Abstract
Background
A new more highly sensitive rapid diagnostic test (HS-RDT) for Plasmodium falciparum malaria (Alere™/Abbott Malaria Ag P.f RDT [05FK140], now called NxTek™ Eliminate Malaria Ag Pf) was launched in 2017. The test has already been used in many research studies in a wide range of geographies and use cases.
Methods
In this study, we collate all published and available unpublished studies that use the HS-RDT and assess its performance in (i) prevalence surveys, (ii) clinical diagnosis, (iii) screening pregnant women, and (iv) active case detection. Two individual-level data sets from asymptomatic populations are used to fit logistic regression models to estimate the probability of HS-RDT positivity based on histidine-rich protein 2 (HRP2) concentration and parasite density. The performance of the HS-RDT in prevalence surveys is estimated by calculating the sensitivity and positive proportion in comparison to polymerase chain reaction (PCR) and conventional malaria RDTs.
Results
We find that across 18 studies, in prevalence surveys, the mean sensitivity of the HS-RDT is estimated to be 56.1% (95% confidence interval [CI] 46.9–65.4%) compared to 44.3% (95% CI 32.6–56.0%) for a conventional RDT (co-RDT) when using nucleic acid amplification techniques as the reference standard. In studies where prevalence was estimated using both the HS-RDT and a co-RDT, we found that prevalence was on average 46% higher using a HS-RDT compared to a co-RDT. For use in clinical diagnosis and screening pregnant women, the HS-RDT was not significantly more sensitive than a co-RDT.
Conclusions
Overall, the evidence presented here suggests that the HS-RDT is more sensitive in asymptomatic populations and could provide a marginal improvement in clinical diagnosis and screening pregnant women. Although the HS-RDT has limited temperature stability and shelf-life claims compared to co-RDTs, there is no evidence to suggest, given this test has the same cost as current RDTs, it would have any negative impacts in terms of malaria misdiagnosis if it were widely used in all four population groups explored here.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-021-07023-5.
Keywords: HS-RDT, Rapid diagnostic test, Malaria diagnosis, Cross-sectional surveys
Background
Rapid diagnostic tests (RDTs) and microscopy are the cornerstone of confirmation of clinical malaria diagnosis in most endemic countries and are also widely used for prevalence surveys. They also have more limited use in other scenarios, including active and reactive case detection and screening pregnant women. RDTs have high sensitivity against clinical infections for Plasmodium falciparum [1, 2] as these are typically associated with higher parasite densities and thus higher levels of antigenemia. However, sensitivity when used for detecting asymptomatic infections is considerably lower.
Recently, a Plasmodium falciparum histidine-rich protein 2 (HRP2)–based RDT (Alere™/Abbott Malaria Ag P.f RDT [05FK140], now called NxTek™ Eliminate Malaria Ag Pf) with a tenfold improved analytical sensitivity as compared to average conventional RDTs (co-RDTs) was prequalified by the World Health Organization (WHO). From here on, we refer to this test as the highly sensitive RDT (HS-RDT).
An unprecedented wide range of studies has been conducted using the HS-RDT in a variety of transmission settings and use cases to investigate the practical benefits of its lower limit of detection (LOD). First and foremost, this test was intended to identify asymptomatic infections in mass screening and active case detection interventions, particularly in low-transmission settings. The diagnostic sensitivity of an RDT is driven primarily by two factors: (i) LOD of the test (i.e., its analytical sensitivity) and (ii) the malaria antigen distribution in the sampled infected population. It has been shown that conventional RDTs are more sensitive in high-transmission settings [3], which is most likely because, on average, individuals have higher parasite densities and thus higher antigenemia [4]. To assess the utility of the HS-RDT, we need to better understand the how it performs in comparison to co-RDTs or more sensitive nucleic acid amplification–based tests (NAATs) (e.g., polymerase chain reaction [PCR]) and in different transmission settings.
Questions remain around the utility of more highly sensitive RDTs for other use cases: for example, given that current RDTs perform well in clinical settings, will a more sensitive test increase the number of people with symptomatic malaria being correctly diagnosed? There may be individuals that test positive due to having chronic asymptomatic infections, but malaria is not the primary cause of their fever. Furthermore, given that HRP2 decays relatively slowly after parasite clearance, there is a risk that HS-RDTs will increase the numbers of false positives in individuals with recently cleared infections. Twenty-five percent of co-RDTs are estimated to remain positive for at least 20 days after the clearance of parasites, and this is expected to be greater for a more sensitive RDT [5].
There has been interest in testing pregnant women for malaria during antenatal care (ANC) visits, where drugs more effective than those used in intermittent preventative therapy during pregnancy (IPTp) could be given upon testing positive [6]. P. falciparum infections are typically harder to detect in pregnant women as the parasites commonly sequester in the placenta [7] and treating asymptomatic infections has been shown to have positive impacts on both the mother and the infant [8]. Therefore, more sensitive diagnostics to identify pregnant women to be treated and cleared of asymptomatic infections has a clear potential public health outcome. In a scenario where pregnant women are intermittently screened, we need to know how much more sensitive the HS-RDT is compared to a co-RDT in this population, and whether using a more sensitive test could reduce malaria burden in pregnant women and improve pregnancy outcomes. The testing of pregnant women at their first ANC visit has been identified as a potential sentinel surveillance strategy for monitoring population-level changes in prevalence, but questions remain as to whether this approach accurately captures these trends, and whether an HS-RDT would improve the accuracy of this strategy.
In this article we summarise published and available unpublished data to evaluate the performance of the HS-RDT across different transmission settings and use cases.
Methods
Systematic review and data description
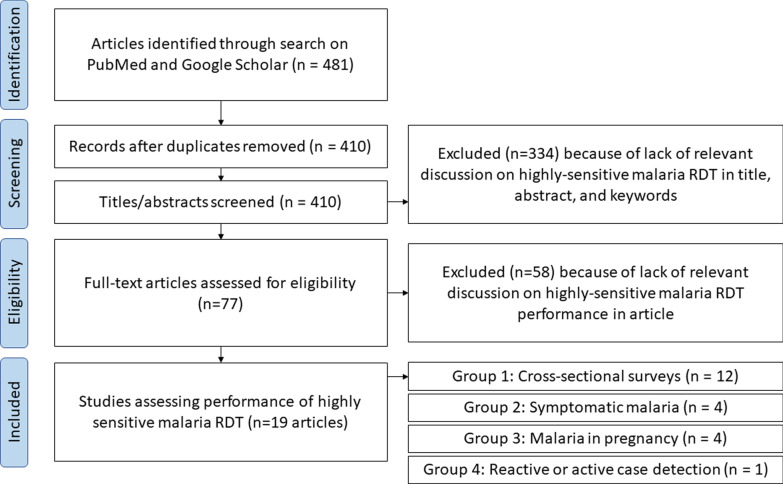
Searches for studies using the Abbott HS-RDT were carried out using PubMed and Google Scholar using the following search terms (“ultra-sensitive” OR “ultra sensitive” OR “ultrasensitive” OR “uRDT” OR “HS-RDT” OR “HS RDT” OR “HSRDT” OR “high-sensitivity RDT” AND “malaria” AND “rapid diagnostic test”) and searching for publications after 2017 (as this was the year the test was launched). This search yielded 481 records, of which 410 were unique records. After screening titles and abstracts, 75 articles were retained for full-text evaluation (Fig. 1). Of these, data were extracted from 18 articles and analysed in this study (Table 1). Studies were retained if they (i) used the HS-RDT (05FK140), (ii) contained performance data with denominators on the HS-RDT and at least one other additional diagnostic method. Investigators of three unpublished studies that were presented at two side meetings on the HS-RDT at the Annual Meeting for the American Society of Tropical Medicine and Hygiene (2017, 2018) were also contacted and agreed to the inclusion of their data in this analysis [9–11].
