Abstract
Mutation of the gene Tafazzin (TAZ) causes Barth syndrome, an X-linked disorder characterized by cardiomyopathy, skeletal muscle weakness, and neutropenia. TAZ is an acyltransferase that catalyzes the remodeling of cardiolipin, the signature phospholipid of the inner mitochondrial membrane. Here we review the major model systems that have been established to study the role of cardiolipin remodeling in mitochondrial function and the pathogenesis of Barth syndrome. We summarize key features of each model and provide examples of how each has contributed to advance our understanding of TAZ function and Barth syndrome pathophysiology.
Introduction
Mutation of the gene Tafazzin (TAZ) causes Barth syndrome1. TAZ is an acyltransferase required for the remodeling of cardiolipin (CL), a hallmark phospholipid of the mitochondrial inner membrane. In normal tissues with high metabolic demand, the four acyl chains of CL have a narrow, characteristic composition (e.g. tetralinoleoyl-CL in striated muscles). Because enzymes in its synthetic pathway lack acyl chain specificity, de novo synthesized CL has greater acyl chain diversity and tends to be more saturated. Remodeling CL into its mature form involves exchange of acyl chains, through phospholipase-catalyzed removal of one acyl chain to form monolysocardiolipin (MLCL) followed by reacylation, or through transacylation, both catalyzed by TAZ.2,3 Patients lacking functional TAZ have decreased total CL, increased CL saturation and diversity of acyl chains, and increased MLCL to CL ratio.2,4 These abnormalities of CL composition impair the function of proteins of the inner mitochondrial membrane, including F1F0 ATPase, components of the electron transport chain, which collectively lead to the manifestations of Barth syndrome. While TAZ is ubiquitously expressed and CL abnormalities in Barth syndrome are widespread, the cardinal disease manifestations -- cardiomyopathy, skeletal myopathy, and neutropenia5,6 -- are tissue restricted. The precise mechanisms by which mutation of TAZ and impaired CL biosynthesis lead to these tissue restricted phenotypes remain imprecisely defined.
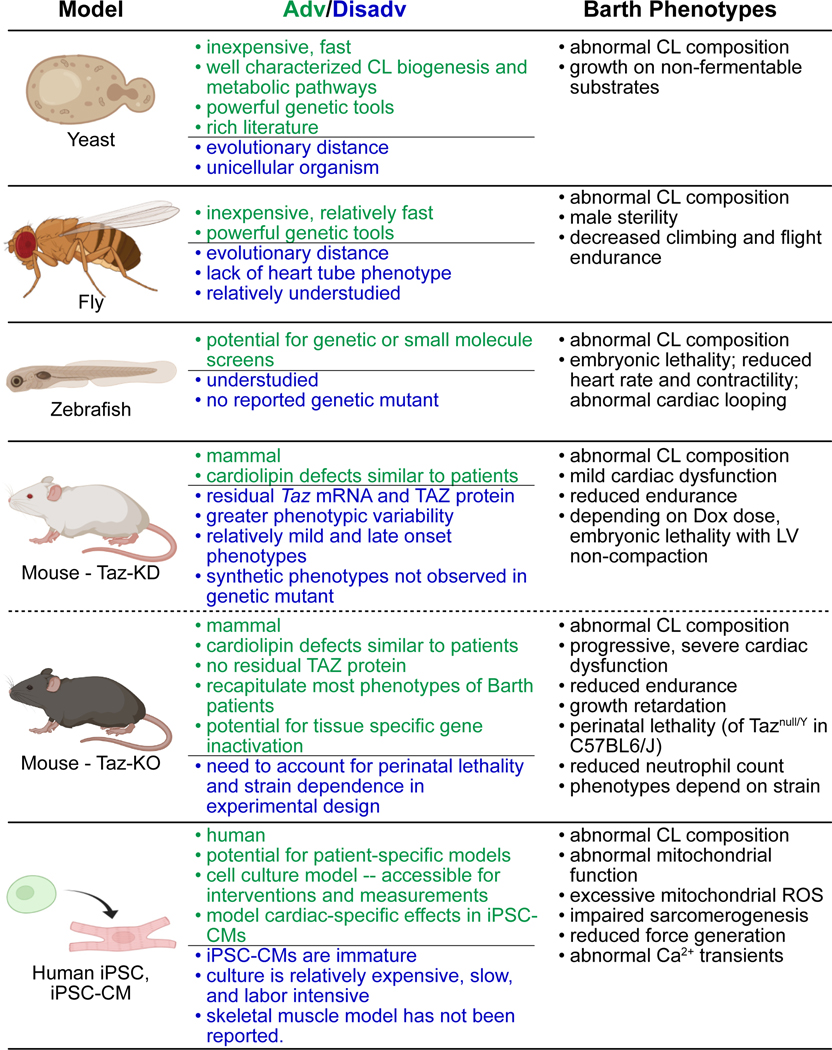
Although CL composition varies between species, the steps of CL biogenesis and its impairment by TAZ mutation are well conserved throughout eukaryotes, from yeast through humans. To improve biological understanding of TAZ function and Barth Syndrome pathogenesis, and to expedite development of effective therapies, diverse model systems have been established to study the consequences of TAZ mutation. Here we review these major model systems and highlight important findings (Fig. 1).
Figure 1. Experimental models of Barth syndrome.
Table lists major model systems, some advantages or disadvantages of each model system, and key phenotypes reportedl.
Yeast
Saccharomyces cerevisiae, baker’s yeast, are eukaryotic cells that are readily cultured and genetically manipulated. Powerful genetic and biochemical tools have been developed to study yeast. These features, combined with their relatively low cost and short experimental timelines, make yeast a superb model system to study gene functions that are highly conserved and manifest in unicellular organisms. At the same time, yeast cannot model aspects of gene function that are less conserved or selectively expressed in multicellular organisms, and it has limited utility as a pre-clinical model to test candidate therapies.
