Abstract
Rationale.
Women with cocaine use disorder have worse treatment outcomes compared to men. Sex differences in cocaine addiction may be driven by differences in neurobiology or stress reactivity. Oxytocin is a potential therapeutic for stress reduction in substance use disorders, but no studies have examined the effect of oxytocin on neural response to drug cues in individuals with cocaine use disorders or potential sex differences in this response.
Objectives.
The goal of this study was to examine the effect of intranasal oxytocin on cocaine cue reactivity in cocaine dependence, modulated by gender and history of childhood trauma.
Methods.
Cocaine-dependent men with (n=24) or without (n=19) a history of childhood trauma, and cocaine-dependent women with (n=16) or without (n=8) a history of childhood trauma completed an fMRI cocaine cue-reactivity task under intranasal placebo or oxytocin (40 IU) on two different days. fMRI response was measured in the right amygdala and dorsomedial prefrontal cortex (DMPFC).
Results.
In the DMPFC, oxytocin reduced fMRI response to cocaine cues across all subject groups. However, in the amygdala, only men with a history of childhood trauma showed a significantly reduced fMRI response to cocaine cues on oxytocin versus placebo, while women with a history of childhood trauma showed an enhanced amygdala response to cocaine cues following oxytocin administration. Cocaine-dependent subjects with no history of childhood trauma showed no effect of oxytocin on amygdala response.
Conclusions.
Oxytocin can reduce cue-reactivity in cocaine dependence, but its effect is modified by sex and childhood trauma history. Whereas men with cocaine dependence may benefit from oxytocin administration, additional studies are needed to determine whether oxytocin can be an effective therapeutic for cocaine-dependent women.
Keywords: cocaine use disorder, functional magnetic resonance imaging, amygdala, sex differences, prefrontal cortex
INTRODUCTION
Evidence indicates important sex differences in symptomatology, trajectory, and treatment of cocaine use disorder (CUD). Of the approximately five million past-year cocaine users in the United States, men represent the majority (64%) (Center for Behavioral Health Statistics and Quality) (2017). Preclinical studies also find sex differences following chronic cocaine self-administration. Across all stages of addiction, female rats demonstrate greater motivated cocaine-seeking than males (for review see (Anker and Carroll 2010a; Anker and Carroll 2010b).
Although numerous factors contribute to observed sex differences in cocaine seeking and relapse, neurobiological response to stress and neuroadaptations due to a history of trauma may play major roles. For example, female rats show greater cocaine- and stress-primed reinstatement, while males rats show greater cue-induced reinstatement (Anker and Carroll 2010a; Anker and Carroll 2010b; Buffalari et al. 2012; Feltenstein et al. 2011). In humans, corticostriatal-limbic hyperactivity is associated primarily with drug cues in men and stress cues in women (Potenza et al. 2012). In addition to sex differences in response to stress cues, women are more likely than men to suffer from PTSD (Garza and Jovanovic 2017). Childhood trauma exacerbates initiation, progression, and maintenance of SUDs in humans (Dube et al. 2003; Enoch 2011) and is associated with relapse, particularly among women (Hyman et al. 2008; Sacks et al. 2008).
Childhood trauma is also associated with structural and functional brain changes in the amygdala, hippocampus, anterior cingulate cortex (ACC), and prefrontal-cortex (PFC) (Arnsten 2009; Hart and Rubia 2012; Lupien et al. 2009; van Harmelen et al. 2014), as well as dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and autonomic stress response systems (DeSantis et al. 2011; Heim et al. 2000). These changes increase neuropsychiatric vulnerability among trauma victims as evidenced by increased rates of psychiatric disorders later in life including post-traumatic stress disorder (PTSD) and substance use disorders (SUD) (Neumann et al. 1996; Ozer et al. 2003; Pirard et al. 2005). Consequently, interventions that target stress neurocircuitry, particularly with consideration of sex differences and trauma history, may improve CUD treatment outcomes.
Oxytocin (OXY) is a hypothalamic neuropeptide that has shown promise as a potential therapeutic for substance use disorders and PTSD. OXY is involved in anxiolytic and natural reward processes and is released peripherally via the posterior pituitary and centrally into the limbic system. Neuroadaptations in the OXY system result from environmental insults including childhood maltreatment (Heim et al. 2009; Wismer Fries et al. 2005) and chronic drug abuse (Lee et al. 2016), particularly in brain regions critical to emotion processing, learning, and memory (e.g. hippocampus, amygdala, hypothalamus; (Georgiou et al. 2015; Sarnyai and Kovacs 1994). Although the anxiolytic effects of OXY have been addressed more extensively in PTSD than in substance use, exogenous OXY administration may reverse drug-induced neuroadaptations (see (Lee et al. 2016) for review). For example, several preclinical studies have demonstrated the potential therapeutic effects of OXY on behavioral and neural correlates in animal models of cocaine addiction (Baracz et al. 2012; Carson et al. 2010; Sarnyai et al. 1991; Weber et al. 2018; Zhou et al. 2014) and stress reactivity (Sabihi et al. 2017), and one study found sex-dependent effects of OXY on cocaine-induced locomotor activity in rodents (Leong et al. 2016). In humans with CUD, childhood adversity has been shown to moderate the effects of OXY on cortisol reactivity during a social stress task (Flanagan et al. 2015). OXY also reduces cue-reactivity in male heavy drinkers in the insula and prefrontal and limbic regions (Hansson et al. 2018). However, (Lee et al. 2014) reported that OXY increased desire to use and cue-induced excitability in a predominantly male sample of CUD subjects in court-ordered treatment. These discrepant findings warrant additional research into effects of OXY on cue-induced craving. In addition, both of these studies used male participants making it difficult to generalize the effects of OXY in females.
It is important not only to examine sex-dependent effects of OXY in humans, but also to understand OXY’s effects within the social context of trauma. Men and women with CUD are likely to respond differently due to the rates and neural consequences of trauma, and sexually dimorphic nature of the oxytonergic system (Boccia et al. 2013; Dumais et al. 2013). In addition, estrogen has been shown to promote OXY release and is required for the synthesis of OXY receptors in the amygdala (Lim and Young 2006). In a review paper, (Borrow and Handa 2017) report several studies showing that estradiol increases OXY levels in plasma, increases OXY mRNA and increases OXY receptor binding. These findings suggest that sex differences in OXY response may be related to estrogen, and warrant further exploration in clinical samples.
This human laboratory study investigates the potential impact of OXY on neural correlates of cue-reactivity in CUD men and women with and without a history of childhood trauma. To our knowledge, this is the first fMRI study of sex differences in effects of OXY on neural cue-reactivity in CUD. The primary study design was a randomized, counterbalanced crossover design where participants were examined under both the OXY and PBO conditions. The primary hypothesis was that OXY would reduce amygdala and prefrontal cortex cue-reactivity in CUD men and women, but attenuation of cue-reactivity would be most pronounced in CUD women due to OXY-enhancing effects of estrogen. We also evaluated whether childhood trauma modifies the effect of OXY on neural correlates of cue-reactivity in male and female subjects with CUD.
METHODS
Participants
Participants took part in a larger study investigating the impact of OXY on subjective and neuroendocrine responses to stressors. The current analysis included data only from the fMRI component of the study. Non-treatment seeking CUD individuals responded to local media advertisements over a 54-month period. Written informed consent was obtained before study assessments were administered. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, and the protocol received Institutional Review Board (IRB) approval. General exclusion criteria included (1) pregnancy, nursing, or plan to become pregnant during the course of the study; (2) women who had a complete hysterectomy, were post-menopausal, or receiving hormone replacement or hormonal contraceptive therapy; (3) history of or current significant hematological, endocrine, cardiovascular, pulmonary, renal, gastrointestinal, or neurological diseases; (4) history of or current psychotic, panic, eating, or bipolar affective disorders; (5) current major depressive disorder and PTSD; (6) history of or current medical conditions that might affect HPA axis activity; (7) synthetic glucocorticoid or exogenous steroid therapy within one month of testing; (8) psychotropic medications (with the exception of selective serotonin reuptake inhibitors), opiates or opiate antagonists, benzodiazepines, antipsychotics, beta-blockers and other medications that might interfere with HPA axis activity or physiologic measurements; (9) acute illness or fever; (10) Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for substance dependence except alcohol, nicotine or marijuana within the past 60 days; or (11) unwillingness or inability to maintain abstinence from cocaine and other drugs of abuse (except nicotine) for three days prior to the cue-reactivity sessions.