Fig. 1.
Flow chart of identification of published studies. The breakdown on studies in the ‘included’ section is greater than the total number of studies as several studies fall into two categories
Table 1.
Details of published and unpublished studies used in this study
| First author, year, reference | Country | Study description | HRP2 | PCR/other NAAT method | qPCR | co-RDT |
|---|---|---|---|---|---|---|
| Acquah et al. 2021 [19] | Ghana | Cross-sectional prevalence | x | x | x | |
| Das et al. 2017 [12] | Myanmar, Uganda | Cross-sectional prevalence | x | x | x | x |
| Druetz et al. 2020 [16] | Haiti | Cross-sectional prevalence | x | |||
| Galatas et al. 2020 [20] | Mozambique | Cross-sectional prevalence | x | x | x | |
| Girma et al. 2019 [21] | Ethiopia | Cross-sectional prevalence | x | x | x | |
| Hofmann et al. 2018 [14] | PNG | Cross-sectional prevalence | x | x | x | |
| Landier et al. 2018 [13] | Myanmar | Cross-sectional prevalence | x | x | x | x |
| Liu et al. 2019 [22] | Myanmar | Cross-sectional prevalence | x | x | ||
| Manjurano et al. 2021 [23] | Tanzania | Cross-sectional prevalence | x | x | ||
| Mwesigwa et al. 2019 [24] | The Gambia | Cross-sectional prevalence | x | |||
| Owalla et al. 2020 [25] | Uganda | Cross-sectional prevalence, Clinical diagnosis | x | x | ||
| Yeung et al. 2020 [15] | Cambodia | Cross-sectional prevalence, Active case detection | x | x | ||
| Hartley et al. 2020 [26] | Tanzania | Clinical diagnosis | x | x | ||
| Hofmann et al. 2019 [27] | Tanzania | Clinical diagnosis | x | x | x | |
| Plucinski et al. 2017 [28] | Angola | Clinical diagnosis | x | x | ||
| Briand et al. 2020 [29] | Benin | Pregnant women | x | |||
| Unwin et al. 2020 [30] | Indonesia | Pregnant women | x | x | ||
| Vásquez et al. 2018 [31] | Colombia | Pregnant women | x | x | ||
| Vásquez et al. 2020 [32] | Colombia | Pregnant women | x | x | x | |
| Unpublished studies | ||||||
| Bridges et al. [10] | Zambia | Cross-sectional prevalence, Active case detection | x | x | ||
| Saad et al. [11] | Cambodia | Cross-sectional prevalence, Active case detection | x | x | ||
| Bennett et al. [9] | Laos | Cross-sectional prevalence, Active case detection | x | x |
The four columns to the right show which other diagnostics were used in each study
Studies were categorised into four groups: Group 1 consisted of studies reporting the prevalence of asymptomatic malaria using the HS-RDT and ideally a molecular NAAT (typically PCR) in cross-sectional population surveys (12 published studies, 3 unpublished studies). Some studies in this group provided multiple prevalence data points as they consisted of multiple population groups [12], used HS-RDTs under both laboratory and field conditions [13], used different PCR methods as the reference standard [14], or used different approaches to sample asymptomatic individuals [15, 16]. This resulted in 20 prevalence data points; of these, all but 2 had PCR as a reference standard, and all but 3 also tested samples using a co-RDT. Group 2 consisted of studies performed in symptomatic individuals presenting for treatment at health facilities (n = 4), although one of these studies only estimated the performance of an HS-RDT based on a hypothetical limit of detection compared to antigen concentrations from febrile patients. Group 3 included studies testing the performance of the test in pregnant women (n = 4), and finally, group 4 included studies using the test in some form of reactive or active case detection (one published study and three unpublished studies). Some studies were included in multiple categories.
Access to full individual-level datasets was available from two studies in Myanmar [13] and Uganda [12]: these were used in the assessment of analytical test performance. The Myanmar dataset consists of data from 1847 specimens from asymptomatic individuals residing in Kayin State, of which 185 have PCR positive P. falciparum infections and 66 have mixed P. falciparum and P. vivax infections. The Uganda dataset consists of data on 607 specimens from asymptomatic individuals residing in Nagongera taken at two time points 3 months apart, of which 249 are PCR positive for P. falciparum only and 12 are PCR positive for P. falciparum mixed infections. The mean prevalence of PCR-positive infection with any P. falciparum is 13.6% (251/1847) in the Myanmar dataset and 43.0% (261/607) in the Uganda dataset. Both sets of samples were also run on the Quansys antigen quantification platform [17, 18] which provides an HRP2 concentration value for each sample. Both studies also tested samples using a co-RDT and quantitative PCR (qPCR). Details of all studies used in this article are shown in Table 1.
Statistical analyses
Two analyses were conducted to assess the analytical performance of the test using two datasets for which we had individual-level data including HRP2 concentrations. Firstly, for each dataset, the data were split into six categories based on HRP2 concentration (< 1; 1–10; 10–100; 100–1000; 1000–10,000; 10,000–100,000 pg/ml), and the sensitivity of the HS-RDT and co-RDT were calculated for each category based on the proportion of PCR-positive samples in that category that were also positive by each RDT. Secondly, logistic regression models were fitted for (i) the probability of RDT positivity (HS-RDT and co-RDT) as a function of HRP2 concentration and (ii) the probability of RDT positivity (HS-RDT and co-RDT) as a function of parasite density as estimated by qPCR. For the HRP2-based logistic regression model, concentration values below the lower limit of detection (LLOD) were set to 0.05 pg/ml as this is half the lowest LLOD of the two studies, and values above the upper limit of detection (ULOD) were set to the respective ULOD value for each study. For the PCR model, in the Myanmar dataset, samples with discordant PCR positivity results (as samples were analysed twice) were excluded from the analysis, as were samples where the Plasmodium species was unable to be identified and samples that were P. vivax–only infections. Mixed P. falciparum/P. vivax, P. falciparum–only, and negative samples were retained. There were no non-falciparum samples in the Uganda dataset, so all samples were retained. Negative samples were set to the lowest value by the PCR method in each study (0.01 parasites/µl in the Uganda data and 0.0000002 parasites/µl in the Myanmar data). Logistic regression models were fitted using a Hamiltonian Monte Carlo procedure implemented in Stan via the brms package in R [31]. The RDT status (0, 1) was the dependent variable, and HRP2 concentration or parasite density were the independent variables. Informative gaussian priors with a mean of 0 and standard deviation of 3 were used for the model parameters. Two chains were run for each model for 5000 iterations after a burn-in of 2500 iterations. Convergence was visually assessed from the traceplots of both parameters. The fitted line and shaded area show the median and the 95% credible interval from the logit-transformed posterior samples from the linear predictor.
