Abstract
Objective:
Symptomatic ureteric stones cause surrounding inflammation-promoting obstruction. C-reactive protein (CRP), white blood cell count (WC), and neutrophil percentage (NP) tend to rise after inflammatory response. Monitoring response during the course of medical expulsive therapy (MET) may help in deciding early intervention, thereby decreasing morbidity. We assessed the role and trends of these markers in predicting the outcome of MET.
Materials and Methods:
One hundred and ninety-two patients with distal ureteric calculus of size >5 mm were included in this prospective study from April 2017 to March 2018 after ethical committee approval. CRP, NP, and WC were measured on day 1, 7, and 14 of MET, and analysis was done.
Results:
On univariate analysis, stone size and mean values of CRP, WC, and NP on day 1, 7 and 14 in stone nonpassers were significantly higher compared to stone passers (P < 0.05). Receiver operator curve analysis showed area under the curve value of 0.798 (P = 0.001) for CRP and cut off value determined was 1.35 mg/dL. Multivariate analysis of different variables showed significant association of higher CRP (>1.35 mg/dL) and larger stone size (>7 mm) with MET failure. Decreasing trend of CRP was seen in both groups, but values were higher in stone nonpassers. WC and NP showed decreasing trend in stone passers but persistently high in stone nonpassers.
Conclusions:
Higher CRP and larger stone size were associated with failure of MET. WC and NP showed decreasing trend in stone passers and persistently higher in nonpassers, which may potentially predict failure of MET, however, their role need to be further studied.
Keywords: C-reactive protein, distal ureteric calculus, inflammatory markers, medical expulsive therapy, neutrophil percentage, total white blood cell count
INTRODUCTION
Worldwide incidence of Urolithiasis is as high as 20%, and the lifetime risk is reported to be 5%–12%.[1,2] Ureteral stones accounts for 20% of all urolithiasis and its prevalence ranges from 3% to 5%.[3,4] The prevalence of urolithiasis is in increasing trend leading to high economic burden to the world population.[5,6] The management of ureteral stone has options such as watchful waiting, medical expulsive therapy (MET), endoscopic removal, extra corporeal shock wave lithotripsy (ESWL), and surgical removal. The choice of treatment and the need for active intervention is still a dilemma in the management of ureteral stone.[7] The European Association of Urology (EAU) guidelines suggest MET with alpha blockers in medically stable, symptom controlled patients with distal ureteric stones >5 mm.[8]
Based on various studies, it has been found that spontaneous stone passage rate for distal ureteric stones to be 70%–75%. Spontaneous stone passage may take up to 4 weeks approximately, during which patient may develop recurrent colic or urinary tract infection increasing the morbidity.[9,10] Symptomatic ureteric stones usually cause surrounding inflammation and edema promoting obstruction.[11] Inflammatory markers like C-reactive protein (CRP), white blood cell count (WC), and neutrophil percentage (NP) were evaluated in various studies to assess their role in obstructing ureteric stones. Based on studies by Aldaqadossi and Park et al., high levels of CRP correlate with lower stone passage rates and need for early intervention.[12,13] Based on a study by Sfoungaristos et al., high white blood cell (WBC) count and NP suggest stone passage hypothesizing passage of stone cause ureteral wall inflammation compared to stones which remain static.[14] In contrast, a study by Park et al. showed low stone passage rates in patients with high NPs.[12] Furthermore, in a recent study by Abushamma et al., high WBC count and high CRP are significantly associated with early intervention during MET.[15] However, a study by Ahmed et al. did not show a significant association of WBC count and stone passage rate.[16] In the present study, we assessed the role of inflammatory markers (CRP, WC and NP) and their trends during MET for distal ureteric calculi of size >5 mm in predicting the outcome of therapy.