Of the 112 participants randomized to treatment order, 12 dropped out (5 males), 7 were excluded because they did not meet eligibility on scanning days (positive drug test: 2 males; positive pregnancy test: 1 female; other medical reasons: 1 male) or were not compliant during scanning (sleeping or unable to complete scans: 2 males, 1 female), leaving 93 who completed both fMRI visits. Of these, 20 participants were excluded due to excessive head motion (8 males) and data from 2 participants (both females) could not be converted to nifti format and were excluded, leaving 71 participants. Four of the 71 individuals did not complete the CTQ (2 males) because that measure was added shortly after initiation of fMRI data collection, leaving a final sample of 67 subjects (43 males).
Assessment
Participants meeting pre-screening criteria were evaluated for study eligibility with the Mini-International Neuropsychiatric Interview (MINI) (Sheehan et al. 1998). The substance use module of the Structured Clinical Interview for DSM-IV (SCID-IV) was used to assess current and lifetime substance use disorders (First et al. 2002). Substance use in the ninety days prior to the study was assessed using the Time-Line Follow-Back (Sobell and Sobell 1992). The Childhood Trauma Questionnaire (CTQ; (Bernstein and Fink 1998)) was used to assess the extent to which individuals experienced five domains of childhood abuse and neglect (sexual abuse, physical abuse, emotional abuse, emotional neglect, and physical neglect). Participants answered each of 25 questions using a 5-point Likert scale ranging from 1 (never true) to 5 (very often true); a total score cut-off of 37 or greater is used to indicate some childhood trauma. A medical history and physical examination were completed to assess for medical exclusions. Participants meeting inclusion criteria and no exclusion criteria were scheduled to complete the study procedures, and instructed to not use cocaine or other drugs of abuse for a minimum of three days prior to the test sessions.
Study Procedures
Participants completed two fMRI cue reactivity sessions on consecutive days. On day 1 of testing, participants arrived at the Medical University of South Carolina’s Addiction Sciences Division research clinic at 10:00 a.m. Upon arrival, urine pregnancy tests were administered. Smokers were provided with a nicotine patch. Self-reports, urine drug screens (Roche Diagnostics, Indianapolis, Indiana), and breathalyzer tests (AlcoSensor III, Intoximeters, Inc., St. Louis, Missouri) were used to assess abstinence. If the pregnancy and drug tests were negative (with the exception of THC), study procedures continued. At 11:30am, subjective ratings were obtained. A modified version of the Within Session Rating Scale was used to assess subjective ratings of craving, mood, anxiety, and stress (Childress et al. 1986). This 1–10 visual analogue scale is anchored with the adjectival modifiers (“not at all,” “mildly,” “moderately,” and “extremely”). The Cocaine Craving Questionnaire (CCQ; (Sussner et al. 2006) Brief was used to assess cocaine craving, but responses from this instrument were not analyzed. Participants were then provided a standardized lunch.
At 1:20pm, participants were administered 40 IUs of OXY nasal spray or matching placebo. This dose was selected based on previous studies using similar doses of OXY (Ditzen et al. 2009; Heinrichs et al. 2003; Kubzansky et al. 2009), as well as our own previous work (Flanagan et al. 2015; McRae-Clark et al. 2013). Doses of OXY between 18–40IU intranasally have been shown to be safe for short-term use in clinical populations (MacDonald et al. 2011). The 40IU dose to be utilized in this study was chosen based on our preliminary data demonstrating an impact on cannabis-related outcomes (McRae-Clark et al. 2013). This dose has also been shown to be effective in reducing cigarette consumption (Van Hedger et al. 2018). Timing of administration was also based on previous studies showing central activity of OXY 40 minutes after intranasal administration (Heinrichs et al. 2009; McRae-Clark et al. 2013).
Intranasal OXY and matching PBO (saline spray) were compounded by the MUSC Investigational Drug Service (IDS). USP certified OXY powder was acquired by the MUSC IDS through the Professional Compounding Centers of America (PCCA). IDS used the potency data provided in the PCCA provided certificate of analysis to prepare the dose required in the final intranasal spray product; the potency of all intranasal batches were further validated by external testing by Eagle Analytics Laboratory. The OXY vehicle consists of glycerin and preserved water; the delivery device utilized was the Professional Compounding Centers of America - PCCA #35–1453 Metered Nasal Spray Pump 20 MM, which delivers 0.1cc per pump. The total volume delivered was based on potency testing; participants administered one pump to each nares, alternating between pumps, and waiting a minimum of 10 seconds between each administration. The range of sprays administered was 9–15 total sprays, with an oxytocin potency range of 27.2 – 49.1 IU/ml.
To achieve balance in sample size with respect to treatment order across sex, a stratified block randomized design with randomly varying block sizes was implemented. Further, the administration of study drug was counterbalanced such that half of the participants were randomized to OXY on day 1 and half to placebo.
Subjective measures were repeated at 1:55pm. Scanning procedures commenced at 2:00pm. The fMRI cue reactivity paradigm has been previously utilized in imaging studies involving cocaine use disordered individuals (Prisciandaro et al. 2014a; Prisciandaro et al. 2014b). The paradigm consisted of pictures of cocaine and related objects (e.g., crack pipe), neutral objects (e.g., furniture), and visual control images that match the cocaine pictures in color and hue, but lack object recognition. Stimulus presentation occurred over eighteen 28.8-second blocks, with 6 additional 28.8-second rest blocks during which subjects fixated on a crosshair. Each task block contained 6 images of either cocaine, neutral objects, or blurred control images, with each picture displayed for 4.8 seconds. Blocks, and stimuli within blocks, were presented in random order. After each task and rest block, participants were asked to rate their craving using a hand pad; ratings ranged from zero (“none”) to four (“severe”), with responses recoded from 1 to 5 for analysis. The rating period lasted 6 seconds. The same pictures were used on both testing days, but the task blocks were in a different order on each day.
fMRI data images were acquired on a Siemens Trio 3.0 Tesla scanner with a 12-channel head coil (Siemens Medical, Erlangen, Germany). During initial scanner tuning, localizing, and structural scanning, participants were shown relaxation images (i.e., 20 scenic pictures, each displayed for 30 seconds, and repeated if necessary). A high-resolution T1-weighted MPRAGE anatomical scan (TR = 2.1 ms, TE = 4.2 ms, flip angle = 12°, field of view = 256 mm, 1.0 mm) covering the entire brain and positioned using a sagittal scout image was acquired for co-registration and normalization of functional images. T2*-weighted gradient echo EPI images were acquired with the following parameters: TR = 2500 ms, TE = 27 ms, flip angle = 77º, 40 axial slices (FOV = 224 × 224 mm, thickness = 3.5 mm voxels with 0.5 mm gap, in interleaved order. A gradient field map image was collected to match the spatial parameters of the EPI images.
Following completion of the first scan, participants returned the following day and completed identical procedures with the opposite treatment condition. At the end of the second scan day, participants were debriefed and compensated.
Data Analysis
fMRI preprocessing
Data were analyzed using FMRI Expert Analysis Tool (FEAT) Version 5.98, part of FMRIB’s Software Library (FSL;www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction using MCFLIRT (Jenkinson et al. 2002), fieldmap-based EPI unwarping using PRELUDE+FUGUE (Jenkinson 2003; Jenkinson 2004); non-brain removal using BET (Smith 2002); spatial smoothing using a Gaussian kernel of FWHM 7.0mm; grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=50.0s). Registration to the MNI template using the high resolution structural scan was carried out using FLIRT (Jenkinson and Smith 2001).
Time-series statistical analysis was carried out using FILM with local autocorrelation correction (Woolrich et al. 2001). The three experimental conditions (cocaine, object, and blur) and 6-second rating periods served as explanatory variables with temporal derivatives; rest blocks were not explicitly modeled. Six head motion parameters (3 translations and 3 rotations) and motion outliers were included as nuisance variables.
fMRI regions-of-interest analysis
Two regions-of-interest (ROIs) were selected for the primary analysis: the right amygdala (MNI coordinates: x=20, y=−2, z=−16) and the dorsomedial prefrontal cortex (MNI coordinates: x=0, y=26, z=44). The right amygdala was selected as an ROI because it is associated with structural and functional changes due to childhood trauma and stress (Hart and Rubia 2012; Lupien et al. 2009). Reviews of fMRI or PET studies of cocaine cue reactivity are not conclusive as to whether the left or right amygdala is more response to cocaine cues (Gordon 2016; Jasinska et al. 2014; Kuhn and Gallinat 2011). However, an activation likelihood estimation meta-analysis by (Chase et al. 2011) reports that the left amygdala shows a stronger response to cocaine cues compared to various control conditions whereas the right amygdala response to cues is more strongly associated with craving. Given that the present study is focused on whether oxytocin can reduce cocaine craving as well as the neural correlate of that craving, the right amygdala was selected as an ROI.