Yeast lacking taz1, the yeast homolog of TAZ, were unable to grow on non-fermentable carbon sources at elevated temperature7,8. Like human TAZ mutant cells, yeast taz1 mutants had reduced overall CL, reduced unsaturated acyl chains, and elevated monolysocardiolipin (MLCL). Human TAZ complemented the yeast taz1 mutation, indicating functional conservation.8. These features make yeast a highly tractable model system to study the function of TAZ and CL.
Several key findings have been made in the yeast system. In yeast, the phospholipase that catalyzes CL deacylation to MLCL is CLD19; the corresponding enzyme(s) in mammals have not been identified. Cld1 deletion blocks CL remodeling such that de novo synthesized, saturated CL predominates. Remarkably, Cld1 deletion rescued the growth defect of Δtaz1 yeast.10,11 Changes in MLCL/CL and total CL level, but not CL saturation, mirrored the severity of the growth defect: compared to wild-type, Δtaz1 had low overall CL level, reduced unsaturation, high MLCL/CL, and impaired growth, whereas Δcld1Δtaz1 had normal overall CL level, reduced unsaturation, low MLCL/CL, and normal growth. These data suggest that saturated CL can fulfill many functions of unsaturated CL, and that elevated MLCL/CL or reduced total CL cause the deleterious effects of TAZ mutation. Studies of yeast with mutations in CL biosynthesis genes also implicated CL in the regulation of mitochondrial iron homeostasis and the iron-sulfur cluster biogenesis12,13, intermediary metabolism including the TCA cycle14,15, and to increased oxidative stress,16 which may contribute to Barth syndrome pathophysiology in humans and mice (see below). Genetic screens in yeast have identified genetic modifiers of taz1 mutation. A synthetic screen for genes whose inactivation results in growth deficiency in combination with taz1 mutation identified Yme1, a mitochondrial quality control protease17 On the other hand, screens for taz1 suppressors identified Odc1p, a conserved carrier of a-ketoglutarate and other Krebs cycle intermediates located in the mitochondrial inner membrane,18 and cycloheximide, a protein synthesis inhibitor.19 It will be interesting to determine if these genes and pathways also modify TAZ deficiency in mammalian models.
The yeast system has also been useful to functionally assess TAZ splice isoforms and human TAZ mutations. Interestingly, of several human TAZ splice isoforms tested, only one, lacking the primate-specific exon 5, complemented, indicating that only this isoform possesses conserved acyltransferase activity in yeast.8 Yeast have also been used to dissect the pathogenic mechanism of human mutations. Based on genetic complementation and biochemistry, twenty one conserved human missense mutants were classified into those that affect TAZ catalytic activity, localization, and stability.20–22
Fruit fly
Drosophila melanogaster is a powerful invertebrate system that has been employed to investigate the pathobiology of Taz mutation. Like yeast, advanced genetic approaches and reagents have been developed to dissect pathogenic mechanisms that are conserved between humans and flies. Flies also have specialized cell types, including muscle cells and a rudimentary heart tube, that are relevant to Barth syndrome, and have matured as a model to study diseases of these organ systems.23,24 However, because of the evolutionary distance between flies and humans, flies are an imperfect model of human pathophysiology.
Taz null flies had reduced total CL and reduced unsaturated acyl chains.25 Mutant indirect wing muscles contained clusters of mitochondria with abnormal cristae.25 Although not grossly apparent, in quantitative assays mutant adult flies had muscle weakness, as demonstrated by reduced ability to fly and to climb against gravity.25,26 Moreover, Taz mutant flies had reduced endurance that failed to improve with exercise training,26 a phenotype highly relevant to patients, who commonly manifest muscle weakness as profound fatigue following minimal exertion.27 However, the beat rate, contractility, and compliance of Taz mutant fly heart tubes was normal25,28 and did not show increased susceptibility to pacing-induced heart failure.26 Flies were also used to test human and Drosophila Taz splice isoforms. Unlike yeast, in which only the isoform lacking primate-specific exon 5 was active,8 in flies isoforms both with and without exon 5 had transacylase activity.29
Male Taz null flies were also sterile as a result of defective spermatid individualization.30 Although male sterility is not a feature of patients with Barth syndrome, this phenotype nevertheless was useful to establish the importance of CL metabolism in mediating the pathogenic effects of Taz mutation in animals. Analogous to the finding that cld1 mutation mitigated the phenotype of taz1 mutant yeast, mutation of Pla2-VIA in flies suppressed the male sterile phenotype of Taz mutant flies. Taz mutation decreased CL levels, increased MLCL/CL ratio, and increased the diversity of CL species. Pla2-VIA; Taz double mutants restored CL levels and MLCL/CL ratio but did not alter the diversity of CL species. However, in wild-type flies Pla2-VIA was not required for CL remodeling. The effect of Pla2-VIA mutation on the muscle phenotypes of Taz mutant flies was not reported. These findings suggest that suppression of the phospholipase(s) that mediate CL deacylation in humans may be a therapeutic strategy to treat Barth syndrome. However, the identity of the responsible phospholipase(s) and their function in other aspects of cellular homeostasis are currently unknown.
The reproducible exercise and sterility phenotypes of Drosophila could be readily adapted to take advantage of the power of fly genetics to identify genes that genetically interact with Taz and modulate the phenotypes of heart, skeletal muscle, and sperm.
Zebrafish
Danio rerio has matured into a potent vertebrate model system amenable to both genetic and small molecule screens. As inexpensive vertebrates with well developed forward and reverse genetics and well established systems to study cardiac and skeletal muscle physiology, zebrafish occupy a unique position among the major model organisms.31,32 Morpholino knockdown of taz in zebrafish embryos yielded among the first animal models of Barth syndrome.33 Morpholinos reduced heart rate, contraction, and looping, and caused embryonic lethality. Specificity of these effects was supported by phenotypic rescue by co-injected morpholino-resistant taz mRNA.
