Abstract
Patient: Female, 43-year-old
Final Diagnosis: Rocky Mountain spotted fever
Symptoms: Acute kidney injury • hyponatremia • rash
Medication: —
Clinical Procedure: —
Specialty: General and Internal Medicine
Objective:
Rare disease
Background:
Rocky Mountain spotted fever (RMSF) is a potentially fatal infectious disease caused by the gram-negative intracellular bacterium Rickettsia rickettsii. The classic triad includes fever, rash, and history of tick exposure; however, the triad presents in only 3% to 18% of cases at the initial visit, and the tick bite is often painless and overlooked. RMSF can present with other manifestations, including hyponatremia, lymphopenia, thrombocytopenia, and coagulopathy. Some of these manifestations can be overlooked if they overlap with manifestations of a patient’s pre-existing conditions.
Case Report:
A 43-year-old woman with RMSF presented with fever and treatment-resistant hyponatremia before developing a rash. Initially, the cause of her hyponatremia was attributed to adrenal insufficiency and dehydration. After appropriate treatments with hormone replacement therapy and intravenous hydration, her laboratory values remained relatively unchanged. A rash later appeared with an atypical RMSF pattern, warranting a detailed integumentary examination, which uncovered the culprit tick in an unusual location. After starting doxycycline, the patient’s signs and symptoms, including her sodium level, improved.
Conclusions:
We conclude that the diagnosis of RMSF is an empiric diagnosis based on clinical signs, symptoms, and appropriate epidemiologic settings for tick exposures. However, typical clinical signs do not always display at presentation. Other manifestations that a patient’s pre-existing conditions can simultaneously cause should not be overlooked and should be examined holistically with other signs, symptoms, laboratory values, and physical examinations. Early treatment with doxycycline is encouraged as evidence strongly suggests that early treatment is correlated with lower mortality.
Keywords: Adrenalectomy, Hyponatremia, Rocky Mountain Spotted Fever
Background
Rocky Mountain spotted fever (RMSF) is a potentially fatal infectious disease caused by the gram-negative intracellular bacterium Rickettsia rickettsii. The classic triad includes fever, rash, and history of tick exposure. However, the triad presents in only 3% to 18% of cases at the initial physician visit because the tick bite is often painless and overlooked [1]. Other manifestations include hyponatremia, lymphopenia, thrombocytopenia, and coagulopathy, but they often occur late in the disease process [2]. We present a case of RMSF in a patient with a history of adrenal cortical carcinoma treated by adrenalectomy and adjuvant mitotane for 3 purposes: (1) to highlight to providers that patients with RMSF can develop hyponatremia before a rash develops. For patients living in the high incidence area (the state of Virginia in our case), RMSF should be included on the differential list when the hyponatremia is resistant to treatments after other obvious causes are addressed; (2) to discuss the difficulty of early diagnosis due to the overlap in signs, symptoms, and laboratory findings between RMSF and patient’s pre-existing conditions; and (3) to stress the importance of a thorough physical examination in patients with suspected tick-borne disease.
Case Report
A 43-year-old woman with a past medical history of adrenal cortical carcinoma (pT2NxM0) treated by adrenalectomy on adjuvant mitotane (500 mg 3 tablets 3 times daily), hydro-cortisone (10 mg 2 tablets in the morning 1 tablet in the evening, with double the dose if patient feels sick), and fludrocortisone (0.1 mg half tablet daily), presented to the hospital Emergency Department with general malaise, a subjective fever, and poor tolerance of oral intake for 3 days. The patient was seen at an urgent care facility when she initially felt ill 3 days earlier, and urinalysis results were reportedly suggestive of a urinary tract infection. She was subsequently given an unknown dose of intramuscular ceftriaxone and oral ciprofloxacin. Vital signs and urinalysis taken upon admission are included in Table 1. All other laboratory findings from 9 days before the admission by a clinic, upon admission, and 21 days after the admission during oncology follow-up are included in Table 2; the laboratory results before and after admission are used as the baseline and the recovery reference, respectively. It is worth noting that a complete physical examination was unremarkable, including an integumentary examination showing no signs of rashes or lesions. Her complete metabolic profile revealed hyponatremia (serum sodium of 123 mmol/L), acute kidney injury (creatinine of 2.28 mg/dL), an elevated urine osmolality of 125 mOsm/kg, and low urine sodium <20 mEq/L, which was consistent with acute kidney injury secondary to hypovolemic hyponatremia. Upon questioning, the patient endorsed that her adrenal cortical carcinoma had been well managed by mitotane, hydrocortisone, and fludrocortisone and she denied taking any stress dose of the glucocorticoid after she became ill 3 days earlier. The hyponatremia was attributed to adrenal insufficiency caused by a lack of stress dose of glucocorticoid in her setting of acute illness. Consequently, her home dose of hydrocortisone was doubled, and her mitotane was held. One liter of intravenous (i.v.) lactated Ringer’s solution was also administered, and her sodium level slightly improved to 131 mmol/L. A computed tomography scan was ordered to rule out any malignancy that may have caused a paraneoplastic syndrome of inappropriate antidiuretic hormone secretion, and it came back negative. In addition, the level of white blood cells was non-elevated, and a repeat urinalysis was inconsistent with urinary tract infection. The patient’s overall presentation made an infectious process unlikely, and she was admitted to be monitored for her sodium level.
