Abstract
An engineered patch of retinal pigment epithelium shows promise in two macular degeneration patients.
Since their original derivation in 1998 (ref.1), the potential of human embryonic stem cells (hESCs) to become any cell type in the body has provided hope for a new class of therapies aimed at replacing diseased or injured tissues. Two decades later, after much progress in learning how to convert pluripotent cells into a variety of cell types2, researchers have brought the first engineered tissue made from hESCs to patients. In this issue, da Cruz et al.3 report transplantation of a ‘patch’ of retinal pigment epithelium (RPE) into the eyes of two individuals with the ‘wet’ form of age-related macular degeneration (AMD). The patch was created by growing hESC-derived RPE on a polyethylene terephthalate scaffold, which aids maturation of the RPE monolayer and provides mechanical support for delivery of the tissue under the retina. The authors demonstrate successful integration of the patch in the eyes of both patients and recovery of retinal function over the area of the patch.
AMD affects over 30 million people world-wide4. Exudative or ‘wet’ AMD is an advanced stage of the condition found in about 10% of the AMD population5. The disease is initiated by degenerative changes in the RPE, a monolayer of cells whose main role is to maintain the health and integrity of photoreceptors. Tight junctions between neighboring RPE cells constitute the outer blood–retina barrier, allowing the RPE to control nutrient and metabolite flow to and from the choroidal blood supply. In addition, the RPE replenishes visual pigments for photoreceptors, recycles worn-out photoreceptor outer segments by phagocytosis, and maintains immune-quiescence in the outer retina5.
In wet AMD, increased VEGF production promotes proliferation of choroidal blood vessels5. The blood vessels break through the degenerating RPE monolayer and leak fluid and blood into the space between the RPE and the photoreceptor layer in the back of the retina, leading to death of photoreceptor cells (Fig. 1). The standard of care for AMD patients is administration of anti-VEGF antibodies. In most cases, this treatment effectively stops fluid leakage, causes proliferating blood vessels to regress, and improves patients’ vision, but it does not repair the degenerative damage to the RPE or cure the disease6.
Figure 1.
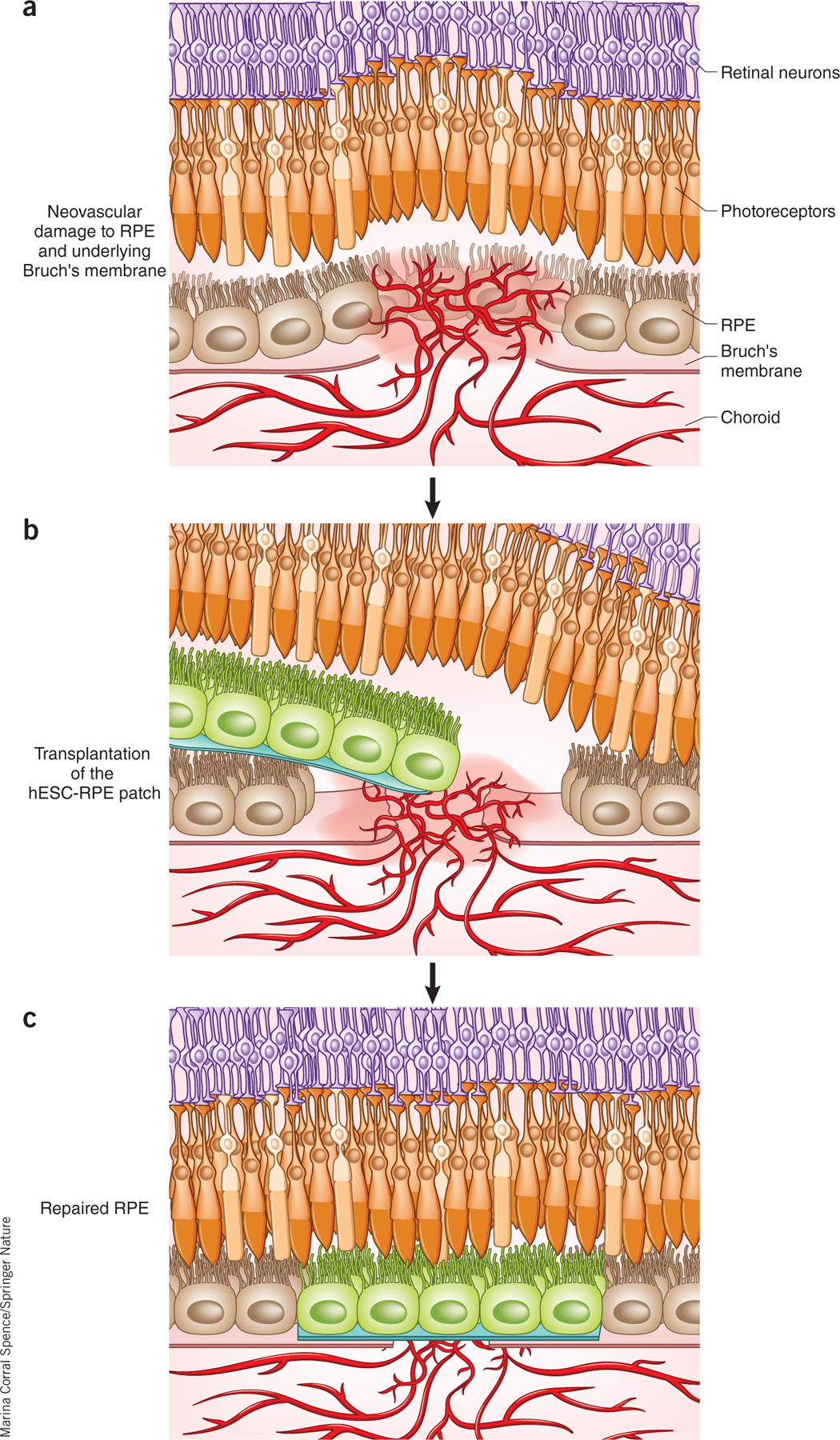
Schematic of the approach used by da Cruz et al.3. (a) In wet AMD, choroidal blood vessels penetrate through the RPE, leaking fluid in the subretinal space between the RPE (light brown) and the retina. (b) An hESC-RPE monolayer on a plastic substrate (green) is delivered to the area of vessel leakage and detachment of the RPE and retina. (c) The expected outcome is that once the retina is flattened on top of the patch, patient photoreceptors (orange) recover function through interaction with transplanted RPE cells, and cytokine cues from the RPE cells cause choroidal vessels to stop proliferating and leaking.
