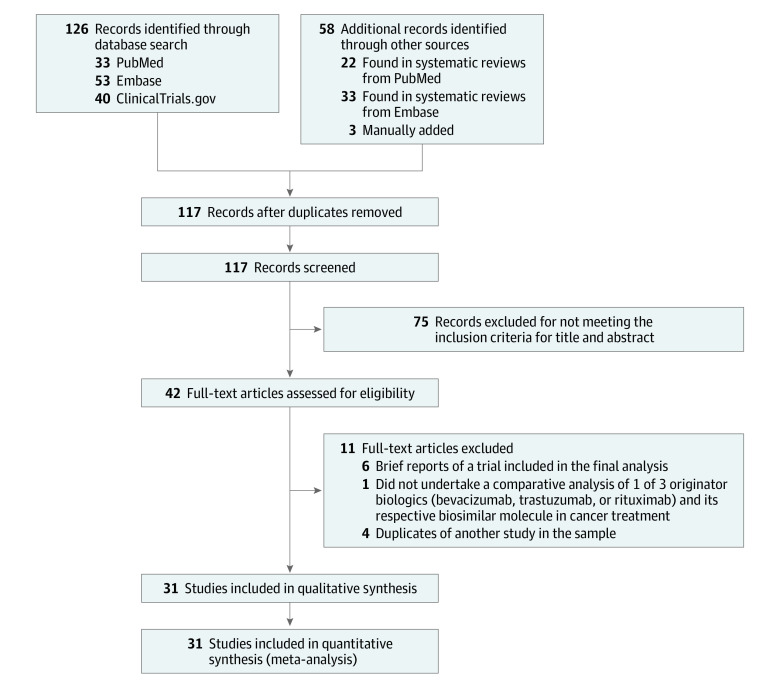
The search identified 184 studies: 33 from PubMed, 53 from Embase, 40 from ClinicalTrials.gov, and 58 from prior systematic reviews or manual addition. After applying our exclusion criteria, we identified 42 studies, and 11 were excluded after final review, leaving 31 studies involving 12 310 patients for detailed analysis (Figure 1; eTable 1 in the Supplement).17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47 Twenty-one studies presented an overall low risk of bias, 2 had a high risk of bias, and for 2 trials, the risk of bias was unclear (eTable 2 in the Supplement). All trials were preregistered. No major sources of publication bias were found (eFigure 1, eFigure 2, eTable 3, and eTable 4 in the Supplement). Six reference drug trials were included, involving 1811 patients. On average, biosimilar studies involved more patients than reference drug trials (mean number of patients, 397 vs 302), were more likely to be randomized clinical trials (RCTs) rather than single-group or observational studies (100% [31 of 31] vs 50% [3 of 6]), and were more likely to be double-blind rather than open-label studies (84% [26 of 31] vs 17% [1 of 6]).
Figure 1. Flow Diagram of 184 Studies Evaluated for the Systematic Review and Meta-analysis.
Although our entry criteria permitted observational studies, all studies of biosimilars in the final sample were multicenter RCTs. All but 5 were double-blinded. Eleven studies compared brand-name bevacizumab with a biosimilar,17,18,19,20,21,22,23,24,25,26,27 9 compared brand-name trastuzumab with a biosimilar,28,29,30,31,32,33,34,35,36 and 11 compared brand-name rituximab with a biosimilar.37,38,39,40,41,42,43,44,45,46,47
All of the identified studies were funded by a biosimilar sponsor seeking regulatory approval, and 12 trials (39%) studied biologics that have subsequently been approved by the FDA. Of these trials, 20% of study authors (99 of 502) were employed by the sponsor, and 48% of studies (15 of 31) in the sample noted the assistance of a medical writer.
Efficacy Meta-analyses
We conducted a meta-analysis (Table 1, Figure 2, and Figure 3) of 3 originator drugs and their respective biosimilars in 6 disease settings: non–small cell lung cancer, metastatic colorectal cancer (mCRC), ERBB2 (formerly HER2 or HER2/neu)–positive early breast cancer, ERBB2-positive metastatic breast cancer, follicular lymphoma, and diffuse large B-cell lymphoma (DLBCL).
Table 1. Results of the Effectiveness of Biosimilars Compared With Their Reference Biologics.
| Outcome and cancer subgroup | RCTs, No. | Line of therapy | Sample size, No.a | Test of heterogeneity | Results of meta-analysis, effect estimates (95% CI) | Noninferiority, No./total No. of RCTsb | ||
|---|---|---|---|---|---|---|---|---|
| Biosimilar | Reference | I2, % | P value | |||||
| Bevacizumab biosimilars vs bevacizumabc | ||||||||
| Overall response rate | ||||||||
| NSCLC | 6 | First | 1673 | 1666 | 0.0 | .75 | 1.02 (0.94-1.10) | 5/6 |
| mCRC | 3 | First | 213 | 159 | 0.0 | .32 | 0.95 (0.72-1.24) | 1/3 |
| Progression-free survival | ||||||||
| mCRC | 2 | First or second | 422 | 381 | 0.0 | .59 | 0.91 (0.80-1.04)d | 2/2 |
| Trastuzumab biosimilars vs trastuzumabe | ||||||||
| Total pathological complete response rate | ||||||||
| ERBB2-positive early BC | 5 | First | 1436 | 1441 | 59.5 | .04 | 1.08 (0.95-1.24) | 4/5 |
| Overall response rate | ||||||||
| ERBB2-positive metastatic BC | 4 | Second | 1040 | 1041 | 18.2 | .30 | 1.01 (0.94-1.08) | 4/4 |
| Rituximab biosimilars vs rituximabf | ||||||||
| Overall response rate | ||||||||
| FL | 7 | First or second | 973 | 970 | 0.0 | .67 | 1.04 (1.00-1.08) | 7/7 |
| DLBCL | 3 | First or second | 411 | 414 | 0.0 | .68 | 1.01 (0.96-1.05) | 2/3 |
| CLL | 1 | First or second | 35 | 35 | NA | NA | 0.99 (0.81-1.22) | 1/1 |
Abbreviations: BC, breast cancer; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; mCRC, metastatic colorectal cancer; NA, not applicable; NSCLC, non–small cell lung cancer; RCTs, randomized clinical trials.