MATERIALS AND METHODS
Study population
Patients who were diagnosed to have distal ureteric calculus in our outpatient department and planned for MET as per guidelines from April 1st 2017, to March 31st 2018, were included consecutively in this study. We conducted this prospective study after approval from Institutional research committee (Approval number: A2-SBMR (2016–2017)/27717/2016/MCT) and Human Ethics Committee (Approval number: 05/32/2017/MCT). Our inclusion criteria were patients who were diagnosed to have distal ureteric calculus of size >5 mm based on noncontrast computed tomography (NCCT), willing for MET and further follow-up. Our exclusion criteria were patients who lost to follow-up, those already taken or undergoing MET, those with altered renal function test and those with documented active urinary tract infection or infection at any other sites clinically.
Study procedure
Patients who were fulfilling the criteria and consenting for the study were started on MET (alpha blocker-Tamsulosin 0.4 mg once daily for 4 weeks). CRP, WC and NP were measured on day 1, 7 and 14 of MET. Patients underwent NCCT to look for passage of stone or impaction at the end of 4 weeks of MET. Outcome of the study was defined as whether stone has passed or not after MET (stone passers or nonpassers). Data collected were analyzed and correlated with the outcome of MET. Trends of CRP, WC and NP during the course of MET were also analyzed for its association with the outcome.
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp. Univariate analysis was done using Chi-square test to compare the association of CRP, TC, and NP with the Stone passers (MET success) and nonpassers (MET failure). Independent t-test was done to compare the mean differences in CRP, WC and NP among stone passers and nonpassers. Multivariate Logistic regression was done and Odds ratio was calculated to assess the association of markers with the outcome. Receiver operator curve (ROC) analysis for area under the curve (AUC) values was done to derive the cut off values for CRP in MET failed patients (stone nonpassers). Repeated measures of ANOVA test was done to compare the mean difference in CRP, WC, and NP at different time intervals. “P” value is considered statistically significant if <0.05.
RESULTS
Flow diagram of study participants is shown in Figure 1. The median age of patients was 36 years (range 15–72). Mean size of calculus was 6.62 mm. Comparison of demographic data, stone characteristics, and inflammatory markers values between stone passers and nonpassers are mentioned in Table 1. After univariate analysis, stone size, CRP, WC, and NP were significantly higher in stone nonpassers compared to stone passers group (P < 0.05).
Figure 1.
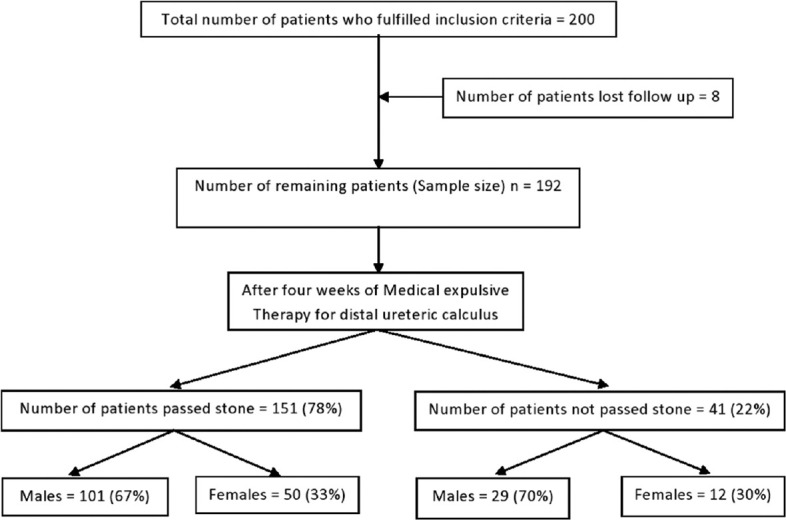
Flow diagram of study participants
Table 1.