Using summary aggregated data from 12 published and 3 unpublished studies that contain information on cross-sectional prevalence (Table 1), the performance of the HS-RDT in this use case was assessed in three ways: (i) comparing HS-RDT prevalence against PCR prevalence and co-RDT prevalence; (ii) calculating sensitivity of the HS-RDT and the co-RDT, calculated as the proportion of PCR-positive samples that are each positive by each RDT. A binomial logistic regression model is used to assess the relationship between PCR prevalence and sensitivity, and the weighted mean sensitivity is calculated for each test. Lastly, (iii) calculating the ratio of HS-RDT prevalence to co-RDT prevalence for each study where both tests were used, and then calculating an aggregated ratio across all studies. The overall ratio was calculated using a random effects model for meta-analysis with the DerSimonian–Laird method using the ‘rmeta’ package in R [32], and the relative risks are presented. One study [25] only used the HS-RDT and PCR on a non-random subset of samples, namely 25 co-RDT-negative individuals and 25 co-RDT-positive individuals. These numbers were converted to population-level prevalence estimates using methods detailed in Additional file 1.
For the four studies that used the HS-RDT to screen pregnant women, the sensitivity of the HS-RDT and co-RDT was calculated as the proportion of PCR-positive samples that tested positive using each RDT.
Results
Sensitivity of the HS-RDT against HRP2 concentration
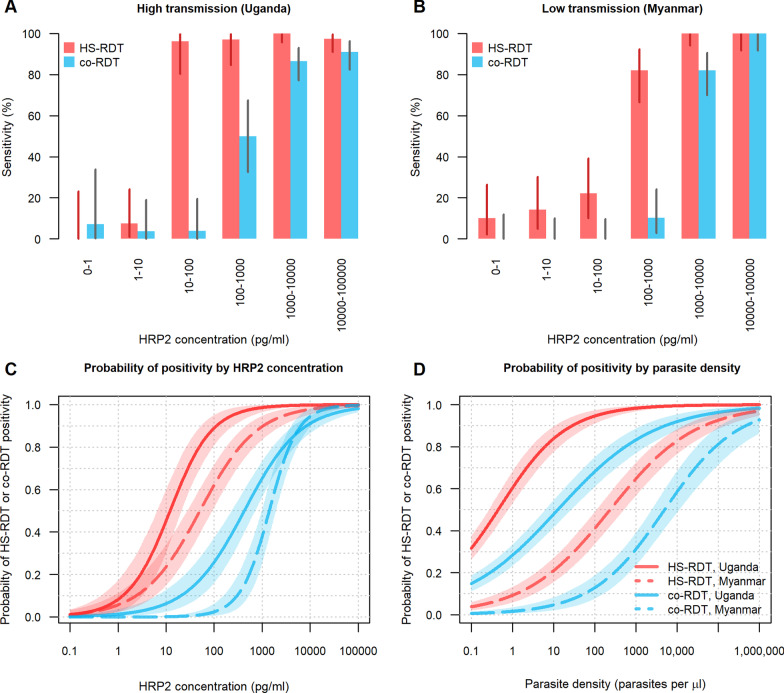
The performance of the HS-RDT on clinical samples based on HRP2 concentration was assessed by calculating the sensitivity amongst samples with different levels of HRP2. In samples with HRP2 concentrations > 1000 pg/ml, the HS-RDT had a mean sensitivity of 99.2% across the high- and low-transmission settings (Fig. 2A and B, respectively) and co-RDT had a sensitivity of > 89.0% in these same samples. The sensitivity of the HS-RDT remained high in samples with HRP2 concentrations 100–1000 pg/ml, with values of 97.1% in Uganda and 82.1% in Myanmar, whereas the sensitivities of the co-RDT were 50.0% and 10.3%, respectively. The sensitivity of the HS-RDT greatly decreased in Myanmar for samples with concentrations of 10–100 pg/ml (sensitivity = 22.2%). In Uganda, sensitivity was still high at this level of HRP2 concentration (sensitivity = 96.2%), but decreased greatly in samples with concentrations of than 1–10 pg/ml (sensitivity = 7.4%). For both settings, the HS-RDT is significantly more sensitive than the co-RDT in samples with HRP2 concentrations that are between 10 and 1000 pg/ml. Figure 2C and D show the probability of positivity either by HRP2 concentration or by parasite density, respectively. The difference in profiles between the Myanmar and Uganda relationships to HRP2 concentration and parasite density may suggest operational differences in the study and differences in the sensitivity of the qPCR method, as these would be anticipated to behave more similarly. The correlation between parasite density by qPCR and HRP2 concentration for both studies is shown in Additional file 4: Fig. S1.
Fig. 2.
Performance of the HS-RDT against HRP2 concentration in PCR-confirmed specimens. Panels A and B show the sensitivity of the HS-RDT and co-RDT in samples grouped by different levels of HRP2 for a high-transmission setting (Uganda, A) and a low-transmission setting (Myanmar, B). Sensitivity is defined as the proportion of PCR-positive samples that are also detected by each RDT. The vertical lines on each bar are 95% binomial confidence intervals for each estimate. Panel C shows the probability of HS-RDT (red lines) and co-RDT (blue lines) positivity as a function of HRP2 concentration and panel D shows the probability of HS-RDT (red lines) and co-RDT (blue lines) as a function of parasite density by quantitative PCR. The shaded region indicates the 95% credible interval of the model fit
Cross-sectional prevalence estimates and sensitivity of the HS-RDT
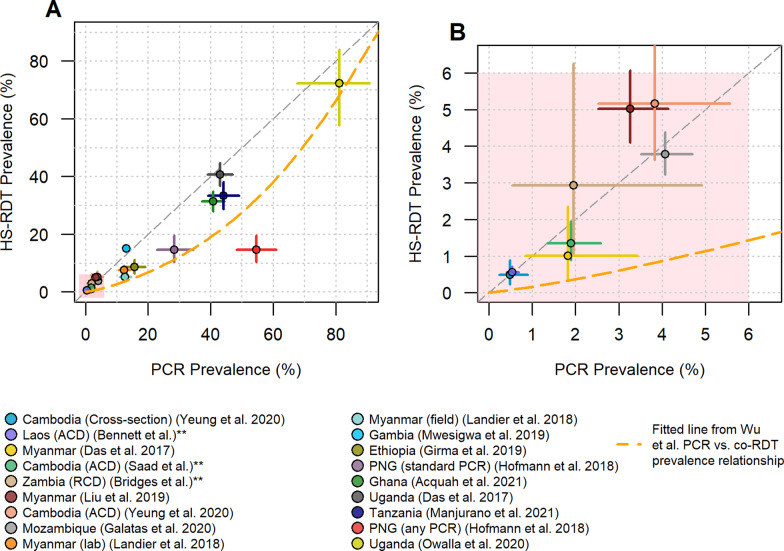
Comparing the prevalence estimates obtained using an HS-RDT and PCR in cross-sectional prevalence surveys (Fig. 3), we see that in all but four of the studies the prevalence falls below the diagonal x = y line, indicating that generally HS-RDT prevalence is lower than PCR prevalence. In the very low-transmission settings, all data points fall close to this line (Fig. 3B), showing good concordance between the two diagnostics. A fitted line from a previously published meta-analysis looking at PCR versus co-RDT prevalence estimates [3] was added to the plots. All the HS-RDT prevalence estimates apart from one lie above line, suggesting the HS-RDT is a more accurate tool than a co-RDT for measuring prevalence across a broad range of transmission settings. In all studies, there is some discordance between the individuals that test positive for PCR and those that test positive by HS-RDT due to the tests measuring two different analytes.