Number of patients at randomization.
Number of RCTs that showed noninferiority for biosimilar drugs based on the margins provided in each individual study.
Bevacizumab biosimilars: ABP 215, PF-06439535, SB8, BCD-021, MB02, and IBI305.
90% CI.
Trastuzumab biosimilars: MYL-1401O, CT-P6, ABP 980, SB3, PF-05280014, PF-05280014, HD201, BCD-022, and HLX02.
Rituximab biosimilars: PF-05280586, GP2013, RTXM83, DRL-rituximab, BCD-020, HLX01, ABP 798, CT-P10, and Zytux.
Figure 2. Meta-analyses of the Association Between Bevacizumab and Trastuzumab Biosimilars and Efficacy Outcomes Compared With Originators Stratified by Cancer Types.
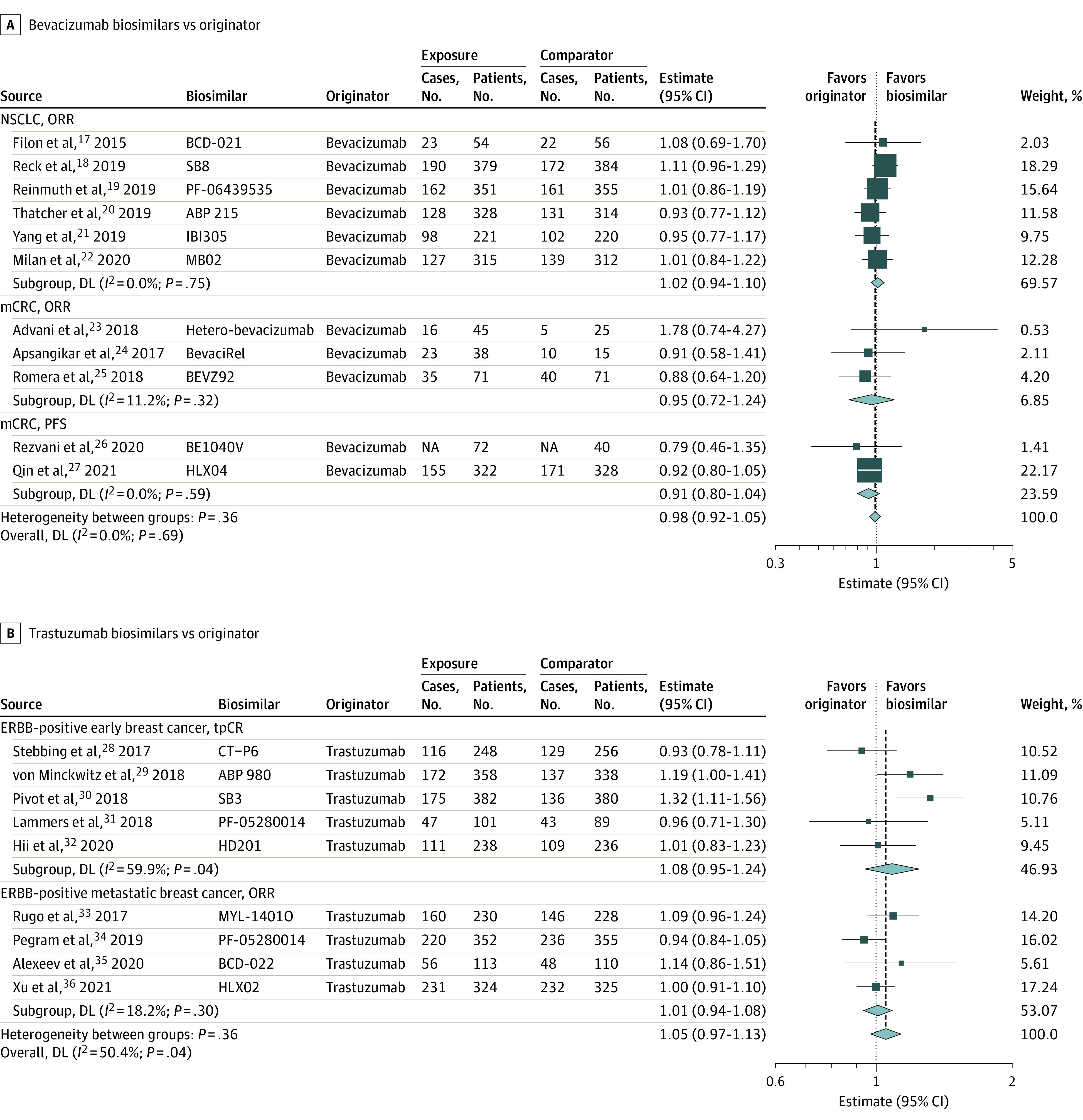
A, Bevacizumab biosimilars vs originator bevacizumab. B, Trastuzumab biosimilars vs originator trastuzumab. If the number of cases was not reported, we calculated them based on the outcome rates when available. The number of events by allocated treatment and the point estimates of the effect sizes are shown for individual trials and subgroups of trials based on the type of cancer and outcome. Weights are from random-effects analysis. Relative estimates for individual trials or subgroups of trials are indicated by the squares and 95% CIs by horizontal lines. Pooled relative estimates and their 95% CIs are indicated by diamonds. The overall pooled estimate is also indicated by a dashed vertical line. The sizes of the squares and the diamonds are proportional to the weight assigned to the relative effect sizes. DL indicates DerSimonian and Laird estimation method; mCRC, metastatic colorectal cancer; NA, not applicable; NSCLC, non–small cell lung cancer; ORR, objective response rate; PFS, progression-free survival; and tpCR, total pathologic complete response.