Comparison of patient characteristics with the outcome of medical expulsive therapy by univariate analysis
| Characteristics (n=192) | MET successful (n=151) | MET failed (n=41) | P |
|---|---|---|---|
| Age (years), mean±SD | 37.68±12.11 | 36.97±10.29 | 0.42 |
| Sex (male/female), n (%) | 101 (66.8)/50 (33.2) | 29 (70.8)/12 (29.2) | 0.64 |
| Side of stone (left/right), n (%) | 74 (49)/77 (51) | 22 (53.6)/19 (46.4) | 0.59 |
| Size of stone (mm), mean±SD | 6.19±1.22 | 8.16±1.35 | 0.0001 |
| Mean value of CRP-day 1/days 7/days 14 of MET (mg/dl) | 3.13±2.73/1.37±1.87/0.31±0.51 | 8.56±7.02/7.04±5.47/6.88±4.99 | 0.0001 |
| Mean value of WC-day 1/days 7/days 14 of MET (cells/mm3) | 7231±2381/6380±1911/5942±1707 | 9814±2348/9554±1855/9645±1826 | 0.0001 |
| Mean value of NP-day 1/days 7/days 14 of MET (%) | 61±13/56±12/53±11 | 70±13/70±11/71±12 | 0.0001 |
Chi square test used for comparing the categorical variables (Age, gender and side of stone), Student t-test used to compare the mean values of size of stone, CRP, WC and NP. Total number of patients n=192, CRP: C-reactive protein, WC: White blood cell count, NP: Neutrophil percentage, SD: Standard deviation, MET: Medical expulsive therapy
ROC analysis was performed showing AUC value of 0.798 (P = 0.001) for CRP [Figure 2]. Cut off value provided by the analysis for CRP was 1.35 mg/dl with 97.6% sensitivity and 70.2% specificity. We could not arrive at a cut off value with optimal sensitivity and specificity for WC and NP after analysis. Univariate analysis of CRP revealed, failure of MET in 2.2% (1/46) patients with lesser CRP (≤1.35 mg/dl) compared to 27.4% (40/146) with CRP >1.35 mg/dl (P = 0.001). Univariate analysis of stone size showed, 6.3% (7/112) of patients with size ≤7 mm compared to 42.5% (34/80) with >7 mm failed to pass the stone (P = 0.001). Multivariate logistic regression analysis was done for study variables such as age (≤40 and >40), sex, side, stone size (≤7 mm and >7 mm), and CRP (≤1.35 mg/dl and >1.35 mg/dl). The results of multivariate analysis are summarized in Table 2. Larger stone size (P = 0.002) and higher CRP values (P = 0.001) were found to be statistically significant in predicting non passage of stone after MET.
Figure 2.
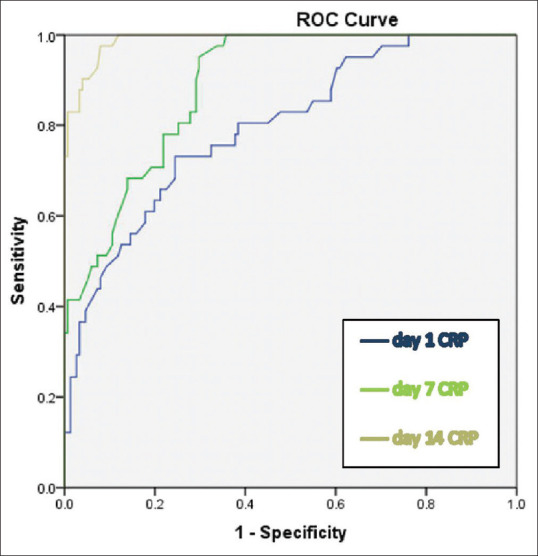
Receiver operator curve analysis for C-reactive protein on day1, 7 and 14 of Medical expulsive therapy
Table 2.
Multivariate analysis of factors predicting the success of medical expulsive therapy
| Variables | OR | 95% CI | P |
|---|---|---|---|
| Age | 3.456 | 0.433-27.566 | 0.242 |
| Sex | 2.127 | 0.322-14.061 | 0.434 |
| Side of stone | 0.571 | 0.099-3.287 | 0.530 |
| Stone size | 0.037 | 0.005-0.289 | 0.002 |
| CRP | 0.013 | 0.003-0.056 | 0.001 |
OR: Odds ratio, CI: Confidence interval, CRP: C-reactive protein
The trends of mean values of inflammatory markers (CRP, WC, NP) on Day 1, 7, and 14 of MET in stone passers and nonpassers were analyzed and represented as charts [Figure 3a-c]. In stone passers group, the mean CRP, WC, and NP values were significantly different between time points with Greenhouse–Geisser effect F values and P values-(F [142.833] P = 0.0005), (F [144.827] P = 0.0005) and (F [107.027] P = 0.0005) respectively, showing a statistically significant downward trend (P < 0.05). In stone nonpassers group, the mean CRP values were significantly different between time points with Greenhouse–Geisser effect F value (7.679) and P = 0.002, showing a statistically significant downward trend (P < 0.05). However, the CRP values remained at a higher level and did not decrease below the estimated cut-off of 1.35. The mean WC and NP values were not statistically different between time points with Greenhouse–Geisser effect F values and P values-(F [0.933] P – 0.375) and (F [1.797] P – 0.182) implying there was no significant fall or raise in WC and NP in stone nonpassers group (P > 0.05).
