Abstract
Background:
Cancer treatment of prepubertal patients impacts future fertility due to the abolition of spermatogonial stem cells (SSCs). In macaques, spermatogenesis could be regenerated by intratesticular transplantation of SSCs, but no studies have involved cytotoxic treatment before puberty and transplantation after puberty, which would be the most likely clinical scenario.
Objectives:
To evaluate donor-derived functional sperm production after SSC transplantation to adult monkeys that had received testicular irradiation during the prepubertal period.
Materials and methods:
We obtained prepubertal testis tissue by unilaterally castrating 6 prepubertal monkeys and 2 weeks later irradiated the remaining testes with 6.9 Gy. However, because spermatogenic recovery was observed, we irradiated them again 14 months later with 7 Gy. Three of the monkeys were treated with GnRH-antagonist (GnRH-ant) for 8 weeks. The cryopreserved testis cells from the castrated testes were then allogeneically transplanted into the intact testes of all monkeys. Tissues were harvested 10 months later for analyses.
Results:
In three of the six monkeys, 61%, 38% and 11% of the epididymal sperm DNA were of the donor genotype. The ability to recover donor-derived sperm production was not enhanced by the GnRH-ant pretreatment. However, the extent of filling seminiferous tubules during the transplantation procedure was correlated with the eventual production of donor sperm. The donor epididymal sperm from the recipient with 61% donor contribution were capable of fertilizing rhesus eggs and forming embryos. Although the transplantation was done into the rete testis, two GnRH-ant treated monkeys, which did not produce donor-derived epididymal sperm, displayed irregular tubular cords in the interstitium containing testicular sperm derived from the transplanted donor cells.
Discussion and Conclusion:
The results further support that sperm production can be restored in non-human primates from tissues cryopreserved prior to prepubertal and postpubertal gonadotoxic treatment by transplantation of these testicular cells after puberty into seminiferous tubules.
Keywords: Transplantation, radiation, spermatogenesis, GnRH-antagonist, ICSI
1. INTRODUCTION
Sustained progress in cancer therapies over the past several decades has led to a rise in pediatric cancer survival rates to approximately 88%.1 However, the gonadotoxicity and risk of infertility from the treatment remains a major health concern in these survivors as it affects the quality of life. Since prepubertal boys are not producing sperm, there are currently no standard-of-care options to preserve their fertility. We estimate that each year an additional 1,400 young men will become sterile due to cancer therapy and myeloablative conditioning therapy for hematopoietic stem cell transplants for non-malignant conditions.2 This is a significant human health concern3,4 and development of new methods of fertility preservation to prevent these effects or restore normal reproductive function after cytotoxic treatment are of great importance to these young male cancer survivors.
If spermatogonial stem cells (SSCs) are completely lost after gonadotoxic therapy, the only way to preserve future fertility of prepubertal males is by harvesting tissue containing SSCs prior to therapy and cryopreservation. With increased awareness and need for fertility preservation, it is the current clinical practice in various centers in the world to cryopreserve the testicular tissues before gonadotoxic therapies in boys,5–8 hoping that a satisfactory technique will be developed to produce sperm from the SSCs present in this tissue. Transplantation of a cell suspension containing SSCs into the seminiferous tubules is one of the techniques that have the potential to restore spermatogenesis and sperm production in vivo. Sperm can be obtained from the testis, epididymis or the ejaculate and have been successfully used to produce live offspring in rodents9–11 and goats,12 and embryos in non-human primates.2,13
Previously, we showed that, in macaques, irradiated during adulthood, autologous14 or allogeneic2 transplantation of SSCs to the testis produced donor-derived sperm in the recipient. These sperm were also competent to fertilize the eggs to produce embryos by ICSI.2 In an attempt to model the prepubertal boys undergoing gonadotoxic therapy and requiring fertility restoration when they reach adulthood, we cryopreserved testis tissue from prepubertal monkeys, irradiated them prepubertally, and planned to transplant the stored cells into the testes after puberty and subsequently test the establishment of donor-derived spermatogenesis and the fertilizing potential of the sperm produced. However, a second dose of irradiation, had to be given to these monkeys, since there was spermatogenic recovery at puberty. Furthermore, although autologous transplantation is desired in the clinical scenario, allogeneic transplantation was used in this study so that donor-derived spermatogenesis and the paternity of embryos produced could be reliably quantified using microsatellites that differed between the donor-recipient pairs.
In an attempt to increase the success of the recovery of spermatogenesis from transplanted cells in a non-human primate, we also tested the effect of gonadotropic and gonadal hormone suppression with a GnRH-antagonist, a method that had proved very successful in rodents.15,16 Although one of our previous studies with macaques14 had indicated that hormonal suppression just prior transplantation enhanced the recovery of spermatogenesis from the donor, a second study2 failed to indicate such a beneficial effect.
Furthermore, we previously reported a single case in which transplantation of a suspension of testicular cells from a prepubertal monkey resulted in the development of donor-derived de novo tubules containing advanced germ cells in the interstitium.17 Since this study also involves transplantation of prepubertal monkey cells into the testes, we scrutinized the transplanted testes tissues for such structures.
2. MATERIALS AND METHODS
2.1. Animals
Six male rhesus monkeys (Macaca mulatta) were purchased from the Michale E. Keeling Center for Comparative Medicine and Research, MD Anderson Cancer Center, Bastrop, Texas as both donor and recipient monkeys for testicular cell transplantation to the testis. They were prepubertal at the time of purchase and were housed in pairs initially at the M.D. Anderson Cancer Center, Houston, Texas in steel cages with a sliding panel between two adjacent compartments to allow social interaction with another companion of the same study group. The animals were fed Harlan TEKLAD Primate diet #7195 with daily enrichment foods, such as seeds, peanuts, fruits, and vegetables; the environment was maintained at a constant temperature (24°C–27°C) and humidity (40%–55%) with a 12-hour light/12-hour dark cycle. During parts of the study when there were minimal interventions for procedures, the monkeys were temporarily housed at the MD Anderson Cancer Center facility in Bastrop, Texas with the same conditions as described above.
All animal care and treatment protocols were approved by the Institutional Animal Care and Use Committees of MD Anderson Cancer Center and Magee-Womens Research Institute.
2.2. Experimental design
Prepubertal monkeys, at 40–41 weeks of age, were unilaterally castrated (Figure 1). The castrated testes were weighed and 6–27 mm3 pieces from the testes were cryopreserved for later allogeneic transplantation. Two weeks later, the remaining testes of the monkeys were given 6.9 Gy of irradiation.
FIGURE 1.

Study design. The monkeys were evaluated before unilateral castration and periodically after exposure to two doses of radiation, hormone suppression, and transplantation. Evaluation included sampling of serum and measurements of testis volume. In addition, periodic semen analysis was performed after the animals reached puberty. Starting immediately after second exposure to testicular irradiation, three monkeys underwent GnRH-ant-mediated hormone suppression for 8 weeks; the other 3 received only sham injections. At the end of the 8-week period, they received allogeneic transplantation of cryopreserved testis tubular cells into one testis, followed by 9 months of immune suppression.
Testis volumes, testosterone levels and sperm counts in the ejaculates were monitored, generally every four weeks, to observe signs of puberty. At about 50 weeks after the first irradiation, the monkeys showed increased testosterone levels, suggesting their entrance to puberty. However, because the testis volumes and sperm counts indicated significant spermatogenic recovery, they were given one more dose of 7-Gy irradiation at 64 weeks from the first dose to deplete endogenous SSCs and the recovering spermatogenesis. The monkeys were then divided into two groups of 3 each; one group was treated with GnRH-antagonist (GnRH-ant) for 8 weeks. At the end of 8 weeks, the testis of each monkey was allogeneically transplanted with cells prepared from the cryopreserved testis pieces of another monkey in the group. To prevent rejection of the transplanted cells, the monkeys were immunosuppressed; the testes and epididymis were harvested 44 weeks after transplantation for analyses as in previous studies.14
2.3. General surgical and post-surgical procedures
For all procedures, the monkeys were first sedated with an IM injection of ketamine (10–25 mg/kg) and then anesthetized with 1–3% isoflurane in oxygen. Before castration surgery, 2% lidocaine was instilled into the spermatic cord to provide local anesthesia. All surgical procedures were performed under aseptic conditions. Each animal received an analgesic (buprenorphine, 0.01–0.03 mg/kg body weight) prior to and at the end of the day of surgery, and 2 times per day for up to 3 days as needed by appearance of the animal under constant monitoring. In addition, at the discretion of the Clinical Veterinarian, daily IM injections of Baytril antibiotic (5 mg/kg) were given for a week post-surgery.