Figure 3. Meta-analyses of the Association Between Rituximab Biosimilars and Efficacy Outcomes Compared With Originator Stratified by Cancer Types.
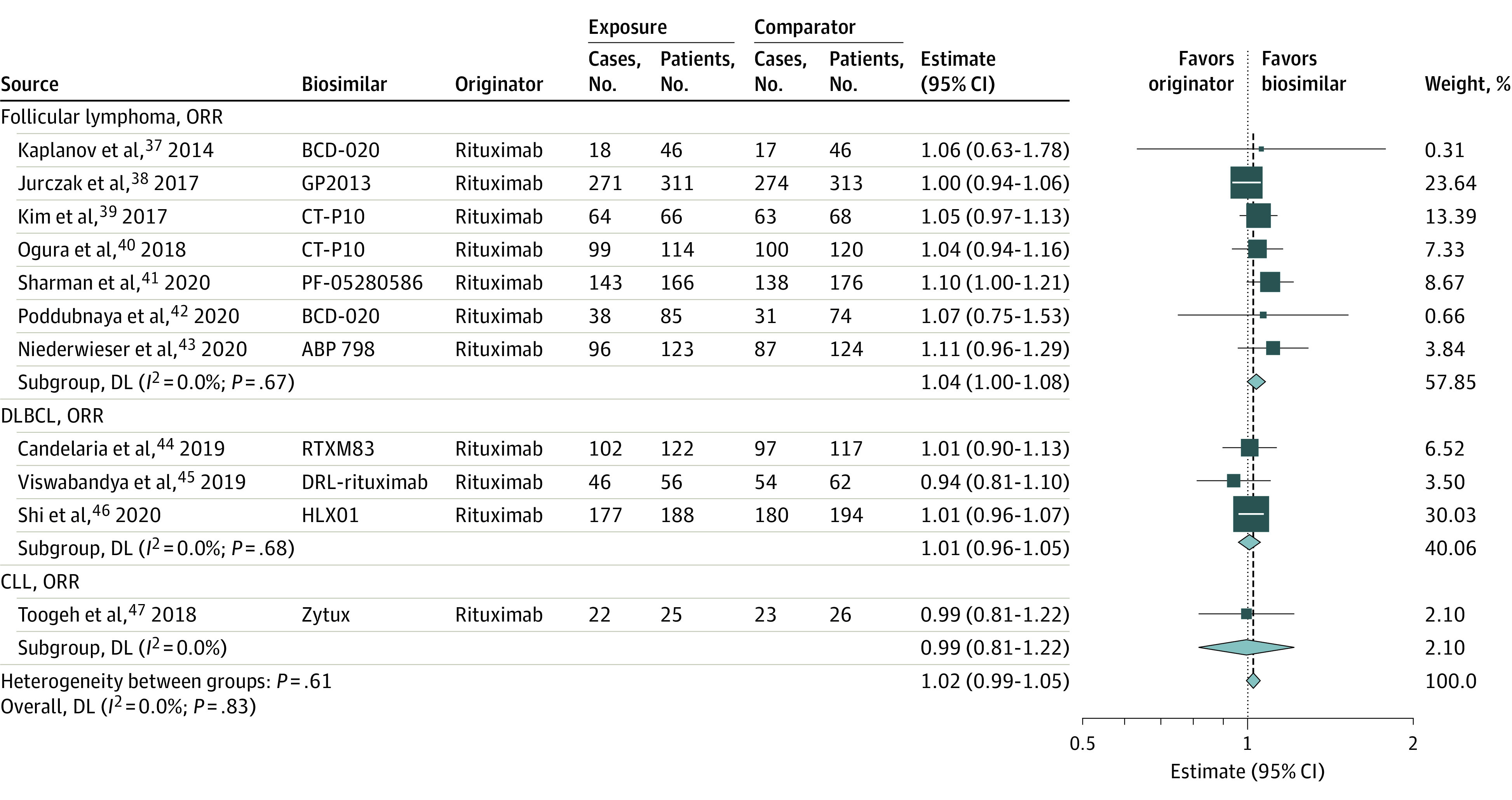
The number of events by allocated treatment and the point estimates of the effect sizes are shown for individual trials and subgroups of trials based on the type of cancer and outcome. Weights are from random-effects analysis. Relative estimates for individual trials or subgroups of trials are indicated by the squares and 95% CIs by horizontal lines. Pooled relative estimates and their 95% CIs are indicated by diamonds. The overall pooled estimate is also indicated by a dashed vertical line. The sizes of the squares and the diamonds are proportional to the weight assigned to the relative effect sizes. CLL indicates chronic lymphocytic leukemia; DL, DerSimonian and Laird estimation method; DLBCL, diffuse large B-cell lymphoma; and ORR, objective response rate.