Despite this promising beginning, zebrafish have not subsequently been used to model Barth syndrome. CRISPR/Cas9 has now enabled generation of targeted mutations in zebrafish,34 and comparison between morpholino and targeted mutant phenotypes has raised questions about morphant specificity.35 Surprisingly, genetic taz mutant fish have not been reported. Such fish could be used to study mature heart phenotypes in taz mutant fish, to perform genetic interaction studies, and to conduct small molecule screens for suppressors of the taz mutant phenotype.36,37
Mice
Mus musculus is the most frequently used mammalian model of disease. Some key advantages of mice are that they are amenable genetic manipulation, their husbandry is less expensive compared to other mammals, and an enormous set of investigative tools, reagents, and reference literature on mice has arisen as a result of their intensive study.
Initial efforts to create genetically modified Taz mutant mice were unsuccessful; in retrospect, this was likely due to male sterility of Taz mutant mice (discussed below), which precluded germline transmission of genetic modifications made in male embryonic stem cells. To circumvent this difficulty, a genetic model was created by developing transgenic mice in which administration of doxycycline (Dox) induced expression of a short hairpin RNA that depleted Taz mRNA by over 90%. This Taz knockdown (Taz-KD) model yielded the first mammalian model of Taz depletion.38,39 Induction of Taz knockdown by administration of Dox in chow at 200–625 mg/kg throughout most of gestation and postnatal life resulted in 90–97% reduction of Taz mRNA in heart. Tetralinoleoyl-CL, the most abundant CL species in mammalian striated muscle, was markedly decreased, MLCL accumulated, total CL was reduced, and unsaturated CL decreased. In younger mice (2 mo), cardiac mitochondrial genome copy number was increased; however significantly more frequent alterations in mitochondrial ultrastructure, most commonly involving cristae morphology, were not evident until 8 months. At 2 months of age, cardiac function was normal. Mild cardiomyopathy was evident at 7–10 months. Skeletal muscle force-frequency was only mild impaired in Taz-KD. However, by 5 months of age Taz-KD mice exhibited a dramatic reduction in maximal exercise capacity. Indirect calorimetry revealed that Taz-KD mice did not differ significantly from controls at baseline. During exercise, Taz-KD mice had markedly impaired oxygen utilization, earlier switch to carbohydrate substrates, and increased lactic acid production,40 which parallel observations made in Barth patients.41 Reduced neutrophil counts were alluded to but not explicitly described in the initial characterization of the Taz-KD model.39 These investigators also noted that Dox itself may affect neutrophil counts, potentially confounding studies of neutropenia using this model.
Another group studied the same Taz-KD mice but used a different Dox dosing regimen -- 2 mg/ml Dox in 10% sucrose drinking water -- which resulted in an estimated 3–10x higher Dox intake42. Taz-KD had reduced fetal survival and those that survived to term had high perinatal lethality. Cardiac histological sections demonstrated myocardial non-compaction and diminished cardiomyocyte proliferation, and electron microscopy revealed that fetal cardiomyocytes had abnormal mitochondrial ultrastructure and sarcomeric organization. Fetal heart systolic function was not significantly reduced, although reduced peak velocity in the dorsal aorta may have been consistent with subtle reduction of systolic function. The doppler blood flow pattern in the dorsal aorta also suggested possible diastolic dysfunction. These data were interpreted to indicate that Taz deficiency in Taz-KD caused abnormal fetal heart development, cardiac non-compaction, and fetal and perinatal death. Notably, fetal loss in this model was more severe than observed in genetic Taz null embryos (see below), suggesting that factors in addition to Taz deficiency likely contribute to this embryonic demise. For example, Dox affects mitochondria43 and metalloproteases44, suggesting the possibility that high Dox interacts with Taz deficiency to impair fetal survival. Although germline Taz null mutation in C57BL6/J mice causes high perinatal loss45, this was not due to inactivation of Taz in fetal cardiomyocytes, since conditional ablation of Taz early after cardiogenesis by Tnnt2-Cre did not impair fetal or perinatal survival (Wang and Pu, unpublished).