2.4. Semen and blood collection
Blood (5–10 ml) was drawn by venipuncture of the saphenous vein of sedated animals. Serum was separated and stored at −20°C. In general, blood sampling was done at monthly intervals, but was drawn more frequently during and immediately after GnRH-ant-treatment, to assess its effects on hormone levels.
Semen was obtained from anaesthetized monkeys by electro-ejaculation using a rectal probe (Beltron Instruments, Longmont, CO, USA), as described previously.14 The sample was allowed to liquefy at 37°C for an hour before spermatozoa were counted in the exudate using a hemocytometer. Sperm counts were expressed per total ejaculate (volume of exudate plus remaining coagulum). Semen collection was done only once before the second irradiation to confirm puberty and assess spermatogenic recovery after the first irradiation. Monthly semen collections were then performed starting at 16 weeks after transplantation.
2.5. Testicular measurements
Individual testis volumes were determined by measuring the length and width of each testis within the scrotum of anesthetized monkey with calipers and modeling the testis as a prolate ellipsoid, applying the following formula: testis volume = π × width2 × length/6.
2.6. Hemicastration and tissue cryopreservation
A scalpel incision was made in the scrotum of anesthetized prepubertal monkeys and the dartos and tunica vaginalis were dissected to expose the left testis. The blood supply to the testis was tied off and the testis along with the epididymis was removed by cutting the spermatic cord, and the incision was closed by suturing.
The removed testis tissues were washed in Petri dishes using Hanks’ balanced salt solution (HBSS) and, using a single edged blade, were cut into small pieces of about 6–27 mm3 and cryopreserved.18 About 5–7 pieces of tissue were placed in a 2 ml cryovial containing 5% DMSO and 5% fetal bovine serum (FBS) in minimal essential medium (MEMα). The vials were placed on ice for 30 min, transferred to −1°C/min containers and placed at −20°C for 90 mins and then these containers were placed overnight at −80°C. Next day, the vials were plunged into liquid nitrogen.
2.7. Irradiation
The testes of anesthetized monkeys were irradiated using a cobalt-60 gamma-irradiator14,17 with a 5×5 cm field size in an antero-posterior direction. Tissue-equivalent bolus material (5-mm thick) was placed over the scrotum to provide a build-up layer. The remaining right testes of prepubertal monkeys were irradiated at a total calculated dose of 6.9 at a rate of 77– 91 cGy/min, with a source-to-skin distance of 80 cm measured to the bolus. This dose was chosen because 7 Gy was previously shown to provide prolonged depletion of spermatogenesis in adult macaque testes.2,14 Although we were aware of reports that this dose might not deplete spermatogenesis in immature macaques,19 higher doses were not given to the prepubertal testis because our preliminary data (not shown) and studies of others20 have shown that irradiation of prepubertal testes with 10 Gy produced failure of the development of the somatic elements of the testis. Instead, it was necessary to give the monkeys a second dose of 7-Gy irradiation when they reached adulthood to eliminate most of the surviving endogenous SSC and the recovering endogenous spermatogenesis.
2.8. GnRH antagonist treatment
The GnRH-ant, Acyline, was obtained from the Contraceptive Development Program of the NICHD, Rockville, MD, USA. Stock solutions of Acyline (2 mg/ml) in 5% aqueous mannitol were prepared and stored at 4°C for a maximum of 1 week. Based on the pharmacokinetics of Acyline,21 and our previous data on hormone suppression in macaques, 14 one group of three monkeys was given twice-weekly subcutaneous injections of Acyline on Mondays and Thursdays at doses of 200 μg/kg and 300 μg/kg, respectively;14,17 the other group of three was sham-injected with bacteriostatic water.
2.9. Allogeneic transplantation
To prepare the cells for transplantation, the cryovials were thawed in a 37°C water bath and washed with HBSS. Tissue pieces were incubated with collagenase IV and DNase I to digest interstitial tissue and the undigested tissue was then incubated with trypsin-DNase to release tubular cells.22,23 The recovered cells were washed, counted and prepared for transplantation as in our previous study.14 The remaining right testes of the unilaterally castrated monkeys were allogeneically transplanted with these cells, choosing the donor-recipient pairs to maximize the unique microsatellite markers between these monkeys.
Transplantation of cells was done essentially as described previously.13,14 Briefly, cells were suspended at 54 −140 × 106 viable cells/ml in MEMα containing 10% FBS, 0.4 mg Trypan blue/ml, 20% (v/v) Optison ultrasound contrast agent (GE Healthcare, Waukesha, WI), 1% antibiotic-antimycotic (a combination of penicillin, streptomycin, and amphotericin B; Gibco), and 0.1 mg DNase I/ml. The cells were transplanted in volumes between 350 and 500 μl via ultrasound-guided injections into the rete testis. A 13 MHz linear superficial probe and a MicroMaxx ultrasound machine (Sonosite, Bothell, WA) were used to visualize the rete testis space and to guide a 25-gauge, 1.5” hypodermic needle into the space. Cells were manually injected under slow constant pressure and chased with saline solution. A transplantation efficiency score was recorded for each transplantation as done previously, based on an ultrasound-visualized estimate of the percentage of the circumference of tubules, going outward from the rete testis, that were filled by donor cell suspension.2 The scores were as follows: 5 = >80%; 4 = 60–80%; 3 = 40–60%; 2 = 20–40%; and 1= <20%. For example, the filling of the tubules recorded in a previous study,13 Movie S1, would be a score of 5. To prevent T cell–mediated rejection of the transplanted allogeneic cells, the recipients were immunosuppressed with human/mouse chimeric anti-CD154 IgG 5C8 (NIH Nonhuman Primate Reagent Resource, University of Massachusetts Medical School, Boston, MA) at 20 mg/kg on days (relative to transplant) −1, 0, 3, 10, 18, 28, and monthly thereafter for an additional 8 months. This treatment of rhesus monkeys was shown to functionally protect renal allografts24 and had been successfully used in allogeneic transplantation of SSCs.2,13
2.10. Hormone assays
Testosterone was assayed using radioimmunoassay (RIA) kit KIR1709 (Immuno-Biological Laboratories America, Minneapolis, MN). We used our own testosterone standard for the assay that was diluted in the zero-standard provided in the kit18. The detection limit of the assay is 0.05 ng/ml. The intra- and inter-assay coefficients of variation were 5% and 16%, respectively.
Circulating concentrations of FSH and luteinizing hormone (LH) were determined by RIA at the Endocrine Technologies Support Core, Oregon National Primate Research Center, Beaverton. The sensitivities of both the FSH and LH assays were 0.05 ng/ml. The intra-assay coefficients of variation were 12.5 % and 8.2%, respectively, for FSH, and LH.
2.11. Histological and immunohistochemical procedures
After harvest the testes were first weighed and pieces of tissue were fixed in either Bouin solution, 4% PFA or 70% ethanol.
For histology, Bouin-fixed pieces were embedded in paraffin, and sections were stained with periodic-acid-Schiff reagent and hematoxylin. For analysis of spermatogenic recovery at the end of the study, at least three sections chosen from different regions of the testis were assessed by systematic scanning across the entire section and a minimum of 2654 tubules were scored per testis. Sertoli cell-only tubules were categorized into two types: those with normal appearing columnar Sertoli cells with a relatively small empty lumen, and those with flatter Sertoli cells with a large empty lumen. The presence of germ cells was scored by calculating the tubule differentiation index (TDI), which is the percentage of seminiferous tubule cross sections containing at least three differentiated germ cell type (B spermatogonia or later stages). In addition, the extent of the progression of germ cell differentiation was assessed by determining the percentages of tubules with germ cells that contained spermatocytes, round spermatids or elongating/elongated spermatids as the most advanced germ cell type present.