When available, we relied on outcomes in the per-protocol populations; otherwise, we used results in the intention-to-treat populations. In all settings, the meta-analysis indicated that the effectiveness of the biosimilars by these measures was statistically indistinguishable from the effectiveness of the originators.
We coded all but 5 trials as finding that the tested biosimilar was noninferior to the originator.17,24,25,31,45 Those 5 trials either failed to publish a predefined noninferiority margin, did not use efficacy as a primary outcome, or both. Viswabandya et al45 compared the biosimilar DRL-rituximab with the originator rituximab in 151 patients with diffuse large B-cell lymphoma using a primary pharmacokinetic end point. Study enrollment was cut short after an unscheduled “blinded sample size reestimation,”45(p3) resulting in a trial underpowered to detect significant differences in overall response rate (ORR). Participants taking the originator rituximab had an ORR of 87.1% compared with 82.1% among those taking DRL-rituximab, a difference of −5.0% (95% CI, −22.8% to 13.2%). The 95% CI lower bound crossed the study’s prespecified −10% noninferiority margin. Similarly, Romera et al25 compared the biosimilar BEVZ92 with the originator bevacizumab in patients with mCRC, in a trial that was also insufficiently powered to produce a reliable efficacy result. There was a numerical difference between the groups, with an ORR of 68% among patients taking the originator and 61% among those taking the biosimilar. Finally, 3 trials reported no predefined noninferiority margin.17,24,31
Bevacizumab
Eleven studies compared the-originator bevacizumab with the biosimilar bevacizumab: 6 among patients with non–small cell lung cancer17,18,19,20,21,22 and 5 among patients with mCRC.23,24,25,26,27 All non–small cell lung cancer studies used the ORR as the primary end point. Three mCRC trials provided ORR data, although 1 trial had a pharmacokinetic end point as its primary outcome. Another mCRC trial used the related metric of disease control rate as the primary end point (the disease control rate includes ORR and counts stable disease). The other 2 mCRC trials used progression-free survival as the primary end point.
Meta-analyses of both conditions showed the studied biosimilars to be indistinguishable from the originator bevacizumab in terms of ORR (non–small cell lung cancer and mCRC) and progression-free survival (mCRC). All 3 meta-analyses showed insignificant heterogeneity in results. In mCRC, removing the 1 study with a pharmacokinetic primary end point as a sensitivity analysis left the results virtually unchanged (eFigure 3 in the Supplement).
Trastuzumab
Nine studies compared the originator trastuzumab and a biosimilar trastuzumab: 4 studies among patients with ERBB2-positive metastatic breast cancer28,29,30,31 and 5 studies among patients with ERBB2-positive early breast cancer.32,33,34,35,36 All trials reported the ORR as their primary end point, and all but 2 early breast cancer trials used the total pathological complete response rate as their primary end point. One trial used breast pathologic complete response rate as its primary end point, although it also reported the total pathological complete response rate. Another trial had a pharmacokinetic outcome as its primary end point.
Both meta-analyses found no difference between originator trastuzumab and biosimilars in the efficacy outcomes. The metastatic breast cancer sample showed no heterogeneity. There was heterogeneity in the full early breast cancer sample (I2 = 59.5%; P = .04). A sensitivity analysis removing the 2 studies that did not use the total pathological complete response rate as a primary end point led to almost identical efficacy results (I2 = 50.4%; P = .13) (eFigure 4 in the Supplement).
Rituximab
Eleven studies in our sample compared the originator rituximab and a biosimilar rituximab: 7 studies among patients with follicular lymphoma,37,38,39,40,41,42,43 3 studies among those with diffuse large B-cell lymphoma,44,45,46 and 1 study among those with chronic lymphocytic leukemia.47 We included the chronic lymphocytic leukemia study in our reported sample but not in a meta-analysis. All studies used the ORR as the primary end point, except 1 trial that reported the ORR but used a pharmacokinetic end point as its primary outcome.
Both meta-analyses found no difference in ORR between originator rituximab and biosimilar groups. Neither had heterogeneity. Excluding the study with the pharmacokinetic primary end point as a sensitivity analysis led to a similar result (eFigure 5 in the Supplement).
Comparison With Originator Pivotal Trials
Table 2 shows a comparison of the study designs of biosimilar efficacy trials with the study designs of the pivotal trials used to secure originator brand-name FDA approval. All 3 brand-name drugs were approved on the basis of 2 trials apiece.48,49,50,51,52,53
Table 2. Study Design Comparison Between Pivotal and Biosimilar Trials.
| Molecule | Cancer setting | Mean sample size, No. | No./total No. | |
|---|---|---|---|---|
| Randomized clinical trials | Double-blind trials | |||
| Rituxan (rituximab) | Follicular lymphoma | 101.5 | 0/2 | 0/2 |
| Rituximab biosimilars | Follicular lymphoma | 277.6 | 7/7 | 5/7 |
| Herceptin (trastuzumab) | Metastatic ERBB2-positive breast cancer | 345.5 | 1/2 | 0/2 |
| Trastuzumab biosimilars | Metastatic ERBB2-positive breast cancer | 520.3 | 4/4 | 4/4 |
| Avastin (bevacizumab) | mCRC | 458.5 | 2/2 | 1/2 |
| Bevacizumab biosimilars | mCRC | 235 | 5/5 | 2/5 |
Abbreviation: mCRC, metastatic colorectal cancer.