Fig. 3.
Comparison of PCR prevalence against HS-RDT prevalence. A shows all data used in this analysis (n = 18), and B shows a zoom-in of the samples with prevalence below 6%. The horizontal and vertical lines from each data point show the binomial confidence intervals associated with the PCR prevalence and HS-RDT prevalence estimates, respectively. The orange dashed line shows the fitted relationship derived from a previous meta-analysis of PCR and co-RDT prevalence surveys [3]. The grey diagonal line shows the x = y equivalence line between the HS-RDT and PCR. Additional details are provided on the sample type where necessary. **Unpublished studies. PNG Papua New Guinea, RCD reactive case detection, ACD active case detection
One study allows observation of the impact of the PCR method used on the comparative prevalence between the HS-RDT and PCR [14]. In Fig. 3, the red and mauve data points are from the same samples from Papua New Guinea (PNG); however, the mauve point shows prevalence as estimated using a standard PCR assay whereas the red point shows prevalence obtained by assuming any one of three PCR assays being positive means the sample is positive, including an ultra-sensitive technique that they found to be 10 × more sensitive than their standard assay [33]. Another study in Myanmar enabled comparison of the performance of the HS-RDT under controlled laboratory settings (orange) versus field conditions (turquoise) [13]; while the prevalence estimate is lower for the HS-RDT run in field conditions versus laboratory conditions, the same drop was also observed with the co-RDT.
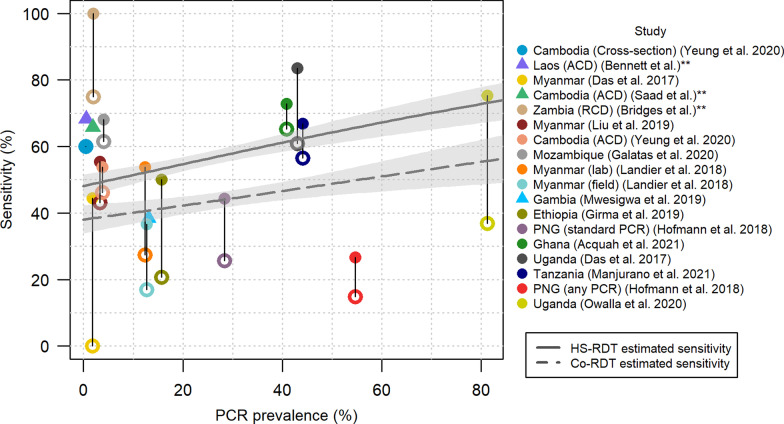
The sensitivity of the HS-RDT and the co-RDT in all the studies (where available) was plotted against PCR prevalence in Fig. 4. A binomial regression model was used to explore whether there was a relationship between PCR prevalence and sensitivity for each RDT. For both the HS-RDT and the co-RDT, there is a statistically significant positive relationship between PCR prevalence and sensitivity (p < 0.05). The weighted mean sensitivity of the HS-RDT across all studies was estimated to be 56.1% (95% confidence interval [CI] from weighted t-test = 46.9–65.4%) compared to 44.3% (95% CI 32.6–56.0%) for the co-RDT. The vertical segments join the sensitivity values from the same study—the length of this segment indicates the percentage point increase in sensitivity of the HS-RDT compared to the co-RDT.
Fig. 4.
Sensitivity of the HS-RDT and co-RDT against PCR prevalence. The filled circles and triangles show the sensitivity of HS-RDT. The unfilled circles joined to the filled circles by a line show the sensitivity of the co-RDT in the same study, if this test was used. The triangles indicate studies where a co-RDT was not used. PCR is the gold-standard diagnostic against which sensitivity is calculated. The solid grey and dashed grey lines show the fit from a binomial generalised linear model of the relationship between PCR prevalence and sensitivity of the HS-RDT and co-RDT respectively. **Unpublished studies. RCD reactive case detection, ACD active case detection
To explore the increase in HS-RDT prevalence estimates compared to those from using a co-RDT, we plotted the ratio of these values in all studies where both tests were used (Fig. 5). The weighted mean estimated ratio of HS-RDT prevalence to co-RDT prevalence is 1.46 (95% CI 1.26–1.70). This means that prevalence estimated using an HS-RDT will be on average 46% higher than if using a co-RDT.
Fig. 5.
Ratio of HS-RDT prevalence to co-RDT prevalence in 16 surveys from 15 studies. The circles show the estimated ratio, and the horizontal lines show the associated binomial 95% confidence intervals. The centre of the blue diamond shows the weighted mean estimated ratio (1.46) and the horizontal extents indicate the 95% confidence interval (1.26–1.70). **Unpublished studies. EAG easy access group, RCD reactive case detection, ACD active case detection
Positive and negative predictive values (PPV and NPV) for the HS-RDT and co-RDT are shown for all studies where data are available in Additional file 2. The unweighted mean PPV across all studies where data were available is 0.75 for the HS-RDT and 0.79 for the co-RDT. The unweighted mean NPV value is 0.91 for the HS-RDT and 0.89 for the co-RDT.
Use for clinical case management
Conventional RDTs are considered to be effective for P. falciparum clinical case management, with high sensitivity against clinical infection [34, 35] that is associated with high parasite densities and HRP2 concentrations. Here we review evidence on the diagnostic performance of the HS-RDT in a clinical setting.
One study has retrospectively evaluated the usefulness of an HS-RDT for clinical diagnosis and fever management compared to a co-RDT [27]. Frozen blood samples from 3000 children and 515 adults presenting with fever to an outpatient clinic in Dar es Salaam, Tanzania, were tested using a co-RDT, an HS-RDT, by ultra-sensitive qPCR, and by enzyme-linked immunosorbent assay (ELISA) to estimate HRP2 concentration. Out of 309 children testing positive by qPCR, 226 (73.1%) and 230 (74.4%) were also positive by co-RDT and HS-RDT, respectively, and out of 48 adults testing positive by qPCR, these values were 35 (72.9%) and 37 (77.1%). Four children and 0 adults were co-RDT positive and qPCR negative, and 9 children and 0 adults were HS-RDT positive and qPCR negative. Of individuals positive for HRP2 by ELISA, 83.1% were positive by a co-RDT and 86.5% were positive by the HS-RDT. This study was conducted in a very low-transmission setting; test positivity rate among febrile individuals (by co-RDT) was only 7.7% in children and 6.8% in adults, which would suggest an even lower population-level prevalence. Therefore, few individuals presenting with non-malarial fevers would be expected to be co- or HS-RDT positive due to having asymptomatic infections or having been recently treated for a malaria infection (and having residual HRP2).
A second study was carried out in the same location [26] and tested 2801 febrile paediatric outpatients. Of the 274 that were PCR positive, 198 and 201 were detected by a co-RDT and an HS-RDT respectively, giving sensitivity values of 72.2% and 73.4%. Furthermore, this study compared the health outcomes up to 28 days after presenting at the health facility with fever. There was no evidence that individuals with PCR-positive, co-RDT-negative infections had worse outcomes compared to PCR-negative individuals in terms of clinical failures (e.g. developing of severe symptoms, having persistent symptoms after 7 days, clinical pneumonia) or secondary hospitalisations.