In some sections, areas packed with irregularly shaped tubular cords containing germ cells, often with incomplete basement membranes were observed. These were readily distinguished from normal seminiferous tubules and appeared identical to the donor-derived de novo tubules we observed in a previous study.17
2.12. Epididymal sperm isolation
The cauda epididymis was minced thoroughly in about 200 μl of pre-warmed modified human tubal fluid (HTF, Cat. No. 90126; Irvine Scientific, Santa Ana, CA) in a 60 mm Petri dish and transferred to a 2 ml microfuge tube. The epididymal mince was incubated thrice with 500 μl of HTF, each time suspending the tissue and allowing it to settle at unit gravity and aspirating the supernatant containing the sperm. The supernatant was filtered through a prewet 100-μm cell strainer basket (BD 352350) into a 50-ml conical tube and the total volume of the filtrate was brought to 2 ml by adding pre-warmed HTF. The number of sperm, their motility and the number of blood cells were counted in the filtrate, which was then divided into two portions: one for genotyping and one for ICSI.
When the level of contaminating somatic cells was <50%, the sperm samples for genotyping were washed in DPBS and pellets were frozen at −80°C. However, when the level of somatic cells was >50%, the sperm were further purified by Percoll gradient separation, reducing the somatic contamination of ~5%, prior to washing and freezing.
The ICSI samples were transferred to 5-ml tubes and equal volumes of pre-warmed Test Yolk Buffer freezing medium were added drop-wise over a 30-second period, mixing thoroughly after each drop of freezing medium was added to avoid osmotic shock to the sperm. The mixture was allowed to equilibrate for 10 minutes at room temperature and then transferred into multiple 2-ml vials. The samples were chilled for 1 hour in the refrigerator (2–5°C), followed by exposure to liquid nitrogen vapor for 30–60 minutes, and then transferred to a liquid nitrogen tank for storage at −196°C.
2.13. Preparation of DNA from the blood, tissue, and sperm
To genotype the monkeys used as donors and recipients, DNA was prepared from non-coagulated blood using the DNeasy Blood & Tissue Kit from Qiagen (Cat No.: 69504).
To extract DNA from sperm, the pellets were suspended in saline sodium citrate buffer and were treated with 0.2% sodium dodecyl sulfate (SDS) to lyse remaining non-sperm cells. In cases in which sperm were not Percoll purified, the sperm were washed one additional time and treated again with SDS; this further eliminated the somatic contaminants and consequently decreased the percentages of recipient DNA in the sperm samples by 1 to 5%. The sperm samples were lysed and digested using proteinase K and dithiothreitol (DTT) at final concentrations of 2 mg/ml and 10 mM, respectively, for 3 hours at 56°C. Then the proteinase K was heat inactivated at 95°C for 15 min, and the extract was directly used for PCR.
For genotyping the suspected de novo regions, we first identified regions with irregularly shaped tubules in PAS-hematoxylin stained, 70%-ethanol fixed testicular sections. These slides were used as guides to identify suspected regions of interest in adjacent unstained serial sections. The surrounding unwanted tissues were scraped off using a razor blade, under a dissection microscope. The proteinase K/DTT lysis solution was carefully dropped on the slide containing the required section, the tissue was released into the solution using a pipette tip and aspirated into a microfuge tube, and processed as was done for sperm above.
2.14. DNA microsatellite analysis
Microsatellite repeat fingerprinting was done with a panel of 29 microsatellites as described previously.17 Microsatellites were amplified and the PCR products were separated by capillary electrophoresis on ABI 3730 DNA Analyzer (Applied Biosystems). Fragment size analysis and genotyping was done with the computer software STRand.
To determine parental origin and sex of ICSI embryos, genotyping was done as above except that before PCR the cells were put through the Whole Genome Amplification (WGA) process using the REPLI-g kit, which contains reagents and primers that will replicate most of the cell genome, producing sufficient DNA for testing: https://www.qiagen.com/us/service-and-support/learning-hub/technologies-and-research-topics/wga/replig-principal-procedure/. In addition to the panel of 29 microsatellites, the primers 5’-CCCTGGGGCTCTGTAAAGAATAGTG-3’ and 5’- ATCAGAGCTTAAACTGGGAAGCTG-3’ were used to amplify sequences from the amelogenin gene which differs on the X and Y chromosomes, to determine the gender of the embryos.
2.15. Intracytoplasmic Sperm Injection (ICSI)
Controlled ovarian stimulation was performed on six female rhesus macaques as previously described.25,26 Oocytes were collected and fertilized with sperm by ICSI, and resulting embryos were cultured as described.27–29 Additional details are provided in the Supplementary Information. Following ICSI and in vitro development, individual embryos were vitrified and sent from the Oregon National Primate Research Center to the Veterinary Genetics Laboratory, University of California, Davis, for microsatellite analysis.
2.16. Statistical analysis
The serum FSH levels, testis volume, and testis weights are presented as arithmetic mean ± SEM. The serum testosterone and LH levels were represented as means ± SEM calculated from log-transformed values. Comparison of the group treated with GnRH-ant and the control group was done using a t-test. When multiple longitudinal measurements were made, the Bonferroni correction for multiple comparisons was applied. Correlations between different endpoints were analyzed using the non-parametric Spearman’s rank-order correlation coefficient. Analyses were performed with the IBM SPSS (version 23) statistical package.
3. Results
3.1. Observations during course of the study
We used the experimental design shown in Figure 1. The monkeys were 40–41 months of age with an average testis volume of 1.5 cm3 and serum testosterone levels of 0.6 ng/ml (Table 1), when the unilateral castration was performed. Histology showed that the castrated testes of all the monkeys were indeed prepubertal containing only spermatogonia, mostly Adark and Apale (Figure S1). The recipient monkeys were monitored to determine when they reached puberty, as indicated by the serum testosterone levels consistently at or above 0.9 ng/ml (Figure 2B, Figure S2; and Table 1) which began at about 40 weeks after the hemicastration and irradiation. The achievement of puberty was confirmed by increases in testis volume resulting from increases in somatic elements and/or development of spermatogenesis (Figure 2A). In 4 of the 6 monkeys, testis volumes increased to at least 10 cm3 (Figure S3), which is greater than that observed in adult monkeys in which spermatogenesis had been well depleted by irradiation.2 The volume increase and the presence of sperm in the ejaculates (Table 1) indicated that much of the volume increase was due to regeneration of endogenous spermatogenesis. Because of this, at 64 weeks after the first irradiation, the now postpubertal monkeys were given another dose of 7-Gy testicular irradiation, which resulted in a decrease in testis volume (Figure 2A) as expected due to the depletion of the germ cells.
Table 1.
Baseline recipient and donor monkey characteristics, treatments, and cells for transplantation.
| Treatment groups | Parameters of monkeys at time of unilateral orchiectomy and first irradiation (6.9 Gy) | Parameters of monkeys at time of second irradiation (7 Gy) | Donor cells and transplantation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Recipient Monkey Number | Age (months)‡ | Serum T (ng/ml)† | Average testis volume (cm3)† | Age (months) | Serum T (ng/ml)** | Testis volume (cm3) | Sperm count/per ejaculate (×106) § | Donor Monkey Number | Testis weight (g) | Total cells injected (millions) | Viability (%) | Transplant efficiency score | |
| No-GnRH-ant control* | 092 | 40.0 | 0.60 | 1.7 | 55.2 | 1.21 | 5.8 | 0 | 120 | 1.02 | 40 | 81% | 3 |
| 114 | 41.0 | 0.45 | 1.5 | 56.1 | 2.57 | 14.0 | 11.24 | 094 | 0.93 | 41 | 92% | 5 | |
| 120 | 40.7 | 0.46 | 1.8 | 55.8 | 3.89 | 13.6 | 1.06 | 124 | 1.46 | 42 | 84% | 3.5 | |
|
| |||||||||||||
| GnRH-ant* | 094 | 40.3 | 0.64 | 1.4 | 55.5 | 2.51 | 9.9 | 6.51 | 122 | 1.12 | 62 | 95% | 4 |
| 122 | 40.9 | 0.48 | 1.5 | 56.0 | 0.90 | 6.8 | NE | 092 | 0.93 | 17 | 89% | 4.5 | |
| 124 | 40.4 | 0.66 | 1.6 | 55.5 | 5.81 | 15.1 | 0.08 | 114 | 1.21 | 32 | 81% | 5 | |
Measured at the time of irradiation.