Avastin (originator bevacizumab) was approved based on 2 RCTs in mCRC, 1 double-blind and 1 open-label, with a mean of 458.5 patients per study. The primary end point for both was overall survival. Of the biosimilar efficacy trials studying bevacizumab in mCRC, 2 were double-blind trials, and 3 were open-label trials, with a mean of 235 patients per study.
Herceptin (originator trastuzumab) was approved based on 2 studies of patients with metastatic ERBB2-positive breast cancer, both open-label trials, 1 of which was an RCT and the other a single-group study, with a mean patient population of 345.5 per trial. The RCT’s primary end point was ORR, and the single-group trial’s primary end point was time to disease progression. Among biosimilar efficacy trials of trastuzumab for patients with metastatic breast cancer, all studies were double blind, with a mean patient population of 520.3.
Rituxan (originator rituximab) was approved based on 2 studies among patients with follicular lymphoma, both single-group, open-label trials. In total, the studies enrolled a mean of 101.5 patients per trial. In 1 study, the primary end point was ORR; in the other, the primary end point was the clinical response rate. Five of the 7 biosimilar trials studying bevacizumab in follicular lymphoma were double blind, and 2 were open label. The trials enrolled a mean of 277.6 patients.
Discussion
In this systematic review and meta-analysis, disease-modifying cancer biosimilars in 3 disease settings were indistinguishable from their reference products in terms of measured effectiveness. Oncology biosimilar efficacy studies had larger sample sizes and were more often conducted as double-blind RCTs compared with the trials used to support their reference products’ initial US approvals.
All studies with predefined noninferiority margins and sufficient power found noninferiority. One study found noninferiority but did not find equivalence because the evidence did not rule out biosimilar superiority.29 All studies in our analysis were preregistered, reducing the concern that the results stemmed from selection bias, and most had an overall low risk of bias. Among the 10 trials with an overall unclear or high risk of bias, 5 were available only as abstracts, limiting a thorough evaluation of their study design.
Biosimilar studies were generally rigorous and large. For trastuzumab and rituximab, the average biosimilar study enrolled substantially more patients than the average originator pivotal trial. Twenty-six of the biosimilar studies (84%) in our sample were double-blind trials, and all were RCTs. By contrast, 5 of the 6 originator pivotal trials were open-label trials, and half were single-group studies. The brand-name bevacizumab trials were an exception, as both were RCTs, and unlike the other pivotal trials in the sample, they assessed overall survival rather than surrogate measures.
The fact that biosimilar pivotal trials had more characteristics that are traditionally associated with rigorous analysis than did originator pivotal trials may be justified by the relative lack of clinical urgency in biosimilar approval. Increased competition, however, comes with its own advantages—lower prices and greater patient access. Conducting large and expensive pivotal trials for biosimilar molecules that have already shown a high degree of similarity in pharmacokinetics and safety in smaller studies creates barriers to entry and reduces expected competition.54 The comparative size and rigor of the biosimilar trials in this study, particularly in light of the near-universal finding of noninferiority, thus raise important questions about how much testing should be required of biosimilars. As the reliability and reproducibility of scientifically assessing biosimilars continue to develop, such similar outcomes will allow regulators to become more flexible with the extent of preapproval testing.
Several systematic reviews of oncology biosimilars have been previously published. The most comprehensive systematic review to date performed a meta-analysis of 23 RCTs published through December 2018 comparing anticancer biologic reference products and their corresponding biosimilars for efficacy and safety.55 Galvão et al56 published a systematic review protocol to survey and analyze oncology biosimilar clinical studies but have not yet published the results. Because more studies have been published since 2018, our sample is the largest yet studied to our knowledge. In addition, our finding that all trials were funded by manufacturers seeking regulatory approval is relevant to the interpretation of our results because manufacturer-sponsored trials have been associated with outcomes favoring the manufacturer’s product being studied.57
Limitations
This study has some limitations. First, all of the biosimilar efficacy measures collected in our analyses were surrogates rather than overall survival. Because these studies compare pharmacologically highly similar products with similar surrogate outcomes, there is no reason to doubt similar overall survival benefits. Second, because we were unable to locate full publications for several trials in our sample, we relied on abstracts, limiting full insight. Third, because we focused our search for biologic drugs with available biosimilars, our sample size was necessarily limited to 3 products made by the same manufacturer, and our search may not have identified trials with negative outcomes leading to nonapproval of a biosimilar, or such trials may not have been published. However, these are 3 very commonly used medications that together accounted for approximately 10% of Medicare Part B spending overall in 2019.2 Fourth, the results should be interpreted in light of biosimilar efficacy only; by contrast, safety, immunogenicity, and toxicity end points were outside the scope of this study. Our results do support patient and physician confidence in the efficacy and extent of testing of currently available biosimilars to treat cancer. More widespread use of these products may help promote price-lowering competition.