Figure 3.
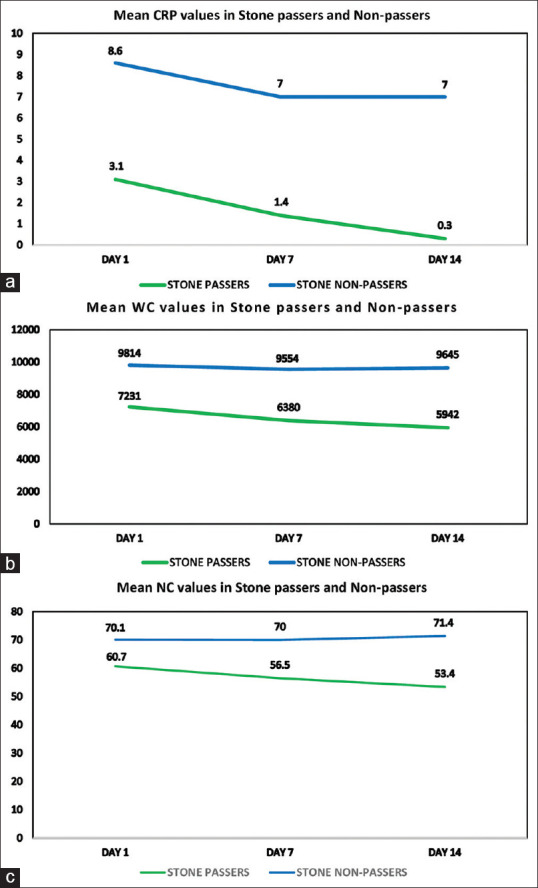
(a-c) Trends of Mean C-reactive protein (in mg/dl), White cell count, Neutrophil percentages among Stone passers and Stone non-passers on day 1, 7 and 14 of Medical expulsive therapy
DISCUSSION
Currently available treatments for ureteric stones are observation (with analgesics with or without adjuvant pharmacotherapy in facilitating spontaneous stone passage), ESWL, ureteroscopy (flexible or semi-rigid), percutaneous nephrolithotomy (PCNL), and open/minimally invasive surgery.[17] Two important factors which decide spontaneous passage of ureteral stones are stone size and location.[18] Ureteral stones <5 mm in any location (upper/mid/lower) have 75% chance of spontaneous passage. Chances of spontaneous passage decrease as stone size increases (60% passage rate for stone size of 5–7 mm, 48% for 7–9 mm and 25% for more than 9 mm). Spontaneous passage rates vary based on stone location (48% for proximal, 60% for mid, 75% for distal and 79% for vesicoureteric junction stones).[9] Spontaneous passage rates were studied by EAU/AUA panel based on recent meta-analysis and found to be 68% for <5 mm stones and 48% for 5–10 mm stones.[19] Observation for spontaneous passage is a good option for smaller ureteric stones with well controlled symptoms, normal renal function, and without infection.[20] Spontaneous passage after observation avoids unnecessary invasive procedure and financial burden to the patient.[21]
MET is described as the administration of pharmacological agent which aid in expulsion of ureteric stones. Various agents such as alpha blockers, calcium channel blockers, corticosteroids, and phosphodiesterase-5 (PDE-5) inhibitors have been studied extensively. Recent guidelines conclude alpha blockers as effective monotherapy for medical expulsion of ureteric stones, whereas calcium channel blockers, corticosteroids, and PDE-5 inhibitors lack adequate evidence to consider as monotherapy.[10] The current EAU guidelines recommend alpha blockers as MET for distal ureteric stones of size more than 5 mm based on the primary outcome of previous trials.[8] Most trials in the literature evaluated MET outcomes at 4 weeks’ period and no data are currently available to support other time intervals.
