Abstract
Background and Aims
Accumulation of visceral adipose tissue is associated with hepatic inflammation and fibrosis, suggestive of its metabolic and inflammatory properties. We aimed to examine the histologic findings of visceral and subcutaneous adipose tissue and to associate these findings with clinical and radiologic characteristics in patients with cirrhosis.
Methods
Included were 55 adults with cirrhosis who underwent liver transplantation from 3/2017–12/2018 and had an abdominal computed tomography (CT) scan within 6 months prior to transplant. Visceral-to-subcutaneous adipose tissue ratio (VSR) was calculated using visceral (VATI) and subcutaneous adipose tissue index (SATI) quantified by CT at the L3-vertebral level and normalized for height (cm2/m2). VAT (greater omentum), SAT (abdominal wall), and skeletal muscle (rectus abdominis) biopsies were collected at transplant.
Results
Majority of patients had VAT inflammation (71%); only one patient (2%) had SAT inflammation. Patients with VAT inflammation had similar median VATI (42 vs 41 cm2/m2), lower median SATI (64 vs 97 cm2/m2), and higher median VSR (0.63 vs 0.37, p = 0.002) than patients without inflammation. In univariable logistic regression, VSR was associated with VAT inflammation (OR 1.47, 95%CI 1.11–1.96); this association remained significant even after adjusting for age, sex, BMI, HCC, or MELD-Na on bivariable analyses.
Conclusion
In patients with cirrhosis undergoing liver transplantation, histologic VAT inflammation was common, but SAT inflammation was not. Increased VSR was independently associated with VAT inflammation. Given the emerging data demonstrating the prognostic value of VSR, our findings support the value of CT-quantified VSR as a prognostic marker for adverse outcomes in the liver transplant setting.
Keywords: Body composition, Obesity, Subcutaneous adipose tissue, Liver transplant, Visceral adiposity
Introduction
Adipose tissue has been recognized as an active endocrine and inflammatory tissue that modulates action and metabolism of surrounding organs via abnormal adipokine and proinflammatory cytokine production. Accumulation of abdominal adipose tissue, particularly the visceral component compared to subcutaneous depots, has been shown to be associated with increased overall mortality as well as obesity-associated morbidities, including liver/gastrointestinal diseases [1–10], cardiovascular diseases [11–14], endocrine/metabolic diseases [15–18], and malignancies [19–23].
Among patients with cirrhosis, classification of body composition by radiologic imaging remains an important area of investigation and potential target for interventions given its objective assessment of patients’ nutritional and metabolic status. Excessive accumulation of visceral adipose tissue (VAT) has been linked to chronic systemic inflammation and associated with increased liver inflammation and fibrosis [24–27]. Furthermore, increased computed tomography (CT)-quantified VAT has been associated with poor pre- and post-transplant survival [3, 8], including increased hepatocellular carcinoma (HCC) risk and recurrence after liver transplantation [1]. Contrary to VAT, an inverse correlation was seen between subcutaneous adipose tissue (SAT) and wait-list mortality [2], further highlighting the prognostic value of varying body composition observed in this population.
Considering the different metabolic and inflammatory effects of VAT versus SAT on clinical outcomes, the relative distribution of body adiposity in the form of visceral-to-subcutaneous adipose tissue ratio (VSR) rather than the absolute area may serve as an important imaging biomarker for prognostication. Among the limited studies evaluating VSR in patients with cirrhosis and/or HCC, elevated VSR was associated with an overall poor prognosis compared to the individual effects of VAT or SAT [28–30]. Although the association between increased VSR and poor outcomes in cirrhosis has not been completely established, a commonality as a result of these abnormal body compositions—particularly increased VAT and VSR—is inflammation, which was observed in prior studies evaluating the histolopathologic relationship between adipose and liver tissue [25, 31–35]. However, these studies were mainly in obese patients undergoing bariatric surgery, and none among patients with cirrhosis. In this study, we leveraged a unique opportunity to obtain VAT and SAT in patients with cirrhosis undergoing liver transplantation to characterize the histologic findings of VAT and SAT and to evaluate their associations with clinical and CT-based measurements of adipose tissues.
Materials and Methods
Study Design and Patient Selection
Our cohort included convenience sampling of adult patients with cirrhosis who underwent liver transplantation at a single transplant center between March 1, 2017, and December 31, 2018, and had available tissue specimens obtained at time of transplant and abdominal CT imaging within 6 months prior to transplant (Supplementary Fig. 1).
Demographic data were extracted from the hepatology clinic visit at time of study enrollment. Clinical evidence of ascites and hepatic encephalopathy was determined based on recorded physical examination or written management plan at time of liver transplantation. Ascites was categorized as either absent, mild/moderate if present on exam and controlled with diuretics, or severe/refractory if they required large-volume paracentesis. Laboratory data were obtained at time of liver transplantation. Patients were considered to have comorbidities such as hypertension, diabetes mellitus, hyperlipidemia, or coronary artery disease if it was reported in their electronic health records.
