Abstract
People living with HIV are living longer, high-quality lives; yet as they age, this population is at increased risk for developing chronic comorbidities, including cardiovascular disease, certain types of cancer (e.g., lung, anal, liver), and diabetes mellitus. The purpose of this state of the science review is to provide an evidence-based summary on common physical comorbidities experienced by people living and aging with HIV. We focus on those chronic conditions that are prevalent and growing, and share behavioral risk factors that are common in people living with HIV. We will discuss the current evidence on the epidemiology, physiology, prevention strategies, screening, and treatment options for people living with HIV across resource settings.
Keywords: Cancer, Cardiovascular Disease, Diabetes Mellitus, HIV, Multimorbidity
Significant progress in the care and treatment of people living with HIV (PLWH) has transformed HIV into a chronic condition. As such, PLWH in every region of the world are currently living near normal lifespans if they are offered antiretroviral treatment and are adherent (Wandeler et al., 2016). While this progress should be celebrated, increased aging is also associated with increased risk for age-related chronic conditions, for which many PLWH are at higher risk (Friedman & Duffus, 2016).
The purpose of this review is to describe the current state of the science on common physical comorbidities experienced by people living and aging with HIV. We focus on those that are prevalent and growing, and share common risk factors, namely smoking, physical inactivity, poor dietary intake, and co-infections. We will discuss the current HIV-specific evidence on atherosclerotic cardiovascular disease, chronic obstructive pulmonary disease; lung, anal, and liver cancer; diabetes mellitus; and chronic kidney disease for PLWH across resource settings and will synthesize the clinical, research, and policy implications of these data.
Atherosclerotic Cardiovascular Disease
Atherosclerotic cardiovascular diseases (ASCVD; e.g., coronary artery disease, myocardial infarctions) have tripled in the past 20 years and are among the leading causes of hospitalizations, disability, and death among PLWH (Fleming et al., 2019; Hart et al., 2018; Shah et al., 2018). ASCVD are caused by a buildup of atherogenic plaque in the artery walls, resulting in coronary artery disease, angina, and myocardial infarctions. It is estimated that PLWH have a 1.5- to 2-fold increased risk of developing ASCVD (Feinstein et al., 2019). With improved treatment and care, the risk of myocardial infarction is decreasing (Klein et al., 2015), yet a recent analysis of a large U.S. dataset (i.e., MarketScan Commercial and Medicare datasets) found an approximately 1.3-fold increase in myocardial infarction, underscoring the need for primary prevention of ASCVD in PLWH (Alonso et al., 2019).
Factors Contributing to ASCVD Risk in PLWH
Increases in ASCVD in PLWH were first observed in the Data Collection on Adverse Events of Anti-HIV Drugs (DAD) study in the early 2000s. In this cohort, combination antiretroviral therapy (cART) was associated with an increased risk for myocardial infarction (Data Collection on Adverse Events of Anti-HIV Drugs [DAD] Study Group, 2003). Later, increased exposure to protease inhibitors (PIs) was implicated as a significant source of this excess risk (DAD Study Group, 2007; Nou et al., 2016) through metabolic side effects, such as dyslipidemia, lipodystrophy, and glucose intolerance. As cART has evolved to have fewer toxic cardiometabolic effects, many have investigated additional sources of increased ASCVD risk in PLWH and have increasingly focused on the role of inflammation.
Increased inflammation among PLWH persists, despite sustained HIV suppression, and is associated with accelerated development of atherosclerosis through vascular inflammation, endothelial dysfunction, hypercoagulability, and disrupted cholesterol transport and capacity (Hart et al., 2018; Nou et al., 2016). Biomarkers of systemic inflammation, including D-dimer, high sensitivity C-reactive protein (hsCRP), and interleukin 6 (IL-6), are among the most studied predictors of ASCVD in HIV and have consistently been associated with increased risk of clinical and subclinical ASCVD (Nou et al., 2016; Subramanya et al., 2019). While HIV public health initiatives, such as test-and-treat and the 90-90-90 goals, have helped to increase the number of PLWH on effective HIV medications, they have also shortened the time between HIV infection and HIV viral suppression. The truncated time of uncontrolled HIV viremia has helped to moderate the cardiometabolic consequences of HIV infection and inflammation, and may help to partially explain the decreasing rates of myocardial infarction in recent years.
While substantial progress has been made in mitigating HIV-specific risk factors for ASCVD, less progress has been made in reducing the traditional risk factors related to the development of ASCVD. Hypertension, hyperlipidemia, obesity, tobacco and other substance use, sedentary lifestyles, and poor diet (high in sodium, high in fat, low in fiber) are elevated in PLWH and are significant contributors to their ASCVD risk. In high resource settings, approximately 42% of PLWH have hypertension and 36% have hyperlipidemia (Olaiya et al., 2018; Wong et al., 2017). Rates of obesity among PLWH range from 8% to 25%, with higher rates of obesity in women, racial and ethnic minorities, and with initiation of some HIV medications (e.g., integrase inhibitors; Bailin et al., 2020; Lake, 2017). Lifestyle factors, including tobacco and other substance use, sedentary behaviors, and diet, influence both traditional ASCVD risk factors and the pathways between inflammation and atherosclerosis. Approximately 37% to 50% of PLWH currently smoke tobacco (Mdodo et al., 2015; Weinberger et al., 2017), 48% of PLWH report a substance use disorder, and 20% report polysubstance use (Hartzler et al., 2017). While the estimated effect of tobacco and substance use on ASCVD varies (Raposeiras-Roubín et al., 2017), there is consensus that they are strongly associated with ASCVD in PLWH, and tailored interventions to mitigate their use are needed. Physical activity can reduce ASCVD by promoting a favorable cholesterol profile by increasing HDL level and function, reducing triglycerides and LDL levels, and reducing insulin sensitivity chronic inflammation (Nystoriak & Bhatnagar, 2018). Yet, on average, only half of PLWH engage in the recommended 150 to 300 minutes of moderate to vigorous physical activity or 75 minutes of vigorous physical activity and 2 days of resistance training per week in accordance with the U.S. Department of Health and Human Services Guidelines. A recent review found PLWH have the lowest levels of physical activity when compared to individuals with other chronic illnesses (Vancampfort et al., 2018). Diets high in fiber and low in salt and processed sugars are associated with improved cardiovascular health. While less is known about dietary intake in PLWH than the other traditional ASCVD risk factors, the limited existing evidence suggests that their diet quality is poor (e.g., high calorie, added sugar, low fiber) and lower than those without HIV (Webel et al., 2017; Weiss et al., 2019).
Preventing CVD in PLWH
The most important CVD prevention strategy is to help PLWH start and adhere to an effective ART regimen as early in their HIV diagnosis as possible (Feinstein et al., 2019). This will minimize inflammation-induced damage to the myocardium and vasculature that may lead to ASCVD. Additionally, strong evidence suggests reducing hypertension (Casey et al., 2019), hypercholesterolemia (Grundy et al., 2019), hyperglycemia (Fox et al., 2015), and stopping all tobacco use (Barua et al., 2018) through non-pharmacological or pharmacological approaches are likely to lead to immediate improvements in ASCVD risk in PLWH (Arnett et al., 2019b); however, because of the potential for drug-drug interactions due to ART (e.g., between some protease inhibitors and lovastatin and simvastatin), non-pharmacological preventative strategies should be prioritized when possible. Additionally, engaging in physical activity, consuming a nutritious diet, and maintaining a healthy weight throughout the lifespan can also reduce the risk of ASCVD. While PLWH may not routinely engage with health care providers until later in their life, it is important to discuss, assess, and emphasize the importance of healthy lifestyle behaviors at each patient encounter. The physical activity recommendations for PLWH are similar to those without HIV infection - 150 to 300 minutes of moderate to vigorous physical activity or 75 minutes of vigorous physical activity per week and 2 days of strength training exercises (Montoya et al., 2019). Recent evidence demonstrated that PLWH mostly fail to meet these guidelines and that low physical activity is associated with increased CVD risk (Vancampfort et al., 2018; Willig et al., 2020). The Academy of Nutrition and Dietetics recently published HIV-specific guidelines for nutrition-related comorbidities (Willig, 2018). To mitigate ASCVD risk, they recommend eating foods rich in fiber, monounsaturated fats, vitamin D, calcium consisting mostly of vegetables, fruits and dairy products, and reducing the consumption of refined sugar, carbohydrates, and sodium (Willig et al., 2018). While dietary intake in PLWH has been less studied, limited data suggest that PLWH in the United States conform to approximately half the daily diet recommendations and consume diets high in refined sugars and carbohydrates (Webel et al., 2017; Weiss et al., 2019).
Clinical Tools to Screen for ASCVD in PLWH
Caring for PLWH with multimorbidity can be challenging. To reduce the overall burden on HIV and primary care providers, there are a number of screening tools that can be implemented in diverse clinical settings. Here we describe two free ASCVD tools that have a strong evidence base and are feasible to conduct during a patient encounter. Additional clinical tools can be found in Table 1.
Table 1.