Studies using the Taz-KD model need to be interpreted with several caveats in mind. First, TAZ is incompletely ablated. Unlike most patients with TAZ loss of function mutations, Taz-KD had 3–10% residual Taz mRNA,38,39 and protein levels as high as 45% of controls.46 Second, the genetic background of the mice in initial reports was “C57BL6/129S6”, and studies of the germline null Taz mouse have now revealed that strain background is a critical variable (see below). Third, the dose of Dox is an important variable47 and can account for differences between studies. Moreover, nursing pups likely receive a low dose of Dox. Although murine data are not available, human data suggest that Dox excretion into milk is low, so that infant serum levels are approximately 6% those of the mother (LactMed: https://www.ncbi.nlm.nih.gov/books/NBK500561/), which may contribute to late onset and mild phenotypes observed in Taz-KD mice. Fourth, there are inconsistencies between the knockdown and knockout models that suggest that some aspects of the Taz-KD phenotype cannot be explained solely by depletion of Taz. High embryonic attrition reported for Taz-KD mice under the high Dox dosing regimen described above is one example. Another is the development of increased left ventricular wall thickness with preserved systolic function reported as the cardiac phenotype in some studies of aged Taz-KD mice.48 To test the hypothesis that increased mitochondrial production of reactive oxygen species (ROS) contributes to the pathogenesis of heart disease in Barth syndrome, Taz-KD mice were crossed to mice that express mitochondrially targeted catalase (MCAT).48 This study found that Taz-KD mice did have elevated levels of reactive oxygen species, but the cardiac phenotype was not altered by the MCAT transgene, leading the authors to conclude that elevated ROS is not required for cardiac pathology in this model. However, the cardiac pathology reported, left ventricular wall thickening, was not observed in the initial description of Taz-KD mice on the same Dox dose, and furthermore increased wall thickness was not observed in Taz knockout mice. Although a hypertrophic cardiomyopathy pattern has been described in some Barth syndrome patients, the inconsistent cardiac phenotype in the Taz-KD model across studies and the possibility that the hypertrophic phenotype is synthetic and not solely attributable to Taz deficiency preclude reaching a clear conclusion about the contribution of ROS to Barth syndrome cardiomyopathy. Notably, another study of the Taz-KD model did not find elevated production of mitochondrial oxidants and questioned its contribution to Barth syndrome pathogenesis.49
Mice with genetically targeted Taz have now been developed. A conditional Taz allele was created by flanking exons 5 to 10 with loxP sites. Chimeric mice transmitted this allele in the germline, yielding the Tazflox allele. Germline Cre-mediated recombination deleted exons 5–10, resulting in the constitutive Taznull allele.45,50 Taznull and Tazflox alleles were extensively backcrossed into the C57BL6/J background, and initial cardiovascular characterization of these mice was performed in this background. Western blotting confirmed that this allele is protein null.45 As expected, TAZnull/Y had elevated MLCL/CL and increased CL diversity. Mutant cardiac mitochondria were smaller but more numerous and had simplified cristae. TAZnull/Y mice had slightly reduced survival to term, but most of these neonates die in the first several days after birth. TAZnull/Y neonates had lower body weight than control littermates, and those most likely to perish had the most severely reduced birth weight. Cardiac selective inactivation of Taz did not impair fetal or neonatal survival, suggesting that perinatal death was non-cardiac (see below). These neonates had reduced motor activity and righting behavior, and less frequent milk spots, suggesting that skeletal muscle weakness, maternal culling behavior, and inability to compete with more vigorous littermates caused postnatal demise.
Taznull/Y mice had lower body weight and length than their littermates, similar to pre-pubertal growth retardation observed in Barth patients. However, in humans this growth delay is often abrogated by late catch up growth,6 whereas Taznull/Y mice were smaller than littermates throughout life.6,51 Taznull/Y mice had progressive dilated cardiomyopathy, with cardiac systolic function becoming measurably depressed by 3 months of age.45 Hearts also exhibited myocardial fibrosis and increased cardiomyocyte apoptosis. These mutant mice also had skeletal muscle disease, as demonstrated by markedly reduced endurance on exercise treadmill testing. Neutrophil counts were lower in Taznull/Y mice than littermate controls but remained within the normal range for mice. Taznull/Y male mice are sterile due to defective spermatogenesis.50,52 Mechanistic investigation revealed that Taz and mitochondria make a novel contribution to acrosomes, specialized structures within the head of sperm.
The perinatal lethality of C57BL6/J Taznull/Y mice can make this model cumbersome for studies of postnatal phenotypes. Conditional mutagenesis can be used to circumvent this difficulty. Myh6-Cre selectively inactivates floxed alleles in cardiomyocytes, usually by embryonic day 12.5. Myh6-Cre; Tazfl/Y mice lack Taz expression in postnatal cardiomyocytes.45 These mice survive normally and develop progressive dilated cardiomyopathy that is slightly more aggressive than observed in Taznull/Y mice, perhaps because the most severely affected Taznull/Y mice die perinatally, resulting in survivor bias.
A critical factor to consider in the design and interpretation of experiments using Taz mutant mice is strain background. By crossing Taznull/WT female mice in the C57BL6/J strain background with mice in other inbred strains, we obtained Taznull/Y F1 males with different strain backgrounds. Survival, cardiomyopathy, and skeletal muscle involvement varied tremendously by strain background, suggestive of strong genetic modifiers (Wang and Pu, unpublished). Identification of these genetic modifiers may lead to novel therapeutic strategies to treat Barth syndrome and may explain the highly variable expression of TAZ mutation in Barth patients. From an experimental standpoint, one can manipulate strain background to adjust survival and the severity of cardiomyopathy or skeletal muscle phenotypes. At the same time, the experimental design must strictly control for effects of genetic background, and greater variability can be expected in mixed genetic backgrounds.
The Taz-KD, Taznull/Y, and Myh6-Cre; Tazflox/Y mouse models have all been used to test the efficacy of AAV-Taz gene therapy.45,46 Taz-KD mice were treated with self-complementary AAV9 that expressed full length human TAZ from the Desmin promoter.46 At a dose of 1E13 vg/kg, the vector increased TAZ protein levels from 45% of control to approximately 115%. The fraction of transduced cardiomyocytes was not measured. This treatment significantly improved cardiac systolic function above untreated levels, but function remained well below control levels. Skeletal muscle function, measured by spontaneous activity measurements after light exercise, were improved to normal levels. Taznull/Y mice were treated with standard or self-complementary AAV9 that expressed full length human TAZ from the synthetic CAG promoter.45 AAV, administered on the second postnatal day at a dose that transduced ~65% of cardiomyocytes and ~60% of skeletal muscle cells, rescued perinatal lethality, and returned cardiac function to normal. However, at 4 months of age, treated mice exhibited declining heart function, likely reflecting loss or dysfunction of non-transduced cardiomyocytes. The cardiac specific Myh6-Cre; Tazflox/Y model was used to further study dose-response. A dose of 2E13 vg/kg, which transduced ~70% of adult cardiomyocytes, prevented cardiac dysfunction for over 4 months, whereas a dose that transduced ~30% of cardiomyocytes had a more variable and less durable effect. Moreover, the higher dose also was able to reverse mild established cardiac dysfunction and improve exercise capacity. Overall, both gene therapy studies provide proof-of-concept that AAV-TAZ gene therapy could be effective therapy for Barth syndrome, although transduction of a large majority of muscle cells is likely required for durable efficacy. One question not yet addressed is the optimal TAZ splice isoform to use for gene therapy. Both full length and exon 5-deleted splice isoforms have transacylase activity, but their relative activity in rescuing BTHS phenotype needs to be established.