A study based in Angola measured the HRP2 concentration of outpatients attending a health facility using the multiplex bead assay [28]. The impact of a hypothetical HS-RDT with a LLOD of 200 pg/ml was then estimated by calculating the proportion of individuals that would have HRP2 concentrations above this threshold. They found that 81% of febrile individuals with detectable HRP2 were detected by a co-RDT and an additional 10–20% of cases would have been identified using this hypothetical HS-RDT. In addition, 52 and 77% of HRP2-positive afebrile individuals were detected using a co-RDT in two separate sites, and an additional 50–60% of individuals would have been detected with the hypothetical HS-RDT.
In Uganda, a study was conducted where the HS-RDT was used alongside a conventional RDT and microscopy for testing febrile children under the age of 5 [25]. During 475 clinic visits, positivity by the HS-RDT was 55.2%, by a co-RDT it was 53.5%, and by microscopy it was 40.6%. The HS-RDT yielded only marginally higher positivity leading to the detection of an additional eight more individuals compared to a co-RDT. The co-RDT and HS-RDT each detected 61 and 69 more cases than microscopy.
Use in screening pregnant women
Four published studies were identified that looked at the performance of the HS-RDT in pregnant women. A study in Benin [29] tested 942 samples in 327 women in the first and third trimesters and at delivery. They found that the overall positivity of the HS-RDT was 16.2% compared to 11.6% by a co-RDT and 18.3% by PCR. Based on 172 PCR-positive samples across all stages of pregnancy, the sensitivity of the HS-RDT was 60.5% compared to 44.2% by a co-RDT. The difference was even more pronounced during the first trimester, where sensitivity by HS-RDT was 57.0% compared to 38.3% by co-RDT.
The authors considered the potential clinical impact of treating women that are positive by HS-RDT but negative by co-RDT (i.e., individuals that would only be detected if an HS-RDT was used) by conducting a multivariate analysis to assess the impact of diagnostic status on maternal and birth outcomes. Individuals in this category (HS-RDT+, co-RDT−) have a 3.4 times higher risk of maternal anaemia during pregnancy compared to uninfected (PCR-negative) women [29]. Co-RDT-positive women had a two times higher risk of anaemia compared to uninfected women. Both these effects were statistically significant, but the difference between them was not. There was a 5.3 times higher risk of low birthweight in the HS-RDT+, co-RDT− group and a 2.3 times higher risk in the co-RDT+ group compared to the uninfected group, but both effects were nonsignificant.
A study in Colombia [31] tested 737 peripheral and placental samples using the HS-RDT as well as light microscopy, nested PCR, a Pf-only RDT and a Pf/Pv RDT. Among all samples, the HS-RDT performed comparably to the best performing co-RDT (sensitivity of 85.7% compared to 82.8%). The authors also disaggregated the data by whether each woman was symptomatic at the time the sample was taken (defined as fever with an axillary temperature ≥ 37.5 °C or history of fever within the last 3 days). The sensitivity was high and exactly the same using all diagnostics among symptomatic women (85.7%, n = 61). Among asymptomatic women (n = 649), sensitivity using the HS-RDT was 71.4% compared to 64.3% with the best performing co-RDT and 50% by light microscopy, although none of the differences were statistically significant.
A second study in Colombia tested 858 pregnant women attending an ANC clinic [32] with an HS-RDT, a co-RDT, microscopy, loop-mediated isothermal amplification (LAMP), and both nested PCR and quantitative reverse transcription PCR (qRT-PCR). The overall prevalence of P. falciparum infection among the participants was 4.5% by the most sensitive diagnostic, qRT-PCR. Using this as the standard reference, the sensitivities of the HS-RDT, co-RDT, microscopy, and LAMP were 64.1%, 53.8%, 59.0%, and 89.7%, respectively. There were four women that were positive by HS-RDT and negative by co-RDT—they all had parasite densities < 100 parasites/µl.
Finally, the performance of the HS-RDT was retrospectively tested against reconstituted stored samples from pregnant women in Indonesia [30]. Based on 158 samples positive for Pf by PCR, the sensitivity of the HS-RDT was 19.6% compared to 22.8% using a co-RDT, indicating that in this population there was no improvement in sensitivity.
Active case detection
One study in Cambodia used the HS-RDT for both reactive and proactive case detection [15]. In the reactive case detection, the households, high-risk neighbours, and co-travellers of passively detected symptomatic ‘index cases’ were screened with an HS-RDT, a co-RDT, and qPCR. In proactive case detection, all high-risk individuals in villages with a high total number of malaria cases were proactively screened. High-risk individuals were defined as those reporting fever in past 48 h or having slept in the forest in the past month. A total of 678 individuals were tested as part of both reactive and proactive case detection. Only 26 individuals were PCR positive, of which 12 were detected by a co-RDT and 14 by the HS-RDT. Furthermore, 12 and 21 PCR-negative individuals were co-RDT and HS-RDT positive, respectively.
A second study in Cambodia used the HS-RDT to conduct active monthly screening of asymptomatic high-risk forest and plantation workers in 11 villages [11]. In this very low-transmission setting, the HS-RDT had a sensitivity of 66.6% with PCR as the gold standard. The maximum parasite density amongst individuals with false negative HS-RDT results was less than 400 parasites/µl and the median parasite density was only 2 parasites/µl, indicating the test missed mostly low-density infections.
In Zambia, the HS-RDT was used in a reactive focal test and treat study where households of index cases detected at a facility were tested with both a co-RDT and HS-RDT [10] (HS-RDT data unpublished). Prevalence was very low in this trial, with only 4/205 individuals testing positive by PCR. The HS-RDT detected all four of these individuals and the co-RDT only detected three.
In another very low transmission setting in Laos [9], the HS-RDT was used in active case detection (HS-RDT data unpublished). Of 11,771 individuals tested by PCR and HS-RDT there were only 63 and 66 positives, respectively. Of the 63 PCR positive samples, 43 of them were also positive by the HS-RDT.
Discussion
The performance of any RDT depends on both the limit of detection of the test and on the distribution of target analyte in the populations where it is being used. In the specific case presented in this review, a test with a given LOD will have lower diagnostic sensitivity in infected populations with lower HRP2 concentrations and higher diagnostic sensitivity in populations with higher HRP2 concentrations. The performance of a test (HS-RDT) with a lower LOD than another test (co-RDT) will be more resilient to fluctuations in the target analyte, in this case HRP2 distribution in a given population. Furthermore, the improvement in sensitivity for a test with a lower LOD will depend on the proportion of the population whose analyte concentrations fall between the LOD of the new test and the LOD of the conventional test.
In asymptomatic cross-sectional surveys, the sensitivity of the HS-RDT in asymptomatic populations is estimated to be 56.1% compared to 44.3% with a co-RDT with PCR as the reference standard. We found a positive relationship between PCR prevalence and the sensitivity of the HS-RDT, indicating that it may perform relatively better in high-transmission settings. This is consistent with evidence that parasite densities are higher in high-transmission settings [4]. The HS-RDT is estimated to detect on average 46% more infections than a co-RDT (Fig. 5). The results presented here show that the HS-RDT consistently outperforms co-RDTs when used for cross-sectional surveys.