Average of last two measurements made on the day of (but just before) unilateral orchiectomy, and a week before. First testicular irradiation of the remaining testis was performed 2 weeks after unilateral orchiectomy.
Average of last 5 measurements.
Collected on 12/7/17; NE indicates no ejaculate was obtained.
No differences were observed between GnRH-ant treatment groups in any parameters (t-test, P>0.05).
FIGURE 2.
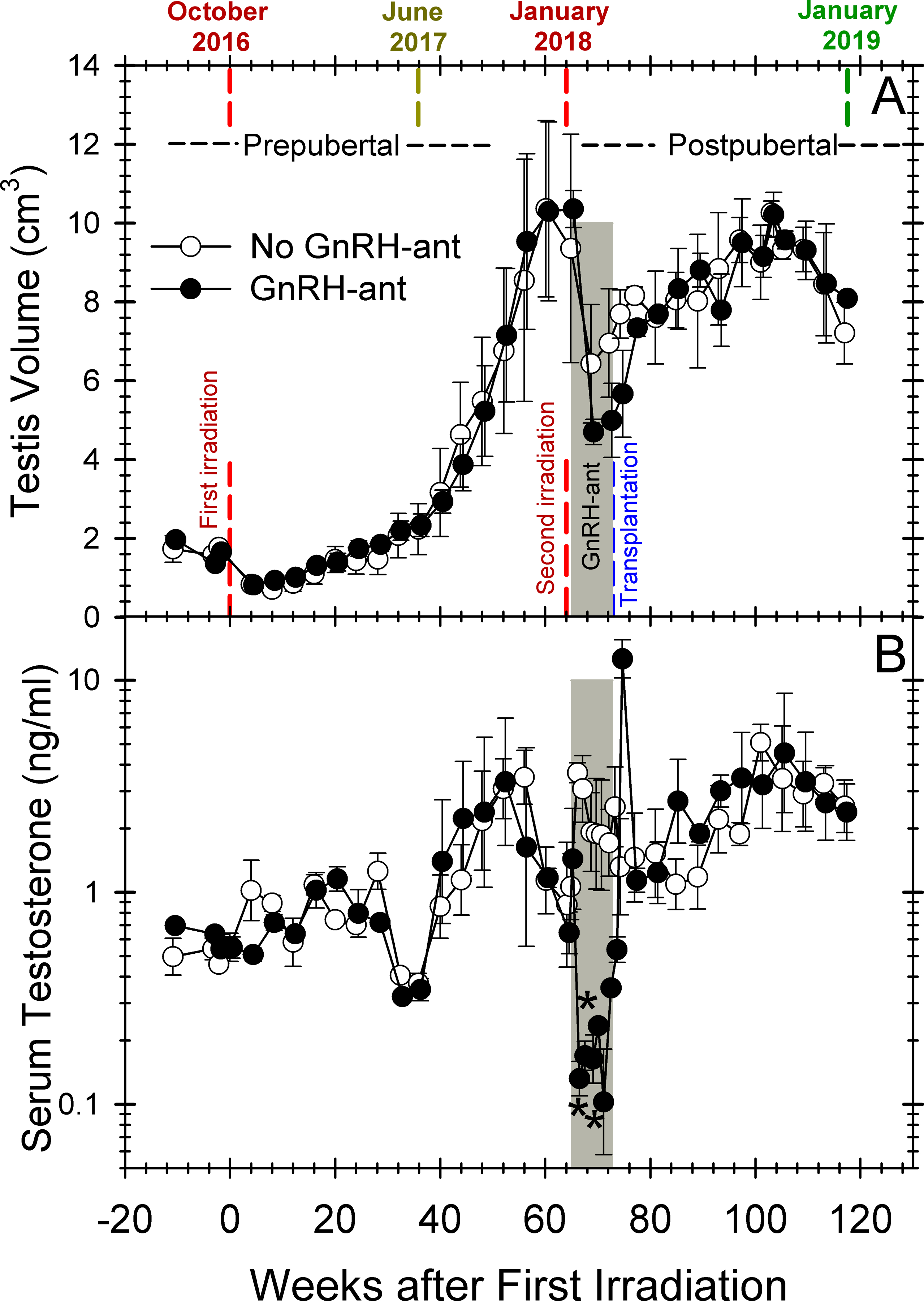
Changes in testis volumes (A) and serum testosterone levels (B) in monkeys during the study. The vertical red and blue dashed lines represent the times of the two doses of irradiation and of transplantation, respectively. The average values for the 3 monkeys receiving GnRH-ant treatment (filled cicle) (n=3) or sham injections (open circle) (n=3) before transplantation are plotted. The grey shaded area represents the duration of the GnRH-ant treatment. For statistical analysis the axis was divided into three time segments, after initial irradiation, after the second irradiation during GnRH-ant treatment, and after transplantation, during which there were 15, 6 and 13 comparisons, respectively. The only statistical difference between the two treatments groups (marked with asterisks) was decreased serum testosterone during the GnRH-ant treatment.
The monkeys were assigned to two treatment groups so that the distributions of ages, testes sizes, and testosterone levels were similar in the two groups (Table 1). One group of 3 monkeys were treated with GnRH-ant for the 8 weeks between the second irradiation and transplantation, and the other 3 monkeys received only sham injections. All 6 monkeys received allogenic transplantation of testis cells from other monkeys in the group at 72 weeks after the first irradiation dose.
As anticipated, 2,14 serum LH and testosterone levels were markedly suppressed during GnRH-ant treatment and, when the treatment was stopped, they reverted to normal levels for irradiated monkeys (Figure 2B and Figure S4). The reductions in testes volumes after the second irradiation (Figure 2A) were consistent with the loss of germ cells due to irradiation; the group treated with GnRH-ant had a tendency towards a greater decrease consistent with the loss of Sertoli and Leydig cell volume seen with hormone suppression in other species.30,31
The ability to obtain ejaculates was not very successful in these monkeys, even during the breeding season of October-February.32,33 After the second irradiation and transplantation, only one ejaculate greater than 1 ml was recorded (Table S1). The ejaculates that were obtained during this period were azoospermic (<6×102/ml), with one exception that had only a few sperm.
3.2. Results from harvested tissues at the end of the study
At the end of the study, 44 weeks after transplantation, the remaining right testis and the cauda epididymis of all the 6 monkeys were harvested.