Histologic Assessment of Visceral, Adipose, and Skeletal Tissue
Tissue specimens included biopsies taken from abdominal VAT (great omentum), abdominal SAT (abdominal wall), and skeletal muscle (rectus abdominis) at the time of liver transplantation. All tissue samples were formalin-fixed and embedded in paraffin by trained pathology technicians. Serial thin sections were taken and mounted on gelatin-coated glass slides and stained with hematoxylin and eosin. Each slide was individually evaluated and scored by a single pathologist blinded to all clinical data. Adipose inflammation was evaluated qualitatively and graded on a scale of 0 to 3, with score 0 assigned to cases with no inflammation or only rare scattered inflammatory cells; score 1 when minimal or focal inflammation was present; score 2 when inflammation was mild but diffuse/multifocal; and score 3 when moderate to severe, diffuse inflammation was seen (Fig. 1).
Fig. 1.
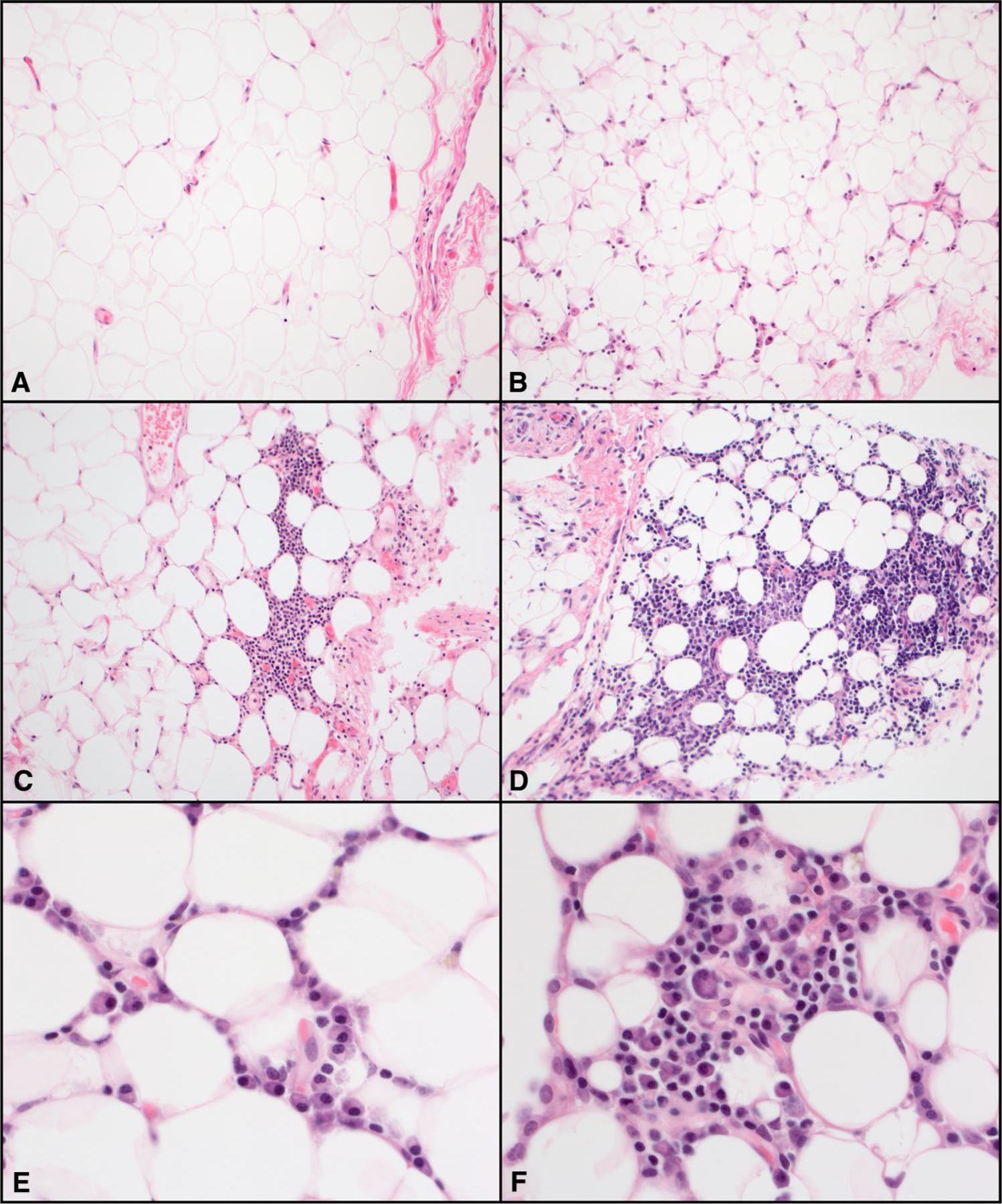
Histologic characteristics of visceral adipose tissue. Representative areas of hematoxylin and eosin-stained visceral adipose tissue inflammation grade (× 200). a Score 0, no inflammation or only rare scattered inflammatory cells. b Score 1, minimal or focal inflammation. c Score 2, diffused or multifocal mild inflammation. d Score 3, diffused moderate or severe inflammation. Higher magnification (× 600) showing numerous plasma cells (e, f) admixed with some lymphocytes (f)
Measurement of Adipose Tissues and Skeletal Muscle Mass
Body composition was assessed using secondary analysis of abdominal CT scans as part of the liver transplant evaluation. CT-based measures of skeletal muscle (psoas, erector spinae, multifidus, quadratus lumborum, rectus abdominis, transverse abdominis, and internal and external oblique), VAT, and SAT, were quantified (cm2) at the lumbar (L3) vertebral level using a post-processing workstation (General Electric Advanced Workstation 3.2, Volume Viewer software, GE Healthcare, Waukesha, WI, USA), which enabled specific tissue demarcation using standard Hounsfield Unit thresholds of -150 to -50 for VAT [36], -190 to -30 for SAT [37], and -29 to 150 for skeletal muscle [38]. As reported in prior studies using these specific Hounsfield Unit thresholds, tissue areas are outlined on an individual CT section/slice by individuals trained in musculoskeletal anatomy resulting in a semiautomatic computed total cross-sectional area (cm2) by summing tissue pixels and multiplying by pixel surface area [39]. All CT images were analyzed by trained personnel in the UCSF 3D Lab, Center for Intelligent Imaging, and were blinded to all clinical data. All values were normalized by height (m2), resulting in a visceral adipose tissue index (VATI, cm2/m2), subcutaneous adipose tissue index (SATI, cm2/m2), and skeletal muscle index (SMI, cm2/m2). VSR was calculated by dividing VATI and SATI.