Prevention, Assessment and Treatment Strategies for Chronic Comorbidities in HIV
| Chronic Condition | HIV Risk | Prevention Strategies | Monitoring/Assessment Strategies | Treatment Strategies | Structural Strategies |
|---|---|---|---|---|---|
| ASCVD | 1.5- to 2-fold greater risk | ↓ Hypertension, ↓Hypocholesteremia ↓ Hyperglycemia ↓ Weight  Smoking Smoking↑Physical activity ↑ Healthy diet |
|
|
Shift to collaborative care models to include cardiovascular specialist, pharmacist, registered dietician, physical therapy |
| COPD | ~10% higher risk |
 Smoking (consider using the 5 As) Smoking (consider using the 5 As)↓ exposure to environmental pollutants
|
|
|
Collaborative care models to include respiratory specialist, pharmacist, smoking cessation counselor Support community and workplace initiatives to reduce tobacco use (e.g., tobacco-free zones, tobacco/vaping taxes, age of purchase) |
| Lung Cancer | ~2-fold greater risk (Hernández-Ramírez et al., 2017) |
 Smoking (consider using the 5 As) Smoking (consider using the 5 As)↑Physical activity ↑ Healthy diet |
|
|
|
| Liver Cancer | 4-fold higher risk | ↓Injection drug use ↓Alcohol & tobacco use ↑Safe sex Administer Hepatitis B vaccine |
|
|
Collaborative care models to include hepatology, pharmacist, substance use/behavioral medicine specialist Support use of needle exchanges |
| Anal Cancer | 19-fold higher risk |
|
|
|
|
| Diabetes Mellitus | ~2-fold reduced risk in HIV, but growing (Herrin et al., 2016) | ↓ Hyperglycemia ↓ Weight ↑Physical Activity ↑ Healthy Diet |
|
|
Adopt collaborative care models to include endocrinology, nephrology, pharmacy, registered dietetics, physical therapy Support increased access to promote food security including SNAP |
Note. ASCVD – Atherosclerotic Cardiovascular Disease, COPD – Chronic Obstructive Pulmonary Disorder, 5 As – Ask, Assess, Advise, Agree, Assist, HPV – Human Papillomavirus, PLWH – People Living with HIV, SNAP - Supplemental Nutrition Assistance Program
Assisting patients to understand their risks is a key, but challenging, principle of shared decision making (Garcia-Retamero & Galesic, 2010; Webel et al., 2020). To simplify risk communication and the resultant decision-making, the American Heart Association and the American College of Cardiology have developed and calibrated their ASCVD risk estimator (Journal of the American College of Cardiology, 2020; Lloyd-Jones et al., 2017). For adults between the ages of 20 and 79 years, this tool can be used to help estimate the 10-year risk of an ASCVD event (e.g., myocardial infarction or stroke), forecast the potential impact of different interventions on that patient’s ASCVD risk, and facilitate shared clinician-patient decision making about ASCVD interventions. Focusing on the most salient and clinically available risk factors (e.g., age, sex, race, blood pressure, smoking status, and cholesterol), this calculator has been studied in PLWH with mixed findings. A large national study found that the ASCVD risk was underestimated in women living with HIV, and suggested the development and calibration of an HIV-specific function to improve estimates (Triant, 2020). Similarly, the Mayo Clinic Statin Choice Decision Aid™ can assist clinicians to help visualize their patient’s risk of ASCVD events and the change in risk due to a cholesterol-lowering intervention (Mayo Clinic, 2020). Unfortunately, this accessible tool does not yet account for blood-pressure-lowering medications or smoking cessation interventions.
The American Heart Association developed a second, more comprehensive tool, My Life Check™, to account for additional lifestyle factors influencing ASCVD (American Heart Association [AHA], 2020). In addition to age, sex, race, blood pressure, smoking status, and cholesterol, this tool assesses weight, diet, blood sugar, physical activity, and zip code. After completing the assessment, participants receive suggested interventions tailored to their unique risk, along with a number of tools to start improving those factors. While the individual components of My Life Check™ are derived from high-level evidence and embedded in clinical guidelines (Arnett et al., 2019a), there is less research on the use of the tool itself to effectively reduce cardiovascular risk in any population.
Treating ASCVD in PLWH
Treatment for ASCVD in PLWH resembles national guidelines for those without HIV with several notable exceptions. First, the most important factor in preventing and treating ASCVD in the context of HIV infection is helping the patient to achieve and maintain viral suppression as soon as possible after HIV infection (Feinstein et al., 2019). After that, an annual assessment of ASCVD and lifestyle optimization with routine follow-up (Figure 1) should be considered for all adults living with HIV, in every setting. While the lifestyle goals are the same for all PLWH throughout their lifespan (smoking cessation; maintaining a healthy weight; regular moderate-to-vigorous physical activity; and consuming a low salt, low sugar, low carbohydrate, high fiber diet), the strategies to achieve these goals must be tailored to the individual and their unique context. These strategies will need to be meaningful and available to the participant, creative, empathetic to their barriers, and dynamic to ensure continual growth and progress toward the individual’s goals. While some participants may respond to mHealth interventions, others may be more comfortable with cognitive behavioral theory-based interventions or social support interventions (Montoya et al., 2019). Nonetheless, given the strong relationships PLWH experience with their health care team (Dawson-Rose et al., 2016), routine assessment, follow-up, and supportive feedback by their health care providers are likely to underscore the importance of lifestyle optimization.
Figure 1. Pragmatic approach to atherosclerotic cardiovascular disease (ASCVD) risk assessment and prevention in treated HIV infection.
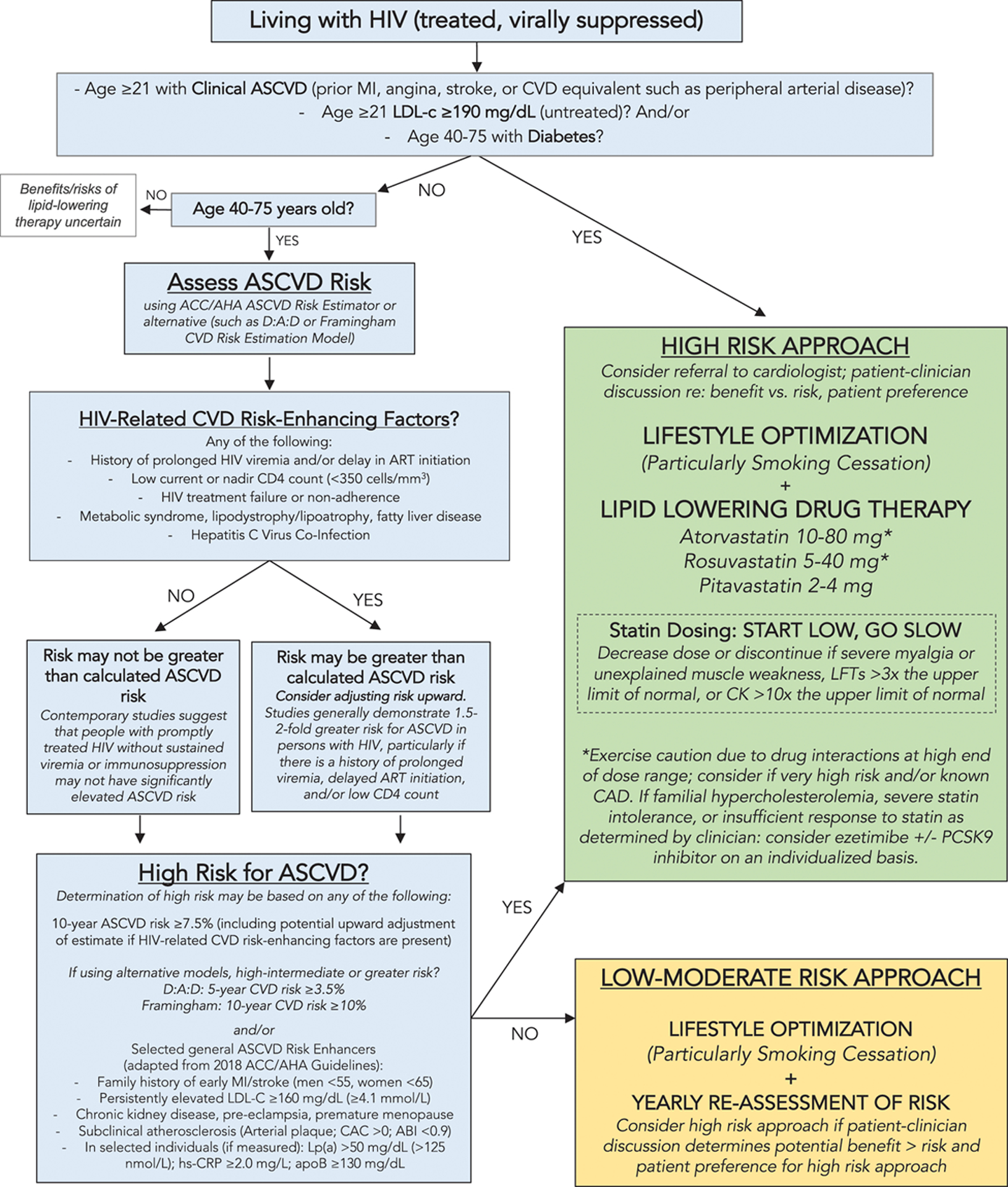
(Reprinted with permission Circulation [2019], 140:e98-e124 © 2019 American Heart Association, Inc.). This figure applies to people with controlled HIV. For people with uncontrolled HIV, the first priority is appropriate HIV therapy to achieve viral suppression. Thresholds based on findings of elevated CVD risk at current or nadir CD4 count <200, <350, and <500 cells/mm3 in Silverberg et al. (2014), Lichtenstein et al., 2010 and Triant et al., 2010. Hazard ratios and incidence rate ratios of 1.4 to 2.1 for myocardial infarction (MI) for people living with HIV (PLWH) vs uninfected people demonstrated in Freiberg et al., (2013) Triant et al., 2007 and Silverberg et al. (2014). Hazard ratio of stroke for PLWH vs. uninfected people was 1.40 in Chow et al. (2012).
Note. ABI indicates ankle-brachial index; ACC/AHA, American College of Cardiology/American Heart Association; apoB, apolipoprotein B; ART, antiretroviral therapy; CAC, coronary artery calcium; CAD, coronary artery disease; CK, creatine kinase; CVD, cardiovascular disease; D:A:D, Data Collection on Adverse Events of Anti-HIV Drugs; hs-CRP, high sensitivity C-reactive protein; LFT, liver function test; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein A; and PCSK9, proprotein convertase subtilisin-kexin type 9.