Apart from stone size and location predicting outcome of MET, various other radiological findings and laboratory values were studied. Based on a study by Lee et al., imaging parameters such as transverse stone diameter, longitudinal stone diameter, ureteral diameter (proximal to stone), ureter to stone diameter ratio are studied and its association with stone expulsion showed significant association with longitudinal stone diameter.[22] In a study by Cilesiz et al., high procalcitonin levels and leukocyturia showed negative effect on spontaneous stone passage suggesting the potential role of inflammatory markers monitoring during MET.[23] Another study by Selvi et al., hypothesized that higher Framingham score may be associated with noncalcium stones with higher stone volumes and functional impairment of ureteral peristalsis due to chronic inflammation in metabolic syndrome resulting in lower spontaneous stone passage rates.[24] In 1930, William Tillet first coined the term CRP, a protein that precipitated streptococcus pneumonia C-polysaccharide. Any inflammatory response stimulates synthesize of CRP from liver. Once the inflammation subsides, CRP values decrease rapidly. This response had been used clinically in various studies to predict infection and inflammation.[25] Inflammatory response caused in ureteric stone lead to elevation of CRP values, which can be used to predict stone passage rate and help in treatment decision.[12] White blood cell count and neutrophil, a component of White blood cells tend to rise in various conditions such as inflammatory response, bacterial infection, and myocardial infarction. We collected the values of inflammatory markers (CRP, WC, NP) at Day 1, 7, and 14 of MET and analyzed the data as well as the trend during the course of therapy. We hypothesized that interim analysis of inflammatory markers and their trend during MET may help in deciding whether to continue MET or plan for active intervention. Based on analysis of the data of 192 patients, males (130 out of 192 patients) had more incidence of ureteric calculus, similar to studies by Park et al. and Ahmed et al.[12,16] In our study, there was no significant difference in the stone expulsion rate between right and left sided stones (P = 0.59) similar to the studies by Puntub and Lerdpraiwan and Jain et al.[26,27] Miller and Kane reported the need of intervention was less likely in right sided stones.[18] In contrast, a study by Sfoungaristos et al., spontaneous passage rate was more on left-sided stones possibly due to no firm attachment of left ureter to parietal peritoneum.[7] The mean age of 37.5 years was comparable with previous studies (ranging from 36 to 46 years).[7,14,16,28] Analyzing age and gender as factors for predicting stone passage after MET were statistically insignificant (“P” =0.42 and 0.64, respectively) similar to previous studies.[26,27] In our study, 78.6% (151/192) patients with distal ureteric calculus of size more than 5 mm passed stone after 4 weeks of MET. Those patients who failed MET underwent ureterorenoscopy (URS). Intraoperative findings such as inflammatory changes in the mucosa surrounding the stone were noted and none had ureteric obstruction distal to the stone preventing spontaneous passage. Four meta-analyses[29,30,31,32] evaluating the outcome of MET with alpha blockers for distal ureteric calculus reported passage rates of 83%, 86.4%, 53%–90%, and 77%–90% respectively compared with our rate of 78.6%. Mean stone size in passers group was 6.19 mm compared to nonpassers group of 8.16 mm and the difference was statistically significant (“P” < 0.01).