Statistical Analysis
Clinical characteristics, laboratory, and radiologic data were reported as numbers and percentages (%) for categorical variables or medians and interquartile ranges (IQR) for continuous variables. Differences in quantitative variables between groups were compared by histologic evidence of VAT inflammation using two-sample t-tests or Wilcoxon rank-sum and Pearson’s chi-square (χ2) tests, as appropriate. We used univariable and bivariable logistic regression models to assess for clinical and imaging predictors of VAT inflammation. Variables of clinical interest or pertinent confounders and variables with a p-value of < 0.1 in univariable analysis were included in bivariable regression analysis. Two-sided p-value < 0.05 was considered statistically significant in all analyses. Analyses were performed using STATA statistical software, version 15.1 (Stata-Corp, College Station, TX, USA).
The study was approved by the Institutional Review Board at the University of California, San Francisco (San Francisco, CA, USA).
Results
Baseline Patient Characteristics
A total of 55 patients were included in this study: 16 (29%) had no histologic evidence of VAT inflammation compared to 39 (71%) with inflammation, further subdivided by minimal (n = 21 or 54%), mild (n = 9 or 23%), and moderate or severe (n = 9 or 23%). One patient (2%) had histologic evidence of SAT inflammation characterized as focal acute inflammation.
Baseline characteristics of the cohort are presented in Table 1, categorized by VAT inflammation. Patients with and without VAT inflammation had similar median age (59 vs 60 years) and body mass index (27.8 vs 29.3 kg/m2). A higher proportion of patients with VAT inflammation were male (59% vs 38%) and had higher rates of prior tobacco use (54% vs 25%) and alcohol use (46% vs 31%), though none were statistically significant. Ethnicity/race categories were similar between the two groups. Patients with VAT inflammation had higher rates of hypertension (46% vs 31%), though they had comparable rates of diabetes mellitus (33% vs 31%), hyperlipidemia (28% vs 31%), and coronary artery disease (8% vs 6%).
Table 1.