In summary, Taznull/Y and Cre; Tazflox/Y mice are excellent models of Barth syndrome to study disease mechanisms and to test the efficacy and safety of proposed therapies.
Human induced pluripotent stem cells
A number of mammalian cell models have been developed to study the effects of TAZ deficiency, including immortalized patient-derived lymphocytes53 or skin fibroblasts54, C2C12 murine skeletal myoblasts with engineered TAZ mutation55, patient-derived induced pluripotent stem cells (iPSCs) and iPSC-derived cardiomyocytes (iPSC-CMs),56,57 and human iPSCs and iPSC-CMs with engineered TAZ mutation.56 Here we focus on human iPSC and iPSC-CM models as these are unique human models of relevant affected cell types.
iPSCs harboring disease-causing mutations can be reprogrammed from patients’ somatic cells or generated from reference wild-type iPSC lines by introduction of patient-derived mutations through genome editing. For studies of biological mechanisms, genome-edited wild-type and mutant lines are often preferable, because the control cells should be isogenic except for the introduced mutation. Patient-derived lines afford the additional opportunity to investigate effects of the patient’s genetic background on disease manifestations and treatment responses. Wild-type reference iPSC lines can be used as non-isogenic controls, or the patient mutations can be corrected through genome editing to yield an isogenic control for each patient-derived mutant line. iPSCs are differentiated into iPSC-CMs by well-established, highly efficient protocols, yielding cardiomyocyte-like cells.58,59 These cells spontaneously beat and develop cardiomyocyte-like action potentials and calcium transients. However, their structural and functional properties are comparable to fetal or neonatal cardiomyocytes, and in most cases these cells lack properties of adult cardiomyocytes, such as rectangular shape, T-tubules, highly ordered sarcomeres, and reliance on oxidative phosphorylation.60 The immaturity of iPSC-CMs is an important caveat that should kept in mind while interpreting the results of experiments using iPSC-CMs.
Patient-derived and genetically engineered iPSCs57 and iPSC-CMs56,61 that lack TAZ have expected derangements in CL biogenesis. Mitochondrial function assays reproducibly demonstrated marked reduction in maximal electron transport chain activity, which has been linked to disassembly of respiratory chain supercomplexes and cardiac-specific succinate dehydrogenase deficiency.61 TAZ mutant iPSC-CMs had high levels of mitochondrial ROS,56 likely reflecting escape of electrons from partially disassembled respiratory chain complexes. Sarcomere assembly was markedly impaired in TAZ mutant iPSC-CMs, and correspondingly engineered heart tissues built from TAZ mutant iPSC-CMs had severely compromised ability to generate contractile force.56 These phenotypes were rescued by a mitochondrially targeted ROS scavenger, but not by culture conditions that normalized ATP levels,56 suggesting that elevated ROS generation participates in the pathogenesis of contractile dysfunction, at least in this cell culture model.
One mechanism by which mitochondrial ROS might affect sarcomere assembly and contractile function is through oxidation and activation of protein kinases. A key ROS-sensitive kinase in the heart is Ca2+-calmodulin-dependent protein kinase II (CaMKII).62 Excessive activation of this kinase is pro-arrhythmic and deleterious to heart function.63 In TAZ mutant iPSC-CMs, ROS activated CaMKII, which increased phosphorylation of RYR2, the main cardiomyocyte intracellular Ca2+ release channel, on serine 2814, resulting in elevated diastolic Ca2+, reduced Ca2+ transient amplitude, and increased frequency of spontaneous Ca2+ release events.64 These findings were suppressed by ROS scavenger, CaMKII inhibitor, and genetic ablation of the RYR2-S2814 phosphorylation site. CaMKII inhibition likewise improved the function of Taz mutant cardiomyocytes isolated from Myh6-Cre; Tazfl/Y mice. These data implicate ROS activation of CaMKII in the pathogenesis of cardiac dysfunction and arrhythmia, at least in the iPSC-CM Barth syndrome model.
These studies show that iPSCs and iPSC-CMs are useful models to dissect disease mechanisms, despite their immaturity. These models are amenable to genetic and small molecule screening approaches, which could be used to further develop mechanistic insights or novel therapies in Barth syndrome. iPSCs can now also be differentiated into human skeletal muscle-like cells.65 Given the importance of muscle fatigue in Barth syndrome manifestations,65 an iPSC-derived skeletal muscle model would appear to be a promising direction for future studies. Similarly, a human iPSC-based model could provide insights into the mechanisms by which TAZ mutation causes neutropenia. Patient-derived iPSCs may be useful to build patient-specific disease models, which it is hoped will enable precision medicine approaches by predicting therapeutic responses of individual patients.66 However, accuracy with which such patient-specific models predict individual responses has not yet been established.