Although numerous prefrontal regions have been associated with cue-reactivity in cocaine dependence, the DMPFC was selected as an ROI in this study given preclinical evidence that it is a critical site for contextual reinstatement of cocaine seeking (Fuchs et al. 2005) and DMPFC FOS activity is associated with anxiety during withdrawal (El Hage et al. 2012). In addition to its role in cocaine seeking and reinstatement, OXY administered to the medial PFC prelimbic region reduced anxiety like behaviors in rats, but not when OXY was delivered to infralimbic or cingulate regions (Sabihi et al. 2017). Moreover, OXY delivery to the prelimbic region increased GABA response in prelimbic PFC and altered neuronal response in amygdala following anxiety testing. These findings suggest a potentially important role of medialPFC regions other than the ACC in OXY-mediated attenuation of anxiety response. However, it should be noted that while some researchers consider the prelimbic region to be part of the dorsomedial PFC, there is considerable debate and inconsistency as to whether these regions are homologous across species; (Laubach et al. 2018). Nevertheless, several findings in humans demonstrate altered DMPFC functioning in trauma exposure or childhood maltreatment. For example, DMPFC response to social exclusion was correlated with severity of childhood maltreatment (van Harmelen et al. 2014). (Harnett et al. 2018) report that the DMPFC may be a site where fear learning is overgeneralized in individuals who report trauma exposure.
The two ROIs were defined as 10-mm (diameter) spheres in MNI space, then spatially transformed into each subject’s native EPI space. The value for the contrast of parameter estimates (cope) for the Cocaine > Object contrast was extracted in each ROI using featquery. The Cocaine > Object contrast reflects the effect of the cocaine cues over and above object cues, which yields the greatest specificity in the fMRI response and rules out lower level visual processing as well as higher level automatic object recognition as contributors to the fMRI effects observed. The cope value in each ROI for this contrast served as the measures of fMRI response.
Generalized linear mixed effects models were developed to specifically assess the effect of treatment condition (OXY, PBO), sex and trauma group (absent, present) on fMRI signal in each ROI separately. The analysis controlled for the effects of visit (1 or 2) and treatment condition order (OXY or PBO first) in each model and these design variables were retained in all models. The modifying effects of sex and trauma group were assessed in the model using appropriate interaction terms and retained when evidence of effect modification or confounding was present.
Cue Response Analysis
To assess whether cocaine cues induced higher levels of craving compared to other cues, a paired t-test tested the effect of cocaine cues versus the other cues and conditions combined (rest, object and blur blocks) on cue craving response in the placebo condition. To assess the effect of study treatment condition on cue craving response, a generalized linear mixed effects model was developed to assess the effect of treatment condition (OXY, PBO), sex and trauma group (absent, present) on interoceptive cocaine cue craving response collected during fMRI scanning. The analysis controlled for the effects of visit (1 or 2) and treatment condition order (OXY or PBO first) in each model as design covariates. The modifying effects of sex and trauma group were assessed in the model using appropriate interaction terms and retained when evidence of effect modification or confounding was present.
Simple correlations between fMRI response and craving response were assessed using Spearman’s rank order correlation coefficient. For these correlations, the difference in fMRI signal (cocaine cue versus object cope value) on PBO minus OXY was correlated with the difference in craving response (for cocaine cues) on PBO minus OXY. These correlations were conducted separately in each Sex x Trauma group.
Generalized linear mixed effects models, paired t-tests, and Spearman correlations were conducted using IBM SPSS Statistics (v. 25, Chicago, IL).
Supplementary Whole-brain fMRI analysis
A supplementary analysis used a whole-brain voxel-wise general linear model approach to examine any regions that were more responsive Cocaine versus Object cues under OXY and PBO conditions. Using FSL, it was not possible to implement the full model used for the ROI analysis (see FSL’s documentation on 2-way mixed effect ANOVA with repeated measures: https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM#ANOVA:_2-groups.2C_2-levels_per_subject_.282-way_Mixed_Effect_ANOVA.29). Therefore, we conducted group analyses separately for the four Sex x Trauma groups. The goal of this analysis was to report patterns of activation that may help to formulate hypotheses for future studies. These higher-level group analyses were conducted using FLAME (FMRIB’s Local Analysis of Mixed Effects) stage 1 (Beckmann et al. 2003; Woolrich 2008; Woolrich et al. 2004). Paired t-tests were also used to directly compare OXY and PBO conditions for the Cocaine > Object contrast separately in each subject group. Z (Gaussianised T/F) statistic images were thresholded using GRF-theory-based maximum height thresholding with a (corrected) significance threshold of P=0.05 (Worsley 2001).
RESULTS
Demographics
Demographic and clinical characteristics for the randomized cohort stratified by sex and trauma group are presented in Table 1. Sixty-seven of the 71 (93%) participants who attended both fMRI study visits had CTQ scores collected; there were no differences in CTQ available data between females and males (females: 24/26=92% vs. males: 43/45=96%). The remaining included participants (n=67) were on average 42 (SD=9.7) years of age with a majority being male (64%) and African American (70%). Female participants were somewhat younger than male participants (39.3±9.0 vs. 43.34±9.8, p=.09), but had similar cocaine use history. Although statistically insignificant, female participants reported numerically higher CTQ scores than males (50.7±21.4 vs. 43.3±15.1, p=.26). The range of CTQ scores in the sample of 67 participants was 25–83 for males and 25–95 for females. Using the cutoff score of 37 or greater to indicate that some trauma was present (Bernstein and Fink 1998) yielded 8 trauma-absent females, 16 trauma-present females, 24 trauma-absent males, and 19 trauma-present males. The four subject groups were not different on any demographic measures.
Table 1.
Demographic and clinical characteristics stratified by sex and trauma group.
| Characteristic | fMRI Data Set (n=67) | Female (n=24) | Male (n=43) | P-Valueb | |||
|---|---|---|---|---|---|---|---|
| Trauma Absent (n=8) | Trauma Present (n=16) | Trauma Absent (n=19) | Trauma Present (n=24) | ||||
| Demographics | |||||||
| Age (yrs) | 41.9 (9.7) | 41.5 (10.7) | 38.6 (8.3) | 45.3 (7.8) | 41.6 (11.2) | 0.19 | |
| Female % (n) | 35.8 (24) | -- | -- | -- | -- | -- | |
| Cigarette Smoker % (n) | 76.1 (51) | 87.5 (7) | 68.8 (11) | 73.7 (14) | 79.2 (19) | 0.75 | |
| African American % (n) | 70.2 (47) | 50.0 (4) | 62.5 (10) | 84.2 (16) | 70.8 (17) | 0.28 | |
| Caucasian % (n) | 29.9 (20) | 50.0 (4) | 37.5 (6) | 15.8 (3) | 29.2 (7) | ||
| Cocaine Use Characteristics | |||||||
| Age at 1st Use | 21.1 (6.2) | 22.7 (7.4) | 21.0 (5.3) | 22.1 (8.5) | 20.0 (4.2) | 0.73 | |
| Total Years of Use | 16.4 (8.2) | 16.6 (7.8) | 13.9 (8.0) | 18.7 (7.9) | 16.2 (8.6) | 0.38 | |
| Age at Dependence Onset | 28.8 (8.8) | 29.7 (11.3) | 26.4 (6.5) | 31.2 (10.1) | 28.0 (8.2) | 0.58 | |
| Using Days per Month | 18.0 (7.8) | 16.8 (8.1) | 17.7 (8.7) | 19.8 (7.7) | 17.2 (7.5) | 0.65 | |
| Baseline Trauma and Subjective Measures | |||||||
| CTQ Total Score | 46.0 (2.6) | 28.5 (4.4) | 61.8 (17.3) | 30.2 (3.0) | 53.7 (12.4) | <0.01 | |
| Trauma Present Group n (%) | 61.2 (41) | -- | -- | -- | -- | -- | |
| Cravinga | 2.8 (2.6) | 3.0 (2.2) | 2.7 (2.9) | 3.1 (2.6) | 2.7 (2.6) | 0.89 | |
| Stressa | 2.3 (2.4) | 1.4 (1.8) | 2.0 (2.5) | 2.5 (2.4) | 2.7 (2.6) | 0.53 | |
| Anxietya | 2.5 (2.3) | 2.3 (1.9) | 2.4 (2.7) | 2.5 (2.5) | 2.6 (2.2) | 0.96 | |
Craving, stress, and anxiety ratings measured on fMRI study day 1 prior to tasks.
Demographic and clinical characteristics were compared across gender and trauma groups using a Kruskal-Wallis test statistic for continuous data and a Chi-Squared test statistic for categorical data.
fMRI regions-of-interest analysis
In the DMPFC, the main effect of treatment with OXY was significant (χ21=4.4, p=.03) indicating fMRI response was reduced under treatment with OXY (Mean=.12, SEM=.04) versus PBO (Mean=.20, SEM=.03). The effect of visit was also significant (χ21=9.6, p=.002), with fMRI response reduced in Visit 2 (Mean=.09, SEM=.03) compared to Visit 1 (Mean=.20, SEM=.04). No other main effects or interactions were significant for the DMPFC.