Baseline characteristics in patients with and without visceral adipose tissue inflammation
| Overall n = 55 | Non-inflammation n = 16 | Inflammation n = 39 | p-value | |
|---|---|---|---|---|
| Age (years) | 60 (53–63) | 60 (56–64) | 59 (53–62) | 0.31 |
| Sex (male) | 29 (53) | 6 (38) | 23 (59) | 0.15 |
| Race | 0.72 | |||
| Non-Hispanic white | 31 (56) | 10 (63) | 21 (54) | |
| Black | 2 (4) | 1 (6) | 1 (3) | |
| Hispanic white | 15 (27) | 4 (25) | 11 (28) | |
| Asian | 7 (13) | 1 (6) | 6 (15) | |
| Body mass index (kg/m2) | 27.8 (24.9–32.3) | 29.3 (24.7–36.2) | 27.8 (24.9–30.3) | 0.53 |
| Weight (kg) | 82.1 (66.7–95.7) | 78.5 (64.5–108.2) | 82.6 (67.6–95.7) | 0.99 |
| Etiology | ||||
| Hepatitis C infection | 18 (33) | 6 (37) | 12 (31) | 0.63 |
| Hepatitis B infection | 5 (9) | 1 (6) | 4 (10) | 0.64 |
| Alcohol | 8 (15) | 3 (19) | 5 (13) | 0.57 |
| NAFLD/NASH | 14 (25) | 2 (13) | 12 (31) | 0.16 |
| AIH/PBC/PSC | 10 (18) | 4 (25) | 6 (15) | 0.40 |
| Tobacco use | 25 (45) | 4 (25) | 21 (54) | 0.05 |
| Alcohol use (moderate/heavy) | 23 (42) | 5 (31) | 18 (46) | 0.31 |
| Hypertension | 23 (42) | 5 (31) | 18 (46) | 0.31 |
| Diabetes mellitus | 18 (33) | 5 (31) | 13 (33) | 0.88 |
| Hyperlipidemia | 16 (29) | 5 (31) | 11 (28) | 0.82 |
| Coronary artery disease | 4 (7) | 1 (6) | 3 (8) | 0.85 |
| Ascites | 0.25 | |||
| None | 19 (35) | 7 (44) | 12 (30) | |
| Mild/moderate | 25 (45) | 8 (50) | 17 (44) | |
| Severe/refractory | 11 (20) | 1 (6) | 10 (26) | |
| Spontaneous bacterial peritonitis | 7 (19) | 1 (11) | 6 (22) | 0.47 |
| Hepatic encephalopathy | 37 (67) | 13 (81) | 24 (62) | 0.16 |
| Hepatocellular carcinoma | 21 (38) | 6 (38) | 15 (38) | 0.95 |
| Native MELD-Na | 19 (11–27) | 14 (11–24) | 19 (11–28) | 0.50 |
| Sodium (mmol/L) | 136 (133–138) | 137 (131–138) | 136 (134–138) | 0.77 |
| Total bilirubin (mg/dL) | 2.5 (1.1–7.7) | 2.6 (1.4–5.3) | 2.5 (1.0–8.9) | 0.81 |
| Creatinine (mg/dL) | 0.82 (0.66–1.25) | 0.90 (0.60–1.27) | 0.82 (0.68–1.16) | 0.68 |
| INR | 1.4 (1.2–2.0) | 1.3 (1.1–1.9) | 1.5 (1.2–2.0) | 0.14 |
Values reported in median (IQR) or number (percentage)
AIH/PBC/PSC autoimmune hepatitis/primary biliary cholangitis/primary sclerosing cholangitis, INR international normalized ratio, MELD-Na model for end-stage liver disease-sodium, NAFLD/NASH nonalcoholic fatty liver disease/nonalcoholic steatohepatitis
There was no significant difference in etiologies among patients with and without VAT inflammation. A similar percentage of patients with VAT inflammation had HCC (38% vs 38%). Patients with VAT inflammation had higher median model for end-stage liver disease-sodium (MELD-Na) (19 vs 14) and similar albumin (3.0 vs 3.1 g/dL). Prevalence of ascites was slightly higher in patients with VAT inflammation (69% vs 56%), with the majority characterized as severe/refractory (26% vs 6%) and had history of spontaneous bacterial peritonitis (22% vs 11%), but were not statistically significant. Additionally, there was no significant association between presence of ascites and/or spontaneous bacterial peritonitis with VAT inflammation on univariable analysis.
Association Between Radiographic Abdominal Adipose Tissue Distribution and Histologic Visceral Adipose Tissue Inflammation
Radiologic findings are presented in Table 2. Median duration from baseline to CT scan was 57 (17–138) days, baseline to transplant was 94 (49–154) days, and CT to transplant was 19 (4–49) days. Patients with VAT inflammation were significantly more likely to have lower median SATI (64 vs 97 cm2/m2) and higher median VSR (0.63 vs 0.37), with similar median VATI (42 vs 41 cm2/m2) and median SMI (47 vs 47 cm2/m2). Median VSR remained significantly higher in patients with VAT inflammation regardless of degree of inflammation: minimal (0.71, p = 0.01), mild (0.64, p = 0.02), and moderate or severe (0.50, p = 0.03), compared to no inflammation (0.37). Additionally, when accounting for gender differences in terms of body composition, median VSR remained significantly higher in male (0.77 vs 0.46, p = 0.046) and female (0.51 vs 0.32, p = 0.039) with VAT inflammation. Patients with HCC had higher median VSR (0.66 vs 0.49, p = 0.09).
Table 2.