Conclusions
In summary, a wealth of model systems have been developed to study the pathological effects of TAZ mutation. These model systems have put researchers in an excellent position to dissect the pathophysiology of Barth syndrome and to discover new therapeutic approaches. While much progress has been made, key questions remain regarding the mechanistic links between TAZ mutation and abnormal CL composition and the manifestations of Barth syndrome. For instance, the contribution of elevated ROS to Barth syndrome pathogenesis remains controversial. Is there a mammalian counterpart of yeast Cld1 and Drosophila Pla2-VIA, and if so could its inhibition ameliorate Barth syndrome manifestations? What are the mechanisms by which TAZ mutation causes neutropenia and growth delay? What is the mechanism that accounts for the wide variation in disease severity observed between Barth patients, and between inbred mouse strains, and could this mechanism be therapeutically exploited? The TAZ-KO model will also be invaluable for preclinical testing of emerging candidate therapies. An important caveat is that mice and humans have considerable physiological differences, and it currently is not known the degree to which therapeutic efficacy in the TAZ-KO model predicts the responses of patients. One important practical hurdle is optimizing this model to study skeletal muscle disease and therapies targeting this indication: in the pure C57BL6/J background, high neonatal loss is likely due to skeletal muscle disease and therefore cannot be circumvented by tissue-specific gene knockout. Potential solutions are temporally controlled gene inactivation or selection of a different inbred strain background that circumvents lethality yet exhibits significant skeletal muscle disease.
Acknowledgements
WTP was supported by funding from the US National Institutes of Health (UH3 HL141798), the US Department of Defense (PR200113), and the Barth Syndrome Foundation. I thank Suya Wang and Mason Sweat for their constructive feedback.
Footnotes
Competing Interests
WTP previously received sponsored research funding from Stealth BioTherapeutics to study elamipretide in Barth syndrome. WTP holds intellectual property on gene therapy for Barth syndrome. WTP is on the Scientific and Medical Advisory Board of the Barth Syndrome Foundation.
References
- 1.Bione S, D’Adamo P, Maestrini E, Gedeon AK, Bolhuis PA & Toniolo D. A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nat. Genet 12, 385–389 (1996). [DOI] [PubMed] [Google Scholar]
- 2.Schlame M & Greenberg ML Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 3–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye C, Shen Z. & Greenberg ML Cardiolipin remodeling: a regulatory hub for modulating cardiolipin metabolism and function. J. Bioenerg. Biomembr 48, 113–123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlame M, Kelley RI, Feigenbaum A, Towbin JA, Heerdt PM, Schieble T, Wanders RJ, DiMauro S. & Blanck TJ Phospholipid abnormalities in children with Barth syndrome. J. Am. Coll. Cardiol 42, 1994–1999 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Clarke SLN, Bowron A, Gonzalez IL, Groves SJ, Newbury-Ecob R, Clayton N, Martin RP, Tsai-Goodman B, Garratt V, Ashworth M, Bowen VM, McCurdy KR, Damin MK, Spencer CT, Toth MJ, Kelley RI & Steward CG Barth syndrome. Orphanet J. Rare Dis 8, 23 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts AE, Nixon C, Steward CG, Gauvreau K, Maisenbacher M, Fletcher M, Geva J, Byrne BJ & Spencer CT The Barth Syndrome Registry: distinguishing disease characteristics and growth data from a longitudinal study. Am. J. Med. Genet. A 158A, 2726–2732 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Gu Z, Valianpour F, Chen S, Vaz FM, Hakkaart GA, Wanders RJA & Greenberg ML Aberrant cardiolipin metabolism in the yeast taz1 mutant: a model for Barth syndrome. Mol. Microbiol 51, 149–158 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Vaz FM, Houtkooper RH, Valianpour F, Barth PG & Wanders RJ Only one splice variant of the human TAZ gene encodes a functional protein with a role in cardiolipin metabolism. J. Biol. Chem 278, 43089–43094 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Beranek A, Rechberger G, Knauer H, Wolinski H, Kohlwein SD & Leber R. Identification of a cardiolipin-specific phospholipase encoded by the gene CLD1 (YGR110W) in yeast. J. Biol. Chem 284, 11572–11578 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye C, Lou W, Li Y, Chatzispyrou IA, Hüttemann M, Lee I, Houtkooper RH, Vaz FM, Chen S. & Greenberg ML Deletion of the cardiolipin-specific phospholipase Cld1 rescues growth and life span defects in the tafazzin mutant: implications for Barth syndrome. J. Biol. Chem 289, 3114–3125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baile MG, Sathappa M, Lu Y-WW, Pryce E, Whited K, McCaffery JM, Han X, Alder NN & Claypool SM Unremodeled and remodeled cardiolipin are functionally indistinguishable in yeast. J. Biol. Chem 289, 1768–1778 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patil VA, Fox JL, Gohil VM, Winge DR & Greenberg ML Loss of cardiolipin leads to perturbation of mitochondrial and cellular iron homeostasis. J. Biol. Chem 288, 1696–1705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Lou W, Grevel A, Böttinger L, Liang Z, Ji J, Patil VA, Liu J, Ye C, Hüttemann M, Becker T. & Greenberg ML Cardiolipin-deficient cells have decreased levels of the iron–sulfur biogenesis protein frataxin. J. Biol. Chem 295, 11928–11937 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raja V. & Greenberg ML The functions of cardiolipin in cellular metabolism-potential modifiers of the Barth syndrome phenotype. Chem. Phys. Lipids 179, 49–56 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raja V, Joshi AS, Li G, Maddipati KR & Greenberg ML Loss of Cardiolipin Leads to Perturbation of Acetyl-CoA Synthesis. Journal of Biological Chemistry 292, 1092–1102 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, He Q. & Greenberg ML Loss of tafazzin in yeast leads to increased oxidative stress during respiratory growth. Mol. Microbiol 68, 1061–1072 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaspard GJ & McMaster CR The mitochondrial quality control protein Yme1 is necessary to prevent defective mitophagy in a yeast model of Barth syndrome. J. Biol. Chem 290, 9284–9298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Taffin de Tilques M, Tribouillard-Tanvier D, Tétaud E, Testet E, di Rago J-P & Lasserre J-P Overexpression of mitochondrial oxodicarboxylate carrier (ODC1) preserves oxidative phosphorylation in a yeast model of Barth syndrome. Dis. Model. Mech 10, 439–450 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Taffin de Tilques M, Lasserre J-P, Godard F, Sardin E, Bouhier M, Le Guedard M, Kucharczyk R, Petit PX, Testet E, di Rago J-P & Tribouillard-Tanvier D. Decreasing cytosolic translation is beneficial to yeast and human Tafazzin-deficient cells. Microb. Cell Fact 5, 220–232 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y-W, Galbraith L, Herndon JD, Lu Y-L, Pras-Raves M, Vervaart M, Van Kampen A, Luyf A, Koehler CM, McCaffery JM, Gottlieb E, Vaz FM & Claypool SM Defining functional classes of Barth syndrome mutation in humans. Hum. Mol. Genet 25, 1754–1770 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whited K, Baile MG, Currier P. & Claypool SM Seven functional classes of Barth syndrome mutation. Hum. Mol. Genet 22, 483–492 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Claypool SM, Whited K, Srijumnong S, Han X. & Koehler CM Barth syndrome mutations that cause tafazzin complex lability. J. Cell Biol 192, 447–462 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ocorr K, Vogler G. & Bodmer R. Methods to assess Drosophila heart development, function and aging. Methods 68, 265–272 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunage RD, Dhanyasi N, Reichert H. & VijayRaghavan K. Drosophila adult muscle development and regeneration. Semin. Cell Dev. Biol 72, 56–66 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Condell M, Plesken H, Edelman-Novemsky I, Ma J, Ren M. & Schlame M. A Drosophila model of Barth syndrome. Proc. Natl. Acad. Sci. U. S. A 103, 11584–11588 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damschroder D, Reynolds C. & Wessells R. Drosophila tafazzin mutants have impaired exercise capacity. Physiol Rep 6, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowen VM, Milligan E, McCormack S, McCurdy K, Smart T, Sherriff L, Toth M. & Valentine J. The Voice of the Patient: Barth Syndrome. A report on the externally-led patient-focused drug development meeting. (Barth Syndrome Foundation, 2019). [Google Scholar]
- 28.Acehan D, Khuchua Z, Houtkooper RH, Malhotra A, Kaufman J, Vaz FM, Ren M, Rockman HA, Stokes DL & Schlame M. Distinct effects of tafazzin deletion in differentiated and undifferentiated mitochondria. Mitochondrion 9, 86–95 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Zhang S, Malhotra A, Edelman-Novemsky I, Ma J, Kruppa A, Cernicica C, Blais S, Neubert TA, Ren M. & Schlame M. Characterization of tafazzin splice variants from humans and fruit flies. J. Biol. Chem 284, 29230–29239 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malhotra A, Edelman-Novemsky I, Xu Y, Plesken H, Ma J, Schlame M. & Ren M. Role of calcium-independent phospholipase A2 in the pathogenesis of Barth syndrome. Proc. Natl. Acad. Sci. U. S. A 106, 2337–2341 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowley G, Kugler E, Wilkinson R, Lawrie A, van Eeden F, Chico TJA, Evans PC, Noël ES & Serbanovic-Canic J. Zebrafish as a tractable model of human cardiovascular disease. Br. J. Pharmacol (2021). doi: 10.1111/bph.15473 [DOI] [PubMed] [Google Scholar]
- 32.Goody MF, Carter EV, Kilroy EA, Maves L. & Henry CA ‘Muscling’ Throughout Life: Integrating Studies of Muscle Development, Homeostasis, and Disease in Zebrafish. Curr. Top. Dev. Biol 124, 197–234 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khuchua Z, Yue Z, Batts L. & Strauss AW A zebrafish model of human Barth syndrome reveals the essential role of tafazzin in cardiac development and function. Circ. Res 99, 201–208 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh J-RJ & Joung JK Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol 31, 227–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kok FO, Shin M, Ni C-W, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, DeSantis DF, Sheppard-Tindell S, Ebarasi L, Betsholtz C, Schulte-Merker S, Wolfe SA & Lawson ND Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 32, 97–108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keßler M, Rottbauer W. & Just S. Recent progress in the use of zebrafish for novel cardiac drug discovery. Expert Opin. Drug Discov 10, 1231–1241 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J, Wu JC, MacRae CA, Kléber AG & Saffitz JE Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci. Transl. Med 6, 240ra74 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A. & Khuchua Z. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J. Biol. Chem 286, 899–908 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soustek MS, Falk DJ, Mah CS, Toth MJ, Schlame M, Lewin AS & Byrne BJ Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency. Hum. Gene Ther 22, 865–871 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powers C, Huang Y, Strauss A. & Khuchua Z. Diminished Exercise Capacity and Mitochondrial bc1 Complex Deficiency in Tafazzin-Knockdown Mice. Front. Physiol 4, 74 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spencer CT, Byrne BJ, Bryant RM, Margossian R, Maisenbacher M, Breitenger P, Benni PB, Redfearn S, Marcus E. & Cade WT Impaired cardiac reserve and severely diminished skeletal muscle O2 utilization mediate exercise intolerance in Barth syndrome. Am. J. Physiol. Heart Circ. Physiol 301, H2122–9 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Phoon CKL, Acehan D, Schlame M, Stokes DL, Edelman-Novemsky I, Yu D, Xu Y, Viswanathan N. & Ren M. Tafazzin knockdown in mice leads to a developmental cardiomyopathy with early diastolic dysfunction preceding myocardial noncompaction. J. Am. Heart Assoc 1, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moullan N, Mouchiroud L, Wang X, Ryu D, Williams EG, Mottis A, Jovaisaite V, Frochaux MV, Quiros PM, Deplancke B, Houtkooper RH & Auwerx J. Tetracyclines Disturb Mitochondrial Function across Eukaryotic Models: A Call for Caution in Biomedical Research. Cell Rep. 10, 1681–1691 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J. & Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv. Dent. Res 12, 12–26 (1998). [DOI] [PubMed] [Google Scholar]