The approach developed by da Cruz et al.3 involves transplantation of a replacement RPE tissue that is meant not only to halt the growth of proliferating vessels but also to repair the area of degenerated RPE (Fig. 1). Their RPE patch was produced using a clinical-grade manufacturing process approved by the UK Medicines and Health Products Regulatory Agency. As a combination of stem cell derivatives and a scaffold intended as a cure, it qualifies as an “advanced regenerative medicine product” according to the US 21st Century Cures Act.
Manufacturing of the RPE patch takes about 55 weeks, which is relatively inefficient and slow compared with some recent work on directed RPE differentiation7. This is because da Cruz et al.3 use a ‘spontaneous’ method in which hESCs that spontaneously differentiate into RPE cell clusters are manually isolated on the basis of their dark pigmentation. The avoidance of added growth factors simplifies the optimization of clinical-grade manufacturing. The authors incorporate several ‘go/no-go’ points in the manufacturing process, including sterility and purity of RPE cells. Although the RPE cells are subjected to structural and functional characterization (electron microscopy, cytokine release and ability to phagocytose photoreceptor outer segments), the final ‘release criteria’ are limited to visual inspection of cells on the scaffold.
Demonstrating the safety of a cell therapy product is of paramount importance to obtain regulatory approval for a phase 1 trial. da Cruz et al.3 tested the safety of their hESC-RPE patch in two animal models. Transplantation of hESC-RPE cells in immunocompromised mice, which allowed long-term survival of the cells, confirmed that the hESC-RPE cells did not form tumors, teratomas, or non-RPE cell types. Transplantation of the clinical product, a 6 × 3 mm RPE patch, in immunosuppressed pigs showed both local and systemic safety of the treatment.
A 6 × 3 mm patch is large enough to cover almost the entire area of a human macula (5 mm in diameter). Delivery of such a large patch to the back of the eye requires a rather complex surgery, with cuts in the sclera and the retina larger than 3 mm. Such large cuts in the eye wall increase the likelihood of surgery-related complications. Nonetheless, da Cruz et al.3 successfully delivered the large patch to the back of the eye in two wet AMD patients who had considerable accumulation of fluid and blood under the retina.
The only long-term immunosuppression used with this allogeneic RPE was local administration of steroids. At one year after surgery, the RPE cells seemed to have survived on the patch, suggesting that the immunosuppression regimen was sufficient to curb the immune response against the transplanted cells. The authors assessed integration of the patch in the back of the eye using several imaging techniques, including optical coherence tomography, fundus photography, adaptive optics, and fluorescein angiography. Most importantly, they found that choroidal vessels underneath the patch were perfused and were no longer leaky, suggesting that disease progression had been halted.