Conclusions
The RCTs evaluated in this systematic review and meta-analysis found that biosimilar disease-modifying oncology drugs had similar efficacy as brand-name reference products. These results should help overcome skepticism that physicians and patients may have in using more cost-effective biosimilar drugs currently available in the US to manage cancer diagnoses.
eMethods. Search Strategy for the Systematic Review and Meta-Analysis
eTable 1. Oncology Biosimilar Efficacy Trials
eTable 2. Quality Assessment Criteria and Risk of Bias Assessment
eFigure 1. Funnel Plot Analysis
eTable 3. Begg’s Test for Small-Study Effects
eTable 4. Egger’s Test for Small-Study Effects
eFigure 2. Funnel Plot by Disease-Measurement Subgroup
eFigure 3. Sensitivity Analysis for ORR, mCRC Removing Romera et al
eFigure 4. Sensitivity Analysis for pCR, ERBB2+ Early Breast Cancer Removing Pivot et al and Lammers et al
eFigure 5. Sensitivity Analysis for ORR, DLBCL Removing Viswabandya et al
References
- 1.US Food and Drug Administration . Remarks from FDA Commissioner Scott Gottlieb, MD, as prepared for delivery at the Brookings Institution on the release of the FDA’s Biosimilars Action Plan. July 18, 2018. Accessed October 5, 2021. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm613881.htm
- 2.Centers for Medicare & Medicaid Services. Medicare part B spending dashboard. December 1, 2021. Accessed December 21, 2021. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Information-on-Prescription-Drugs/MedicarePartB
- 3.Sarpatwari A, Barenie R, Curfman G, Darrow JJ, Kesselheim AS. The US biosimilar market: stunted growth and possible reforms. Clin Pharmacol Ther. 2019;105(1):92-100. doi: 10.1002/cpt.1285 [DOI] [PubMed] [Google Scholar]
- 4.Dave CV, Kesselheim AS, Fox ER, Qiu P, Hartzema A. High generic drug prices and market competition: a retrospective cohort study. Ann Intern Med. 2017;167(3):145-151. doi: 10.7326/M16-1432 [DOI] [PubMed] [Google Scholar]
- 5.Nabhan C, Parsad S, Mato AR, Feinberg BA. Biosimilars in oncology in the United States: a review. JAMA Oncol. 2018;4(2):241-247. doi: 10.1001/jamaoncol.2017.2004 [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration . Scientific considerations in demonstrating biosimilarity to a reference product: guidance for industry. 2015. Accessed October 5, 2021. https://www.fda.gov/media/82647/download
- 7.Lee CC, Kesselheim AS, Sarpatwari A. Clinical development times for biosimilars in the United States. Mayo Clin Proc. 2020;95(10):2152-2154. doi: 10.1016/j.mayocp.2020.06.039 [DOI] [PubMed] [Google Scholar]
- 8.Zhai MZ, Sarpatwari A, Kesselheim AS. Why are biosimilars not living up to their promise in the US? AMA J Ethics. 2019;21(8):E668-E678. doi: 10.1001/amajethics.2019.668 [DOI] [PubMed] [Google Scholar]
- 9.Frank RG, Shahzad M, Feldman WB, Kesselheim AS. Biosimilar competition: early learnings. NBER working paper series. National Bureau of Economic Research. 2021. Accessed October 4, 2021. https://www.nber.org/system/files/working_papers/w28460/w28460.pdf
- 10.US Food and Drug Administration. Biosimilars action plan: balancing innovation and competition. July 2018. Accessed January 4, 2022. https://www.fda.gov/media/114574/download
- 11.US Food and Drug Administration. Drugs@FDA: FDA-approved drugs. Last accessed October 4, 2021. https://www.accessdata.fda.gov/scripts/cder/daf/
- 12.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Andrea E, Kesselheim AS, Franklin JM, Jung EH, Hey SP, Patorno E. Heterogeneity of antidiabetic treatment effect on the risk of major adverse cardiovascular events in type 2 diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2020;19(1):154. doi: 10.1186/s12933-020-01133-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Andrea E, Hey SP, Ramirez CL, Kesselheim AS. Assessment of the role of niacin in managing cardiovascular disease outcomes: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(4):e192224. doi: 10.1001/jamanetworkopen.2019.2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 17.Filon O, Orlov S, Burdaeva O, et al. Efficacy and safety of BCD-021, bevacizumab biosimilar candidate, compared to Avastin: results of international multicenter randomized double blind phase III study in patients with advanced non-squamous NSCLC. J Clin Oncol. 2015;33(15):8057. doi: 10.1200/jco.2015.33.15_suppl.8057 [DOI] [Google Scholar]
- 18.Reck M, Luft A, Bondarenko I, et al. A phase III study comparing SB8, a proposed bevacizumab biosimilar, and reference bevacizumab in patients with metastatic or recurrent non-squamous NSCLC. Ann Oncol. 2019;30(suppl 5):V644-V645. doi: 10.1093/annonc/mdz260.087 [DOI] [Google Scholar]
- 19.Reinmuth N, Bryl M, Bondarenko I, et al. PF-06439535 (a bevacizumab biosimilar) compared with reference bevacizumab (Avastin), both plus paclitaxel and carboplatin, as first-line treatment for advanced non-squamous non–small-cell lung cancer: a randomized, double-blind study. BioDrugs. 2019;33(5):555-570. doi: 10.1007/s40259-019-00363-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thatcher N, Goldschmidt JH, Thomas M, et al. Efficacy and safety of the biosimilar ABP 215 compared with bevacizumab in patients with advanced nonsquamous non–small cell lung cancer (MAPLE): a randomized, double-blind, phase III study. Clin Cancer Res. 2019;25(7):2088-2095. doi: 10.1158/1078-0432.CCR-18-2702 [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Wu B, Huang L, et al. Biosimilar candidate IBI305 plus paclitaxel/carboplatin for the treatment of non-squamous non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(6):989-999. doi: 10.21037/tlcr.2019.12.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millan S, Trukhin D, Kolesnik O, et al. Bevacizumab biosimilar and reference bevacizumab in subjects with stage IIIB/IV no squamous non-small cell lung cancer (NSCLC) (STELLA study): results for the primary endpoint in a confirmatory, double-blind, randomized, controlled study. J Clin Oncol. 2020;38(suppl 15):e21543. [Google Scholar]