The second unique consideration for PLWH is the increased risk for their HIV antiretroviral medication to interact with common cardiovascular medications. While modern ART has a lower potential for drug-drug interactions compared with earlier generation medications, cardiovascular medications are common sources of these interactions (Courlet et al., 2019). To prevent adverse effects, each patient’s unique medical and medication history will need to be considered in the context of their health goals; however, there are some general guidelines that should be considered when prescribing cardiovascular medications. The lipid-lowering medication class, statins, are highly effective at reducing ASCVD, yet several of these medications are contraindicated with some ART, including simvastatin (when taking DRV/r, DRV/c, EVG/c, EFV) and lovastatin (when taking DRV/r, DRV/c, EVG/C, EFV). Atorvastatin, rosuvastatin, pravastatin, and pitavastatin can induce pharmacokinetic effects when taken with ART but have a lower risk for interactions. Using the lowest dose statin to reduce lipid levels is recommended (Devanathan et al., 2019). Additionally, calcium channel blockers (e.g., amlodipine, verapamil) can interact with DRV/r, DRV/c, EVG/c, EFV, and ETR, and may require dose adjustment (Devanathan et al., 2019). Additionally emerging evidence suggests that antiplatelet medications (e.g., clopidogrel, prasugrel) may interact with RTV and COBI (Bravo et al., 2018). Given the increasing complexity of ASCVD treatment in PLWH, working closely with a pharmacist and utilizing the University of Liverpool HIV Drug Interaction Checker can help minimize the risk of drug-drug interactions or adverse events (Liverpool, 2020).
As PLWH continue to age, they will not only be diagnosed with ASCVD but also with heart failure, stroke, and peripheral arterial disease. While these conditions share prevention and screening similarities with ASCVD, they have unique HIV-specific screening, prevention, and treatment considerations. These cardiovascular conditions were excluded from this review for the sake of parsimony, not to minimize their importance. To learn more about the incidence, prevention, and management of these conditions, we refer the reader to these recent reviews (Beckman et al., 2018; Nguyen et al., 2020; Sinha & Feinstein, 2020).
Chronic Obstructive Pulmonary Disease and HIV
Chronic obstructive pulmonary disease (COPD) has emerged as a major cause of morbidity in PLWH. COPD is characterized by progressive airflow limitation and persistent respiratory symptoms, including shortness of breath, cough, and sputum production (Vogelmeier et al., 2017). Although population-based estimates of the prevalence of COPD in the United States are lacking, it has been demonstrated that COPD is more prevalent in PLWH compared to those without HIV (OR 1.14, 95%CI, 1.05–1.25; Antoniou et al., 2020), with as much as 25% of PLWH estimated to have COPD (Lalloo et al., 2016); however, this may underestimate the true prevalence, as many PLWH remain undiagnosed due to the lack of adequate screening, especially among women.
Physiologically, COPD is characterized by small airway obstruction (e.g., obstructive bronchiolitis) and parenchymal destruction (e.g., emphysema). Although smoking is the major risk factor for the development of COPD, nearly 25% of patients with COPD report having never smoked (Labaki & Rosenberg, 2020). Other risk factors may include exposure to environmental pollutants and lung irritants (including secondhand smoke), and α−1 antitrypsin deficiency. Recently, HIV was identified as an independent risk factor for COPD, and this risk may be due to changes in immune function, direct effects of HIV, increased susceptibility to infection, and inflammatory factors (MacDonald et al., 2018).
Screening for COPD
Screening for COPD is recommended for anyone older than age 40 who has risk factors (e.g., smoking, respiratory symptoms). A diagnosis of COPD is made by conducting pulmonary function testing. Initially, forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) are measured, followed by calculation of the ratio of FEV1/FVC (Labaki & Rosenberg, 2020). In patients with COPD, the FEV1/FVC ratio is low (less than 0.70), demonstrating limited expiratory airflow. COPD may be classified based on the FEV1 percent predicted value, with FEV1 >80% predicted, FEV1 of 50–79% predicted, and FEV1 30–49% predicted, or FEV1 < 30% predicted, corresponding to mild, moderate, severe, or very severe, respectively. While a certain level of reduction in FEV1 is normal due to aging, people with COPD show a rapid decline (defined using a common cutoff of >40 mL/year), which is associated with respiratory disease and all-cause mortality (Makinson et al., 2018; Ronit et al., 2018).
While there are few clinical trials examining respiratory decline in PLWH, the Strategic Timing of ART Treatment (START) study, a large randomized clinical trial with patients from 20 countries, conducted a substudy that examined the effect of smoking on lung function and health status among PLWH (MacDonald et al., 2018). The START Pulmonary Substudy examined annual rate of FEV1 decline in more than 1,000 participants living with HIV over nearly 4 years (MacDonald et al., 2018). As expected, smokers had a significantly faster rate of FEV1 decline compared to nonsmokers. Importantly, there was no significant difference between continuous (daily) smokers and intermittent smokers, emphasizing the importance of smoking cessation for all PLWH regardless of their level of smoking.
Treating and Preventing COPD in HIV
Pharmacotherapy, in the form of respiratory inhalers, smoking cessation, pulmonary rehabilitation, administration of influenza and pneumococcal vaccines, and oxygen therapy (when needed) are the mainstays of treatment for patients with COPD, regardless of HIV status. Pharmacotherapy for COPD may include inhaled bronchodilators and corticosteroids.
Cigarette smoking is the most common and significant risk factor for COPD. Therefore, efforts to reduce smoking prevalence and improve smoking cessation among PLWH are critical. Please refer to an upcoming section on lung cancer prevention for a discussion of smoking cessation among PLWH.
Cancer among PLWH
Epidemiology in the United States and Around the Globe
As HIV has transitioned to a chronic illness, cancer has emerged as a leading cause of morbidity and mortality in PLWH (Ehren et al., 2014; Shiels et al., 2011). From 2011–2015, one in six deaths among PLWH in the United States was attributed to cancer (Horner et al., 2020), which exceeded cancer-related deaths in the general population for all age groups. Cancers in PLWH are typically divided into AIDS-defining cancers, such as Kaposi’s sarcoma, non-Hodgkin lymphoma, and cervical cancer, which may be associated with advanced immunosuppression, or non-AIDS–defining cancers (NADCs), such as lung, liver, and anal cancers, which may be associated with inflammation and certain risk behaviors (Engels, 2017). While combined data from national surveys demonstrated a decrease in the proportion of deaths attributed to AIDS-defining cancers between 2000 and 2010, the proportion of deaths attributed to NADCs increased significantly over the same period of time (Vandenhende et al., 2015). In the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) study, nearly 10% of deaths in PLWH were due to cancer (Engels, 2017).
Lung Cancer in PLWH
Factors contributing to increased lung cancer risk in PLWH.
Lung cancer has emerged as a leading cause of morbidity and mortality in the United States (Ehren et al., 2014; Shiels et al., 2011). Although lung cancer in PLWH may be related to the effects of chronic inflammation and immunomodulation (Sigel et al., 2016), the increased risk of lung cancer in PLWH is primarily due to the high prevalence of tobacco use. Smoking prevalence (~40%) is substantially higher in PLWH (Ledgerwood & Yskes, 2016) compared with the general population (15%; CDC, 2018) and is associated with increased rates of lung cancers (Clifford et al., 2012; Sigel et al., 2016; Sigel et al., 2012), and other malignancies (Antiretroviral Therapy Cohort, 2010; Niaura et al., 2000). In the era of cART, PLWH lose more years due to smoking than to HIV infection itself (Helleberg et al., 2015; Mamary et al., 2002; Reddy et al., 2016). One study found that the incidence rate of smoking-related cancers among PLWH smokers was more than five times higher compared with general population smokers (Helleberg et al., 2014). It has been estimated that at least 90% of lung cancers and 20% of all other cancers in PLWH could be prevented by eliminating smoking (Altekruse et al., 2018).
Smoking cessation and lung cancer prevention among PLWH.
Smoking cessation studies in PLWH have demonstrated disappointing outcomes, with low quit rates, poor adherence to therapy, and a lack of sustained abstinence (Cioe, 2013; Cui et al., 2012; Gritz et al., 2013; Ingersoll et al., 2009; Lloyd-Richardson et al., 2009; Matthews et al., 2013; Pacek & Cioe, 2015; Vidrine et al., 2012). Forty percent of smokers living with HIV express a willingness to quit (Benard et al., 2007) and two-thirds are interested in quitting when asked (Mamary et al., 2002). Pacek, Latkin, et al. (2014) found that among 267 PLWH who smoked, 74% were interested in quitting, 59% had used smoking cessation pharmacotherapy in the past, and 32% of those who hadn’t used smoking pharmacotherapy previously were willing to try pharmacotherapy if it were prescribed (Pacek, Latkin, et al., 2014). Although they desire to quit and are motivated to quit, PLWH are less likely to quit compared to smokers in the general population, and few are able to achieve long-term abstinence (Mdodo et al., 2015; Pacek, Harrell, et al., 2014; Pacek, Latkin, et al., 2014; Tami-Maury et al., 2013). On average, PWLH smoke heavily (15–19 cigarettes/day), are moderately or highly nicotine dependent (67%), and use multiple forms of tobacco, including cigars, pipes, and chewing tobacco (Shuter et al., 2012; Tami-Maury et al., 2013); all factors that reduce the success of smoking cessation attempts.