- 45.Wang S, Li Y, Xu Y, Ma Q, Lin Z, Schlame M, Bezzerides VJ, Strathdee D. & Pu WT AAV Gene Therapy Prevents and Reverses Heart Failure in a Murine Knockout Model of Barth Syndrome. Circ. Res 126, 1024–1039 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki-Hatano S, Saha M, Rizzo SA, Witko RL, Gosiker BJ, Ramanathan M, Soustek MS, Jones MD, Kang PB, Byrne BJ, Cade WT & Pacak CA AAV-Mediated TAZ Gene Replacement Restores Mitochondrial and Cardioskeletal Function in Barth Syndrome. Hum. Gene Ther (2018). doi: 10.1089/hum.2018.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren M, Miller PC, Schlame M. & Phoon CKL A critical appraisal of the tafazzin knockdown mouse model of Barth syndrome: what have we learned about pathogenesis and potential treatments? Am. J. Physiol. Heart Circ. Physiol 317, H1183–H1193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson JM, Ferrara PJ, Verkerke ARP, Coleman CB, Wentzler EJ, Neufer PD, Kew KA, de Castro Brás LE & Funai K. Targeted overexpression of catalase to mitochondria does not prevent cardioskeletal myopathy in Barth syndrome. J. Mol. Cell. Cardiol 121, 94–102 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goncalves RLS, Schlame M, Bartelt A, Brand MD & Hotamışlıgil GS Cardiolipin deficiency in Barth syndrome is not associated with increased superoxide/H2 O2 production in heart and skeletal muscle mitochondria. FEBS Lett. 595, 415–432 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren M, Xu Y, Erdjument-Bromage H, Donelian A, Phoon CKL, Terada N, Strathdee D, Neubert TA & Schlame M. Extramitochondrial cardiolipin suggests a novel function of mitochondria in spermatogenesis. J. Cell Biol 218, 1491–1502 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spencer CT, Bryant RM, Day J, Gonzalez IL, Colan SD, Thompson WR, Berthy J, Redfearn SP & Byrne BJ Cardiac and clinical phenotype in Barth syndrome. Pediatrics 118, e337–46 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Cadalbert LC, Ghaffar FN, Stevenson D, Bryson S, Vaz FM, Gottlieb E. & Strathdee D. Mouse Tafazzin Is Required for Male Germ Cell Meiosis and Spermatogenesis. PLoS One 10, e0131066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnston J, Kelley RI, Feigenbaum A, Cox GF, Iyer GS, Funanage VL & Proujansky R. Mutation characterization and genotype-phenotype correlation in Barth syndrome. Am. J. Hum. Genet 61, 1053–1058 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valianpour F, Wanders RJA, Overmars H, Vreken P, Van Gennip AH, Baas F, Plecko B, Santer R, Becker K. & Barth PG Cardiolipin deficiency in X-linked cardioskeletal myopathy and neutropenia (Barth syndrome, MIM 302060): a study in cultured skin fibroblasts. J. Pediatr 141, 729–733 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Lou W, Reynolds CA, Li Y, Liu J, Hüttemann M, Schlame M, Stevenson D, Strathdee D. & Greenberg ML Loss of tafazzin results in decreased myoblast differentiation in C2C12 cells: A myoblast model of Barth syndrome and cardiolipin deficiency. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 1863, 857–865 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang D-Z, Li K, Wang J, Wanders RJA, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK & Pu WT Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med 20, 616–623 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dudek J, Cheng I-F, Balleininger M, Vaz FM, Streckfuss-Bömeke K, Hübscher D, Vukotic M, Wanders RJA, Rehling P. & Guan K. Cardiolipin deficiency affects respiratory chain function and organization in an induced pluripotent stem cell model of Barth syndrome. Stem Cell Res. 11, 806–819 (2013). [DOI] [PubMed] [Google Scholar]
- 58.Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ & Palecek SP Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc 8, 162–175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD & Wu JC Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galdos FX, Guo Y, Paige SL, VanDusen NJ, Wu SM & Pu WT Cardiac Regeneration: Lessons From Development. Circ. Res 120, 941–959 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dudek J, Cheng I-F, Chowdhury A, Wozny K, Balleininger M, Reinhold R, Grunau S, Callegari S, Toischer K, Wanders RJ, Hasenfuß G, Brügger B, Guan K. & Rehling P. Cardiac-specific succinate dehydrogenase deficiency in Barth syndrome. EMBO Mol. Med 8, 139–154 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erickson JR, Joiner M-LAL, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham A-JLJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ & Anderson ME A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133, 462–474 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson ME, Brown JH & Bers DM CaMKII in myocardial hypertrophy and heart failure. J. Mol. Cell. Cardiol 51, 468–473 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Wang S, Guo X, Li Y, Ogurlu R, Lu F, Prondzynski M, de la Serna Buzon S,Ma Q, Zhang D, Wang G, Cotton J, Guo Y, Xiao L, Milan DJ, Xu Y, Schlame M, Bezzerides VJ & Pu WT Increased ROS-Mediated CaMKII Activation Contributes to Calcium Handling Abnormalities and Impaired Contraction in Barth Syndrome. Circulation (2021). doi: 10.1161/CIRCULATIONAHA.120.048698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Wal E, Herrero-Hernandez P, Wan R, Broeders M, In ‘t Groen SLM, van Gestel TJM, van IJcken WFJ, Cheung TH, van der Ploeg AT, Schaaf GJ & Pijnappel WWMP Large-Scale Expansion of Human iPSC-Derived Skeletal Muscle Cells for Disease Modeling and Cell-Based Therapeutic Strategies. Stem Cell Reports 10, 1975–1990 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inoue H, Nagata N, Kurokawa H. & Yamanaka S. iPS cells: a game changer for future medicine. EMBO J. 33, 409–417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]