Table 1.
Vital signs and urinalysis from patient on admission.
| Vital signs on admission | |||||||
| Ht 155cm | Wt 72 Kg | BMI 30 | T 36.5°C | BP 120/84 mmHg | HR 84 | RR 16 | SpO2 96% |
| Urinalysis on admission | |||||||
| Color | Yellow | ||||||
| Transparency | Clear | ||||||
| Protein | Negative | ||||||
| Leukocyte | Negative | ||||||
| Nitrite | Negative | ||||||
| Blood | Negative | ||||||
| Glucose | Negative | ||||||
| Ketone | Trace | ||||||
| pH | 5.0 | ||||||
| Specific gravity | 1.008 | ||||||
| Bilirubin | Negative | ||||||
| Urobilinogen | <2.0 mg/dL | ||||||
| RBC/HPF | 4/HPF | ||||||
| WBC/HPF | 7/HPF | ||||||
| Bacteria/HPF | Few | ||||||
| Budding yeast/HPF | Negative | ||||||
| Squamous epithelial/HPF | 10/HPF | ||||||
Ht – height; wt – weight; BMI – body mass index; T – temperature; BP – blood pressure; HR – heart rate; RR – respiratory rate; SpO2 – oxygen saturation.
Table 2.
Complete laboratory testing performed before, upon, and after admission.
| Laboratory finding | Before admission* | On admission | 2 weeks after discharge** |
|---|---|---|---|
| Sodium | 135 mmol/L | 123 mmol/L | 140 mmol/L |
| Potassium | 4.4 mmol/L | 4.0 mmol/L | 3.6 mmol/L |
| Chloride | 106 mmol/L | 94 mmol/L | 109 mmol/L |
| Carbon dioxide | 24 mmol/L | 16 mmol/L | 27 mmol/L |
| Anion gap | 5 mmol/L | 13 mmol/L | 4 mmol/L |
| Osmolality | N/A | 278 mOsm/kg | N/A |
| Glucose | 85 mg/dL | 91 mmol/L | 77 mg/dL |
| BUN | 14 mg/dL | 35 mmol/L | 15 mg/dL |
| Creatinine | 0.89 mg/dL | 2.28 mmol/L | 0.81 mg/dL |
| GFR | 80 mL/min/1.73 m2 | 26 mL/min/1.73 m2 | 89 mL/min/1.73 m2 |
| AST | 23 unit(s)/L | 62 unit(s)/L | 21 unit(s)/L |
| ALT | 8 unit(s)/L | 7 unit(s)/L | 12 unit(s)/L |
| Alk phos | 93 unit(s)/L | 90 unit(s)/L | 112 unit(s)/L |
| Bilirubin, total | 0.2 mg/dL | 0.4 mg/dL | 0.3 mg/dL |
| Bilirubin, conjugated | 0.1 mg/dL | 0.3 mg/dL | 0.2 mg/dL |
| Protein, total | 6.4 g/dL | 5.1 g/dL | 5.9 g/dL |
| Albumin | 3.6 g/dL | 2.7 g/dL | 3.1 g/dL |
| Globulin | 2.8 g/dL | 2.4 g/dL | 2.8 g/dL |
| Calcium | 8.6 mg/dL | 8.7 mg/dL | 9.1 mg/dL |
| Magnesium | N/A | N/A | 1.5 mg/dL |
| Creatinine, urine | N/A | 21.6 mg/dL | N/A |
| Osmolality, urine | N/A | 125 mOsm/kg | N/A |
| Potassium urine | N/A | 9.4 mmol/L | N/A |
| Sodium, urine | N/A | <20 mmol/L | N/A |
| WBC | 7.3×109/L | 9.9×109/L | 7.7×109/L |
| RBC | 4.47×1012/L | 4.05×1012/L | 3.80×1012/L |
| Hemoglobin | 13.2 g/dL | 11.8 g/dL | 10.9 g/dL |
| HCT | 40.8% | 35.0% | 33.6% |
| MCV | 91.3 fL | 86.4 fL | 88.4 fL |
| MCH | 29.5 pg | 29.1 pg | 28.7 pg |
| MCHC | 32.4 g/dL | 33.7 g/dL | 32.4 g/dL |
| RDW | 13.4% | 13.5% | 14.6% |
| PLT | 206×109/L | 153×109/L | 163×109/L |