In the right amygdala, the Treatment Group x Sex interaction was significant (χ23=4.0, p=.05) indicating that males and females had differential right amygdala fMRI response to the treatment. Further, this relationship was modified by the presence of trauma (Treatment Group x Sex x Trauma Group interaction χ27=7.1, p=.008). Group level comparisons revealed that OXY had no effect on amygdala response for the trauma absent group for females or males, but that it significantly reduced amygdala response for males with trauma present (p=.006) and conversely increased amygdala response for females with trauma present (See Figure 1; p=.012). To determine whether this interaction in the trauma present subjects was driven by a sex difference in fMRI response to cocaine cues, an independent-samples t-test was conducted for cocaine cues alone, collapsed over treatment, to compare responses in males (Mean = .38, SEM = .09) and females (Mean = .33, SEM = .08), but this was not significant, t(38) = .38, p = .71.
Figure 1.
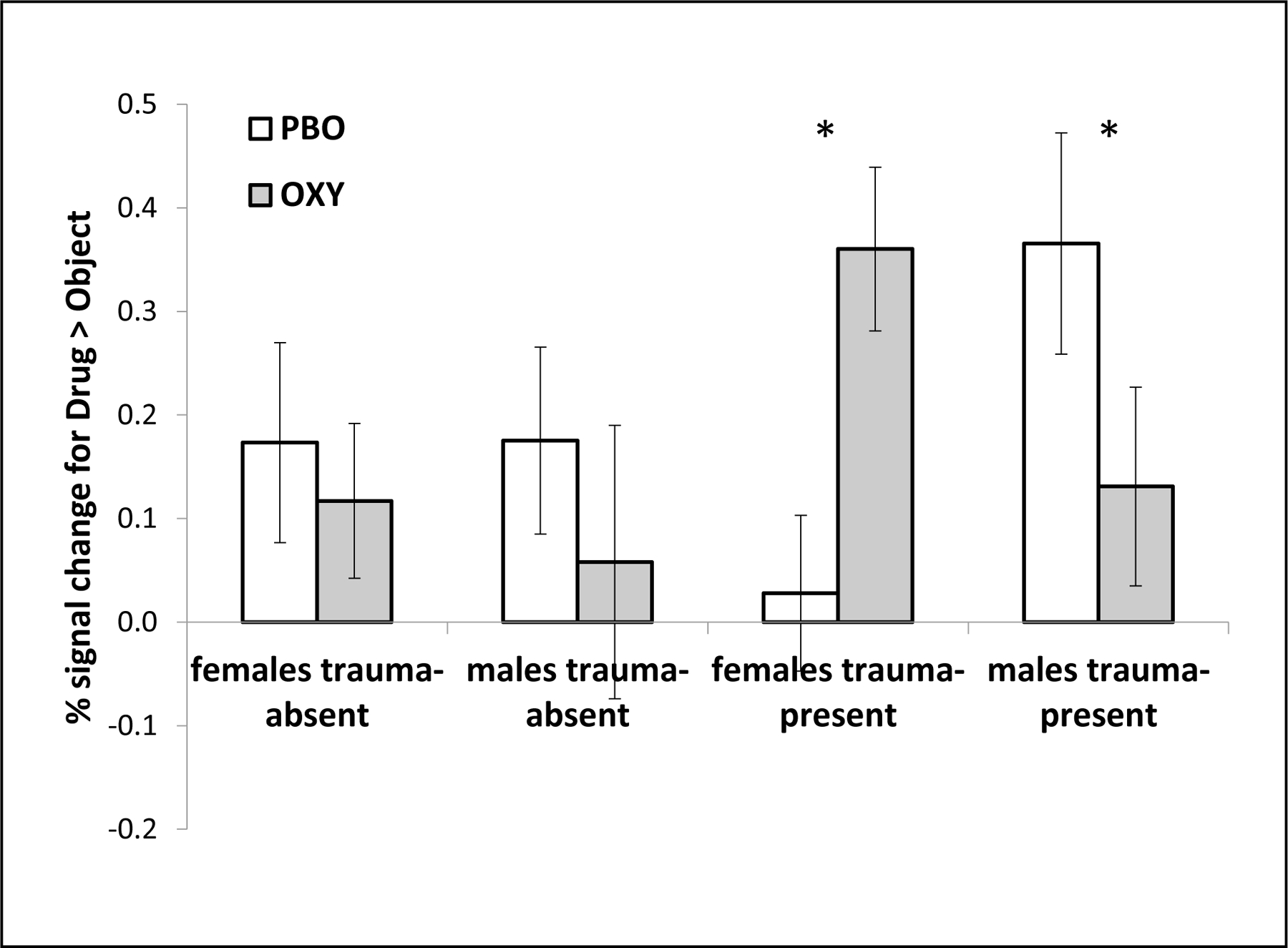
Significant three-way interaction in the right amygdala region-of-interest. fMRI signal for the Drug > Object contrast is shown as a function of sex, trauma group and drug condition (PBO = placebo; OXY = oxytocin). Significant pairwise contrasts are indicated with an asterisk. Error bars are standard error of the mean. Response in females with no childhood trauma was not different on PBO (M = .17; SE = .10) versus OXY (M = .12; SE = .07). Response in males with no childhood trauma was not different on PBO (M = .18; SE = .08) versus OXY (M = .06; SE = .08). Response in females with childhood trauma was lower on PBO (M = .03; SE = .09) versus OXY (M = .36; SE = .13). Response in males with childhood trauma was higher on PBO (M = .37; SE = .11) versus OXY (M = .13; SE = .10)
Cue Response Analysis
Cocaine cues induced higher levels of craving (Mean=2.43, SEM=0.15) than other cues (Mean = 1.69, SEM = 0.10) in the placebo condition, t(69) = 7.56, p = .0001, demonstrating that cocaines cues were effective at inducing craving. There were no significant main effects of treatment condition (OXY v. PBO), sex, treatment order, trauma group or interactions on craving ratings. The only effect that was statistically significant for craving ratings in the cocaine condition was study visit (χ21= 15.0, p = .01) where ratings were lower in Visit 2 (Mean= 2.21, SEM=.14) than in Visit 1 (Mean= 2.53, SEM=.15).
The correlation between change in fMRI response in the amygdala or DMPFC and change in cocaine cue craving rating due to OXY as compared to PBO was assessed and stratified by sex and trauma group. Correlations were not significant for the DMPFC. However, the correlation between right amygdala fMRI response and reduction in cue craving was significant in trauma-present males (See Figure 2; rho=.45, p=.03) but not in any other subject group (p’s > .11). The positive correlation for trauma-present males indicates that a greater reduction in fMRI signal was associated with a greater reduction in craving.
Figure 2.