Computed tomography-quantified body composition according to visceral adipose tissue inflammation
| Overall n = 55 | Non-inflammation n = 16 | Inflammation n = 39 | p-value | |
|---|---|---|---|---|
| SMI (cm2/m2) | 47 (41–52) | 47 (43–54) | 47 (40–52) | 0.64 |
| Male | 49 (45–54) | 52 (45–65) | 49 (41–54) | 0.42 |
| Female | 45 (40–51) | 47 (40–50) | 43 (39–51) | 0.71 |
| VATI (cm2/m2) | 41 (22–65) | 41 (18–57) | 42 (22–69) | 0.54 |
| Male | 49 (24–69) | 52 (46–59) | 46 (24–72) | 0.63 |
| Female | 34 (17–56) | 23 (15–45) | 38 (21–60) | 0.34 |
| SATI (cm2/m2) | 67 (44–99) | 97 (64–127) | 64 (40–82) | 0.01 |
| Male | 64 (40–110) | 127 (110–150) | 53 (32–73) | 0.02 |
| Female | 73 (59–96) | 75 (63–99) | 70 (49–90) | 0.40 |
| VSR | 0.55 (0.38–0.89) | 0.37 (0.25–0.65) | 0.63 (0.49–1.00) | 0.002 |
| Male | 0.75 (0.50–1.06) | 0.46 (0.31–0.78) | 0.77 (0.55–1.12) | 0.046 |
| Female | 0.45 (0.32–0.55) | 0.32 (0.19–0.46) | 0.51 (0.41–0.61) | 0.039 |
Values reported in median (IQR)
SATI subcutaneous adipose tissue index, SMI skeletal muscle index, VATI visceral adipose tissue index, VSR visceral-to-subcutaneous adipose tissue ratio
Regarding timing of imaging to transplant, sub-analyses including only patients with imaging within 90 days prior to transplant (n = 49) had similar results, in which patients with VAT inflammation had higher median VSR (0.61 vs 0.35, p = 0.003) and lower median SATI (58 vs 96 cm2/m2, p = 0.009), with similar median VATI (39 vs 37 cm2/m2, p = 0.74) and median SMI (47 vs 47 cm2/m2, p = 0.49).
We analyzed clinical, laboratory, and radiologic factors associated with VAT inflammation (Table 3). In univariable logistic regression, only increased CT-quantified VSR (per 0.1 unit) was significantly associated with histologic VAT inflammation (OR 1.47, 95% CI 1.11–1.96, p = 0.008). In bivariable logistic regression, after adjusting for age, sex, BMI, HCC, or MELD-Na, CT-quantified VSR remained independently associated with VAT inflammation. Given gender-associated differences in body composition, additional bivariable logistic regression analyses were conducted separately in men and women, also adjusting for age, BMI, HCC, or MELD-Na. In men, VSR was statistical significantly associated with histologic VAT inflammation after adjusting for HCC (OR 2.07, 95% CI 1.03–4.15, p = 0.04) or MELD-Na (OR 1.68, 95% CI 1.01–2.79, p = 0.045) as shown in Supplementary Table 1. In women, VSR was not statistical significantly associated with histologic VAT inflammation after adjusting for age, BMI, HCC, or MELD-Na (Supplementary Table 2).
Table 3.
Univariable and bivariable logistic regression for predictors of visceral adipose tissue inflammation
| Univariable analysis | Bivariable analysis | Bivariable analysis | Bivariable analysis | Bivariable analysis | Bivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| VSR (per 0.1 unit) | 1.47 (1.11–1.96) | 0.008 | 1.53 (1.13–2.07) | 0.006 | 1.46 (1.08–1.98) | 0.01 | 1.48 (1.10–1.97) | 0.009 | 1.51 (1.12–2.03) | 0.007 | 1.50 (1.12–2.00) | 0.006 |
| Age (year) | 0.98 (0.92–1.05) | 0.63 | 0.95 (0.87–1.03) | 0.21 | – | – | – | – | – | – | – | – |
| Sex (male) | 2.40 (0.72–7.93) | 0.15 | – | – | 1.12 (0.28–4.39) | 0.87 | – | – | – | – | – | – |
| BMI (kg/m2) | 0.96 (0.86–1.06) | 0.39 | – | – | – | – | 0.97 (0.87–1.07) | 0.51 | – | – | – | – |
| HCC | 1.29 (0.39–4.25) | 0.68 | – | – | – | – | – | – | 0.57 (0.14–2.32) | 0.43 | – | – |
| MELD-Na (per 1 point) | 1.03 (0.96–1.09) | 0.43 | – | – | – | – | – | – | – | – | 1.05 (0.97–1.13) | 0.20 |
BMI body mass index, HCC hepatocellular carcinoma, MELD-Na model for end-stage liver disease-sodium, VSR visceral-to-subcutaneous adipose tissue ratio
Discussion
CT-based measures of body composition (e.g., skeletal muscle mass, subcutaneous fat, visceral fat) have emerged as strong predictors of outcomes in patients with cirrhosis [1, 2, 4, 8, 9, 40–44]. Although measures of muscle mass (e.g., psoas muscle, total abdominal skeletal muscle) are the most well-studied [40–45], abdominal adipose tissue—particularly VAT and VSR—are gaining increased recognition for their prognostic value particularly in light of rising rates of obesity [1, 3, 8]. However, little is known in the applicability of these CT-based biomarkers of adipose tissue to their histologic findings. In this study of patients with cirrhosis who underwent liver transplantation, we observed that nearly three-quarters of our cohort displayed histologic VAT inflammation characterized by a spectrum of lymphoplasmacytic and perivascular lymphocytic aggregates. Furthermore, histologic VAT inflammation was independently associated with increased VSR quantified on CT imaging, providing clues to the mechanism by which VAT, and its imbalance relative to SAT, might lead to or be a biomarker for adverse outcomes in this population.