Previous studies measured one or two inflammatory markers (CRP, WC, and NP) at the diagnosis of ureteric stones and used its association with spontaneous stone passage rate (7,12–14,16). Our methodology was to serially (day 1, 7, and 14 of MET) measure all three values and used for analysis to predict the outcome of MET which was not studied previously. By serially measuring these values, the inflammatory response caused by stone impaction or passage during the course of MET can be studied. The mean CRP values of day 1 were 3.13 (in stone passers) and 8.16 (in stone non passers) and the difference was statistically significant. Özcan et al. study also revealed high CRP level was significantly associated with nonexpulsion of distal ureteric calculi of size 4–10 mm with a cut off value of 0.506 mg/dl.[33] The finding was in concurrence with Aldaqadossi, Hada et al., and Jain et al. with their calculated CRP cutoff values of 2.19 mg/dl, 2.45 mg/dl, and 0.41 mg/dl, respectively.[13,26,34] In our study, the cut off value was 1.35 mg/dL. The low cut off value of CRP in Jain et al.'s study could be possibly due to the exclusion of the confounding factor of elderly age as CRP increases with age and their study population included patients <50 years of age.[26]
On analysis, the mean values of CRP, WC, and NP in stone passers showed serially decreasing trend over the course of MET possibly due to the resolution of ureteric mucosal inflammation after passage of stone. Although in stone nonpassers group, mean CRP showed decreasing trend, the values remained higher at all three time points compared to stone passers as shown in Figure 3a. Persistently elevated levels of CRP could be due to the continuing inflammation around the stone. Previous studies too support our findings that CRP level increases with inflammation in impacted stones and decreases once stone is passed out.[12,25] The mean values WC and NP in stone nonpassers did not show a significant increase or decrease over the course of MET but the values at all three time points were higher compared to stone passers, as shown in Figure 3b and c. These findings could possibly infer those stones with surrounding mucosal inflammation caused the persistently higher values of WC and NP.
On univariate analysis, all three inflammatory markers studied showed significant difference between stone passers and nonpassers, but we could not arrive at an optimal cut off values for WC and NP due to poor specificity. Hence, WC and NP were not included in the multivariate analysis. With the estimated cut off for CRP by ROC analysis, multivariate analysis showed significant association between high values and failure of MET. This finding was similar to previous studies by Park et al., Puntub and Lerdpraiwan, and Jain et al.[12,26,27] Hence, we propose CRP values can be used as a surrogate inflammatory marker to predict the outcome of MET. In contrast, a study by Shah et al. analyzed single initial values of CRP, WBC count, and neutrophil values in patients started on conservative management and found to have no association with spontaneous stone passage. However, this study has limitations such as retrospective nature and unmeasured confounding factors.[35] Furthermore, higher the stone size (>7 mm), lesser the chance of spontaneous stone passage similar to previous studies.[12,26,27,33]
Limitations and recommendations
Even though the mean values of WC and NP were higher in stone non-passers group compared to the other group, the values were within the normal reference range of the laboratory. This inferred the doubtful significance of WC and NP as inflammatory markers in predicting the outcome of MET. The observation of the trend of WC and NP at regular intervals during MET may assist in deciding to continue MET (downward trend) or proceed with early intervention (static/upward trend). However, there is no available literature for trend analysis and needs further studies to affirm this hypothesis.
CONCLUSIONS
Decision-making regarding when and whom to intervene early during MET to avoid complications is still confusing. Higher CRP and larger stone size were associated with failure of MET. No previous studies to our knowledge evaluated the trend of inflammatory markers during the course of MET. Our study revealed decreasing trend of CRP in both stone passers and nonpassers, but the values were higher in nonpassers. WC and NP showed decreasing trend in stone passers and persistently higher in nonpassers, which may potentially predict failure of MET, however, their role need to be further studied.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Picozzi SC, Marenghi C, Casellato S, Ricci C, Gaeta M, Carmignani L. Management of ureteral calculi and medical expulsive therapy in emergency departments. J Emerg Trauma Shock. 2011;4:70–6. doi: 10.4103/0974-2700.76840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johri N, Cooper B, Robertson W, Choong S, Rickards D, Unwin R. An update and practical guide to renal stone management. Nephron Clin Pract. 2010;116:c159–71. doi: 10.1159/000317196. [DOI] [PubMed] [Google Scholar]
- 3.Parmar MS. Kidney stones. BMJ. 2004;328:1420–4. doi: 10.1136/bmj.328.7453.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed AF, Al-Sayed AY. Tamsulosin versus alfuzosin in the treatment of patients with distal ureteral stones: Prospective, randomized, comparative study. Korean J Urol. 2010;51:193–7. doi: 10.4111/kju.2010.51.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int. 2003;63:1817–23. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 6.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–5. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sfoungaristos S, Kavouras A, Perimenis P. Predictors for spontaneous stone passage in patients with renal colic secondary to ureteral calculi. Int Urol Nephrol. 2012;44:71–9. doi: 10.1007/s11255-011-9971-4. [DOI] [PubMed] [Google Scholar]
- 8.Türk C, Knoll T, Seitz C, Skolarikos A, Chapple C, McClinton S, et al. Medical expulsive therapy for ureterolithiasis: The EAU recommendations in 2016. Eur Urol. 2017;71:504–7. doi: 10.1016/j.eururo.2016.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Coll DM, Varanelli MJ, Smith RC. Relationship of spontaneous passage of ureteral calculi to stone size and location as revealed by unenhanced helical CT. AJR Am J Roentgenol. 2002;178:101–3. doi: 10.2214/ajr.178.1.1780101. [DOI] [PubMed] [Google Scholar]
- 10.Türk C, Knoll T, Petrik A. EAU Guidelines on Urolithiasis. 2014. [Last accessed on 2018 Apr 04]. Available from: https://www.uroweb.org .