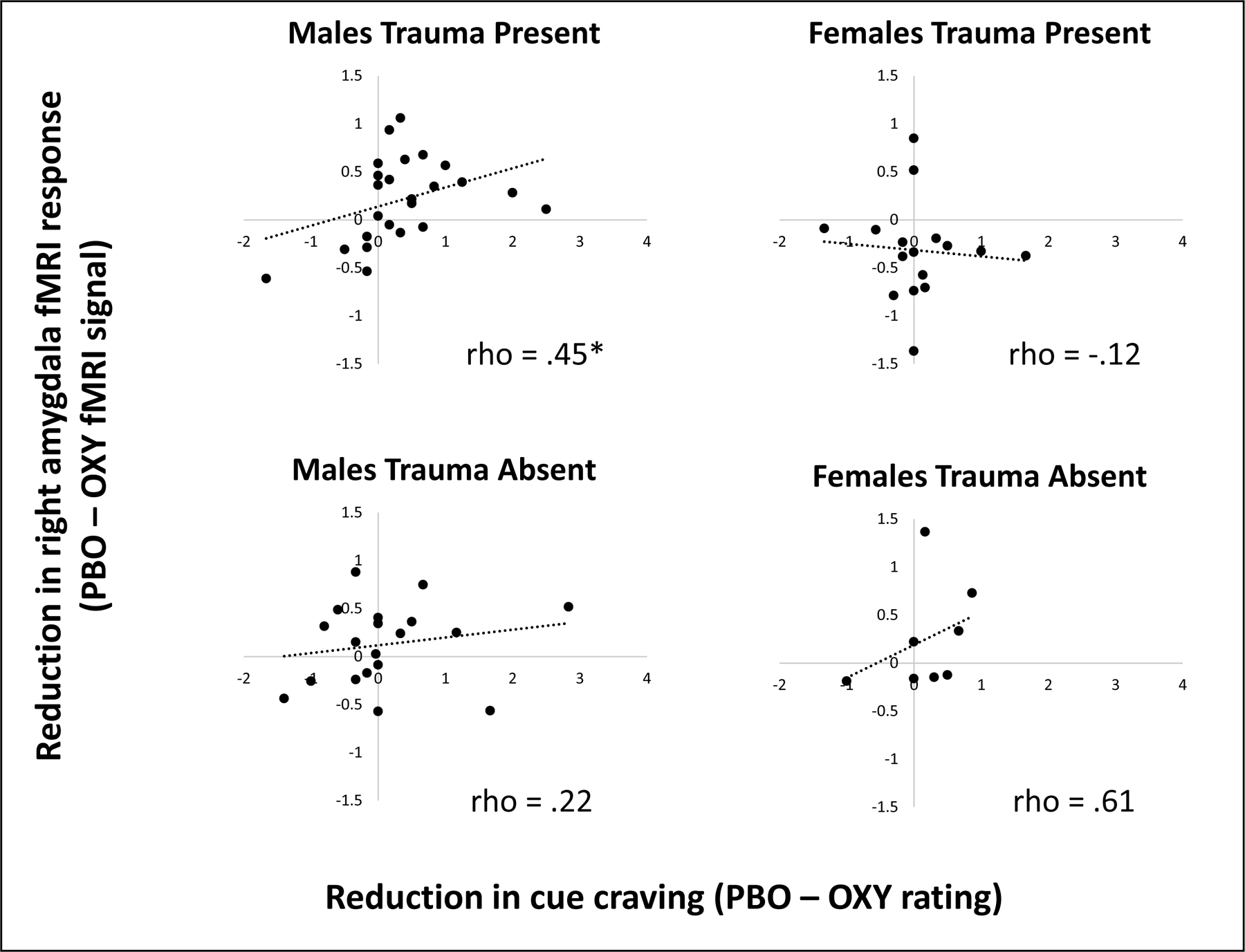
Correlations between reduction in cue craving (x-axis) and reduction in fMRI signal due to OXY in the right amygdala region of interest as a function of sex (males, females) and trauma group (absent present). Spearman rank correlations were only significant for the male trauma-present group (indicated by *).
Supplementary Whole-brain fMRI Analysis
Activation maps for the Cocaine versus Object contrast for the four Sex x Trauma groups stratified by treatment condition are shown in Figure 3. Activation reflects significantly greater fMRI signal for cocaine cues versus object cues at a cluster-corrected p-value of .05. Paired t-tests comparing OXY and PBO conditions directly in each subject group yielded no significant activation at a corrected threshold. However, we note several patterns that not only confirm the ROI analyses but suggest interesting patterns that were not addressed in the ROI analysis. One pattern is that some regions that are activated in the PBO condition are not activated or are reduced in extent in the OXY condition, especially in prefrontal (female trauma-present and male trauma-absent groups) and parietal cortex (all groups show reduced extent of activation). In addition, the male trauma-present group shows left insula activation on PBO which is not present on OXY (white arrow). Activation patterns also reflect the ROI finding in the right amygdala where the male trauma-present group shows activation on PBO but this activation is absent on OXY, whereas the female trauma-present group shows no amygdala activation on PBO but this activation is present on OXY (green arrow). The whole-brain analysis also echos the ROI finding that the DMPFC shows a pronounced response on PBO but this response is absent or reduced in extent on OXY. This effect is mostly found in the female trauma-present and male trauma-absent groups (turquoise arrows; however, note that no interactions were significant in the ROI analysis). While the activation patterns within each subject group and treatment condition were significant at a cluster-corrected level, the comparison across treatment conditions yielded no significant activation; thus, the patterns described above should be interpreted with caution.
Figure 3.
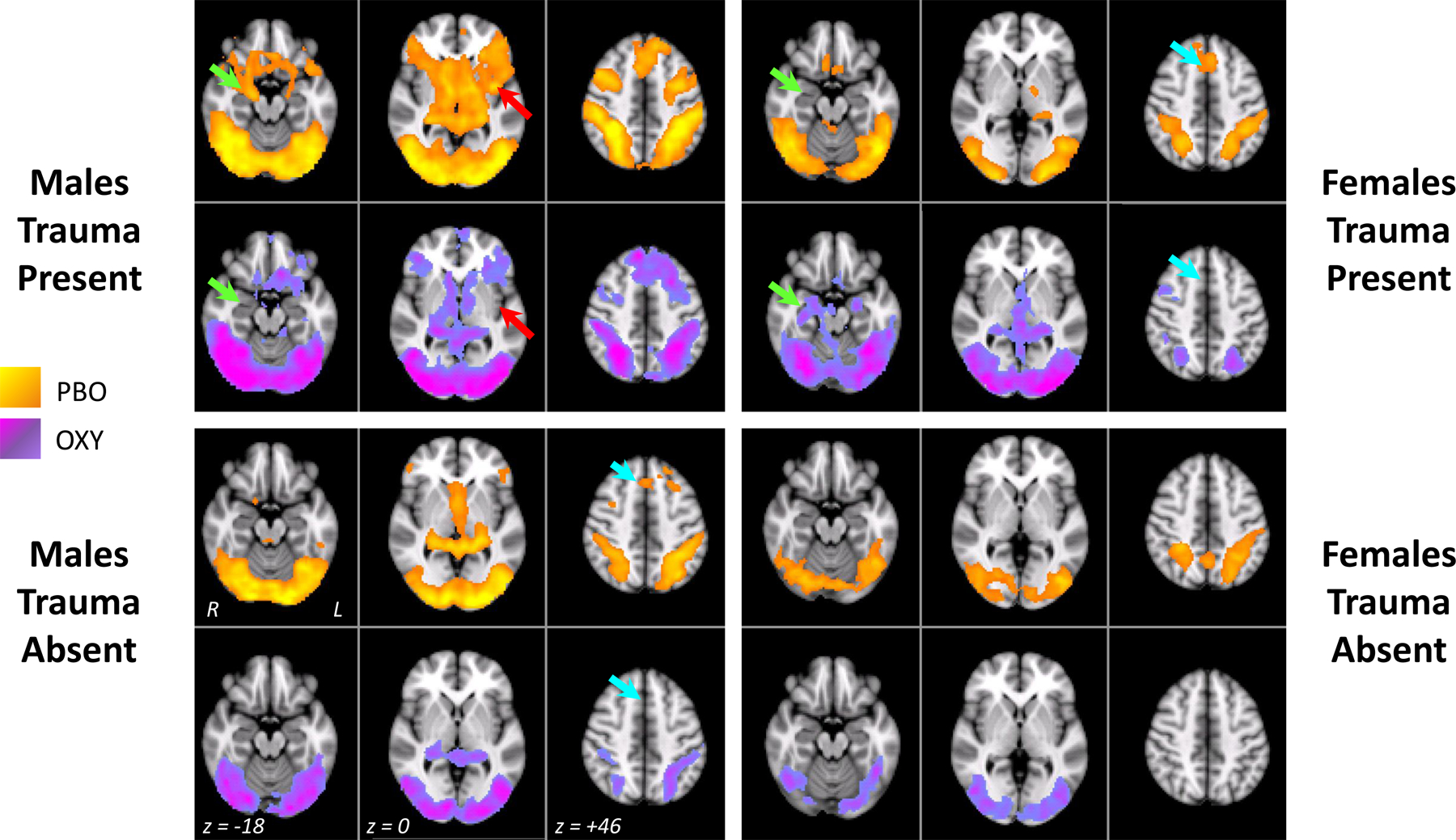
Results from the general linear model voxel-wise whole-brain analysis for the four Sex x Trauma groups in each drug condition (PBO = placebo, shown in yellow-orange; OXY=oxytocin, shown in blue-purple). Three axial slices at MNI z coordinates of −18, 0 or +46 are shown for each Sex x Trauma Group x Drug combination, with the left hemisphere shown on the right of each brain image. Green arrows indicate the approximate location for the right amygdala region-of-interest. Turquoise arrows indicate the approximate location for the DMPFC region. Red arrow points to a left insula region in males trauma-present. Activation maps reflect the contrast of Cocaine > Object cues and were cluster corrected at Z>2.33, p = .05.
DISCUSSION
In the present fMRI study, intranasal OXY reduced cocaine cue reactivity in the DMPFC, but this reduction was not further qualified by sex or childhood trauma. In contrast, reduced cue reactivity in the amygdala was further qualified by both sex and trauma group. Only males who had reported childhood trauma showed a significant decrease in amygdala cue reactivity, and these decreases were associated with decreased craving on OXY. Males and females with no trauma showed no reduction, whereas females with trauma showed significantly increased amygdala cue reactivity on OXY.