Our results extend the findings of previous studies evaluating the histologic characteristics of adipose tissues though in a population of patients with cirrhosis, in which similar findings of increased inflammation within VAT versus SAT were reported [25, 31–33]. The observed histologic discrepancy between the two depots is due to differences in their metabolic activity, lipolytic activity, and adipocytokine profile [24, 25, 46, 47]. In studies utilizing immunohistochemistry, one major difference was the abundance of immune cells including macrophages and plasma cells within VAT as compared to SAT [31–33]. The histologic findings of VAT inflammation in this study were the presence of lymphoplasmacytic aggregates. This was also described in a prior study, suggesting a possible difference in plasma cell recruitment by adipocytokines between the two depots in which interactions with the adaptive immune system are more pronounced within VAT [48].
In our current study, increased VSR quantified on CT was independently associated with VAT inflammation. This observed difference in VSR was predominately driven by decreased CT-quantified SAT, rather than an increased in VAT, perhaps due to a limitation of the small cohort size. It is well recognized that the accumulation of VAT is mainly responsible for the majority of liver- and obesity-related complications in comparison with SAT. Although the role of SAT remains to be fully elucidated, limited prior studies have shown an inverse association with mortality, further suggesting that perhaps SAT may have metabolic protective properties through its different inflammatory and adipocytokine profile as previously discussed [2, 9, 24]. Moreover, only 2% of patients had SAT inflammation compared to 71% of patients with VAT inflammation. These findings further suggest that the distribution of adipose tissue rather than the absolute value of its different depots is a major determinant of disease severity. Thus, elevated VSR may serve as an important imaging biomarker in inflammation and disease progression, while aiding in understanding the relationship between visceral adiposity and liver disease, in addition to obesity-related conditions.
The findings of this study should be considered in light of several limitations. Our cohort was relatively small, reducing our power to adjust for multiple confounders. However, we believe that this limitation is balanced by the novelty of our findings in a unique cohort given the difficulty of procuring visceral and subcutaneous adipose tissue from patients undergoing liver transplantation, which are generally obtained among those undergoing abdominal surgery for other clinical reasons. Our cohort was assembled as a convenience sampling of patients undergoing liver transplantation at our center which may have introduced selection bias. However, inclusion into this cohort was based on availability of the tissue procurement team, which was independent of clinically relevant patient characteristics. Additionally, adipose tissue inflammation was evaluated by a single pathologist blinded to all clinical data. Adipose tissue inflammation was evaluated qualitatively based on the degree of inflammatory cell aggregates given no available standardized scale. This was similar to the histologic method done on a previous article assessing visceral adipose tissue inflammation in mice, in which the same pathologist had to also derive a histologic inflammation scale [49]. Furthermore, we were unable to evaluate the metabolic and adipocytokine profile of adipose tissues due to the retrospective nature of the study. Moreover, we did not conduct immunohistochemistry analysis which could help differentiate the type of inflammatory cells; however, this has been evaluated in the several aforementioned studies [31–33]. Unfortunately, the only anthropometric measurement available was BMI, and not waist circumference or waist-to-hip ratio. Given that the majority of our patients had ascites (65%), anthropometric measurements such as BMI and waist-to-hip ratio are limited in their ability to adjust for fluid retention (e.g., ascites and/or systemic edema) and inability to discriminate between different components of body composition. Another limitation was the timing and availability of CT-quantified adipose tissue and skeletal muscle mass, which was done at a single time point. Although the median (IQR) time from body composition imaging to transplant was 19 (4–49) days, there were 18 patients with imaging completed within 7 days from transplant, in which both skeletal muscle wasting and loss of VAT have the potential to occur in the setting of critical illness; however, there was no statistically significant differences in timing of imaging on VAT inflammation (p = 0.88) or VSR (p = 0.96). Lastly, the small cohort size limited our ability to evaluate the association between adipose tissue inflammation and post-transplant clinical outcomes, such as acute rejection, infections, or mortality (which are relatively rare events), but our findings lay the groundwork for future studies to evaluate these outcomes.
In conclusion, we have demonstrated that the majority of patients with cirrhosis displayed histologic VAT inflammation across a spectrum characterized as lymphoplasmacytic and perivascular lymphocytic aggregates, compared to minimal inflammation seen on SAT. Importantly, VAT inflammation was shown to be associated with increased VSR, which is emerging as an important imaging biomarker and predictor of adverse outcomes in both patients with cirrhosis and the general population. Our data offers further evidence in the clinical relevance of visceral adiposity and provides a rational basis for future studies evaluating the association between adipose tissue inflammation and outcomes in patients with cirrhosis, with visceral obesity as an important target for future interventions to improve clinical outcomes.
Supplementary Material
Funding
This study was funded by NIH K23AG048337 (Lai), NIH R01AG059183 (Lai), NIH P30DK026743 (Lai, Maher), NIH R21AG067554 (Lai), and NIH 5T32DK060414–18 (Ha). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
Abbreviations
- VAT
Visceral adipose tissue
- CT
Computed tomography
- HCC
Hepatocellular carcinoma
- SAT
Subcutaneous adipose tissue
- VSR
Visceral-to-subcutaneous adipose tissue ratio
- VATI
Visceral adipose tissue index
- SATI
Subcutaneous adipose tissue index
- SMI
Skeletal muscle index
- MELD-Na
Model for end-stage liver disease-sodium
- BMI
Body mass index
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10620-021-07099-8.