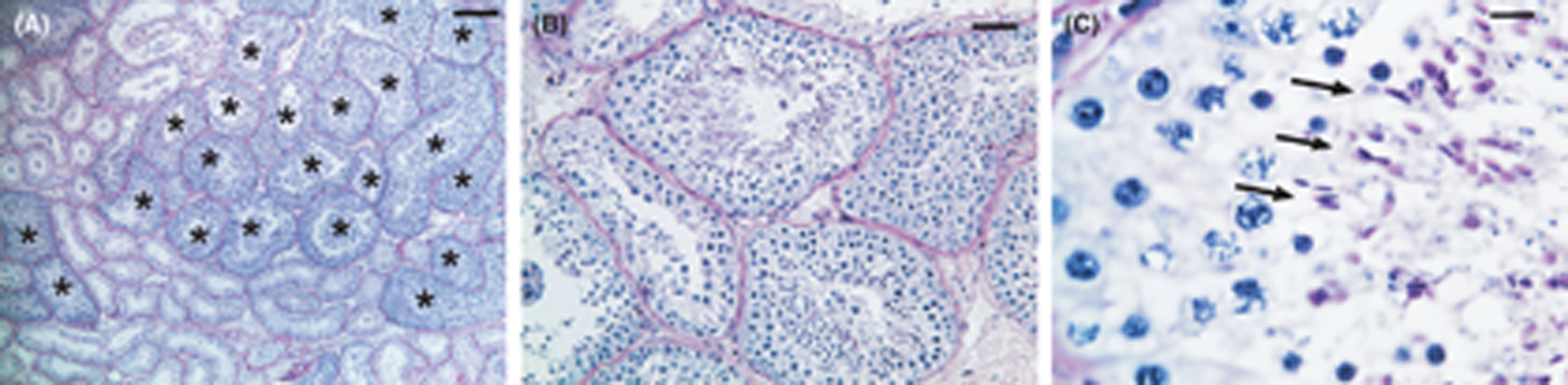
The testis weights in these monkeys varied between 3.3 and 7.0 g (Table 2, Figure 3A). Histology, as expected, showed that the majority of the tubule cross-sections contained only Sertoli cells with the complete absence of germ cells (Figure 4A). In 5 out of 6 monkeys, Sertoli cells were mostly columnar with a small empty lumen (asterisks in Figure 4B, C, D), which were considered normal for irradiated macaque testes. However, in one of the monkeys (#092), 99% of the Sertoli-only tubules displayed large empty lumens and lower epithelial height of the Sertoli cells (Table 2; Figure 4E, F). This tubule dilation is likely a consequence of damage from the first prepubertal irradiation, as this monkey showed very little increase in testis size after the first irradiation (Figure S3A). Low numbers of such dilated tubules (< 1% of tubules) were also observed in three monkeys: #094, #122 and #124 (Table 2, Figure 4C, D).
Table 2.
Parameters of spermatogenic recovery in the recipient testis/epididymis.
| Treatment groups | Recipient Monkey Number | Testis volume (cm3)† | Testis weight (g)* | TDI (%)* | Percent of differentiating tubules with late spermatids* | Percentage of dilated tubules * | Presence of de novo cords in testis | Cauda epididymal sperm (×106)* | Percentage of donor sperm in cauda epididymis* | Donor sperm in cauda epididymis (×106)* |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| No GnRH-ant control | 092 | 5.7 | 3.6 | 0.03% | 0% | 98.7% | No | 0 | NA | 0 |
| 114 | 7.9 | 7.0 | 33.1% | 76% | 0% | No | 26 | 38% | 9.9 | |
| 120 | 8.1 | 6.2 | 1.6% | 56% | 0.0% | No | 0.6 | 11% | 0.07 | |
|
| ||||||||||
| GnRH-ant | 094 | 8.3 | 5.2 | 20.1% | 82% | 0.9% | Yes | 4.7 | 0% | 0 |
| 122 | 7.8 | 3.3 | 3.0% | 64% | 0.4% | Yes | 0.9 | 0% | 0 | |
| 124 | 8.1 | 6.1 | 27.9% | 66% | 0.1% | No | 13 | 61% | 7.9 | |
No differences were observed between hormone treatment groups (t-test, P>0.05).
Measurements include scrotal wall thickness
FIGURE 3.

Spermatogenic endpoints in individual monkeys. Testis weights (A), tubule differentiation indices (B) and yield of sperm from the cauda epididymis (C) are shown for the monkeys treated with GnRH-ant (hatched bars) and those receiving only sham injections. Testes with abnormal tubules (dilated or de novo) are indicated in (B). The portion of the columns filled with green in (C) shows the numbers of spermatozoa that were donor-derived.
FIGURE 4.

Testis histology at tissue harvest, 44 weeks after transplantation. (A) Most tubules only contain Sertoli cells (B) Normal Sertoli-only tubules (*) and tubules showing regeneration of spermatogenesis (†). Note that the Sertoli cells in normal tubules have columnar appearance with a small lumen often with the presence of cytoplasmic processes. (C-F) Abnormal dilated Sertoli-only tubules (‡) with low epithelial heights and large empty lumens and some adjacent normal Sertoli-only tubules (*). Monkey numbers are indicated beside panels. Scale bars: A: 200 μm; C, E: 100 μm; B,D,F: 50 μm.
Germ cell differentiation, identified by nuclear morphology and location, was observed in the seminiferous tubules of all monkeys (Figure 5). In monkey #092 with the extensive dilated tubules, only one normal tubule showed spermatogenic cell differentiation. In the other five monkeys, germ cell differentiation, quantified by the tubule differentiation index (TDI), was observed in 2% to 33% of the normal tubules (Figure 3B). Spermatogenesis proceeded to the late spermatid stage in 70% of the differentiating tubules (Figure 5C). In addition, two monkeys (#094 and #122) displayed irregular tubule-like cords, as will be discussed below.
FIGURE 5.
Histology of the testis of a monkey that showed donor-derived sperm in the epididymis. Representative PAS-Hematoxylin stained testis sections at the end of the study from monkey #114. Tubules showing differentiating germ cells in (A) are indicated by asterisks. Note the presence mature spermatids (arrows in C) indicating complete spermatogenesis. Scale bars: A: 200 μm; B: 50 μm; C:10 μm.
Epididymal sperm counts in five of the monkeys varied between 0.6 and 26×106; one monkey (#092) had no sperm in the epididymis (Figure 3C, Table 2). As expected, there was a perfect positive correlation between the cauda epididymal sperm count and the TDI (Spearman coefficient 1.0, P<0.01) among the different animals. Microsatellite analysis revealed that, in three of the recipient monkeys, 61%, 38%, and 11% of the epididymal sperm were of the donor genotype, but in the other two of the monkeys with epididymal sperm, there were no donor-derived sperm (Table 3, Figure S5). The monkeys with 61% and 38% donor sperm had high epididymal sperm numbers, with 8 and 10 million donor sperm, respectively, indicating the potential for fertility preservation.
Table 3.
Microsatellite analysis of the cauda epididymal spermatozoa, or testicular cells (retrieved from regions of the section suspected to contain de novo cords), from testicular irradiated monkeys transplanted with testicular cells containing SSCs. Wherever chimerism was observed, the percent donor DNA was calculated from the heights of donor and recipient peaks, and the results are averages and SEM of analyses of at least 4 microsatellite loci.
| Treatment groups | Recipient monkey # | Donor monkey # | Percentage of donor genotype transplanted | Unique microsatellite loci analyzed | |
|---|---|---|---|---|---|
| Epididymal sperm | Testicular cells from suspected de novo region | ||||
| No GnRH-ant control | 092 | 120 | No sperm | No de novo | NA |
| 114 | 094 | 37.7±1.2 | No de novo | D2S1333, D3S1768, D4S2365, D6S501 | |
| 120 | 124 | 10.7± 0.3 | No de novo | D2S1333, D3S1768, D6S501, D11S2002 | |
| GnRH-ant | 094 | 122 | 0 | 65.1± 0.8% | D2S1333, D3S1768, D6S501, D7S794 |
| 122 | 092 | 0 | 80.4± 1.6% | D2S1333, D3S1768, D6S501, D8S1106 | |
| 124 | 114 | 61.1± 2.0% | No de novo | D3S1768, D4S2365, D11S2002, D12S364 | |
NA: Not applicable
We next assessed the factors that might be responsible for the variability in the success of the transplantation as measured by the numbers of donor sperm in the epididymis (Table 2, Figure 3). The GnRH-ant treatment before transplantation had no significant effect on donor spermatogenic output (t-test P>0.6). The numbers of donor cells injected and donor cell viability were unrelated to the extent of donor spermatogenesis (Spearman correlation, P>0.9). However, there were trends or significance that the testis volume (P=0.02) and serum T (P=0.08) measured at the time or second irradiation (8 weeks before transplantation) and the transplantation efficiency score (P=0.14) were positively related to the numbers of donor sperm in the epididymis. For instance, the two monkeys with a transplantation score of 5 had the highest numbers of donor sperm in their epididymis. On the other hand, the presence of dilated tubules (P=0.04) and the irregular tubule-like cords (P=0.15) appeared to be negatively related to the success of the transplantation. Testicular damage from the first irradiation, as evidenced by small testis volumes and low levels of serum T in monkey #092, likely contributed to the dilated tubules and extremely low levels of both endogenous and donor spermatogenic recovery observed in this monkey at tissue harvest.