Although the prosocial effects of OXY on neural function have been widely investigated, fewer studies have examined OXY’s role in reducing neural response to drug cues in substance users. Hannson and colleagues (Hansson et al. 2018) showed that OXY reduced cue-reactivity in male heavy drinkers in insula, prefrontal, and limbic regions. The present study similarly showed reduced cue-reactivity in male cocaine users in limbic (amygdala) and prefrontal (DMPFC) regions and reduced cue reactivity in the right amygdala was associated with decreased craving response due to OXY in males with childhood trauma. A recent resting-state connectivity study with the same sample of subjects as the present study examined oxytocin-induced changes in connectome measures (Joseph et al. 2019). Interestingly, both males and females showed changes in anterior cingulate cortex connectivity on oxytocin, but only males showed these changes in the amygdala. These findings are similar to the present study in which only males with childhood trauma showed reduced cue-reactivty in the amygdala on oxytocin, but both males and females showed reduced DMPFC cue reactivity on oxytocin. Together, these findings suggest that higher order regulatory brain regons like the DMPFC and anterior cingulate are affected by oxytocin in CUD, but not further modulated by gender. Limbic regions like the amygdala, in contrast, may show sex-specific responses.
The finding that effects of OXY emerged in the amygdala in the present study is not necessarily surprising given that the amygdala is rich in oxytocin receptors in humans (Boccia et al. 2013) and is a major locus for hyper-reactivity to negative emotion and stress in anxiety disorders and PTSD. Several neuroimaging studies have reported that OXY reduced neural reactivity in the amygdala to negative valence or stimuli that invoke stress or anxiety in typical subjects and individuals with PTSD (Domes et al. 2007; Flanagan et al. in press; Kirsch et al. 2005; Koch et al. 2016), or that OXY modifies amygdala functional connectivity in typical subjects or individuals with social anxiety disorder (Dodhia et al. 2014; Kumar et al. 2014). While the present study did not use stress cues, presentation of cocaine cues to cocaine users may invoke a state of negative affect, particularly during abstinence. OXY acted to reduce the amygdala response to these cues in a similar manner as reported in prior studies that manipulated stress cues.
Many prior studies have focused on the amygdala as a target for anxiolytic effects of OXY, but other regions are also potential targets. The present study showed that OXY reduced DMPFC response to cocaine cues, suggesting it may be another target brain region. The DMPFC not only shows an increased response to drug cues (see review by (Jasinska et al. 2014) and shows stronger functional connectivity with the insula in response to cocaine cues (Cisler et al. 2013), but it is also a site where learned fear may be overgeneralized (Harnett et al. 2018) and where childhood trauma modulates response to social exclusion (van Harmelen et al. 2014). Connectivity of the DMPFC with limbic circuitry (amygdala and hippocampus) likely reflects effortful emotional regulation and may be a critical variable that distinguishes childhood trauma exposure from PTSD (Birn et al. 2014). Taken together, these findings not only implicate the DMPFC in cocaine cue reactivity, but also point to this region as a site where trauma symptomology may manifest. However, in the present study, the OXY response on DMPFC was not further qualified by sex or childhood trauma, suggesting it may be a potential therapeutic that targets SUD symptoms (rather than post-traumatic stress symptoms) in men and women with CUD.
The present finding that OXY enhanced amygdala cue-reactivity in females with childhood trauma was not predicted. However, on placebo, females with childhood trauma showed a minimal response to cocaine cues versus objects, compared with the other subject groups. Two other studies have also reported reduced neural response to arousing and salient stimuli in cocaine-dependent females. One study showed a dampened neural response to naturally arousing stimuli in female cocaine users compared to male cocaine users (Canterberry et al. 2016). Also, compared to men, cocaine-dependent women showed less regional cerebral blood flow in the amygdala during script-guided cue-induced craving (Kilts et al. 2004). Kilts et al.(2004) suggested that the decreased amygdala response in cocaine-dependent women in their study might be related to learned associations between trauma and cocaine use, given prior findings of blunted amygdala response in studies of trauma exposure and PTSD (Britton et al. 2005; Diener et al. 2016). The blunted amygdala response in the placebo condition observed in the present study in female cocaine users with childhood trauma could reflect a vigilance-avoidance mechanism whereby an initial emotional response to cocaine cues is rapidly suppressed to avoid experiencing an associated fear response related to trauma (Diener et al. 2016). OXY may reduce this suppression leading to an exacerbated amygdala response to cocaine cues. However, why this occurs only in females with childhood trauma, and not in males, needs further exploration.
One possibility is that incentive salience interacts with gender, trauma history, or both. A prior study in cocaine-dependent men and women reported that corticostriatal-limbic hyperactivity was associated primarily with drug cues in men and stress cues in women (Potenza et al. 2012). Also, among cocaine-dependent women and men there was a correlation between craving and peak stress response to cocaine cues, but only women show this pattern in response to a social stressor (Waldrop et al. 2010). These findings suggest that drug cues are more salient to men and stress cues are more salient to women. Because only drug cues were used in the present study, the ameliorative effects of OXY were only observed in cocaine-dependent men. If stress cues are more salient to cocaine-dependent women and lead to greater corticolimbic reactivity compared to drug cues, effects of OXY may in turn be more pronounced, particularly in cocaine-dependent women with a history of childhood trauma.
Other factors such as interactions among childhood trauma, OXY, and gonadal hormones like estrogen and progesterone (Garza and Jovanovic 2017; Lim and Young 2006; Weber et al. 2018) could explain the exacerbated amygdala response due to OXY in cocaine-dependent women with a history of childhood trauma. For example, estradiol is associated with enhanced drug effects in cocaine dependence (Evans et al. 2002; Sofuoglu et al. 1999; Sofuoglu et al. 2004). OXY plays an important role in emotion, memory, and learning and may further enhance these drug effects. However, future studies are needed to examine potential mechanisms driving the interaction between sex and childhood trauma in cocaine dependence.
Limitations
Although OXY shows promise in reducing stress response and neural reactivity in substance users (Lee et al. 2016), there are many factors that can affect oxytocin’s response. Here, we examined the role of sex and childhood trauma, but genetic factors, timing of traumatic events, gonadal hormones, contextual variables and interindividual factors may also play a role (Donadon et al. 2018; Garza and Jovanovic 2017; Olff et al. 2013). In addition, the present study did not examine or control for the effect of menstrual cycle phase or estrogen levels, so no conclusions could be reached about how gonadal hormones may influence oxytocin function.
The sample size in the present study, when broken down by sex and trauma group, may limit the interpretation and generalizability of study results. Analyses that were conducted within each group separately (i.e., correlations between fMRI signal and craving response and whole-brain fMRI analyses) should be interpreted with caution. For this reason, we presented those analyses not to test particular hypotheses, but rather, to present descriptives that may be helpful in formulating hypotheses for future studies with larger samples. For example, the whole-brain analysis finding that the left insula was activated in response to cocaine versus object cues in males with some childhood trauma on PBO but not on OXY suggests that future studies may want to focus analyses on salience network regions.
The number of ROIs examined in the present study was limited to only two in order to avoid multiple simultaneous statistical tests. Additional ROIs such as the insula, striatum, hippocampus, anterior cingulate cortex, and others, are likely of interest when examining effects of oxytocin in SUD. Also, it is not clear whether there is a lateralized response in some of these regions. However, in order to be modulated by oxytocin a given brain region should be responsive to cocaine cues under placebo in a given subject group. The exploratory whole-brain analyses provide some preliminary clues as to which regions may be most of interest for future studies.
Another limitation was that the present study used the same stimuli on both days of fMRI scanning, which could potentially lead to habituation or neural adapation to the stimuli. This was potentially the case in the DMPFC where fMRI response to cocaine cues was greater on Day 1 than on Day 2. Although a main effect of OXY treatment emerged in the DMPFC even controlling for visit, it is possible that having repeated stimuli underestimated its effect.
In conclusion, the present study demonstrated that OXY can reduce amygdala reactivity to cocaine cues in cocaine-dependent men with a history of childhood trauma. The reduced amygdala reactivity was also behaviorally relevant in that decreased craving due to OXY was correlated with the magnitude of fMRI response reduction. OXY did not reduce amygdala reactivity in cocaine-dependent men or women with no history of childhood trauma and OXY enhanced amygdala response in cocaine-dependent women with a history of childhood trauma. These findings suggest that OXY may affect craving response in cocaine-dependence, but factors such as gender and childhood trauma history need to be carefully considered in future studies of oxytocin.