- 23.Advani S, Biswas G, Sinha S, et al. A prospective, randomized, multiple-dose, multi-center, comparative clinical study to evaluate the efficacy, safety, immunogenicity of a biosimilar bevacizumab (test product, Hetero) and reference medicinal product (bevacizumab, Roche) in patients of metastatic colorectal cancer. J Assoc Physicians India. 2018;66(6):55-59. [PubMed] [Google Scholar]
- 24.Apsangikar PD, Chaudhry SR, Naik MM, Deoghare SB, Joseph J. Comparative pharmacokinetics, efficacy, and safety of bevacizumab biosimilar to reference bevacizumab in patients with metastatic colorectal cancer. Indian J Cancer. 2017;54(3):535-538. doi: 10.4103/ijc.IJC_394_17 [DOI] [PubMed] [Google Scholar]
- 25.Romera A, Peredpaya S, Shparyk Y, et al. Bevacizumab biosimilar BEVZ92 versus reference bevacizumab in combination with FOLFOX or FOLFIRI as first-line treatment for metastatic colorectal cancer: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018;3(12):845-855. doi: 10.1016/S2468-1253(18)30269-3 [DOI] [PubMed] [Google Scholar]
- 26.Rezvani H, Mortazavizadeh SM, Allahyari A, et al. Efficacy and safety of proposed bevacizumab biosimilar BE1040V in patients with metastatic colorectal cancer: a phase III, randomized, double-blind, noninferiority clinical trial. Clin Ther. 2020;42(5):848-859. doi: 10.1016/j.clinthera.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 27.Qin S, Li J, Bai Y, et al. ; HLX04-mCRC03 Investigators . Efficacy, safety, and immunogenicity of HLX04 versus reference bevacizumab in combination with XELOX or mFOLFOX6 as first-line treatment for metastatic colorectal cancer: results of a randomized, double-blind phase III study. BioDrugs. 2021;35(4):445-458. doi: 10.1007/s40259-021-00484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stebbing J, Baranau Y, Baryash V, et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017;18(7):917-928. doi: 10.1016/S1470-2045(17)30434-5 [DOI] [PubMed] [Google Scholar]
- 29.von Minckwitz G, Colleoni M, Kolberg HC, et al. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19(7):987-998. doi: 10.1016/S1470-2045(18)30241-9 [DOI] [PubMed] [Google Scholar]
- 30.Pivot X, Bondarenko I, Nowecki Z, et al. Phase III, randomized, double-blind study comparing the efficacy, safety, and immunogenicity of SB3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2–positive early breast cancer. J Clin Oncol. 2018;36(10):968-974. doi: 10.1200/JCO.2017.74.0126 [DOI] [PubMed] [Google Scholar]
- 31.Lammers PE, Dank M, Masetti R, et al. Neoadjuvant PF-05280014 (a potential trastuzumab biosimilar) versus trastuzumab for operable HER2+ breast cancer. Br J Cancer. 2018;119(3):266-273. doi: 10.1038/s41416-018-0147-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hii J, Pivot X, Mclendon K, et al. Establishing analytical and clinical similarity between HD201 and herceptin. J Clin Oncol. 2020;38(suppl 15):579. [Google Scholar]
- 33.Rugo HS, Barve A, Waller CF, et al. ; Heritage Study Investigators . Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: a randomized clinical trial. JAMA. 2017;317(1):37-47. doi: 10.1001/jama.2016.18305 [DOI] [PubMed] [Google Scholar]
- 34.Pegram MD, Bondarenko I, Zorzetto MMC, et al. PF-05280014 (a trastuzumab biosimilar) plus paclitaxel compared with reference trastuzumab plus paclitaxel for HER2-positive metastatic breast cancer: a randomised, double-blind study. Br J Cancer. 2019;120(2):172-182. doi: 10.1038/s41416-018-0340-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexeev SM, Khorinko AV, Mukhametshina GZ, et al. Randomized double-blind clinical trial comparing safety and efficacy of the biosimilar BCD-022 with reference trastuzumab. BMC Cancer. 2020;20(1):783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu B, Zhang Q, Sun T, et al. ; HLX02-BC01 Investigators . Efficacy, safety, and immunogenicity of HLX02 compared with reference trastuzumab in patients with recurrent or metastatic HER2-positive breast cancer: a randomized phase III equivalence trial. BioDrugs. 2021;35(3):337-350. doi: 10.1007/s40259-021-00475-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplanov K, Zaritskiy A, Alexeev S, et al. Key results of international randomized open-label clinical study of BCD-020 (rituximab biosimilar candidate) in patients with B-cell non-Hodgkin’s lymphoma. Blood. 2014;124(21):5467. doi: 10.1182/blood.V124.21.5467.5467 [DOI] [Google Scholar]
- 38.Jurczak W, Moreira I, Kanakasetty GB, et al. Rituximab biosimilar and reference rituximab in patients with previously untreated advanced follicular lymphoma (ASSIST-FL): primary results from a confirmatory phase 3, double-blind, randomised, controlled study. Lancet Haematol. 2017;4(8):e350-e361. doi: 10.1016/S2352-3026(17)30106-0 [DOI] [PubMed] [Google Scholar]