| MOV | 12.4 fL | 12.7 fL | 12.5 fL |
| %Neu | 49.8% | 51.9% | 50.2% |
| %Lym | 42.3% | 36.2% | 40.7% |
| %Mono | 5.3% | 11.5% | 6.5% |
| %Eos | 1.8% | 0.1% | 2.1% |
| %Baso | 0.8% | 0.3% | 0.5% |
| NEU | 3.6×109/L | 4.1×109/L | 3.8×109/L |
| LYM | 3.1×109/L | 2.7×109/L | 3.1×109/L |
| MONO | 0.4×109/L | 1.1×109/L | 0.5×109/L |
| EOS | 0.1×109/L | 0.0×109/L | 0.2×109/L |
| BASO | 0.1×109/L | 0.0×109/L | 0.0×109/L |
This laboratory result was obtained 9 days prior to the admission and used as a baseline;
this laboratory result was obtained 21 days after the admission during an oncology follow-up.
Unexpectedly, on the next day, the patient’s fever spiked to 39.3°C. On physical examination, newly-developed, diffuse small erythematous blanching papules with some coalescences were noted on the patient’s shoulder, bilaterally extending to the scapula and humerus region, sparing the palmar and plantar surfaces, raising concern for a drug reaction because a rash starting on the shoulder is atypical for RMSF. A dermatology consultation was requested and a complete integumentary examination was performed, showing dissemination of the rash on the back, shoulders, and abdomen; the palmer and plantar surfaces were uninvolved. A tiny 4-legged tick with a central white spot on the dorsum was discovered in the left inframammary fold, likely an Amblyomma/Lone Star tick by appearance, and raised clinical suspicion for tick-borne illness. A serology for tick-borne illness was ordered and was positive for RMSF IgG, confirming the diagnosis of RMSF. The positive RMSF IgG finding was consistent with laboratory values that the patient presented with upon admission, such as a low serum osmolality at 278 mOsm/Kg, low serum albumin level at 2.7 g/dL, and the critically low sodium level at 123 mmol/L. These laboratory values suggested the body’s appropriate response to hypovolemia secondary to vascular injury caused by RMSF infection and decreased tissue perfusion by releasing antidiuretic hormone. Unfortunately, the level of antidiuretic hormone (ADH) was not obtained during the course of treatment. The patient was started on oral doxycycline (100 mg every 12 h) for 10 days.
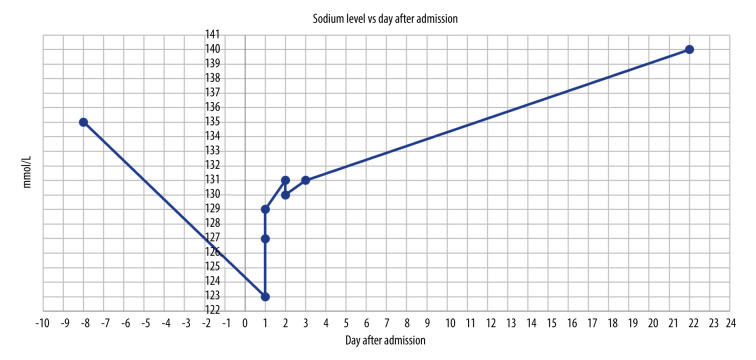
One day later, the patient was discharged as she became hemodynamically stable. She was next seen for an oncology follow-up 21 days after the discharge to discuss the option of restarting her mitotane therapy. Per the clinical notes, she reported a dramatic improvement of her symptoms, including the fever and the rash, except for some residual fatigue. Per laboratory results, her hyponatremia had been resolved completely (Figure 1).
Figure 1.