For clinical diagnosis, the incremental benefit of using the HS-RDT compared to a co-RDT to test febrile individuals appears to be marginal, with all studies reporting a small number of additional PCR-positive cases detected with the more sensitive test. This is likely because the antigen concentrations of individuals with febrile malaria are higher than in asymptomatic populations and are in the range where the co-RDT already performs well (Fig. 2A, B). The HS-RDT did detect more false positives (PCR negative, HS-RDT positive) compared to the co-RDT, highlighting the importance of proper clinical management to investigate a range of causes of the fever for RDT-positive individuals. This is particularly relevant in settings where proper diagnostic skills to identify other causes of fever may be limited, as is often the case among community health workers and basically trained health staff. This is especially a concern in high-transmission areas with a high probability of having recent malaria episodes with persistent antigens. Conversely, the HS-RDT may also detect new infections sooner [12] which is a clear benefit in highly susceptible populations such as infants. The risk–benefit of a more sensitive test for malaria case management needs to be better understood.
For screening pregnant women for the malaria, the results here indicate that the HS-RDT may be more sensitive than a co-RDT for detecting malaria in pregnant women in all but one study, and that the additional infections detected by the HS-RDT may have clinical significance for the mother and child. However, only the data aggregated across all stages of pregnancy from the Benin trial [29] produced a statistically significant result. Currently, WHO recommends IPTp in areas with moderate to high transmission. However, there is widespread resistance to sulfadoxine-pyrimethamine, the antimalarial used in this intervention [36]. This has led to calls for a screening-based approach where women are screened with an RDT regardless of symptoms and are treated with a more efficacious antimalarial if positive. Initial trials of this approach using a co-RDT have had mixed results and as such this intervention is not recommended by WHO [37–41]. However, it has been considered that a more sensitive screening tool could improve the effectiveness of this approach [6]. Additionally, trials have investigated the safety of giving antimalarials to women in the first trimester, which if recommended by WHO, would be more impactful if a highly sensitive and specific RDT was available in antenatal clinics.
The Senegal National Malaria Control Programme has recently started to evaluate the use the HS-RDT in reactive case detection strategy. However, there is currently limited direct evidence on the added value of an HS-RDT in active case detection. Results presented here indicate that the HS-RDT is more sensitive than the co-RDT in asymptomatic individuals, suggesting that its use in active case detection would likely improve the effectiveness of the intervention compared to using a co-RDT.
The HS-RDT has a more limited stability claim than most co-RDTs; it claims storage stability to 30 °C versus 40 °C for most of the WHO prequalified RDTs. It also has a more restricted shelf life of 12 months, compared to 24 months for other prequalified co-RDTs [42]. A survey of many of the investigators of the studies described here suggests that many were aware of these limitations and managed them, but many studies also did not track temperature exposure. There is a possibility that HS-RDTs in some of the studies were compromised, perhaps leading to reduced incremental benefits of the HS-RDT over the co-RDT. Some studies compared the performance of the tests on frozen samples and in one case reconstituted specimens by combining retrospectively blood pellets with plasma [30]. The impact of this on the performance of any given RDT is not properly characterized and may have influenced some of the study results. Conversely, different studies used different co-RDTs, which will have had different LOD for HRP2 and consequently the difference in comparative test performance may be affected by this. The LOD of HRP2-based RDTs has been shown to vary up to fourfold depending on the product and parasite culture strain investigated [43]. This, together with lot-to-lot performance variations, could confound the gain in clinical sensitivity observed between the HS-RDT and co-RDTs. Furthermore, PCR is typically used as the reference assay for assessing the performance of a new RDT, however, there is known to be large variation in the sensitivity of different PCR assays. For example, a highly sensitive PCR assay may detect very low density infections that also have very low levels of HRP2. RDTs may miss these infections and as a result have lower estimated sensitivity values. An investigation of the impact of PCR sensitivity is presented in Additional file 3. Results indicate that the HS-RDT sensitivity estimates were relatively robust to the PCR assay sensitivity, however the co-RDT performed significantly worse in studies where a sensitive PCR assay was used.
The Alere™/Abbott Malaria Ag P.f RDT is the first malaria RDT that has been launched with the claim of being ‘highly sensitive’. However, incremental improvements to RDT performance have been continuous and ongoing since development of these tools began in the 1990s. The HS-RDT has been submitted to unprecedented evaluation, with dozens of peer-reviewed articles published since its launch in 2017. The HS-RDT has catalysed a better understanding of how to evaluate RDTs and even the development and adoption of quantitative antigen tests to support their evaluation [17, 18, 43, 44]. The lessons learnt with the HS-RDT will help more quickly assess the implications of improvements in LOD for other tests in terms of sensitivity in different population groups and use cases.
Conclusions
The evidence presented here indicates that the HS-RDT is more sensitive than a conventional RDT for testing asymptomatic populations and could provide a marginal improvement in clinical diagnosis and screening pregnant women. Provided proper clinical management of fevers is possible, and the HS-RDT has comparable costs, heat stability and shelf-life to co-RDTs, there is no evidence to suggest it would have any negative impacts in terms of malaria misdiagnosis if it were widely used in all four population groups explored here.
Supplementary Information
Additional file 1. Estimating population-level prevalence by HS-RDT and PCR in Owalla et al.
Additional file 2. Positive and negative predictive values for the HS-RDT and co-RDT.
Additional file 3. Sub-analyses on the potential biases associated with PCR sensitivity.
Additional file 4. Correlation between parasite density and HRP2 concentration, and positivity by HS-RDT and co-RDT.
Acknowledgements
We would like the thank all studies participants who contributed to all the studies used in this analysis. We would also like to thank Abigail Pratt and Scott Miller at the Bill and Melinda Gates Foundation, Jane Cunningham and Andrea Bosman at the World Health Organisation, and Jordi Landier at Institut de Recherche pour le Développement for their constructive feedback on this analysis. We would like to thank Mulenga Mwenda-Chimfwembe, Brenda Mambwe and Conceptor Mulube for performing the PCR experiments in [28]. We thank Christine Waresak for her assistance in manuscript proofing and editing and David Boyle for his critical reading of the entire manuscript.
Abbreviations
- ACD
Active case detection
- ANC
Antenatal care
- CI
Confidence interval
- Co-RDT
Conventional RDT
- ELISA
Enzyme-linked immunosorbent assay
- HRP2
Histidine-rich protein 2
- HS-RDT
Highly sensitive rapid diagnostic test
- LAMP
Loop-mediated isothermal amplification
- LLOD
Lower limit of detection
- LOD
Limit of detection
- NAAT
Nucleic acid amplification–based tests
- NPV
Negative predictive value
- PCR
Polymerase chain reaction
- PNG
Papua New Guinea
- PPV
Positive predictive value
- qPCR
Quantitative polymerase chain reaction
- qRT-PCR
Quantitative reverse transcription polymerase chain reaction
- RCD
Reactive case detection
- RDT
Rapid diagnostic test
- ULOD
Upper limit of detection
- WHO
World Health Organization
Authors' contributions
HS, XD, GD conceived the study. HS and GD wrote the first draft of the manuscript and all authors provided substantial edits. SK and HS conducted the systematic review and data extraction. HS conducted the data analysis. DB, HM, NS, MS, AB provided unpublished data and interpretation of results. All authors read and approved the final manuscript.