To date, tobacco cessation clinical trials in PLWH have included the use of a transdermal nicotine patch (Cropsey et al., 2013; Ingersoll et al., 2009; Lloyd-Richardson et al., 2009; Manuel et al., 2013; Moadel et al., 2012); varenicline, a prescription drug to treat nicotine addiction (Chew et al., 2014; Cropsey et al., 2015; Cui et al., 2012; Ferketich et al., 2013); cognitive behavioral therapy (Matthews et al., 2013); in-person or computer-based counseling; web-based interventions; and brief advice to quit. Quit rates and sustained abstinence were generally low (8% to 20%), and differences between intervention and control groups were often non-significant (Humfleet et al., 2013; Lloyd-Richardson et al., 2009; Moadel et al., 2012; Stanton et al., 2015; Vidrine et al., 2012). When examining factors associated with successful quitting, however, Stanton (2015) and Vidrine et al. (2015) found that self-efficacy for smoking cessation mediated the effect of the smoking cessation intervention; while Browning and colleagues (2016) concluded that adherence to treatment was positively associated with sustained abstinence (Browning et al., 2016; Stanton et al., 2015; Vidrine et al., 2015). In addition, de Dios and colleagues (2016) found that social support for smoking cessation was positively associated with nicotine patch adherence and indirectly associated with 7-day point prevalence abstinence. In a systematic review, Cooperman (2016) suggested that further research was needed to develop tailored interventions for PLWH that target adherence to smoking cessation pharmacotherapy, self-efficacy for quitting, and social support for smoking cessation (Cooperman, 2016).
Screening and treatment for lung cancer in PLWH.
Screening for lung cancer in PLWH does not differ from that of the general population (Sigel et al., 2017). The U.S. Preventive Services Task Force recommends annual screening for lung cancer with low-dose computed tomography (LDCT) in adults, ages 55 to 80 years, who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. The American Cancer Society has similar recommendations (Smith et al., 2019). Two clinical trials for lung cancer screening have been conducted with PLWH. In the first trial, adherence to study protocol was low and only one lung cancer was detected (Hulbert et al., 2014). In the second trial, adherence was better and lung cancer was detected in participants younger than 55 years of age (the recommended age for initial screening in smokers; Makinson et al., 2016).
Surgery is the standard of care for early stage lung cancer. The effect of HIV on lung cancer treatment tolerability, toxicity, and effectiveness is not well described; however, the prognosis for PLWH who develop lung cancer has been shown to be worse than uninfected persons (Sigel et al., 2017). It is not known if the poorer outcomes are related to lung cancer treatment disparities, decreased tolerance to treatment, increased risk of treatment toxicity, or competing risk from HIV-associated morbidities (Sigel et al., 2012). While few well-controlled studies exist, one study that compared PLWH to those without HIV found more surgical complications and poorer survival following surgery in those with HIV (Marcus et al., 2015). Clearly, a focus is needed on prevention, early detection, and adequate treatment of primary lung cancers in PLWH to improve overall outcomes.
Preventing Lung Cancer in PLWH.
Smoking cessation is the key focus of lung cancer preventive efforts for PLWH, due to their high prevalence of cigarette smoking. Nurses have an important role in prevention by implementing the recommended 5As in clinical settings (Fiore et al., 2008). The 5As describe an effective and brief intervention that is feasible in HIV clinical settings. It consists of (a) Ask every patient about smoking, (b) Advise each smoker to quit, (c) Assess readiness to quit, (d) Assist by offering medication and providing or referring for counseling, and (e) Arrange for follow-up to prevent relapse and promote success. There are seven first-line FDA-approved medications (5 forms of nicotine-replacement therapy [patch, lozenge, gum, inhaler, nasal spray] and 2 non-nicotine [varenicline, bupropion]) for smoking cessation that have demonstrated safety and efficacy for all smokers, including PLWH. A comprehensive approach to smoking cessation, including some form of pharmacotherapy with behavioral counseling, has been shown to produce the best outcomes (Fiore et al., 2008).
Liver Cancer in PLWH
Epidemiology in the United States and around the globe.
Liver cancer is the fourth most common NADC in the United States (Robbins et al. 2015; Horner et al., 2020). Hepatocellular carcinoma (HCC) is the most common histologic form of liver cancer accounting for approximately 75% of all diagnoses (Petrick et al., 2020). Incident HCC occurs more frequently in PLWH as compared to the general population andeceiving an AIDS diagnosis in particular is associated with a 4-fold increased risk of incident HCC (Sahasrabuddhe et al., 2012). The increased risk of HCC in PLWH is primarily due to their disproportionate prevalence of conditions that are associated with chronic liver disease (Sahasrabuddhe et al., 2012).. In the NA-ACCORD study, liver cancer accounted for 0.9% of deaths in PLWH between 1995–2009, second only to lung cancer as a proportion of NADC-related deaths (Engels, 2017).
Globally, liver cancer is the fourth leading cause of cancer mortality worldwide (Bray et al., 2018). It is estimated that 85% of HCC diagnoses occur in low-resource or middle-resource countries (Yang et al., 2019). Regional variations in liver cancer incidence and mortality are attributed to multiple factors, including differences in the incidence of conditions leading to chronic liver disease, availability of liver cancer screening programs, and access to treatment.
Factors Contributing to Liver Cancer Risk in PLWH.
HCC usually occurs against a background of oxidative stress and inflammation due to chronic liver disease. Regardless of the agent triggering chronic liver disease, liver cirrhosis is the most important predisposing risk factor for HCC. Pre-existing cirrhosis is present in 80% of individuals with an HCC diagnosis (Marrero et al., 2018).
The role of HIV mono-infection in the etiology of HCC remains unclear. Findings from the Veterans Aging Cohort Study provide the strongest evidence to date implicating HIV viremia as an independent contributing factor to HCC risk (Torgersen et al., 2020). In contrast, prior studies failed to establish relationships between HIV viremia and HCC (Kowalkowski et al., 2014; Kramer et al., 2015; Park et al., 2018). Mixed evidence on the associations between low absolute CD4+ T-cell counts and HCC comes primarily from studies on HIV and HCV or HBV co-infection (Bruyand et al., 2011; Gjærde et al., 2016).
In the United States, the most common causes of HCC are HCV infection, followed by alcohol-associated liver disease, non-alcoholic fatty liver disease (NAFLD), and HBV (Younossi et al., 2015). Less-prevalent conditions, such as hereditary hemochromatosis, primary biliary cholangitis, and Wilson’s disease have also been associated with HCC development. HCC incidence (Marrero et al., 2018) and clinical features (Piscaglia et al., 2016) differ according to the underlying condition. For the most common causes of HCC, we summarize information on prevalence and disease progression in the general population and, as available, in PLWH.
Chronic HCV infection is the most important cause of HCC in high-income countries including the United States. It is associated with a 10‐ to 20‐fold increased risk for HCC (McGlynn et al., 2020). HIV/HCV coinfection is associated with increases in overall cirrhosis risk (~2-fold increased risk) and with accelerated HCV disease progression as compared to HCV mono-infection (Graham et al., 2001; Thein et al., 2008). The annual incidence of HCC in people with HCV-related cirrhosis ranges from 1% to 8% (El-Serag & Kanwal, 2014). In the United States, the prevalence of HCV infection (past or present) is estimated at 1.7% for the general population (Hofmeister et al., 2019) and between 6% and 25% in PLWH (Bosh et al., 2018; Crowell et al., 2015; Kim et al., 2013; Prussing et al., 2015; Weber et al., 2010).
Alcohol-associated liver disease is a general term used to refer to a spectrum of alcohol-related liver injuries including fatty liver, alcoholic hepatitis, and cirrhosis. In the United States, one “drink” is defined as a beverage containing about 14 g of alcohol. A daily alcohol intake of 80 grams for ≥ 10 years increases HCC risk approximately 5-fold over that of nondrinkers (Morgan et al., 2004). The annual incidence of HCC in alcohol-associated cirrhosis ranges from 1–4% (Pocha & Xie, 2019). In the United States, the prevalence of alcohol-associated liver disease is estimated at 8% to 9% for the general population (Dang et al., 2020). There are limited data on disease prevalence in PLWH. In a cross-sectional analysis of PLWH who were Medicare beneficiaries between 2006 and 2016, the prevalence of alcohol associated liver disease was estimated at 2.3% (Dang et al., 2020); however, prevalence estimates of alcohol use disorder in PLWH (12% to 76%) appear to exceed that of the general population (Duko et al., 2019).
NAFLD, including its advanced form non-alcoholic steatohepatitis, has emerged as the leading cause of chronic liver disease globally (Younossi, 2019). The American Association for the Study of Liver Diseases (AASLD) defines NAFLD as the presence of ≥ 5% liver fat without evidence of hepatocellular injury in the form of hepatocyte ballooning in those without significant alcohol consumption (Chalasani et al., 2018). Non-alcoholic steatohepatitis is defined as the presence of ≥ 5% liver fat and inflammation with hepatocyte injury, with or without liver fibrosis in those without significant alcohol consumption (Chalasani et al., 2018). NAFLD is associated with diabetes mellitus, obesity, and metabolic syndrome, including in PLWH (Maurice et al., 2017). The role of HIV and ART in development of NAFLD continues to be investigated (Seth & Sherman, 2019). NAFLD is associated with a 2.6-fold increased risk of HCC (Younossi et al., 2015). The annual incidence of HCC in NAFLD cirrhosis is estimated to range between 1–3% (Marrero et al., 2018). Depending on the study, NAFLD prevalence in the United States ranges from 11% to 46% in the general population (Younossi et al., 2016) and between 23% and 73% in PLWH (Dang et al., 2020; Maurice et al., 2017).
Chronic hepatitis B infection (HBV) is the most important cause of HCC in Eastern Asian and most North African countries where HBV is endemic (Yang et al., 2019). Multiple case control studies have compared HCC risk in HBV infected and uninfected groups. Chronic HBV infection is associated with between a 5- and 103-fold increased risk of developing HCC (McGlynn et al., 2020). There is strong evidence indicating that hepatitis B viral load is directly correlated with liver disease progression and the risk of HCC (Iloeje et al., 2006). HIV/HBV coinfection is associated with higher hepatitis B viral loads and accelerated HBV disease progression. The annual incidence of HCC in HBV cirrhosis is estimated at 3% to 8% (Marrero et al., 2018). The most recent estimates of HBV infection in the United States are 4.2% in the general population (Shing et al., 2020), and 5.3% in PLWH (Leumi et al., 2020).