ACKNOWLEDGEMENTS
This study was sponsored by a National Institute on Drug Abuse grants P50DA016511 (K. Brady), K23DA045099 (B. Sherman), and K24DA038240 (A. McRae-Clark), with additional support from the National Center for Advancing Translational Sciences grant UL1TR001450 (K. Brady). We thank Laura Lohnes for assistance with the fMRI data analysis.
Footnotes
DISCLOSURES AND CONFLICTS OF INTEREST
No authors have financial disclosures or conflicts of interest to declare.
REFERENCES
- Anker JJ, Carroll ME (2010a) Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats Psychopharmacology (Berl) 208:211–222 doi: 10.1007/s00213-009-1721-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME (2010b) The Role of Progestins in the Behavioral Effects of Cocaine and Other Drugs of Abuse: Human and Animal Research Neurosci Biobehav Rev 35:315–333 doi: 10.1016/j.neubiorev.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF (2009) Stress signalling pathways that impair prefrontal cortex structure and function Nat Rev Neurosci 10:410–422 doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracz SJ, Rourke PI, Pardey MC, Hunt GE, McGregor IS, Cornish JL (2012) Oxytocin directly administered into the nucleus accumbens core or subthalamic nucleus attenuates methamphetamine-induced conditioned place preference Behav Brain Res 228:185–193 doi: 10.1016/j.bbr.2011.11.038 [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM (2003) General multilevel linear modeling for group analysis in FMRI Neuroimage 20:1052–1063 doi: 10.1016/s1053-8119(03)00435-x [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L (1998) Childhood Trauma Questionnaire: A retrospective self-report manual The Psychological Corporation, San Antonio, TX [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ (2014) Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity Depress Anxiety 31:880–892 doi: 10.1002/da.22291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, Petrusz P, Suzuki K, Marson L, Pedersen CA (2013) Immunohistochemical localization of oxytocin receptors in human brain Neuroscience 253:155–164 doi: 10.1016/j.neuroscience.2013.08.048 [DOI] [PubMed] [Google Scholar]
- Borrow AP, Handa RJ (2017) Estrogen Receptors Modulation of Anxiety-Like Behavior Vitamins and hormones 103:27–52 doi: 10.1016/bs.vh.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I (2005) Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery Biol Psychiatry 57:832–840 doi: 10.1016/j.biopsych.2004.12.025 [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Baldwin CK, Feltenstein MW, See RE (2012) Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats Physiol Behav 105:209–214 doi: 10.1016/j.physbeh.2011.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canterberry M, Peltier MR, Brady KT, Hanlon CA (2016) Attenuated neural response to emotional cues in cocaine-dependence: a preliminary analysis of gender differences Am J Drug Alcohol Abuse 42:577–586 doi: 10.1080/00952990.2016.1192183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS, Cornish JL, Guastella AJ, Hunt GE, McGregor IS (2010) Oxytocin decreases methamphetamine self-administration, methamphetamine hyperactivity, and relapse to methamphetamine-seeking behaviour in rats Neuropharmacology 58:38–43 doi: 10.1016/j.neuropharm.2009.06.018 [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L (2011) The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis Biol Psychiatry 70:785–793 doi: 10.1016/j.biopsych.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP (1986) Conditioned responses in a methadone population: A comparison of laboratory, clinic, and natural settings Journal of Substance Abuse Treatment 3:173–179 [DOI] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, Andrew James G, Kilts CD (2013) Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction Psychiatry Res 213:39–46 doi: 10.1016/j.pscychresns.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis SM et al. (2011) Gender differences in the effect of early life trauma on hypothalamic-pituitary-adrenal axis functioning Depress Anxiety 28:383–392 doi: 10.1002/da.20795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener SJ et al. (2016) Reduced amygdala responsivity during conditioning to trauma-related stimuli in posttraumatic stress disorder Psychophysiology 53:1460–1471 doi: 10.1111/psyp.12699 [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M (2009) Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict Biol Psychiatry 65:728–731 [DOI] [PubMed] [Google Scholar]
- Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ, Phan KL (2014) Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder Neuropsychopharmacology 39:2061–2069 doi: 10.1038/npp.2014.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC (2007) Oxytocin attenuates amygdala responses to emotional faces regardless of valence Biol Psychiatry 62:1187–1190 doi: 10.1016/j.biopsych.2007.03.025 [DOI] [PubMed] [Google Scholar]
- Donadon MF, Martin-Santos R, Osorio FL (2018) The Associations Between Oxytocin and Trauma in Humans: A Systematic Review Frontiers in pharmacology 9:154 doi: 10.3389/fphar.2018.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF (2003) Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study Pediatrics 111:564–572 [DOI] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH (2013) Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways Horm Behav 64:693–701 doi: 10.1016/j.yhbeh.2013.08.012 [DOI] [PubMed] [Google Scholar]
- El Hage C, Rappeneau V, Etievant A, Morel AL, Scarna H, Zimmer L, Berod A (2012) Enhanced anxiety observed in cocaine withdrawn rats is associated with altered reactivity of the dorsomedial prefrontal cortex PLoS One 7:e43535 doi: 10.1371/journal.pone.0043535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA (2011) The role of early life stress as a predictor for alcohol and drug dependence Psychopharmacology (Berl) 214:17–31 doi: 10.1007/s00213-010-1916-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW (2002) The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women Psychopharmacology 159:397–406 [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE (2011) Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle Psychopharmacology (Berl) 216:53–62 doi: 10.1007/s00213-011-2187-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition SCID-I/P, [Google Scholar]
- Flanagan J, Sippel LM, Moran-Santa Maria M, Hartwell K, Brady KT, Joseph JE (in press) Impact of Oxytocin on the Neural Correlates of Fearful Face Processing in PTSD Related to Childhood Trauma European Journal of Psychotraumatology [DOI] [PMC free article] [PubMed]
- Flanagan JC, Baker NL, McRae-Clark AL, Brady KT, Moran-Santa Maria MM (2015) Effects of adverse childhood experiences on the association between intranasal oxytocin and social stress reactivity among individuals with cocaine dependence Psychiatry Res 229:94–100 doi: 10.1016/j.psychres.2015.07.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE (2005) The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats Neuropsychopharmacology 30:296–309 doi: 10.1038/sj.npp.1300579 [DOI] [PubMed] [Google Scholar]
- Garza K, Jovanovic T (2017) Impact of Gender on Child and Adolescent PTSD Current psychiatry reports 19:87 doi: 10.1007/s11920-017-0830-6 [DOI] [PubMed] [Google Scholar]
- Georgiou P et al. (2015) The oxytocin analogue carbetocin prevents priming-induced reinstatement of morphine-seeking: Involvement of dopaminergic, noradrenergic and MOPr systems Eur Neuropsychopharmacol 25:2459–2464 doi: 10.1016/j.euroneuro.2015.09.015 [DOI] [PubMed] [Google Scholar]
- Gordon HW (2016) Laterality of Brain Activation for Risk Factors of Addiction Current drug abuse reviews 9:1–18 doi: 10.2174/1874473709666151217121309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC et al. (2018) Oxytocin Reduces Alcohol Cue-Reactivity in Alcohol-Dependent Rats and Humans Neuropsychopharmacology 43:1235–1246 doi: 10.1038/npp.2017.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett NG, Ference EW 3rd, Wood KH, Wheelock MD, Knight AJ, Knight DC (2018) Trauma exposure acutely alters neural function during Pavlovian fear conditioning Cortex 109:1–13 doi: 10.1016/j.cortex.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Rubia K (2012) Neuroimaging of child abuse: a critical review Front Hum Neurosci 6:52 doi: 10.3389/fnhum.2012.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C et al. (2009) Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene Front Behav Neurosci 3:41 doi: 10.3389/neuro.08.041.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C et al. (2000) Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood Jama 284:592–597 [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U (2003) Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress Biol Psychiatry 54:1389–1398 [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G (2009) Oxytocin, vasopressin, and human social behavior Frontiers in neuroendocrinology 30:548–557 [DOI] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R (2008) Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men Drug Alcohol Depend 92:208–216 doi: 10.1016/j.drugalcdep.2007.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y (2014) Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies Neurosci Biobehav Rev 38:1–16 doi:S0149–7634(13)00247–9 [pii]10.1016/j.neubiorev.2013.10.013 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M (2003) Fast, automated, N-dimensional phase-unwrapping algorithm Magn Reson Med 49:193–197 doi: 10.1002/mrm.10354 [DOI] [PubMed] [Google Scholar]