The irregular-tubule-like cords observed in monkeys #094 and #122 appeared to be identical to the de novo tubular cords we have described previously (Figure 6A,B).17 These abnormal cords filled an estimated 1.5–4.2% of the testis volume. They possessed incomplete basal laminae and contained germ cells up to and including round spermatids and, although rarely, mature spermatids (Figure 6B). Immunostaining for Vasa and acrosin confirmed the identification of germ cells and spermatids, respectively, in these cords (data not shown). In both of these cases, these cords were observed in the interstitium adjacent to the rete testis (Figure 6C). Microsatellite analysis of the DNA extracted from the regions containing these abnormal cord structures showed 65% and 80% of donor genotypes in the two monkey (Table 3), confirming that they indeed originated de novo from transplanted donor cells (Figure S6). The remaining percentages were likely contributed mostly by the recipient interstitial cells in addition to any possible minor contaminants from the endogenous tubular area.
FIGURE 6.

(A) Region of irregular de novo tubular cords with interspersed endogenous Sertoli-cell only tubules (*). De novo cords with spermatogenic development to the spermatocyte (†) and spermatid (‡) stages are indicated. (B) Higher magnification of region from A showing round spermatids (arrowheads) and elongated spermatids (arrows). (C) Region of de novo cords (DN) showing that it is adjacent to the rete testis area. Interspersed normal tubules that are Sertoli-cell-only (*) and with recovery spermatogenesis (¶) are indicated. Scale bars: A &B: 50 μm; C: 200 μm.
3.3. Intracytoplasmic Sperm Injection (ICSI) Results
To test whether the donor-derived sperm obtained after transplantation were functional, we injected the cryopreserved sperm from the recipients into in vivo matured rhesus oocytes. A total of 85 ova were injected with sperm from recipients #124 and #114; 14 developed into zygotes (16% of injected ova) and were maintained for 8 days in culture (Table S2). Six reached the compact morula stage (Figure 7A) and one reached early blastocyst. In the first set of injections using the epididymal sperm from recipient #124, which had 61% donor contribution, 4 embryos were successfully genotyped by microsatellite analysis and 2 had the paternal genotype of the transplant donor and 2 had the genotype of the transplant recipient (Table S2, Figure 7B). These results confirmed that the sperm produced from the transplanted SSCs are fertilization competent and can produce embryos. Three embryos from the second set of injections were transferred into timed recipients (Supporting Methods), but no pregnancies were established. In the third set of injections, when the recipient (#114) with 38% donor sperm was used, embryo genotyping was successful in 4 embryos; 2 had developed parthenogenetically and 2 were derived from endogenous sperm produced by the recipient male. It was not known why embryo development was suboptimal and no pregnancies were achieved; it was not specific to possible quality problems with the donor-derived sperm, since ICSI with sperm derived from endogenous SSC did not yield any better results.
FIGURE 7.

Embryo produced by ICSI with epididymal sperm from a monkey (#124) with high percentage of donor-derived sperm. (A) Compact morula resulting from in vitro culture of the fertilized oocyte. (B) Microsatellite DNA analysis of one donor-derived embryo and comparison with the oocyte, SSC donor and recipient male profiles. Alleles specific for the oocyte donor (represented by purple font and arrow), transplant donor (represented by black font and arrow), and transplant recipient (represented by green font and arrow) are indicated on the electropherogram panels. The presence of the alleles at 201 and 292 nucleotide pairs in the embryo demonstrates the paternal origin as being from the donor.
4. DISCUSSION
The most important finding in the current study was that, in a model that closely relates to gonadotoxic cancer treatment before puberty and transplantation of prepubertal testis cells back into the testis after puberty, significant donor-derived spermatogenesis was obtained in 3 out of 6 monkeys. In two of these monkeys, 13 and 26 million sperm were recovered from the cauda epididymis and 61% and 38% of these sperm, respectively, were donor derived. The high percentages of donor sperm demonstrate the success of the transplantation. The observation that the tubule differentiation indices in these two monkeys were 28% and 33%, respectively, as compared to 0.03– 20% in the remaining 4 monkeys, supports the conclusion that this was a result of enhanced donor cell colonization. A third monkey had 11% donor representation in the epididymal sperm, but there were only 0.6 million sperm
Comparison with previous studies (Table 4) emphasizes that this is the only study in which some of the gonadotoxic treatment was delivered prepubertally, and the transplantation was done after puberty, which will most likely be the clinically used strategy in humans. Unfortunately, because the 6.9-Gy radiation dose was insufficient for the desired level of SSC depletion in the prepubertal testis, it was also necessary to give another radiation dose after they reached puberty, which deviates from the usual clinical scenario. Nevertheless, the result that 3 of 6 recipient monkeys produced donor sperm is within the range of most of the previous studies.
Table 4.
Comparison with Previous Studies of Spermatogonial Stem Cell Transplantation in Non-human Primates.
| Species of Macaque | Recipient Prep | Age Cytotoxic Treatment | Age Transplant | Donor SSCs | Donor Marking | Enhanced recovery in transplant testis a | Donor sperm a | Percent Donor (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Cyno-molgus | Irradiation (2 Gy) | Adult | Adult | Autologous | None | 2/5 | ND b | – – | 43 |
| Rhesus | Irradiation (10 Gy) | Prepubertal | Prepubertal | Autologous | None | 1/4 | ND b | – – | 20 |
| Rhesus | Irradiation (10 Gy) | Pubertal | Pubertal | Autologous | None | 0/2 | ND b | – – | 20 |
| Rhesus | Busulfan (8–12 mg/kg) | Prepubertal | Prepubertal c | Autologous | Lentivirus | NA d | 3/5 | – – | 13 |
| Rhesus | Busulfan (8–12 mg/kg) | Adult | Adult | Autologous | Lentivirus | NA d | 9/12 | – – | 13 |
| Rhesus | Busulfan (8–11 mg/kg) | Adult | Adult | Allogeneic | Microsatellites | NR e | 2/6 | 10, 1.1 | 13 |
| Cyno-molgus | Irradiation (7 Gy) | Adult | Adult | Autologous | Lentivirus | 2/11 g | 6/12 h | – – | 14 |
| Rhesus | Irradiation (7 Gy) | Adult | Adult | Allogeneic | Microsatellites | 2/15 i | 5/15 j | 93, 84, 1.7, 0.4, 1.0 | 2 |
| Rhesus | Irradiation (6.9+7 Gy) | Prepubertal & Adult f | Adult | Allogeneic | Microsatellites | NA | 3/6 k | 61, 38, 11 | Present Study |
Number positive results/number transplanted.
ND: Not detectable because autologous transplantation was done with unmarked cells.
Transplantation was done 9–15 weeks after busulfan treatment. The pubertal status at this time was not reported.
NA: Not applicable because all or nearly all of the recipients were hemicastrated.
NR: Not reported.
6.9 Gy was given prepubertally and an additional 7 Gy was given after puberty because of recovery of spermatogenesis.
2/6 for GnRH-ant-treated, 0/5 for no GnRH-ant
5/6 for GnRH-ant-treated, 1/6 for no GnRH-ant
1/10 for GnRH-ant-treated, 1/5 for no GnRH-ant
3/10 for GnRH-ant-treated, 2/5 for no GnRH-ant
1/3 for GnRH-ant-treated, 2/3 for no GnRH-ant.