Preventing Liver Cancer in PLWH.
Nurses have an essential role in HCC prevention, which is largely focused on preventing and treating HCV and HBV infections, alcohol-associated liver disease, and NAFLD. Individuals at risk for HCV infection should receive counseling on injection hygiene and safe-sex practices that can prevent transmission. For those with HIV/HCV coinfection, treatment with direct-acting antiviral agents in multiple clinical trials resulted in sustained virologic response rates of ≥ 95%, irrespective of genotype and cirrhosis status (Naggie et al., 2015; Rockstroh et al., 2015; Rockstroh et al., 2018; Wyles et al., 2017). Achieving a sustained virologic response significantly reduces the risk of developing HCC (Ioannou et al., 2018). Nursing care coordination and case management can facilitate linkage to HCV infection treatment (Starbird et al., 2020) and treatment completion (Sherbuk et al., 2019).
HIV nurses can also integrate screening, brief intervention, and referral to treatment (SBIRT) for hazardous alcohol use into their clinical routines (Finnell et al., 2014). AASLD recommends that all patients should be routinely screened for alcohol use using validated questionnaires (Lucey et al., 2020). The U.S. Preventive Services Task Force recommends use of 1- to 3-item instruments, including the Alcohol Use Disorders Identification Test-Concise (AUDIT-C) or the National Institute of Alcohol Abuse and Alcoholism recommended Single Alcohol Screening Question (SASQ) as preliminary screening tools. (U.S. Preventive Services Taskforce, 2018). Positive screenings should be followed by screening with an instrument with greater sensitivity and specificity (e.g., AUDIT) to confirm hazardous alcohol use. Pharmacotherapy and referral to treatment are indicated for all individuals engaged in hazardous drinking. In the context of ALD, alcohol abstinence continues to be the mainstay of treatment. The effectiveness of abstinence can be enhanced with behavioral interventions that can be delivered by nurses, social workers, and other members of the HIV care team (Addolorato et al., 2016; Scott-Sheldon et al., 2017; Singal et al., 2018).
The American Gastroenterological Association recommends that patients with NAFLD cirrhosis receive counseling on abstaining from alcohol and tobacco use (Loomba et al., 2020). Optimal management of diabetes, dyslipidemia, and obesity using pharmacologic approaches and lifestyle modification is indicated for those with NAFLD and advanced liver fibrosis (Loomba et al., 2020).
Finally, vaccination can effectively prevent HBV infection and is recommended for all PLWH without evidence of past or current HBV infection (Schillie et al., 2018). All individuals with HIV/HBV coinfection should receive an antiretroviral therapy (ART) regimen that includes 2 drugs with activity against HBV: tenofovir (TAF or TDF) plus lamivudine or emtricitabine (Panel on Antiretroviral Guidelines for Adults and Adolescents, 2019; Terrault et al., 2018). If tenofovir (TAF or TDF) cannot be safely used, entecavir with a fully suppressive ART regimen may be used as an alternative. The goal of HBV treatment is to suppress hepatitis B viremia, thereby preventing progression of liver disease. Discontinuation of anti-HBV drugs should be avoided due to the risk of hepatitis flares (Dore et al., 2010). Treatment is continued indefinitely with monitoring of virologic response (HBV DNA levels) as part of routine medical care.
Clinical tools to Screen for Liver Cancer in PLWH.
Screening for HCC in PLWH does not differ from that of the general population. The goal of screening is to reduce HCC-related mortality by diagnosing the malignancy earlier in the disease course. Decisions to initiate HCC surveillance are guided by cirrhosis status and underlying HCC risk factors. AASLD recommends HCC screening every 6 months for all adults with cirrhosis, and certain populations at higher risk of HCC (without evidence of cirrhosis), using ultrasonography with or without alpha-fetoprotein (AFP) laboratory tests (Marrero et al., 2018). Patients with a lesion ≥ 1 cm on ultrasound or AFP > 20 ng/mL should undergo diagnostic evaluation with a multiphasic CT or MRI. The American Gastroenterological Association recently published similar guidance, but additionally recommended screening for those with NAFLD and evidence of advanced fibrosis (Loomba et al., 2020).
Treating liver cancer in PLWH.
HCC treatment is categorized into curative and non-curative treatment options. Treatment selection is based on multiple factors including (but not limited to) tumor stage and cirrhosis status. Early-stage HCC is amenable to curative treatment. Curative treatment approaches include local ablation, surgical resection, or liver transplantation. Noncurative treatment approaches may be used to slow tumor progression and include transarterial chemoembolization, transarterial radioembolization, stereotactic body radiation therapy, and systemic chemotherapy. The effect of HIV on HCC treatment tolerability, toxicity, and effectiveness is not well described; however, in general, the prognosis for PLWH who develop HCC has been shown to be worse than for persons without HIV (Pinato et al., 2019; Torgersen et al., 2020). In the United States, 5-year HCC survival is estimated at 10% in the general population (Golabi et al., 2017). Early detection HCC is essential to maximize survival.
Anal Cancer and HIV
Epidemiology of anal cancer among PLWH in the United States and around the globe.
Anal cancer has emerged as a common malignancy in PLWH, ranking third in incidence among NADCs (Horner et al., 2020). It is relatively rare in the general population, accounting for 0.5% of all new cancer diagnoses, and fewer than 9,000 cases annually in the United States (National Cancer Institute, 2020). Although multiple epidemiological studies reported increases in U.S. anal cancer rates between 1997–2007 (Colón-López et al., 2018; Islami et al., 2017; Nelson et al., 2013), incidence rates have declined since 2008 (Shiels et al., 2018). PLWH have a 19-fold increased risk of incident anal cancer as compared to the general population (Colón-López et al., 2018). The highest incidence occurs in men who have sex with men (MSM) living with HIV, and in people with an AIDS diagnosis.
Globally, anal cancer trends differ by country income status, with increases in incidence reported through 2007 in Australia, Canada, Denmark, France, Italy, the Netherlands, and the United Kingdom (Islami et al., 2017). As in the United States, it is a relatively rare diagnosis, ranking thirtieth among all new cancer cases, and thirty-first in deaths (Bray et al., 2018). Women are disproportionately affected, representing 58% of all new anal cancer diagnoses in 2018 (Bray et al., 2018).
Factors contributing to increased anal cancer risk in PLWH.
There is strong evidence implicating persistent human papilloma virus (HPV) infection as a cause of anal squamous cell carcinoma (ASCC) — the dominant histologic type of anal cancer (Islami et al., 2017). Although more than 100 HPV types have been identified (Tong et al., 2014), HPV types 16 and 18 have the greatest oncogenic potential. These high-risk HPV types are detected in anal tissues in approximately 90% of ASCC cases (Martel, 2017; Alemany et al., 2015; Saraiya et al., 2015). Behaviors that increase the risk of HPV acquisition (e.g., number of sexual partners, anal intercourse) are commonly associated with increased anal cancer risk (Wasserman et al., 2017).
Most adults acquire HPV at some point in their lives, but the virus is usually cleared spontaneously. Living with HIV effectively doubles the risk of HPV acquisition and halves the rate of HPV clearance as compared to not living with HIV (Looker et al., 2018). Suppressive ART appears to be important for reducing HIV persistence and, therefore, reducing anal cancer risk (Kelly et al., 2020). In a recent meta-analysis, those receiving ART had a 35% lower high-risk HPV prevalence than individuals who were ART naïve, and ART users with a sustained undetectable HIV viral load had a 44% reduced risk of anal cancer compared to those without (Kelly et al., 2020).
Other risk factors associated with HPV persistence and anal cancer include conditions associated with immunosuppression (e.g., with use of immunosuppressive therapies following organ transplantation; Larsen et al., 2020) and to a lesser extent smoking (Wasserman et al., 2017). The latter is of particular significance given the high prevalence of tobacco use that is observed in PLWH. (We refer the reader to the section on lung cancer above.)
Preventing anal cancer in PLWH.
Recommended strategies for primary anal cancer prevention include HPV vaccination, behavioral strategies to reduce HPV exposure (e.g., condom use), smoking cessation, and treatment of pre-cancerous squamous intraepithelial lesions (SIL). These lesions are classified as low-grade squamous intraepithelial lesions (LSIL) or high-grade squamous intraepithelial lesions (HSIL) based on their malignancy potential (Roberts et al., 2017). Nurses are well-positioned to counsel patients on these strategies and to administer appropriate vaccinations. The Advisory Committee on Immunization Practices now recommends catch-up vaccinations for all people through age 26 years, regardless of HIV status (Meites et al., 2019). The 9-valent HPV vaccine (Guardasil-9) is the only FDA-approved vaccine currently available in the United States. It provides protection against high-risk HPA types that are implicated in the development of anal cancer. The HPV vaccine is FDA-approved for prevention, but not therapeutic use.
The Centers for Disease Control and Prevention (CDC) strongly recommend use of male latex condoms for preventing transmission or acquisition of HPV infection in PLWH (Panel on Opportunistic Infections in Adults and Adolescents with HIV, 2019); however, patients should be counseled that HPV can still be transmitted by skin to skin contact with HPV-infected areas of the body that are not covered by a condom. Smoking cessation is broadly recommended for PLWH. Those with or at high-risk for anal cancer should be counseled on its association with both primary anal cancer and recurrence.
Currently, topical imiquimod, fluorouracil, trichloroacetic acid and cidofovir, and ablative therapies are recommended options for the treatment of LSIL or HSIL (Stewart et al., 2018). Comparisons of the various treatment protocols are limited. One ongoing study, the Anal Cancer HSIL Outcomes Research (ANCHOR) trial (NCT02135419), aims to determine whether screening and treatment of HSIL is effective in reducing subsequent anal cancer in PLWH compared with active monitoring (including anal cytology combined with high resolution anoscopy [HRA] and biopsy of any concerning lesions).
Screening tools for the detection of anal cancer.