- Jenkinson M (2004) improving the Registration of B0-disorted Epi Images using Calculated Cost Function Weights: we 202 Neuroimage 22:e1544–e1545 [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images Neuroimage 17:825–841 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM (2001) A global optimisation method for robust affine registration of brain images Medical image analysis 5:143–156 [DOI] [PubMed] [Google Scholar]
- Joseph JE et al. (2019) Oxytocin-Induced Changes in Intrinsic Network Connectivity in Cocaine Use Disorder: Modulation by Gender, Childhood Trauma, and Years of Use Front Psychiatry 10:502 doi: 10.3389/fpsyt.2019.00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP (2004) The neural correlates of cue-induced craving in cocaine-dependent women Am J Psychiatry 161:233–241 doi: 10.1176/appi.ajp.161.2.233 [DOI] [PubMed] [Google Scholar]
- Kirsch P et al. (2005) Oxytocin modulates neural circuitry for social cognition and fear in humans J Neurosci 25:11489–11493 doi: 10.1523/JNEUROSCI.3984-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2016) Intranasal Oxytocin Normalizes Amygdala Functional Connectivity in Posttraumatic Stress Disorder Neuropsychopharmacology 41:2041–2051 doi: 10.1038/npp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky LD, Mendes WB, Appleton A, Block J, Adler GK (2009) Protocol for an experimental investigation of the roles of oxytocin and social support in neuroendocrine, cardiovascular, and subjective responses to stress across age and gender BMC Public Health 9:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J (2011) Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response Eur J Neurosci 33:1318–1326 doi: 10.1111/j.1460-9568.2010.07590.x [DOI] [PubMed] [Google Scholar]
- Kumar J, Vollm B, Palaniyappan L (2014) Oxytocin affects the connectivity of the precuneus and the amygdala: a randomized, double-blinded, placebo-controlled neuroimaging trial The international journal of neuropsychopharmacology 18 doi: 10.1093/ijnp/pyu051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubach M, Amarante LM, Swanson K, White SR (2018) What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 5 doi: 10.1523/eneuro.0315-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Glassman M, King-Casas B, Kelly DL, Stein EA, Schroeder J, Salmeron BJ (2014) Complexity of oxytocins effects in a chronic cocaine dependent population Eur Neuropsychopharmacol 24:1483–1491 doi: 10.1016/j.euroneuro.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Rohn MC, Tanda G, Leggio L (2016) Targeting the Oxytocin System to Treat Addictive Disorders: Rationale and Progress to Date CNS drugs 30:109–123 doi: 10.1007/s40263-016-0313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Zhou L, Ghee SM, See RE, Reichel CM (2016) Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats Exp Clin Psychopharmacol 24:55–64 doi: 10.1037/pha0000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Young LJ (2006) Neuropeptidergic regulation of affiliative behavior and social bonding in animals Horm Behav 50:506–517 doi:S0018–506X(06)00180–2 [pii]10.1016/j.yhbeh.2006.06.028 [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition Nat Rev Neurosci 10:434–445 doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ (2011) A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research Psychoneuroendocrinology 36:1114–1126 doi: 10.1016/j.psyneuen.2011.02.015 [DOI] [PubMed] [Google Scholar]
- McRae-Clark AL, Baker NL, Moran-Santa Maria M, Brady KT (2013) Effect of oxytocin on craving and stress response in marijuana-dependent individuals: a pilot study Psychopharmacology 228:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann DA, Houskamp BM, Pollock VE, Briere J (1996) The Long-Term Sequelae of Childhood Sexual Abuse in Women: A Meta-Analytic Review Child Maltreatment 1:6–16 doi: 10.1177/1077559596001001002 [DOI] [Google Scholar]
- Olff M et al. (2013) The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences Psychoneuroendocrinology 38:1883–1894 doi: 10.1016/j.psyneuen.2013.06.019 [DOI] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DS (2003) Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis Psychol Bull 129:52–73 [DOI] [PubMed] [Google Scholar]
- Pirard S, Sharon E, Kang SK, Angarita GA, Gastfriend DR (2005) Prevalence of physical and sexual abuse among substance abuse patients and impact on treatment outcomes Drug Alcohol Depend 78:57–64 doi: 10.1016/j.drugalcdep.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R (2012) Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence Am J Psychiatry 169:406–414 doi: 10.1176/appi.ajp.2011.11020289 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisciandaro JJ, Joseph JE, Myrick H, McRae-Clark AL, Henderson S, Pfeifer J, Brady KT (2014a) The relationship between years of cocaine use and brain activation to cocaine and response inhibition cues Addiction doi: 10.1111/add.12666 [DOI] [PMC free article] [PubMed]
- Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT (2014b) Brain activation to cocaine cues and motivation/treatment status Addict Biol 19:240–249 doi: 10.1111/j.1369-1600.2012.00446.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quality CfBHSa (2017) Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health (HHS Publication No. SMA 17–5044, NSDUH Series H-52) Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/, Rockville, MD [Google Scholar]
- Sabihi S, Dong SM, Maurer SD, Post C, Leuner B (2017) Oxytocin in the medial prefrontal cortex attenuates anxiety: Anatomical and receptor specificity and mechanism of action Neuropharmacology 125:1–12 doi: 10.1016/j.neuropharm.2017.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JY, McKendrick K, Banks S (2008) The impact of early trauma and abuse on residential substance abuse treatment outcomes for women J Subst Abuse Treat 34:90–100 doi: 10.1016/j.jsat.2007.01.010 [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Babarczy E, Krivan M, Szabo G, Kovacs GL, Barth T, Telegdy G (1991) Selective attenuation of cocaine-induced stereotyped behaviour by oxytocin: putative role of basal forebrain target sites Neuropeptides 19:51–56 [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Kovacs GL (1994) Role of oxytocin in the neuroadaptation to drugs of abuse Psychoneuroendocrinology 19:85–117 [DOI] [PubMed] [Google Scholar]
- Sheehan DV et al. (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10 J Clin Psychiatry 59 Suppl 20:22–33;quiz 34–57 [PubMed] [Google Scholar]
- Smith SM (2002) Fast robust automated brain extraction Hum Brain Mapp 17:143–155 doi: 10.1002/hbm.10062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow-back: A technique for assessing self-reported alcohol consumption In: Litten RZ, Allen JP (eds) Measuring alcohol consumption: Psychosocial and biomedical methods Humana Press, Totawa, NJ, pp 41–72 [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK (1999) Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans Experimental and clinical psychopharmacology 7:274. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR (2004) Effects of progesterone treatment on cocaine responses in male and female cocaine users Pharmacology Biochemistry and Behavior 78:699–705 [DOI] [PubMed] [Google Scholar]
- Sussner BD, Smelson DA, Rodrigues S, Kline A, Losonczy M, Ziedonis D (2006) The validity and reliability of a brief measure of cocaine craving Drug Alcohol Depend 83:233–237 doi: 10.1016/j.drugalcdep.2005.11.022 [DOI] [PubMed] [Google Scholar]
- van Harmelen AL, Hauber K, Gunther Moor B, Spinhoven P, Boon AE, Crone EA, Elzinga BM (2014) Childhood emotional maltreatment severity is associated with dorsal medial prefrontal cortex responsivity to social exclusion in young adults PLoS One 9:e85107 doi: 10.1371/journal.pone.0085107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hedger K, Kushner MJ, Lee R, de Wit H (2018) Oxytocin Reduces Cigarette Consumption in Daily Smokers Nicotine Tob Res doi: 10.1093/ntr/nty080 [DOI] [PMC free article] [PubMed]
- Waldrop AE et al. (2010) Community-dwelling cocaine-dependent men and women respond differently to social stressors versus cocaine cues Psychoneuroendocrinology 35:798–806 doi: 10.1016/j.psyneuen.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber RA, Logan CN, Leong KC, Peris J, Knackstedt L, Reichel CM (2018) Regionally Specific Effects of Oxytocin on Reinstatement of Cocaine Seeking in Male and Female Rats The international journal of neuropsychopharmacology 21:677–686 doi: 10.1093/ijnp/pyy025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD (2005) Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior Proc Natl Acad Sci U S A 102:17237–17240 doi: 10.1073/pnas.0504767102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M (2008) Robust group analysis using outlier inference NeuroImage 41:286–301 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM (2004) Multilevel linear modelling for FMRI group analysis using Bayesian inference Neuroimage 21:1732–1747 doi: 10.1016/j.neuroimage.2003.12.023 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM (2001) Temporal autocorrelation in univariate linear modeling of FMRI data Neuroimage 14:1370–1386 doi: 10.1006/nimg.2001.0931 [DOI] [PubMed] [Google Scholar]
- Worsley KJ (2001) Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM (eds) Functional MRI: An Introduction to Methods OUP, [Google Scholar]
- Zhou L, Sun WL, Young AB, Lee K, McGinty JF, See RE (2014) Oxytocin reduces cocaine seeking and reverses chronic cocaine-induced changes in glutamate receptor function The international journal of neuropsychopharmacology 18 doi: 10.1093/ijnp/pyu009 [DOI] [PMC free article] [PubMed] [Google Scholar]