Funding
This work was supported, in part, by the Bill & Melinda Gates Foundation [Grant No. OPP1135840]. Under the grant conditions of the Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the Author Accepted Manuscript version that might arise from this submission. The funding body had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The published datasets analysed, and all code written during the current study are available from https://github.com/PATH-Global-Health/HS-RDT_analysis. Individual-level datasets from Myanmar and Uganda are available from the corresponding author on request.
Declarations
Ethics approval and consent to participate
The data used in this work were provided by previous published studies that acquired proper ethical approval from relevant organisations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
^Martin De Smet: Deceased
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mweu MM, Wambua J, Njuga F, Bejon P, Mwanga D. Bayesian evaluation of the performance of three diagnostic tests for Plasmodium falciparum infection in a low-transmission setting in Kilifi County, Kenya. Wellcome Open Res. 2019 doi: 10.12688/wellcomeopenres.15204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coldiron ME, Assao B, Langendorf C, Sayinzoga-Makombe N, Ciglenecki I, de la Tour R, et al. Clinical diagnostic evaluation of HRP2 and pLDH-based rapid diagnostic tests for malaria in an area receiving seasonal malaria chemoprevention in Niger. Malar J. 2019;18(1):443. doi: 10.1186/s12936-019-3079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu L, van den Hoogen LL, Slater H, Walker PGT, Ghani AC, Drakeley CJ, et al. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature. 2015;528(7580):S86–93. doi: 10.1038/nature16039. [DOI] [PubMed] [Google Scholar]
- 4.Slater HC, Ross A, Felger I, Hofmann NE, Robinson L, Cook J, et al. The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat Commun. 2019;10(1):1433. doi: 10.1038/s41467-019-09441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalrymple U, Arambepola R, Gething PW, Cameron E. How long do rapid diagnostic tests remain positive after anti-malarial treatment? Malar J. 2018;17(1):228. doi: 10.1186/s12936-018-2371-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker PGT, Cairns M, Slater H, Gutman J, Kayentao K, Williams JE, et al. Modelling the incremental benefit of introducing malaria screening strategies to antenatal care in Africa. Nat Commun. 2020;11(1):3799. doi: 10.1038/s41467-020-17528-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zakama AK, Ozarslan N, Gaw SL. Placental malaria. Curr Trop Med Rep. 2020 doi: 10.1007/s40475-020-00213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yimam Y, Nateghpour M, Mohebali M, Afshar MJA. A systematic review and meta-analysis of asymptomatic malaria infection in pregnant women in Sub-Saharan Africa: a challenge for malaria elimination efforts. PLoS ONE. 2021;16(4):e0248245. doi: 10.1371/journal.pone.0248245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.University of California, San Francisco. Assessing the effectiveness of targeted active case detection among high risk populations in Southern Lao PDR [Internet]. clinicaltrials.gov; 2021 Feb [cited 2021 Apr 18]. Report No.: NCT03783299. Available from: https://clinicaltrials.gov/ct2/show/NCT03783299.
- 10.Bridges DJ, Miller JM, Chalwe V, Moonga H, Hamainza B, Steketee R, et al. Community-led Responses for Elimination (CoRE): a study protocol for a community randomized controlled trial assessing the effectiveness of community-level, reactive focal drug administration for reducing Plasmodium falciparum infection prevalence and incidence in Southern Province, Zambia. Trials. 2017;18(1):511. doi: 10.1186/s13063-017-2249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saad N, De Cramer C, Vernaeve L, Etienne W, Huy R, Nguon C. The performance of Alere Malaria Ag P.f®, a highly sensitive malaria RDT (hsRDT) for screening, against PCR in Cambodia. In New Orleans; 2018.
- 12.Das S, Jang IK, Barney B, Peck R, Rek JC, Arinaitwe E, et al. Performance of a high-sensitivity rapid diagnostic test for Plasmodium falciparum malaria in asymptomatic individuals from Uganda and Myanmar and naive human challenge infections. Am J Trop Med Hyg. 2017;97(5):1540–1550. doi: 10.4269/ajtmh.17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landier J, Haohankhunnatham W, Das S, Konghahong K, Christensen P, Raksuansak J, et al. Operational performance of a Plasmodium falciparum ultrasensitive rapid diagnostic test for detection of asymptomatic infections in eastern Myanmar. J Clin Microbiol. 2018 doi: 10.1128/JCM.00565-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann NE, Gruenberg M, Nate E, Ura A, Rodriguez-Rodriguez D, Salib M, et al. Assessment of ultra-sensitive malaria diagnosis versus standard molecular diagnostics for malaria elimination: an in-depth molecular community cross-sectional study. Lancet Infect Dis. 2018;18(10):1108–1116. doi: 10.1016/S1473-3099(18)30411-0. [DOI] [PubMed] [Google Scholar]
- 15.Yeung S, McGregor D, James N, Kheang ST, Kim S, Khim N, et al. Performance of ultrasensitive rapid diagnostic tests for detecting asymptomatic Plasmodium falciparum. Am J Trop Med Hyg. 2019;102(2):307–309. doi: 10.4269/ajtmh.19-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Druetz T, Stresman G, Ashton RA, van den Hoogen LL, Joseph V, Fayette C, et al. Programmatic options for monitoring malaria in elimination settings: easy access group surveys to investigate Plasmodium falciparum epidemiology in two regions with differing endemicity in Haiti. BMC Med. 2020;18(1):141. doi: 10.1186/s12916-020-01611-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang IK, Tyler A, Lyman C, Rek JC, Arinaitwe E, Adrama H, et al. Multiplex human malaria array: quantifying antigens for malaria rapid diagnostics. Am J Trop Med Hyg. 2020;102(6):1366–1369. doi: 10.4269/ajtmh.19-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang IK, Tyler A, Lyman C, Kahn M, Kalnoky M, Rek JC, et al. Simultaneous quantification of plasmodium antigens and host factor C-reactive protein in asymptomatic individuals with confirmed malaria by use of a novel multiplex immunoassay. J Clin Microbiol. 2019 doi: 10.1128/JCM.00948-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acquah FK, Donu D, Obboh EK, Bredu D, Mawuli B, Amponsah JA, et al. Diagnostic performance of an ultrasensitive HRP2-based malaria rapid diagnostic test kit used in surveys of afebrile people living in Southern Ghana. Malar J. 2021;20(1):125. doi: 10.1186/s12936-021-03665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galatas B, Mayor A, Gupta H, Balanza N, Jang IK, Nhamussua L, et al. Field performance of ultrasensitive and conventional malaria rapid diagnostic tests in southern Mozambique. Malar J. 2020;19(1):451. doi: 10.1186/s12936-020-03526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girma S, Cheaveau J, Mohon AN, Marasinghe D, Legese R, Balasingam N, et al. Prevalence and epidemiological characteristics of asymptomatic malaria based on ultrasensitive diagnostics: a cross-sectional study. Clin Infect Dis. 2019;69(6):1003–1010. doi: 10.1093/cid/ciy1005. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Soe TN, Zhao Y, Than A, Cho C, Aung PL, et al. Geographical heterogeneity in prevalence of subclinical malaria infections at sentinel endemic sites of Myanmar. Parasites Vectors. 