Data on the benefits of routine anal cancer screening are not yet definitive, and there are currently no national recommendations for routine anal cancer screening in PLWH.. In the United States, the New York State Department of Health AIDS Institute recommends routine anal cancer screening using digital rectal examination and anal cytology for MSM living with HIV, cisgender women, transgender women, and transgender men beginning at age 35 (New York State Department of Health AIDS Institute, 2020). The goal of anal dysplasia screening is to identify and treat HSIL before they progress to irreversible high-grade lesions or local invasive disease (New York State Department of Health AIDS Institute, 2020). The CDC acknowledge that some specialists recommend anal cytology for PLWH, but recommend that it only be considered if there is access to HRA services (Panel on Opportunistic Infections in Adults and Adolescents with HIV, 2019). This is because abnormal cytology results require follow-up with direct visualization of the anal canal using HRA and biopsy of suspicious lesions.
Pending definitive randomized trials, a recent observational study provides some evidence for the benefit of structured screening programs using anal cytology in PLWH. In a single-center retrospective analysis of 3,111 PLWH receiving outpatient HIV care, participation in an annual anal cancer screening program was associated with a significant reduction in invasive ASCC incidence (HR, 0.20; 95% CI, .04–.97; Revollo et al., 2020). The median length of follow-up was 4.6 years in the screening group and 4.8 years in the non-screening group (Revollo et al., 2020).
Treating anal cancer.
Treatment modalities for ASCC may include radiation therapy, chemotherapy, excision, or combined modalities. Survival rates at five years exceed 80% in early stage cancer, with worsening survival in later stages (Leeds, 2016). Recurrence is common and optimal surveillance regimens have yet to be established. Data are mixed on whether PLWH experience greater treatment toxicities than those living without HIV, although overall survival appears to be similar (Bryant et al., 2018).
Diabetes and Chronic Kidney Disease among People with HIV
Epidemiology of Diabetes Mellitus in the United States and Around the Globe
Globally, it is estimated that 8.8% of adults have diabetes mellitus, and that number is expected to increase to 10.4% by 2040 (Ogurtsova et al., 2017). Developing countries may experience an increase in diabetes as high as 20% over the next 10 years (Cho et al., 2018). The prevalence of diabetes in PLWH in the United States is approximately 12% (Kalra et al., 2011), compared to a prevalence of 10.5% in the general population (CDC, 2020), and a global prevalence of 8.5% (Emerging Risk Factors Collaboration, 2010). A similar pattern of slightly increased prevalence has been reported in other parts of the world, including Sub-Saharan Africa and Asia (Kagaruki et al., 2014). As the prevalence of diabetes continues to increase worldwide (Emerging Risk Factors Collaboration, 2010), it can be assumed that the prevalence in PLWH will also increase (Kalra et al., 2011).
Diabetes mellitus physiology and its impact on PLWH.
Diabetes is a group of metabolic disorders manifested in the body’s ability to produce and/or use insulin at the cellular level. The causes and risk factors for diabetes type 2 in PLWH are similar to those without HIV: aging, obesity, genetic disposition, and inflammation (Sarkar & Brown, 2019). Obesity leads to increased levels of circulating insulin which, if prolonged, can lead to insufficient production of insulin (Van Greevenbroek et al., 2013). PLWH are at slightly higher risk for developing diabetes, which is associated with a longer time living with HIV, chronic low levels of inflammation, oxidative stress, and mitochondrial damage caused by HIV treatment (Blas-Garcia et al., 2011; Brown et al., 2010; Tingstedt et al., 2019). For PLWH, an additional diagnosis of diabetes decreases life expectancy (Park et al., 2019). Poorly controlled diabetes leads to end organ damage, including kidney damage, due to the deleterious effects of excess glucose on the vascular system (Gregg et al., 2016). In one study of PLWH (N = 10,043), the only participants with acute kidney disease were those also diagnosed with diabetes (Park et al., 2019).
As Black, indigenous populations, and people of color (BIPOC) are over-represented in PLWH, racism can negatively affect diabetes mellitus-related health outcomes in these populations by inducing chronic stress and inequities in health care systems (Harrell et al., 2011). Notably, higher rates of discrimination have been associated with higher levels of glucose (Wagner et al., 2015). Accordingly, BIPOC experience worse diabetes-related health outcomes, such as amputations, when compared to the White population (Osborn et al., 2013). As disparities continue, health care providers need to actively advocate for equitable diabetes screening and medical treatment.
Preventing and screening for diabetes in PLWH.
People at risk for developing diabetes should be encouraged to control their weight, eat a low carbohydrate diet, and exercise. Losing a small amount of weight (5%−7%) and increasing weekly exercise decreases the risk of diabetes by over 50% (Tabák et al., 2012; CDC, 2018). As time on HIV treatment is a non-modifiable variable associated with the development of diabetes, close monitoring and early treatment would be appropriate in this population.
Screening tools include laboratory tests of hemoglobin A1C (A1C) and fasting glucose. Diabetes is diagnosed with an A1C greater than or equal to 6.5% or an abnormal oral glucose tolerance test (OGTT), which, while more sensitive, is less commonly used in the clinical setting for adults (Olson et al., 2010). For PLWH, A1C may be artificially lower due to ART-induced hemolysis that decreases the lifespan of hemoglobin cells (Slama et al., 2014). In contrast, the sensitivity of the OGTT to detect diabetes is not affected by HIV medications (Gianotti et al., 2011).
Treating diabetes in PLWH.
The introduction of new oral diabetes medications have improved glycymic control in people with diabetes. Current diabetes treatment recommendations for PLWH include an initial oral anti-diabetes medication (such as metformin) with the addition of a second medication if treatment goals are not obtained (such as a sulfonylurea, a thiazolidinedione, a sodium-glucose cotransporter-2 [SGLT-2] inhibitor, or a dipeptidyl peptidase – 4 [DPP-4] inhibitor; Qaseem et al., 2017). In one study of PLWH with diabetes, participants who were using insulin to manage glucose were more likely to reach A1C goals than those on oral anti-diabetes medications (ZuÑiga et al., 2016); however, it is important to note that many patients prefer oral over injectable treatment (Qaseem et al., 2017).
Recommendations for glucose self-monitoring vary from a few times a day to continuous monitoring, depending upon use and intensity of insulin treatment (American Diabetes Association, 2017). Barriers to monitoring include reluctance to test in a work place, cost, and stigma (Ong et al., 2014). Among PLWH, stigma may be a greater barrier due to concerns of exposing co-workers who are aware of the HIV status to a blood-borne pathogen.
Diets need to be based on patients’ personal preferences and cultural norms to promote sustained adherence (MacLeod et al., 2017). Interventions that include carbohydrate counting have shown efficacy for decreasing A1C (MacLeod et al., 2017). For PLWH with diabetes mellitus, a low carbohydrate diet can be compatible with the need to consume vitamin-rich foods to support the immune system (Botros et al., 2012).
Food insecurity can interfere with diabetes management. In non-HIV-infected populations, food insecurity has been associated with lower rates of glucose self-monitoring (Seligman et al., 2010), perhaps due to the price of testing strips and competing financial priorities (Gucciardi et al., 2014). Food insecurity may be alarmingly high among PLWH, with one study reporting a prevalence of 71% among adults living in a resource-rich setting (Anema et al., 2011). Food insecurity may also affect HIV treatment outcomes as it has been associated with decreased antiretroviral adherence and incomplete viral suppression (Weiser et al., 2013).
Diabetes and HIV in low- and middle-income countries.
Diabetes has become a global epidemic, driven by a combination of Western diet and sedentary lifestyle, with cases projected to reach 438 million by 2030 (Emerging Risk Factors Collaboration, 2010). Rates of diabetes among PLWH in low- to middle-income countries (LMIC) have been difficult to assess because treatment is often siloed and there may be no screening for chronic conditions. In some LMIC, food insecurity among PLWH may be as high as 90% (Benzekri et al., 2015). Successfully managing, supporting, and monitoring for the development of diabetes will be key for decreasing morbidity and mortality. Patient-centered interventions that are tailored to culture and personal preferences, combined with addressing social determinants of health, will improve patients’ attainment of diabetes goals.
Epidemiology of chronic kidney disease in the United States and around the globe.
One potential consequence of poorly managed diabetes is chronic kidney disease (CKD). Approximately 10–20% of PLWH have an additional diagnosis of CKD, presenting with proteinuria and reduced glomerular filtration rate (GFR; Park and ZuÑiga, 2018). While it is one of the most common causes of HIV-related morbidity and mortality (Adedeji et al., 2015; Kim et al., 2011; Szczech et al., 2004), less than 1% of PLWH (1.9 per 1,000 patients) with CKD will progress to end stage renal disease (ESRD), a reversible stage of kidney disease that requires dialysis (Bickel et al., 2013).
Unlike many other chronic conditions, CKD is often a negative sequela of other chronic, nephrotoxic conditions, such as hypertension, diabetes, or hepatitis. Additional risk factors include older age, Black race, and injection drug use; high CD4+ T cell counts are protective (Bickel et al., 2013; Bonjoch et al., 2014). Black PLWH are at higher risk for developing CKD than non-Hispanic, White PLWH due to a combination of predisposing genetic polymorphisms and health care inequities, such as delayed referrals (Norton et al., 2016). For all PLWH, an additional risk factor is use of nucleoside reverse transcriptase inhibitors (NRTI) which can damage renal tubules and reduce kidney function; however, the viral suppressive benefits of NRTI may outweigh the risk of developing CKD (Scherzer & Shlipak, 2015).
Prevalence of CKD in LMIC has not been well-characterized due to a paucity of studies in these regions (Stanifer et al., 2018). In LMIC, the prevalence of CKD in PLWH ranges between 4.7% to 12.3%, which is similar to people without HIV (Ekrikpo et al., 2018; Neuen et al., 2017; Stanifer et al., 2016). As Westernized diets become more common and obesity is increasing in LMIC, the rates of CKD are increasing in tandem with diabetes (Neuen et al., 2017). Additional risk factors for CKD in LMIC include poor sanitation and waste disposal, and heavy environmental pollution (Stanifer et al., 2016); in one systematic review, CD4+ T cell count and ART did not significantly contribute to the development of CKD (Ekrikpo et al., 2018).