Patient’s serum sodium level before, during, and after admission. This graph shows the level of the patient’s sodium levels throughout her hospital stay from day 1 to day 3. The patient was discharged on day 3 and was seen again on day 22 for an oncology follow-up. Despite aggressive management of her adrenal insufficiency, her sodium level did not correct appropriately from day 1 to day 3. After being given doxycycline for RMSF on day 3, her sodium level started to improve steadily and returned to normal by the time she was followed up in the oncology clinic on day 22. The normal sodium level of 135 mmol/L on day -8 is her sodium level 8 days prior to the admission obtained by the oncology clinic and used as the baseline for sodium level.
Discussion
RMSF cases have risen steadily, from 495 cases in 2000 to a peak of 6248 cases in 2017, with 5 states (Arkansas, Missouri, North Carolina, Tennessee, and Virginia) accounting for over 50% of the total cases [3]. The etiologic agent for RMSF is Rickettsia rickettsii, which causes direct vascular injury that can manifest in 3 ways: (1) endothelial cells produce prostaglandins and increase vascular permeability [4]; (2) damaged endothelial cells can activate coagulation pathways and consume platelets and clotting factors; and (3) decreased effective circulating volume, caused by increased vascular permeability, triggers the kidney to release ADH as an appropriate response to hypovolemia [5].
As a result of the vascular injury, if treated late, RMSF can cause severe kidney injury or lead to potentially fatal complications, such as the development of severe cerebral edema [6]. The diagnosis of RMSF is empirically based on consistent clinical signs and an appropriate epidemiologic setting. Early treatment is preferred, and clinicians should not wait for the skin rash to develop before initiating treatment [7].
As an essential piece of social history, camping has been associated with the diagnosis of RMSF; however, our patient did not report any recent history of camping. The principal vector of RMSF in the eastern United States is Dermacentor variabilis, a dog tick [8]. Consequently, we need to raise clinical suspicion for RMSF when the patient lives in suburban, wooded areas with outdoor pets or even plays with neighbors’ outdoor pets. In addition to fever and tick exposure, the classic triad of RMSF also includes rash, which classically starts on extremities and progresses toward the trunk. However, many patients do not have the classic pattern for rash progression (including our patient) or the rash, especially as an initial presentation [9]. Other common manifestations of RMSF include nonspecific abdominal pain, cough, bleeding, edema, confusion, and neurologic signs [8,10]; typical laboratory findings in RMSF include hyponatremia, thrombocytopenia, coagulopathy, and increased liver enzymes [6].
However, the diagnosis of RMSF can be challenging even in areas with more frequent cases, especially when the clinical presentations of RMSF can resemble the signs and symptoms of a patient’s pre-existing conditions, which, in our case, was hyponatremia secondary to chronic iatrogenic adrenal insufficiency. When our patient presented in the Emergency Department, her adrenal insufficiency was conveniently believed to be the only cause of her hyponatremia. Further, the repeat urinalysis and non-elevated white blood cell count made an infectious process unlikely. However, it became clear later on that her adrenal insufficiency was not the sole contributor to the hyponatremia, as the sodium level failed to improve appropriately after aggressive i.v. fluid therapy and mineralocorticoid replacement therapy. After she was started on doxycycline, the patient showed a steady improvement of her sodium level, which returned to the normal (140 mmol/L) range upon follow-up. This confirms that an active infection of RMSF was partially responsible for the hyponatremia that the patient presented with.
The routine diagnosis of RMSF is by indirect fluorescent antibody (IFA) testing for the IgM and IgG antibodies to Rickettsia rickettsii. However, most patients need a sufficient amount of time to mount an antibody response after exposure. Additional time is required for IgG antibodies to appear owing to the need for class switching. A review article suggests that IgM titers remain positive for months after tick exposure and IgM titer alone is insufficient for diagnosing acute infection [11]. In all, IFA testing for IgG antibodies is more definitive for RMSF and can be negative during acute infection; additional testing should be done 1 to 6 weeks later to allow for a positive IgG titer study. In the meantime, empirical first-line treatment of doxycycline [12] should be initiated to stop the RMSF from progressing to more advanced stages because definitive laboratory testing can take several days to weeks. In 1 study of RMSF, the mortality was reported to drop from 22.9% if doxycycline was given later than 5 days of symptom onset to 6.5% when given within 5 days [9]. Our patient developed a rash on day 2 of her hospital stay, which eventually raised our clinical suspicion of drug reactions and infectious diseases and led to a decision for dermatological consultation. A thorough integumentary examination revealed the culprit tick in the left inframammary fold. The inoculation site of the tick bite in RMSF is often painless to begin with [1]; a concealed location as presented in our patient makes it even more prone to being overlook.