It is important to determine what specific factors might be associated with good colonization with donor cells. The effect of GnRH-ant treatment on the presence and yield of donor sperm was evaluated. Whereas two of the three control monkeys not treated with GnRH-ant produced donor sperm, only one of the three GnRH-ant treated monkeys produced donor sperm (Table 2), and we concluded that overall the GnRH-ant treatment showed no correlation with spermatogenic recovery from donor SSCs. This result is similar to that observed recently in a study involving allogeneic transplantation in rhesus monkeys,2 but differs from the stimulation of recovery of donor spermatogenesis by GnRH-ant-treatment observed earlier in a study involving autologous transplantation in cynomolgus macaques (Table 4).14 The inability to see any favorable effects of GnRH-ant in these allogeneic transplantation studies might be due to a possible enhancement of immune responses when testosterone is suppressed,34 resulting in the immune suppression being inadequate. This possibly could offset any benefit the hormone and immune suppression might have on colonization and recovery, but could also be a result of the species difference.
The effect of the efficiency of the filling of seminiferous tubules with the donor cell suspension on the production of donor sperm was assessed. The two cases with a transplantation efficiency score of 5 resulted in the highest levels of donor sperm in the epididymis. Although these data suggested a trend that transplantation efficiency was important for the success of the transplant, the correlation was not statistically significant (P=0.14). A similar trend towards higher donor sperm production with better transplantation efficiency was also observed in our previous study2, and when we combined the data of both studies, the association was highly statistically significant (Spearman’s correlation, ρ=0.58, P=0.006). Thus, efficient transfer of the injected cell suspension to the seminiferous tubules is indeed an important factor in successful transplantation.
The characteristics and functional integrity of the recipient testis may be factors in the ability to colonize, especially after the damaging prepubertal cytotoxic treatment.35 Both the serum T levels and testis volumes, measured at the time of the second irradiation, 8 weeks before transplantation, seemed to be correlated with the donor sperm production. The serum T level is a measure of pubertal development and testis volume is a measure of both pubertal development and recovery of spermatogenesis after the first irradiation. These results indicate that success from transplantation is dependent on the somatic cells of the testis going through relatively normal pubertal development despite the prepubertal irradiation. Further studies of transplantation are needed with a model of more complete spermatogenic cell depletion, because the transplantation is only needed when there is a failure of endogenous spermatogenic recovery.
Also the presence of dilated tubules, observed in the final histological samples taken 10 months after transplantation, was negatively correlated with the yield of donor sperm and appeared to be a factor limiting the development of donor spermatogenesis. Whereas in previous studies we have never observed such structural damage to the seminiferous tubule from 7 Gy testicular irradiation of adult monkeys,2,14 four of the six monkeys receiving 6.9 Gy prepubertally had dilated tubules in the final histological sample taken 10 months after transplantation, and in one (#092) of them, nearly all the tubules were dilated. It is likely that this was due to damage incurred from the first dose of 6.9 Gy since this monkey failed to show the increase in testicular volume (Figure S3A) that would be expected from maturation of the somatic elements of the testis during puberty. Dilated tubules in adult rhesus monkeys after prepubertal irradiation had also been observed previously, but no dose-response was reported,19 and we have also observed such tubule dilation (7%, 24%) in two rhesus monkeys that had received only 1 dose of 6.9 Gy before puberty (data not shown). The immature Sertoli cells in prepubertal testis, which are expected to be still proliferating,36 are likely one of the targets for such sensitivity of the somatic structure of the testis in these juvenile monkeys. Future studies of molecular markers of Sertoli cell functional status in such cases are important for further development SSC transplantation.
It has been suggested that it might be possible to restore tubular function and the SSC niche by donor Sertoli cells, as was demonstrated after chemical ablation of Sertoli cells in mice. 37 Our previous studies 38 showed that transplanted donor Sertoli cells colonized irradiated rat tubules but did not restore the somatic environment to support differentiation of endogenous spermatogonia, which were otherwise blocked from differentiation. Although transplantation of Sertoli cells as a niche replacement strategy may be beneficial to enhance recovery from transplanted cells in prepubertally irradiated monkeys, we as yet have no data as to whether or not the rhesus Sertoli cells in the transplantation suspension colonize the tubules of these irradiated monkeys.
However, in two of the six recipients, we observed the formation of de novo tubular cords containing somatic and germ cells, derived from the donor, in the interstitial space (Figure 6). This result extends our previous observation on a single irradiated adult monkey transplanted with prepubertal testis cells in which we confirmed that both the germ and somatic components of the de novo tubules were of donor-origin.17 As was the case in the previous study, the ultrasound visualization of the transplantation demonstrated that the cells were indeed injected into the rete testis and entered the tubules (transplant efficiency score ≥4) (Table 1). However, various studies have shown that even when injection is done into the rete, there is significant leakage of the cells to the interstitium.39,40 The location of de novo cords adjacent to the rete testis suggest that the rete itself may be the source of the leakage. Based on the observation that all 3 monkeys, in which we observed de novo cords in the interstitium, were treated with GnRH-ant, we suggest that the hormone suppression might increase the leakage of transplanted cells into the interstitium and/or create an environment favorable to the development of these de novo cords. It was noteworthy that none of these three monkeys showed any evidence of donor spermatozoa in the epididymis, indicating the there was no intratubular development of transplanted cells. Although the leakage and formation of de novo cords in the interstitium appears negatively correlated with intratubular donor spermatogenesis, the production of donor spermatozoa in these cords potentially can be used for fertilization. Thus, if the seminiferous tubules do not support donor spermatogenesis from the cryopreserved SSCs due to endogenous Sertoli cells rendered defective by gonadotoxic therapies, spermatogenesis from such de novo derived cords may be an alternative strategy for fertility preservation.
Since there have been no reports as to whether chemotherapy treatment also results in similar damage to the Sertoli cells, we reanalyzed the testicular tissues from a previous study 13, in which 5 prepubertal monkeys were treated with 8–12 mg/kg busulfan when they were prepubertal and then given autologous transplantation of lentivirus transduced testicular cells. There were no dilated tubules nor any morphological abnormalities in these testes that were harvested about 3 years after busulfan exposure. Even in the few Sertoli-only tubules, the Sertoli cells had a regular columnar appearance. However it should be noted that even at these high doses of busulfan, 97% of tubules showed recovering spermatogenesis, 83% of which progressed to the spermatid stage. Most of this recovery must be from endogenous surviving SSC since two of the monkeys were negative for production of lentivirus marked sperm. This is in contrast to our results with two prepubertal monkeys (not shown) irradiated with 6.9 Gy that showed spermatogenic recovery in only 33% of tubules at 2 years after irradiation. Thus although busulfan does not produce the damage to the somatic testis tissue that irradiation does, busulfan in not as effective at producing prolonged loss of spermatogenesis in prepubertal animals. Since even 6.9 Gy did not fully eliminate endogenous spermatogenesis, there is a need for a better model that would kill SSCs with minimal somatic testicular tissue damage.
In conclusion, we have demonstrated that SSC transplantation after puberty can restore spermatogenesis and fertilization-competent sperm production after prepubertal and postpubertal irradiation and have characterized the factors that may be related to the success of the technique. Particularly the precise delivery of cells and filling of tubules at the injection appears to be an important factor. However, complete depletion of germ cells without causing somatic damage was not possible with single doses of radiation during the prepubertal period, and improvements in the treatment paradigm are necessary. Since the spermatogenic function of the human testis is more sensitive to fractionated doses of radiation 41 or combining radiation with alkylating agents, such as busulfan 42, than to single doses of radiation, these may be better models to deplete the germ cells. It is hoped that these procedures will more closely model the cohort of patients treated prepubertally with gonadotoxic cancer therapies, who have normal tubular somatic cells but with spermatogenic depletion, and are in need of fertility preservation procedures.
Supplementary Material
Acknowledgements
This work was supported by research grants P01 HD075795 from NIH/NICHD to KO, R01 HD100197 from NIH/NICHD to KO and MLM, Cancer Center Support Grant P30 CA016672 from NIH/NCI to the University of Texas MD Anderson Cancer Center and P51 OD011092 from NIH to the Oregon National Primate Research Center. We would like to acknowledge the outstanding work of Jennifer M. Meyer who assisted in managing the treatment of the monkeys, sample collections, and animal health issues and also Cathy Ramsey and Fernanda de Carvalho for their valuable assistance in the ovarian-stimulation, embryo culture and ICSI procedures. We sincerely thank Dr. Min S. Lee, National Institute for Child Health and Human Development, for providing the Acyline.