Screening, prevention and treatment of CKD in PLWH.
Early identification of those at risk for CKD is critical to preserving kidney function in PLWH. Screening labs include eGFR <60 mL/min/1.73 m2 and/or presence of protein in the urine (Park & Zuniga, 2018). Kidney function is monitored using albuminuria, which is flagged for concern when the albumin/creatine ratio is ≥30 mg/g. A first morning urine catch is the more effective method for screening for CKD than a random urine test (Saydah et al., 2013). Mocroft and colleagues (2015) developed a CKD risk assessment score based on 13 traditional and HIV-related risk factors, that can help identify patients who are not good candidates for certain nephrotoxic HIV medications, such as tenofovir disoproxil fumarate.
The cornerstone for CKD prevention and treatment is the management of chronic conditions that lead to CKD. Limited access to nephrology care is associated with progression to end stage renal disease, which is frequently common in LMIC; linkage to care and referral to specialists will improve outcomes for PLWH (Gillespie et al., 2015; Lucas et al., 2014).
If CKD progresses to ESRD, patients may require renal replacement therapy, dialysis, or renal transplantation. Dialysis for PLWH does not require isolation or precautions above standard precautions. It is recommended that PLWH have an arterial venous fistula placed for vascular access instead of peripheral or subclavian catheters to reduce the risk for a central line associated blood-stream infection. It is recommended that ART dosages be reduced due to the nephrotoxicity, although this does not apply to medications excreted by extra-renal routes (Trullas et al., 2011). NRTI are filtered from the blood during dialysis, therefore, they must be administered after completion of dialysis (Diana & Naicker, 2016).
In 2013, the U.S. Congress passed, and President Obama signed, the HIV Organ Policy Equity Act, authorizing PLWH to donate organs, including kidneys. PLWH are eligible for renal transplant in most countries; however, in addition to the normal restrictions, PLWH must also have a CD4+ T cell count over 200 cells/ml3, no neoplasms or immune reconstitution, no opportunistic infections in the previous year, and viral suppression (Trullas et al., 2011). Post-transplant, PLWH need to be monitored for potential drug-drug interactions between immunosuppressive medications and ART (Lucas et al., 2014) and for changes in viral load (Trullas et al., 2011). PLWH show a higher rate of rejection and mortality compared to those without HIV (Stock et al., 2010).
Intersections
It is rare that aging PLWH experience just one additional comorbidity (Friedman & Duffus, 2016; Kim et al., 2012). Increasingly, PLWH experience multimorbidities characterized by shared pathways that can interact and lead to the development of other conditions (Wong et al., 2017). For example, increased weight and a poor diet can lead to hypertension and diabetes, vascular disease, and ultimately kidney disease. While prevention of conditions through the non-pharmacological mitigation of these shared pathophysiological pathways is the ideal clinical approach, PLWH often face many challenges to early prevention. If pharmacotherapy is used to treat one or more of these chronic conditions, it is imperative that the clinician recognize the increased risk of polypharmacy–related adverse events in this population (Courlet et al., 2019; Devanathan et al., 2019), particularly as they age. Working with a pharmacist to recognize and reduce these potential drug-drug interactions and adverse events is a critical strategy to maintaining the health and well-being of PLWH confronting multiple comorbidities.
Globally, PLWH often confront social challenges that increase their probability of developing multiple chronic health conditions. Poverty, low socioeconomic position, institutional racism, homophobia, ageism, and sexism can intersect throughout the lifespan to create conditions (e.g., discrimination, segregation, increased domestic work, gender norms that discourage physical activity, sexual abuse) that increase risk for many of the chronic conditions we reviewed (Caceres et al., 2019; Chrisler et al., 2016; O’Neil et al., 2018; Walsemann et al., 2016; Williams et al., 2019) and for HIV.
Preventing and mitigating these deeply ingrained societal conditions is complex, but also necessary to optimize the health of PLWH. As nurse clinicians, scientists, educators, and activists, we are uniquely positioned at the patients’ bedsides, in their communities, and due to the growth of virtual spaces, in their homes, to do what we do best. Assess and ask explicitly, but gently, about poverty, discrimination, sexual and other forms of abuse, and then listen to the patient, student, or the participant (Hardeman et al., 2018). While there are few evidence-based treatments for societal ills, the trauma-informed care model can be a guide when working with individuals (Purkey et al., 2018). At a structural level, we can work with our professional associations and local communities to learn more about these injustices and act to change the policies and systems that perpetuate them.
Finally, as aging PLWH are increasingly diagnosed with chronic conditions, it will be important to re-visit patients’ goals for their care. Simply asking them what matters the most to them now can facilitate shared-decision making around the treatment plan. Each encounter is a chance to update their goals of care and, if the patient is willing, to complete advance directives. An individual’s preferences can evolve throughout the aging process and a candid conversation about what matters to them is important. This can also be an opportune time to discuss socioeconomic challenges that may limit patients’ abilities to achieve their own health goals and strategize ways to overcome those challenges.
Conclusions
Nurses will increasingly encounter PLWH who are also living with one, or multiple, chronic physical comorbidities. Providing excellent, patient-centered care to this growing population will require nurses in all settings to recognize the unique risk HIV poses to the development of these conditions and to understand how to prevent, screen, and potentially treat these often intersecting conditions. While our review provides a summary of this current evidence, it is also clear that the best care will need to be delivered by a coordinated multidisciplinary team. In many settings, HIV care has led the way in detection, treatment, adherence, and self-management – all of which requires a multidisciplinary, egalitarian team approach. We believe that the HIV community comprising nurses, providers, researchers, and advocates can do the same for chronic comorbidity care.
KEY CONSIDERATIONS.
People living with HIV are aging and are at elevated risk for developing multiple chronic health conditions, including cardiovascular diseases, COPD, cancers, chronic kidney disease, and diabetes mellitus.
Similar to HIV, each chronic health condition requires its own prevention, screening, and treatment management strategies; yet these strategies often interact with the others, putting PLWH at risk for suboptimal chronic disease management.
Nurse clinicians, scientists, educators, and activists are uniquely positioned at the patients’ bedsides, in their communities, and increasingly in their homes to help mitigate the complex societal and structural challenges that increase risk for these chronic health conditions.
By adopting multidisciplinary, collaborative care and incorporating evidence-based findings, teams may more effectively address the growing multimorbidity burden in the growing population of people aging with HIV.
Funding Information:
The work was funded, in part, by the National Institutes of Health (NR018391, U01HL142099, AG066562; PI: A. Webel). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: The authors report no real or perceived vested interests related to this article that could be construed as a conflict of interest.
Compliance with Human Subjects: This is a state of the science review and no human subjects were involved in this manuscript. As such, IRB approval was not sought or obtained.
Contributor Information
Allison Webel, Associate Professor of Nursing, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA, and Associate Editor, Journal of the Association of Nurses in AIDS Care..
Julie Schexnayder, Frances Payne Bolton School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA..
Patricia A. Cioe, Behavioral and Social Sciences, School of Public Health, Brown University, Providence, Rhode Island, USA..
Julie A. Zuñiga, School of Nursing, University of Texas at Austin, Austin, Texas, USA..