During an attempt to review the management of this patient, it was believed that obtaining and trending the level of ADH was indicated when the patient presented with a decreased level of sodium. This could be incorporated in the initial work-up of patients presenting with hyponatremia. It was also argued that being a cancer patient with glucocorticoid replacement made the patient prone to infectious disease; however, a literature review suggests that among 7738 cases of patients with RMSF, only 325 (4.2%) cases reported an immunosuppressive condition and only 33 (0.4%) cases were cancer, although immunosuppressed patients are 4.4 times as likely to die from RMSF [13].
Conclusions
In summary, RMSF is an empiric diagnosis based on clinical signs, symptoms, and appropriate epidemiologic settings for tick exposures. Early treatment with doxycycline is encouraged as evidence strongly suggests that early treatment is correlated with lower mortality. Symptoms (in our case, hyponatremia) that a patient’s pre-existing conditions can simultaneously cause should not be overlooked. If a patient fails to respond to treatment, the presenting signs should be examined holistically with other signs, symptoms, and laboratory findings. Last but not least, a thorough physical examination can often unveil more clues for the underlying cause of the patient’s disease. In our case, the culprit tick had always been on the patient’s skin and hiding in the left inframammary fold.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Thorner AR, Walker DH, Petri WA., Jr Rocky mountain spotted fever. Clin Infect Dis. 1998;27(6):1353–59. doi: 10.1086/515037. [DOI] [PubMed] [Google Scholar]
- 2.Regan J, Traeger M, Humpherys D, et al. Risk factors for fatal outcome from Rocky Mountain spotted fever in a highly endemic area – Arizona, 2002–2011. Clin Infect Dis. 2015;60(11):1659–66. doi: 10.1093/cid/civ116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Center for Disease Control Prevention . Rocky Mountain spotted fever (RMSF), Epidemiology and statistics. Centers for Disease Control and Prevention; [Cited 2021 Dec 6] Available from URL: https://www.cdc.gov/rmsf/stats/index.html. [Google Scholar]
- 4.Rydkina E, Sahni A, Baggs R, et al. Infection of human endothelial cells with spotted fever group rickettsiae stimulates cyclooxygenase 2 expression and release of vasoactive prostaglandins. Infect Immun. 2006;74(9):5067–74. doi: 10.1128/IAI.00182-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplowitz L. Hyponatremia in Rocky Mountain spotted fever: Role of antidiuretic hormone. Ann Intern Med. 1983;98(3):334–35. doi: 10.7326/0003-4819-98-3-334. [DOI] [PubMed] [Google Scholar]
- 6.Rhodes S, Teagarden A, Graner B, et al. Brain death secondary to Rocky Mountain spotted fever encephalitis. Case Rep Crit Care. 2020;2020:5329420. doi: 10.1155/2020/5329420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker D. Rocky Mountain spotted fever: A seasonal alert. Clin Infect Dis. 1995;20(5):1111–17. doi: 10.1093/clinids/20.5.1111. [DOI] [PubMed] [Google Scholar]
- 8.Sexton D, McClain M. Clinical manifestations and diagnosis of Rocky Mountain spotted fever. n.d. UpToDate. [Cited 2021 December 4], Available from URL: https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-rocky-mountain-spotted-fever.
- 9.Kirkland K, Wilkinson W, Sexton D. Therapeutic delay and mortality in cases of Rocky Mountain spotted fever. Clin Infect Dis. 1995;20(5):1118–21. doi: 10.1093/clinids/20.5.1118. [DOI] [PubMed] [Google Scholar]
- 10.Buckingham SC, Marshall GS, Schutze GE, et al. Tick-borne Infections in Children Study Group. Clinical and laboratory features, hospital course, and outcome of Rocky Mountain spotted fever in children. J Pediatr. 2007;150(2):180–4(184.e1). doi: 10.1016/j.jpeds.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 11.McQuiston J, Dunn J, Morris K, et al. Inadequacy of IgM antibody tests for diagnosis of Rocky Mountain spotted fever. Am J Trop Med Hyg. 2014;91(4):767–70. doi: 10.4269/ajtmh.14-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7(11):724–32. doi: 10.1016/S1473-3099(07)70261-X. [DOI] [PubMed] [Google Scholar]
- 13.Dahlgren F, Holman R, Paddock C, et al. Fatal rocky mountain spotted fever in the United States, 1999–2007. Am J Trop Med Hyg. 2012;86(4):713–19. doi: 10.4269/ajtmh.2012.11-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]