- 11.Crowley AR, Byrne JC, Darracott Vaughan E, Marion DN. The effect of acute obstruction on ureteral function. J Urol. 1990;143:596–9. doi: 10.1016/s0022-5347(17)40037-1. [DOI] [PubMed] [Google Scholar]
- 12.Park CH, Ha JY, Park CH, Kim CI, Kim KS, Kim BH. Relationship between spontaneous passage rates of ureteral stones less than 8 mm and serum C-reactive protein levels and neutrophil percentages. Korean J Urol. 2013;54:615–8. doi: 10.4111/kju.2013.54.9.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldaqadossi HA. Stone expulsion rate of small distal ureteric calculi could be predicted with plasma C-reactive protein. Urolithiasis. 2013;41:235–9. doi: 10.1007/s00240-013-0551-1. [DOI] [PubMed] [Google Scholar]
- 14.Sfoungaristos S, Kavouras A, Katafigiotis I, Perimenis P. Role of white blood cell and neutrophil counts in predicting spontaneous stone passage in patients with renal colic. BJU Int. 2012;110:E339–45. doi: 10.1111/j.1464-410X.2012.11014.x. [DOI] [PubMed] [Google Scholar]
- 15.Abushamma F, Ktaifan M, Abdallah A, Alkarajeh M, Maree M, Awadghanem A, et al. Clinical and Radiological predictors of early intervention in acute ureteral colic. Int J Gen Med. 2021;14:4051–9. doi: 10.2147/IJGM.S322170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed AF, Gabr AH, Emara AA, Ali M, Abdel-Aziz AS, Alshahrani S. Factors predicting the spontaneous passage of a ureteric calculus of ≤10 mm. Arab J Urol. 2015;13:84–90. doi: 10.1016/j.aju.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. J Nephrol. 2000;13(Suppl 3):S45–50. [PubMed] [Google Scholar]
- 18.Miller OF, Kane CJ. Time to stone passage for observed ureteral calculi: A guide for patient education. J Urol. 1999;162:688–90. doi: 10.1097/00005392-199909010-00014. [DOI] [PubMed] [Google Scholar]
- 19.Preminger GM, Tiselius HG, Assimos DG, Alken P, Buck C, Gallucci M, et al. 2007 guideline for the management of ureteral calculi. J Urol. 2007;178:2418–34. doi: 10.1016/j.juro.2007.09.107. [DOI] [PubMed] [Google Scholar]
- 20.Wood KD, Gorbachinsky I, Gutierrez J. Medical expulsive therapy. Indian J Urol. 2014;30:60–4. doi: 10.4103/0970-1591.124209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotan Y, Gettman MT, Roehrborn CG, Cadeddu JA, Pearle MS. Management of ureteral calculi: A cost comparison and decision making analysis. J Urol. 2002;167:1621–9. [PubMed] [Google Scholar]
- 22.Lee SR, Jeon HG, Park DS, Choi YD. Longitudinal stone diameter on coronal reconstruction of computed tomography as a predictor of ureteral stone expulsion in medical expulsive therapy. Urology. 2012;80:784–9. doi: 10.1016/j.urology.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Cilesiz NC, Ozkan A, Kalkanli A, Eroglu A, Gezmis CT, Simsek B, et al. Can serum procalcitonin levels be useful in predicting spontaneous ureteral stone passage? BMC Urol. 2020;20:1–6. doi: 10.1186/s12894-020-00608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvi I, Baydilli N, Tokmak TT, Akinsal EC, Basar H. CT-related parameters and Framingham score as predictors of spontaneous passage of ureteral stones≤10 mm: Results from a prospective, observational, multicenter study. Urolithiasis. 2021;49:227–37. doi: 10.1007/s00240-020-01214-6. [DOI] [PubMed] [Google Scholar]