Declarations
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Montano-Loza AJ, Mazurak VC, Ebadi M et al. Visceral adiposity increases risk for hepatocellular carcinoma in male patients with cirrhosis and recurrence after liver transplant. Hepatology 2018;67:914–923. 10.1002/hep.29578. [DOI] [PubMed] [Google Scholar]
- 2.Ebadi M, Tandon P, Moctezuma-Velazquez C et al. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J Hepatol 2018;69:608–616. 10.1016/j.jhep.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 3.Terjimanian MN, Harbaugh CM, Hussain A et al. Abdominal adiposity, body composition and survival after liver transplantation. Clin Transplant 2016;30:289–294. 10.1111/ctr.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamaguchi Y, Kaido T, Okumura S et al. Impact of skeletal muscle mass index, intramuscular adipose tissue content, and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation 2017;101:565–574. 10.1097/TP.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 5.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14:336–341. 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 6.Usui G, Shinozaki T, Jinno T et al. Association between visceral abdominal obesity and long-segment Barrett’s esophagus in a Japanese population. J Gastroenterol 2020;55:189–197. 10.1007/s00535-019-01640-3. [DOI] [PubMed] [Google Scholar]
- 7.Jung CH, Rhee EJ, Kwon H, Chang Y, Ryu S, Lee WY. Visceral-to-subcutaneous abdominal fat ratio is associated with nonalcoholic fatty liver disease and liver fibrosis. Endocrinol Metab (Seoul) 2020;35:165–176. 10.3803/EnM.2020.35.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura N, Tsuchiya A, Oda C et al. Visceral adipose tissue index and hepatocellular carcinoma are independent predictors of out-come in patients with cirrhosis having endoscopic treatment for esophageal varices. Dig Dis 2020. 10.1159/000508867. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues SG, Brabandt B, Stirnimann G, Maurer MH, Berzigotti A. Adipopenia correlates with higher portal pressure in patients with cirrhosis. Liver Int 2019;39:1672–1681. 10.1111/liv.14175. [DOI] [PubMed] [Google Scholar]
- 10.Ohki T, Tateishi R, Shiina S et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut 2009;58:839–844. 10.1136/gut.2008.164053. [DOI] [PubMed] [Google Scholar]
- 11.Diaz AA, Young TP, Kurugol S et al. Abdominal visceral adipose tissue is associated with myocardial infarction in patients with COPD. Chronic Obstr Pulm Dis 2015;2:8–16. 10.15326/jcopdf.2.1.2014.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Y, Wang YC, Lu CQ, Zeng C, Chang D, Ju S. Correlations between the abdominal fat-related parameters and severity of coronary artery disease assessed by computed tomography. Quant Imaging Med Surg 2018;8:579–587. 10.21037/qims.2018.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab 2010;95:5419–5426. 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pou KM, Massaro JM, Hoffmann U et al. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes Care 2009;32:481–485. 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borel AL, Nazare JA, Smith J et al. Visceral, subcutaneous abdominal adiposity and liver fat content distribution in normal glucose tolerance, impaired fasting glucose and/or impaired glucose tolerance. Int J Obes (Lond) 2015;39:495–501. 10.1038/ijo.2014.163. [DOI] [PubMed] [Google Scholar]
- 16.Kantartzis K, Machann J, Schick F, Fritsche A, Haring HU, Stefan N. The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia 2010;53:882–889. 10.1007/s00125-010-1663-6. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen-Duy TB, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab 2003;284:E1065–E1071. 10.1152/ajpendo.00442.2002. [DOI] [PubMed] [Google Scholar]
- 18.Oh YH, Moon JH, Kim HJ, Kong MH. Visceral-to-subcutaneous fat ratio as a predictor of the multiple metabolic risk factors for subjects with normal waist circumference in Korea. Diabetes Metab Syndr Obes 2017;10:505–511. 10.2147/DMSO.S150914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grignol VP, Smith AD, Shlapak D, Zhang X, Del Campo SM, Carson WE. Increased visceral to subcutaneous fat ratio is associated with decreased overall survival in patients with metastatic melanoma receiving anti-angiogenic therapy. Surg Oncol 2015;24:353–358. 10.1016/j.suronc.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang HW, Kim D, Kim HJ et al. Visceral obesity and insulin resistance as risk factors for colorectal adenoma: a cross-sectional, case-control study. Am J Gastroenterol 2010;105:178–187. 10.1038/ajg.2009.541. [DOI] [PubMed] [Google Scholar]
- 21.Ryan AM, Duong M, Healy L, Ryan SA, Parekh N, Reynolds JV et al. Obesity, metabolic syndrome and esophageal adenocarcinoma: epidemiology, etiology and new targets. Cancer Epidemiol 2011;35:309–319. 10.1016/j.canep.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Schapira DV, Clark RA, Wolff PA, Jarrett AR, Kumar NB, Aziz NM. Visceral obesity and breast cancer risk. Cancer 1994;74:632–639. . [DOI] [PubMed] [Google Scholar]