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Shetty G, Mitchell JM, Meyer JM, et al. Restoration of functional sperm production in irradiated pubertal rhesus monkeys by spermatogonial stem cell transplantation. Andrology. 2020;8:1428–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenney LB, Antal Z, Ginsberg JP, et al. Improving male reproductive health after childhood, adolescent, and young adult cancer: Progress and future directions for survivorship research. J Clin Oncol. 2018;36:2160–2168. [DOI] [PubMed] [Google Scholar]
- 4.Green D, Galvin H, Horne B. The psycho-social impact of infertility on young male cancer survivors: a qualitative investigation. Psychooncology. 2003;12:141–152. [DOI] [PubMed] [Google Scholar]
- 5.Valli-Pulaski H, Peters KA, Gassei K, et al. Testicular tissue cryopreservation: 8 years of experience from a coordinated network of academic centers. Hum Reprod. 2019;34:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gassei K, Orwig KE. Experimental methods to preserve male fertility and treat male factor infertility. Fertil Steril. 2016;105:256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goossens E, Jahnukainen K, Mitchell RT, et al. Fertility preservation in boys: recent developments and new insights (dagger). Hum Reprod Open. 2020;2020:hoaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giudice MG, de Michele F, Poels J, Vermeulen M, Wyns C. Update on fertility restoration from prepubertal spermatogonial stem cells: How far are we from clinical practice? Stem cell research. 2017;21:171–177. [DOI] [PubMed] [Google Scholar]
- 9.Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Renfree MB, Short RV. Successful intra- and interspecific male germ cell transplantation in the rat. Biol Reprod. 2003;68:961–967. [DOI] [PubMed] [Google Scholar]
- 11.Ryu BY, Orwig KE, Avarbock MR, Brinster RL. Stem cell and niche development in the postnatal rat testis. Dev Biol. 2003;263:253–263. [DOI] [PubMed] [Google Scholar]
- 12.Honaramooz A, Behboodi E, Megee SO, et al. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260–1264. [DOI] [PubMed] [Google Scholar]
- 13.Hermann BP, Sukhwani M, Winkler F, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shetty G, Uthamanthil RK, Zhou W, et al. Hormone suppression with GnRH antagonist promotes spermatogenic recovery from transplanted spermatogonial stem cells in irradiated cynomolgus monkeys. Andrology. 2013;1:886–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Leuprolide, a gonadotropin-releasing hormone agonist, enhances colonization after spermatogonial transplantation into mouse testes. Tissue Cell. 1998;30:583–588. [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Shao SH, Weng CC, Wei C, Meistrich ML. Hormonal suppression restores fertility in irradiated mice from both endogenous and donor-derived stem spermatogonia. Toxicol Sci. 2010;117:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shetty G, Mitchell JM, Lam TNA, et al. Donor spermatogenesis in de novo formed seminiferous tubules from transplanted testicular cells in rhesus monkey testis. Hum Reprod. 2018;33:2249–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fayomi AP, Peters K, Sukhwani M, et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019;363:1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Rooij DG, van de Kant HJ, Dol R, et al. Long-term effects of irradiation before adulthood on reproductive function in the male rhesus monkey. Biol Reprod. 2002;66:486–494. [DOI] [PubMed] [Google Scholar]
- 20.Jahnukainen K, Ehmcke J, Quader MA, et al. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011;26:1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbst KL, Coviello AD, Page S, Amory JK, Anawalt BD, Bremner WJ. A single dose of the potent gonadotropin-releasing hormone antagonist acyline suppresses gonadotropins and testosterone for 2 weeks in healthy young men. J Clin Endocrinol Metab. 2004;89:5959–5965. [DOI] [PubMed] [Google Scholar]
- 22.Hermann BP, Sukhwani M, Lin CC, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in Rhesus macaques. Hum Reprod. 2009;24:1704–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. [DOI] [PubMed] [Google Scholar]
- 25.Byrne JA, Pedersen DA, Clepper LL, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. [DOI] [PubMed] [Google Scholar]
- 26.Bishop CV, Reiter TE, Erikson DW, et al. Chronically elevated androgen and/or consumption of a Western-style diet impairs oocyte quality and granulosa cell function in the nonhuman primate periovulatory follicle. J Assist Reprod Genet. 2019;36:1497–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewitson L, Dominko T, Takahashi D, et al. Unique checkpoints during the first cell cycle of fertilization after intracytoplasmic sperm injection in rhesus monkeys. Nat Med. 1999;5:431–433. [DOI] [PubMed] [Google Scholar]
- 28.Mitalipov S, Kuo HC, Byrne J, et al. Isolation and characterization of novel rhesus monkey embryonic stem cell lines. Stem Cells. 2006;24:2177–2186. [DOI] [PubMed] [Google Scholar]
- 29.Ramsey C, Hanna C. In Vitro Culture of Rhesus Macaque (Macaca mulatta) Embryos. Methods Mol Biol. 2019;2006:341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chemes HE, Dym M, Raj HG. Hormonal regulation of Sertoli cell differentiation. Biol Reprod. 1979;21:251–262. [DOI] [PubMed] [Google Scholar]
- 31.Meistrich ML, Wilson G, Shuttlesworth G, Huhtaniemi I, Reissmann T. GnRH agonists and antagonists stimulate recovery of fertility in irradiated LBNF1 rats. J Androl. 2001;22:809–817. [PubMed] [Google Scholar]
- 32.Gordon TP, Rose RM, Bernstein IS. Seasonal rhythm in plasma testosterone levels in the rhesus monkey (Macaca mulatta): a three year study. Horm Behav. 1976;7:229–243. [DOI] [PubMed] [Google Scholar]
- 33.Wickings EJ, Nieschlag E. Seasonality in endocrine and exocrine testicular function of the adult rhesus monkey (Macaca mulatta) maintained in a controlled laboratory environment. Int J Androl. 1980;3:87–104. [DOI] [PubMed] [Google Scholar]
- 34.Gubbels Bupp MR, Jorgensen TN. Androgen-Induced Immunosuppression. Front Immunol. 2018;9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trondle I, Westernstroer B, Wistuba J, Terwort N, Schlatt S, Neuhaus N. Irradiation affects germ and somatic cells in prepubertal monkey testis xenografts. Mol Hum Reprod. 2017;23:141–154. [DOI] [PubMed] [Google Scholar]
- 36.Simorangkir DR, Ramaswamy S, Marshall GR, Roslund R, Plant TM. Sertoli cell differentiation in rhesus monkey (Macaca mulatta) is an early event in puberty and precedes attainment of the adult complement of undifferentiated spermatogonia. Reproduction. 2012;143:513–522. [DOI] [PubMed] [Google Scholar]
- 37.Yokonishi T, McKey J, Ide S, Capel B. Sertoli cell ablation and replacement of the spermatogonial niche in mouse. Nat Commun. 2020;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Shao S, Shetty G, Meistrich ML. Donor Sertoli cells transplanted into irradiated rat testes stimulate partial recovery of endogenous spermatogenesis. Reproduction. 2009;137:497–508. [DOI] [PubMed] [Google Scholar]
- 39.Faes K, Lahoutte T, Hoorens A, Tournaye H, Goossens E. In search of an improved injection technique for the clinical application of spermatogonial stem cell transplantation. Reprod Biomed Online. 2017;34:291–297. [DOI] [PubMed] [Google Scholar]
- 40.Gul M, Hildorf S, Dong L, et al. Review of injection techniques for spermatogonial stem cell transplantation. Hum Reprod Update. 2020;26:368–391. [DOI] [PubMed] [Google Scholar]
- 41.Meistrich ML, van Beek MEAB. Radiation sensitivity of the human testis. Adv Radiat Biol. 1990;14:227–268. [Google Scholar]
- 42.Sanders JE, Buckner CD, Leonard JM, et al. Late effects on gonadal function of cyclophosphamide, total-body irradiation, and marrow transplantation. Transplantation. 1983;36:252–255. [DOI] [PubMed] [Google Scholar]
- 43.Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod. 2002;17:55–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