Microperimetry and visual acuity measurements showed that the retina over the area of the patch regained sensitivity and that the vision of both patients improved during the one-year interval. The two patients went from barely being able to read to being able to read 48–83 words per minute. Although the improvement seen in visual acuity was suggested to be due to the RPE patch, contributions from the surgical procedure and local steroids cannot be completely ruled out at this stage.
da Cruz et al.3 noted several adverse events associated with the patch or the surgical procedure: (i) exposure of the suture to the implant that delivered the immunosuppressive drug (ii) worsening of diabetes in one patient, caused by short-term oral steroids; and (iii) retinal detachment and associated proliferative vitreoretinopathy (PVR), both likely due to disease-associated hemorrhage concomitant with retinal surgery. In addition, one patient had the unexpected outcome of areas of ‘double-thickness’ RPE, probably because the native RPE had not completely degenerated across the entire area of the patch. While all three adverse events were addressed surgically or medically, the effects of retinal detachment, PVR and double-thickness RPE could compromise the long-term survival and functioning of the implanted RPE cells and must be closely monitored.
As the RPE patch is advanced to further clinical trials, several key issues should be considered. Confirming RPE quality by visual inspection requires highly trained technicians and limits the possibility of scaling up manufacturing in commercial settings. A potency assay for the RPE patch that is not solely dependent on user experience and that correlates directly with in vivo functionality will be needed in order to reach a phase 3 trial. Furthermore, given the long manufacturing process, it would be highly desirable to implement a cryopreservation step close to the final product. This would make possible an off-the-shelf cell therapy product that does not require a new manufacturing run for each patient. Such a strategic approach would reduce costs and facilitate commercialization.
Assuming that most of the photoreceptors were still alive when the RPE patch was transplanted into the two patients, the potential of the patch to cure patients of wet AMD will depend on the long-term ability of the RPE cells to support photoreceptor function and suppress new blood vessel growth, and the ability of the plastic scaffold to effectively replace Bruch’s membrane (Fig. 1) to allow nutrient and metabolite exchange with the choroidal blood supply. If the patch is not curative, regulators may require a comparison of this therapy to the anti-VEGF therapy currently in use.
The work of da Cruz et al.3 raises many interesting questions for future investigation. Is an allogeneic hESC-based therapy immunologically feasible in the long term, or is the autologous induced pluripotent stem cell (iPSC)-based approach tested by Mandai et al.7 and further developed by the National Eye Institute (NEI)/NIH more likely to succeed? Can an RPE patch also provide a replacement tissue for atrophied RPE in the ‘dry’ form of AMD (an approach currently being tested at the University of Southern California8)? Is a plastic non-degradable scaffold an appropriate substitute for Bruch’s membrane, or is a biodegradable scaffold (such as the one under development by the NEI/NIH8 team) preferable? Several clinical trials in progress should help elucidate these questions.
The work of da Cruz et al.3—carried out in the UK—illustrates the kind of innovation expected from the regenerative medicine initiative of the 21st Century Cures Act signed by President Barack Obama in 2016 (ref. 9). Taken together with similar recent studies that used allogeneic hESC-RPE cells in suspension10 and an autologous RPE sheet derived from iPSCs (shown in one patient)7, it lends further support to the safety and feasibility of cell therapies produced from pluripotent stem cells for a group of eye diseases that represents a major cause of blindness in older adults.
Footnotes
COMPETING INTERESTS
The author declares no competing interests.
References
- 1.Thomson JA et al. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Martin U Front. Med 4, 229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Cruz L et al. Nat. Biotechnol 36, 328–337 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Ambati J & Fowler BJ Neuron 75, 26–39 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villegas VM, Aranguren LA, Kovach JL, Schwartz SG & Flynn HW Jr. Expert Opin. Drug Deliv 14, 273–282 (2017). [DOI] [PubMed] [Google Scholar]
- 6.May-Simera HL et al. Cell Reports 22, 189–205 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandai M et al. N. Engl. J. Med 376, 1038–1046 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Bharti K et al. Invest. Ophthalmol. Vis. Sci 55, 1191–1202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson KL & Collins FS N. Engl. J. Med 376, 111–113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz SD et al. Lancet 385, 509–516 (2015). [DOI] [PubMed] [Google Scholar]