- 23.von Hafe P, Pina F, Perez A, Tavares M, Barros H. Visceral fat accumulation as a risk factor for prostate cancer. Obes Res 2004;12:1930–1935. 10.1038/oby.2004.242. [DOI] [PubMed] [Google Scholar]
- 24.van der Poorten D, Milner KL, Hui J et al. Visceral fat: a key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008;48:449–457. 10.1002/hep.22350. [DOI] [PubMed] [Google Scholar]
- 25.du Plessis J, van Pelt J, Korf H et al. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology 2015;149:e14. 10.1053/j.gastro.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 26.Yu SJ, Kim W, Kim D, Yoon JH, Lee K, Kim JH et al. Visceral obesity predicts significant fibrosis in patients with nonalcoholic fatty liver disease. Medicine (Baltimore) 2015;94:e2159. 10.1097/MD.0000000000002159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004;145:2273–2282. 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara N, Nakagawa H, Kudo Y et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 2015;63:131–140. 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Hamaguchi Y, Kaido T, Okumura S et al. Preoperative visceral adiposity and muscularity predict poor outcomes after hepatectomy for hepatocellular carcinoma. Liver Cancer 2019;8:92–109. 10.1159/000488779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou L, Deng Y, Fan X et al. A sex-stratified prognostic nomogram incorporating body compositions for long-term mortality in cirrhosis. JPEN J Parenter Enteral Nutr 2020. 10.1002/jpen.1841. [DOI] [PubMed] [Google Scholar]
- 31.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes 2006;55:1554–1561. 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 32.Tordjman J, Divoux A, Prifti E et al. Structural and inflammatory heterogeneity in subcutaneous adipose tissue: relation with liver histopathology in morbid obesity. J Hepatol 2012;56:1152–1158. 10.1016/j.jhep.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Tordjman J, Poitou C, Hugol D et al. Association between omental adipose tissue macrophages and liver histopathology in morbid obesity: influence of glycemic status. J Hepatol 2009;51:354–362. 10.1016/j.jhep.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 34.van Beek L, Lips MA, Visser A et al. Increased systemic and adipose tissue inflammation differentiates obese women with T2DM from obese women with normal glucose tolerance. Metabolism 2014;63:492–501. 10.1016/j.metabol.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Wree A, Schlattjan M, Bechmann LP et al. Adipocyte cell size, free fatty acids and apolipoproteins are associated with non-alcoholic liver injury progression in severely obese patients. Metabolism 2014;63:1542–1552. 10.1016/j.metabol.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Vehmas T, Kairemo KJ, Taavitsainen MJ. Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes Relat Metab Disord 1996;20:570–573. [PubMed] [Google Scholar]
- 37.Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes 1986;10:53–67. [PubMed] [Google Scholar]
- 38.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1985;1998:115–122. 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 39.Meza-Junco J, Montano-Loza AJ, Baracos VE et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47:861–870. 10.1097/MCG.0b013e318293a825. [DOI] [PubMed] [Google Scholar]
- 40.Carey EJ, Lai JC, Wang CW et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23:625–633. 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo SZ, Ahmad M, Dunn MA et al. Sarcopenia predicts post-transplant mortality in acutely ill men undergoing urgent evaluation and liver transplantation. Transplantation 2019;103:2312–2317. 10.1097/TP.0000000000002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montano-Loza AJ, Meza-Junco J, Baracos VE et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl 2014;20:640–648. 10.1002/lt.23863. [DOI] [PubMed] [Google Scholar]
- 43.Montano-Loza AJ, Meza-Junco J, Prado CM et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10:166–173, 73 e1. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 44.van Vugt JLA, Alferink LJM, Buettner S et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol 2018;68:707–714. 10.1016/j.jhep.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 45.Carey EJ, Lai JC, Sonnenday C et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology 2019;70:1816–1829. 10.1002/hep.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghigliotti G, Barisione C, Garibaldi S et al. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation 2014;37:1337–1353. 10.1007/s10753-014-9914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology 2009;50:957–969. 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 48.Palming J, Gabrielsson BG, Jennische E et al. Plasma cells and Fc receptors in human adipose tissue–lipogenic and anti-inflammatory effects of immunoglobulins on adipocytes. Biochem Biophys Res Commun 2006;343:43–48. 10.1016/j.bbrc.2006.02.114. [DOI] [PubMed] [Google Scholar]
- 49.Duwaerts CC, Amin AM, Siao K et al. Specific macronutrients exert unique influences on the adipose-liver axis to promote hepatic steatosis in Mice. Cell Mol Gastroenterol Hepatol 2017;4:223–236. 10.1016/j.jcmgh.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