2019;12(1):83. doi: 10.1186/s13071-019-3330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manjurano A, Omolo JJ, Lyimo E, Miyaye D, Kishamawe C, Matemba LE, et al. Performance evaluation of the highly sensitive histidine-rich protein 2 rapid test for Plasmodium falciparum malaria in North-West Tanzania. Malar J. 2021;20(1):58. doi: 10.1186/s12936-020-03568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mwesigwa J, Slater H, Bradley J, Saidy B, Ceesay F, Whittaker C, et al. Field performance of the malaria highly sensitive rapid diagnostic test in a setting of varying malaria transmission. Malar J. 2019;18(1):288. doi: 10.1186/s12936-019-2929-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Owalla TJ, Okurut E, Apungia G, Ojakol B, Lema J, Murphy SC, et al. Using the ultrasensitive Alere Plasmodium falciparum malaria Ag HRP-2TM rapid diagnostic test in the field and clinic in northeastern Uganda. Am J Trop Med Hyg. 2020;103(2):778–84. doi: 10.4269/ajtmh.19-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartley M-A, Hofmann N, Keitel K, Kagoro F, Moniz CA, Mlaganile T, et al. Clinical relevance of low-density Plasmodium falciparum parasitemia in untreated febrile children: a cohort study. PLoS Med. 2020;17(9):e1003318. doi: 10.1371/journal.pmed.1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann NE, Antunes Moniz C, Holzschuh A, Keitel K, Boillat-Blanco N, Kagoro F, et al. Diagnostic performance of conventional and ultrasensitive rapid diagnostic tests for malaria in febrile outpatients in Tanzania. J Infect Dis. 2019;219(9):1490–1498. doi: 10.1093/infdis/jiy676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plucinski MM, Rogier E, Dimbu PR, Fortes F, Halsey ES, Aidoo M. Estimating the added utility of highly sensitive histidine-rich protein 2 detection in outpatient clinics in sub-Saharan Africa. Am J Trop Med Hyg. 2017;97(4):1159–1162. doi: 10.4269/ajtmh.17-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Briand V, Cottrell G, Tuike Ndam N, Martiáñez-Vendrell X, Vianou B, Mama A, et al. Prevalence and clinical impact of malaria infections detected with a highly sensitive HRP2 rapid diagnostic test in Beninese pregnant women. Malar J. 2020;19(1):188. doi: 10.1186/s12936-020-03261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unwin VT, Ahmed R, Noviyanti R, Puspitasari AM, Utami RAS, Trianty L, et al. Use of a highly-sensitive rapid diagnostic test to screen for malaria in pregnancy in Indonesia. Malar J. 2020;19(1):28. doi: 10.1186/s12936-020-3110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vásquez AM, Medina AC, Tobón-Castaño A, Posada M, Vélez GJ, Campillo A, et al. Performance of a highly sensitive rapid diagnostic test (HS-RDT) for detecting malaria in peripheral and placental blood samples from pregnant women in Colombia. PLoS ONE. 2018;13(8):e0201769. doi: 10.1371/journal.pone.0201769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vásquez AM, Vélez G, Medina A, Serra-Casas E, Campillo A, Gonzalez IJ, et al. Evaluation of highly sensitive diagnostic tools for the detection of P. falciparum in pregnant women attending antenatal care visits in Colombia. BMC Pregnancy Childbirth. 2020;20(1):440. doi: 10.1186/s12884-020-03114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLOS Med. 2015;12(3):e1001788. doi: 10.1371/journal.pmed.1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abba K, Deeks JJ, Olliaro PL, Naing C-M, Jackson SM, Takwoingi Y, et al. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev [Internet]. 2011 [cited 2021 Jun 18];(7). Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008122.pub2/abstract. [DOI] [PMC free article] [PubMed]
- 35.Osanjo GO, Onyango IA, Kimani J, Ochanda J, Oyugi J. Evaluation of malaria rapid diagnostic tests among children in a malaria endemic region in coastal Kenya. Afr J Pharmacol Ther [Internet]. 2017 Jul 6 [cited 2021 Jun 18];6(2). Available from: http://journals.uonbi.ac.ke/ajpt/article/view/1589.
- 36.Amimo F, Lambert B, Magit A, Sacarlal J, Hashizume M, Shibuya K. Plasmodium falciparum resistance to sulfadoxine-pyrimethamine in Africa: a systematic analysis of national trends. BMJ Glob Health. 2020 doi: 10.1136/bmjgh-2020-003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.WHO Malaria Policy Advisory Committee and Secretariat Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of eighth biannual meeting (September 2015) Malar J. 2016;15(1):117. doi: 10.1186/s12936-016-1169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tagbor H, Bruce J, Agbo M, Greenwood B, Chandramohan D. Intermittent screening and treatment versus intermittent preventive treatment of malaria in pregnancy: a randomised controlled non-inferiority trial. PLoS ONE. 2010;5(12):e14425. doi: 10.1371/journal.pone.0014425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tagbor H, Cairns M, Bojang K, Coulibaly SO, Kayentao K, Williams J, et al. A non-inferiority, individually randomized trial of intermittent screening and treatment versus intermittent preventive treatment in the control of malaria in pregnancy. PLoS ONE. 2015;10(8):e0132247. doi: 10.1371/journal.pone.0132247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desai M, Gutman J, L’lanziva A, Otieno K, Juma E, Kariuki S, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet. 2015;386(10012):2507–19. doi: 10.1016/S0140-6736(15)00310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madanitsa M, Kalilani L, Mwapasa V, van Eijk AM, Khairallah C, Ali D, et al. Scheduled intermittent screening with rapid diagnostic tests and treatment with dihydroartemisinin-piperaquine versus intermittent preventive therapy with sulfadoxine-pyrimethamine for malaria in pregnancy in Malawi: an open-label randomized controlled trial. PLOS Med. 2016;13(9):e1002124. doi: 10.1371/journal.pmed.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The Global Fund. List of Rapid Diagnostic Test (RDT) kits for Malaria classified according to the Global Fund Quality Assurance Policy [Internet]. 2021. Available from: https://www.theglobalfund.org/media/5891/psm_qadiagnosticsmalaria_list_en.pdf.
- 43.Jimenez A, Rees-Channer RR, Perera R, Gamboa D, Chiodini PL, González IJ, et al. Analytical sensitivity of current best-in-class malaria rapid diagnostic tests. Malar J. 2017;16(1):128. doi: 10.1186/s12936-017-1780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martiáñez-Vendrell X, Jiménez A, Vásquez A, Campillo A, Incardona S, González R, et al. Quantification of malaria antigens PfHRP2 and pLDH by quantitative suspension array technology in whole blood, dried blood spot and plasma. Malar J. 2020;19(1):12. doi: 10.1186/s12936-019-3083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Estimating population-level prevalence by HS-RDT and PCR in Owalla et al.
Additional file 2. Positive and negative predictive values for the HS-RDT and co-RDT.
Additional file 3. Sub-analyses on the potential biases associated with PCR sensitivity.
Additional file 4. Correlation between parasite density and HRP2 concentration, and positivity by HS-RDT and co-RDT.
Data Availability Statement
The published datasets analysed, and all code written during the current study are available from https://github.com/PATH-Global-Health/HS-RDT_analysis. Individual-level datasets from Myanmar and Uganda are available from the corresponding author on request.