- 25.Pepys MB, Baltz ML. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- 26.Jain A, Sreenivasan SK, Manikandan R, Dorairajan LN, Sistla S, Adithan S. Association of spontaneous expulsion with C-reactive protein and other clinico-demographic factors in patients with lower ureteric stone. Urolithiasis. 2020;48:117–22. doi: 10.1007/s00240-019-01137-x. [DOI] [PubMed] [Google Scholar]
- 27.Puntub A, Lerdpraiwan W. Relationship between the spontaneous passage rates of ureteral stones less than 10 mm and serum C-reactive protein levels, white blood cell count and neutrophil percentages. Insight Urol. 2018;39:42–9. [Google Scholar]
- 28.Moon YJ, Kim HW, Kim JB, Kim HJ, Chang YS. Distribution of ureteral stones and factors affecting their location and expulsion in patients with renal colic. Korean J Urol. 2015;56:717–21. doi: 10.4111/kju.2015.56.10.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amer T, Osman B, Johnstone A, Mariappan M, Gupta A, Brattis N, et al. Medical expulsive therapy for ureteric stones: Analysing the evidence from systematic reviews and meta-analysis of powered double-blinded randomised controlled trials. Arab J Urol. 2017;15:83–93. doi: 10.1016/j.aju.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye Z, Zeng G, Yang H, Tang K, Zhang X, Li H, et al. Efficacy and safety of tamsulosin in medical expulsive therapy for distal ureteral stones with renal colic: A multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2018;73:385–91. doi: 10.1016/j.eururo.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 31.Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, et al. Medical therapy to facilitate urinary stone passage: A meta-analysis. Lancet. 2006;368:1171–9. doi: 10.1016/S0140-6736(06)69474-9. [DOI] [PubMed] [Google Scholar]
- 32.Seitz C. Medical expulsive therapy of ureteral calculi and supportive therapy after extracorporeal shock wave lithotripsy. Eur Urol Suppl. 2010;9:807–13. [Google Scholar]
- 33.Özcan C, Aydoğdu O, Senocak C, Damar E, Eraslan A, Oztuna D, et al. Predictive factors for spontaneous stone passage and the potential role of serum C-reactive protein in patients with 4 to 10 mm distal ureteral stones: A prospective clinical study. J Urol. 2015;194:1009–13. doi: 10.1016/j.juro.2015.04.104. [DOI] [PubMed] [Google Scholar]
- 34.Hada A, Yadav SS, Tomar V, Priyadarshi S, Agarwal N, Gulani A. Assessment of factors affecting the spontaneous passage of lower ureteric calculus on the basis of lower ureteric calculus diameter, density, and plasma C- reactive protein level. Urol Ann. 2018;10:302–7. doi: 10.4103/UA.UA_89_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah TT, Gao C, Peters M, Manning T, Cashman S, Nambiar A, et al. Factors associated with spontaneous stone passage in a contemporary cohort of patients presenting with acute ureteric colic: Results from the Multi-centre cohort study evaluating the role of Inflammatory Markers In patients presenting with acute ureteric Colic (MIMIC) study. BJU Int. 2019;124:504–13. doi: 10.1111/bju.14777. [DOI] [PubMed] [Google Scholar]