- 39.Kim WS, Jurczak W, Sancho J-M, et al. Double-blind, randomized phase 3 study to compare efficacy and safety of the biosimilar CT-P10 to rituximab combined with CVP therapy in patients with previously untreated advanced-stage follicular lymphoma. J Clin Oncol. 2017;35(suppl 15):7532. doi: 10.1200/JCO.2017.35.15_suppl.7532 [DOI] [Google Scholar]
- 40.Ogura M, Sancho JM, Cho SG, et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 in comparison with rituximab in patients with previously untreated low-tumour-burden follicular lymphoma: a randomised, double-blind, parallel-group, phase 3 trial. Lancet Haematol. 2018;5(11):e543-e553. doi: 10.1016/S2352-3026(18)30157-1 [DOI] [PubMed] [Google Scholar]
- 41.Sharman JP, Liberati AM, Ishizawa K, et al. A randomized, double-blind, efficacy and safety study of PF-05280586 (a rituximab biosimilar) compared with rituximab reference product (MabThera) in subjects with previously untreated cd20-positive, low-tumor-burden follicular lymphoma (LTB-FL). BioDrugs. 2020;34(2):171-181. doi: 10.1007/s40259-019-00398-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poddubnaya IV, Alekseev SM, Kaplanov KD, et al. Proposed rituximab biosimilar BCD-020 versus reference rituximab for treatment of patients with indolent non-Hodgkin lymphomas: an international multicenter randomized trial. Hematol Oncol. 2020;38(1):67-73. doi: 10.1002/hon.2693 [DOI] [PubMed] [Google Scholar]
- 43.Niederwieser D, Hamm C, Cobb P, et al. Efficacy and safety of ABP 798: results from the JASMINE trial in patients with follicular lymphoma in comparison with rituximab reference product. Target Oncol. 2020;15(5):599-611. doi: 10.1007/s11523-020-00748-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Candelaria M, González DE, Delamain MT, et al. ; RTXM83 study . Rituximab biosimilar RTXM83 versus reference rituximab in combination with CHOP as first-line treatment for diffuse large B-cell lymphoma: a randomized, double-blind study. Leuk Lymphoma. 2019;60(14):3375-3385. doi: 10.1080/10428194.2019.1633632 [DOI] [PubMed] [Google Scholar]
- 45.Viswabandya A, Shah S, Mukhopadhyay A, et al. Randomized, double-blind, pharmacokinetic equivalence trial comparing DRL-rituximab with MabThera in patients with diffuse large B-cell lymphoma. J Glob Oncol. 2019;5:1-13. doi: 10.1200/JGO.19.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi Y, Song Y, Qin Y, et al. A phase 3 study of rituximab biosimilar HLX01 in patients with diffuse large B-cell lymphoma. J Hematol Oncol. 2020;13(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toogeh G, Faranoush M, Razavi SM, et al. A double-blind, randomized comparison study between Zytux vs MabThera in treatment of CLL with FCR regimen: non-inferiority clinical trial. Int J Hematol Oncol Stem Cell Res. 2018;12(2):84-91. [PMC free article] [PubMed] [Google Scholar]
- 48.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-792. doi: 10.1056/NEJM200103153441101 [DOI] [PubMed] [Google Scholar]
- 49.McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825-2833. doi: 10.1200/JCO.1998.16.8.2825 [DOI] [PubMed] [Google Scholar]
- 50.Maloney DG, Grillo-López AJ, White CA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90(6):2188-2195. doi: 10.1182/blood.V90.6.2188 [DOI] [PubMed] [Google Scholar]
- 51.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21(1):60-65. doi: 10.1200/JCO.2003.10.066 [DOI] [PubMed] [Google Scholar]
- 52.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335-2342. doi: 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 53.Cobleigh MA, Vogel CL, Tripathy D, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639-2648. doi: 10.1200/JCO.1999.17.9.2639 [DOI] [PubMed] [Google Scholar]
- 54.Moore TJ, Mouslim MC, Blunt JL, Alexander GC; Shermock KM. Assessment of availability, clinical testing, and US Food and Drug Administration review of biosimilar biologic products. JAMA Intern Med. 2021;181(1):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Yu S, Yang Z, et al. Efficacy and safety of anti-cancer biosimilars compared to reference biologics in oncology: a systematic review and meta-analysis of randomized controlled trials. BioDrugs. 2019;33(4):357-371. doi: 10.1007/s40259-019-00358-1 [DOI] [PubMed] [Google Scholar]
- 56.Galvão TF, Livinalli A, Lopes LC, Zimmermann IR, Silva MT. Biosimilar monoclonal antibodies for cancer treatment. Cochrane Database Syst Rev. 2020;(2):CD013539 doi: 10.1002/14651858.CD013539 [DOI] [Google Scholar]
- 57.Ridker PM, Torres J. Reported outcomes in major cardiovascular clinical trials funded by for-profit and not-for-profit organizations: 2000-2005. JAMA. 2006;295(19):2270-2274. doi: 10.1001/jama.295.19.2270 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Strategy for the Systematic Review and Meta-Analysis
eTable 1. Oncology Biosimilar Efficacy Trials
eTable 2. Quality Assessment Criteria and Risk of Bias Assessment
eFigure 1. Funnel Plot Analysis
eTable 3. Begg’s Test for Small-Study Effects
eTable 4. Egger’s Test for Small-Study Effects
eFigure 2. Funnel Plot by Disease-Measurement Subgroup
eFigure 3. Sensitivity Analysis for ORR, mCRC Removing Romera et al
eFigure 4. Sensitivity Analysis for pCR, ERBB2+ Early Breast Cancer Removing Pivot et al and Lammers et al
eFigure 5. Sensitivity Analysis for ORR, DLBCL Removing Viswabandya et al