REFERENCES
- Addolorato G, Mirijello A, Barrio P, & Gual A (2016). Treatment of alcohol use disorders in patients with alcoholic liver disease. Journal of Hepatology, 65(3), 618–630). 10.1016/j.jhep.2016.04.029 [DOI] [PubMed] [Google Scholar]
- Adedeji TA, Adedeji NO, Adebisi SA, Idowu AA, Fawale MB, & Jimoh KA (2015). Prevalence and Pattern of Chronic Kidney Disease in Antiretroviral-I Patients with HIV/AIDS. Journal of the International Association of Providers of AIDS Care (JIAPAC), 2325957415587570. doi.org/ 10.1177/2325957415587570 [DOI] [PubMed] [Google Scholar]
- AHA. (2020). My Life Check. Retrieved from https://mlc.heart.org
- Alemany L, Elle Saunier M, Alvarado-Cabrero I, Quir Os B, Salmeron J, Shin H-R, Pirog EC, Uria Guimer N, Hernandez-Suarez G, Felix A, Clavero O, Lloveras B, Kasamatsu E, Goodman MT, Hernandez BY, Laco J, Tinoco L, Geraets DT, Lynch CF, … De Sanjos S (2015). Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. International Journal of Cancer, 136, 98–107. 10.1002/ijc.28963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. (2017). Standards of medical care in diabetes—2017 abridged for primary care providers. Clinical diabetes: a publication of the American Diabetes Association, 35(1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Barnes AE, Guest JL, Shah A, Shao IY, & Marconi V (2019). HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. Journal of the American Heart Association, 8(14), e012241. doi: 10.1161/JAHA.119.012241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altekruse SF, Shiels MS, Modur SP, Land SR, Crothers KA, Kitahata MM, … Engels EA (2018). Cancer burden attributable to cigarette smoking among HIV-infected people in North America. AIDS, 32(4), 513–521. doi: 10.1097/qad.0000000000001721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anema A, Weiser SD, Fernandes KA, Ding E, Brandson EK, Palmer A, … Hogg RS (2011). High prevalence of food insecurity among HIV-infected individuals receiving HAART in a resource-rich setting. AIDS Care, 23(2), 221–230.DOI: 10.1080/09540121.2010.498908. [DOI] [PubMed] [Google Scholar]
- Antiretroviral Therapy Cohort C (2010). Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis, 50(10), 1387–1396. doi: 10.1086/652283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou T, Yao Z, Raboud J, & Gershon AS (2020). Incidence of chronic obstructive pulmonary disease in people with HIV in Ontario, 1996–2015: a retrospective population-based cohort study. CMAJ Open, 8(1), E83–E89. doi: 10.9778/cmajo.20190028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, … Ziaeian B (2019a). 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 140(11), e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, … Ziaeian B (2019b). 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 140(11), e563–e595. doi: 10.1161/CIR.0000000000000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailin SS, Gabriel CL, Wanjalla CN, & Koethe JR (2020). Obesity and Weight Gain in Persons with HIV. Current HIV/AIDS Reports, 17(2), 138–150. doi: 10.1007/s11904-020-00483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua RS, Rigotti NA, Benowitz NL, Cummings KM, Jazayeri M-A, Morris PB, … Wiggins BS (2018). 2018 ACC Expert Consensus Decision Pathway on Tobacco Cessation Treatment. A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents, 72(25), 3332–3365. doi: 10.1016/j.jacc.2018.10.027 [DOI] [PubMed] [Google Scholar]
- Beckman JA, Duncan MS, Alcorn CW, So-Armah K, Butt AA, Goetz MB, … Freiberg MS (2018). Association of Human Immunodeficiency Virus Infection and Risk of Peripheral Artery Disease. Circulation, 138(3), 255–265. doi: 10.1161/CIRCULATIONAHA.117.032647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard A, Bonnet F, Tessier JF, Fossoux H, Dupon M, Mercie P, … Chene G (2007). Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care STDS, 21(7), 458–468. doi: 10.1089/apc.2006.0142 [DOI] [PubMed] [Google Scholar]
- Benzekri NA, Sambou J, Diaw B, Sall F, Niang A, Ba S, … Seydi M (2015). High prevalence of severe food insecurity and malnutrition among HIV-infected adults in Senegal, West Africa. PLOS ONE, 10(11), e0141819. doi: 10.1371/journal.pone.0141819.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel M, Marben W, Betz C, Khaykin P, Stephan C, Gute P, … Brodt H (2013). End‐stage renal disease and dialysis in HIV‐positive patients: observations from a long‐term cohort study with a follow‐up of 22 years. HIV Medicine, 14(3), 127–135. doi: 10.1111/j.1468-1293.2012.01045 [DOI] [PubMed] [Google Scholar]
- Blas-Garcia A, Apostolova N, & V Esplugues J (2011). Oxidative stress and mitochondrial impairment after treatment with anti-HIV drugs: clinical implications. Current pharmaceutical design, 17(36), 4076–4086. DOI: 10.2174/138161211798764951. [DOI] [PubMed] [Google Scholar]
- Bonjoch A, Juega J, Puig J, Pérez-Alvarez N, Aiestarán A, Echeverria P, … Bonet J (2014). High prevalence of signs of renal damage despite normal renal function in a cohort of HIV-infected patients: evaluation of associated factors. AIDS Patient Care and STDs, 28(10), 524–529. Retrieved from http://online.liebertpub.com/doi/abs/10.1089/apc.2014.0172?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub%3Dpubmed [DOI] [PubMed] [Google Scholar]
- Bosh KA, Coyle JR, Hansen V, Kim EM, Speers S, Comer M, Maddox LM, Khuwaja S, Zhou W, Jatta A, Mayer R, Brantley AD, Muriithi NW, Bhattacharjee R, Flynn C, Bouton L, John B, Keusch J, Barber CA, … Hall HI (2018). HIV and viral hepatitis coinfection analysis using surveillance data from 15 US states and two cities. Epidemiology and Infection, 146(7), 920–930. 10.1017/S0950268818000766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botros D, Somarriba G, Neri D, & Miller TL (2012). Interventions to address chronic disease and HIV: strategies to promote exercise and nutrition among HIV-infected individuals. Current HIV/AIDS Reports, 9(4), 351–363. DOI: 10.1007/s11904-012-0135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo I, Álvarez H, Mariño A, Clotet B, & Moltó J (2018). Recurrent coronary disease in HIV-infected patients: role of drug-drug interactions. Br J Clin Pharmacol, 84(7), 1617–1619. doi: 10.1111/bcp.13583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 68(6), 394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, & McComsey GA (2010). Association between systemic inflammation and incident diabetes mellitus in HIV-infected patients after initiation of antiretroviral therapy. Diabetes care. DOI: 10.2337/dc10-0633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KK, Wewers ME, Ferketich AK, Diaz P, Koletar SL, & Reynolds NR (2016). Adherence to Tobacco Dependence Treatment Among HIV-Infected Smokers. AIDS Behav, 20(3), 608–621. doi: 10.1007/s10461-015-1059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyand M, Dabis F, Vandenhende MA, Lazaro E, Neau D, Leleux O, Geffard S, Morlat P, Chêne G, & Bonnet F (2011). HIV-induced immune deficiency is associated with a higher risk of hepatocarcinoma, ANRS CO3 Aquitaine Cohort, France, 1998–2008. Journal of Hepatology, 55(5), 1058–1062. 10.1016/j.jhep.2011.02.017 [DOI] [PubMed] [Google Scholar]
- Bryant AK, Huynh-Le MP, Simpson DR, Gupta S, Sharabi AB, & Murphy JD (2018). Association of HIV status with outcomes of anal squamous cell carcinoma in the era of highly active antiretroviral therapy. JAMA Oncology, 4(1), 120–122. 10.1001/jamaoncol.2017.2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres BA, Markovic N, Edmondson D, & Hughes TL (2019). Sexual Identity, Adverse Life Experiences, and Cardiovascular Health in Women. Journal of Cardiovascular Nursing, 34(5), 380–389. doi: 10.1097/jcn.0000000000000588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey DE, Thomas RJ, Bhalla V, Commodore-Mensah Y, Heidenreich PA, Kolte D, … Ziaeian B (2019). 2019 AHA/ACC Clinical Performance and Quality Measures for Adults With High Blood Pressure: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circulation: Cardiovascular Quality and Outcomes, 12(11), e000057. doi: 10.1161/HCQ.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2018). Current Cigarette Smoking Among Adults in the United States. Retrieved from https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm
- Centers for Disease Control Prevention. (2020). National diabetes statistics report, 2020. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services. https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, & Sanyal AJ (2018). The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology, 67(1), 328–357. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- Chew D, Steinberg MB, Thomas P, Swaminathan S, & Hodder SL (2014). Evaluation of a Smoking Cessation Program for HIV Infected Individuals in an Urban HIV Clinic: Challenges and Lessons Learned. AIDS Res Treat, 2014, 237834. doi: 10.1155/2014/237834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N, Shaw J, Karuranga S, Huang Y, da Rocha Fernandes J, Ohlrogge A, & Malanda B (2018). IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes research and clinical practice, 138, 271–281. DOI: 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012; 60:351–358. doi: 10.1097/QAI.0b013e31825c7f24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrisler JC, Barney A, & Palatino B (2016). Ageism can be Hazardous to Women’s Health: Ageism, Sexism, and Stereotypes of Older Women in the Healthcare System. Journal of Social Issues, 72(1), 86–104. doi: 10.1111/josi.12157 [DOI] [Google Scholar]
- Cioe P (2013). Smoking Cessation Interventions in HIV-Infected Adults in North America: A Literature Review. J Addict Behav Ther Rehabil, 2(3), 1000112. doi: 10.4172/2324-9005.1000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford GM, Lise M, Franceschi S, Egger M, Bouchardy C, Korol D, … Schoni-Affolter F (2012). Lung cancer in the Swiss HIV Cohort Study: role of smoking, immunodeficiency and pulmonary infection. Br J Cancer, 106(3), 447–452. doi: 10.1038/bjc.2011.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-López V, Shiels MS, Machin M, Ortiz AP, Strickler H, Castle PE, Pfeiffer RM, & Engels EA (2018). Anal cancer risk among people with HIV infection in the United States. Journal of Clinical Oncology, 36(1), 68–75. 10.1200/JCO.2017.74.9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell TA, Berry SA, Fleishman JA, LaRue RW, Korthuis PT, Nijhawan AE, Moore RD, & Gebo KA (2015). Impact of hepatitis coinfection on healthcare utilization among persons living with HIV. Journal of Acquired Immune Deficiency Syndromes, 68(4), 425–431. 10.1097/QAI.0000000000000490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo Clinic. (2020). Statin Choice Decision Aid. Retrieved from https://statindecisionaid.mayoclinic.org/index.php/statin/index
- Cooperman N (2016). Current research on cigarette smoking among people with HIV. Current Addiction Reports, 3, 19–26. doi: 10.1007/s40429-016-0090-2 [DOI] [Google Scholar]
- Courlet P, Livio F, Guidi M, Cavassini M, Battegay M, Stoeckle M, … Study SHC (2019). Polypharmacy, Drug–Drug Interactions, and Inappropriate Drugs: New Challenges in the Aging Population With HIV. Open forum infectious diseases, 6(12). doi: 10.1093/ofid/ofz531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Hendricks PS, Jardin B, Clark CB, Katiyar N, Willig J, … Carpenter MJ (2013). A pilot study of screening, brief intervention, and referral for treatment (SBIRT) in non-treatment seeking smokers with HIV. Addict Behav, 38(10), 2541–2546. doi: 10.1016/j.addbeh.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropsey KL, Jardin BF, Burkholder GA, Clark CB, Raper JL, & Saag MS (2015). An Algorithm Approach to Determining Smoking Cessation Treatment for Persons Living With HIV/AIDS: Results of a Pilot Trial. Journal of acquired immune deficiency syndromes (1999), 69(3), 291–298. doi: 10.1097/qai.0000000000000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Robinson L, Elston D, Smaill F, Cohen J, Quan C, … Smieja M (2012). Safety and tolerability of varenicline tartrate (ChaIx((R))/Intix((R))) for smoking cessation in HIV-infected subjects: a pilot open-label study. AIDS Patient Care STDS, 26(1), 12–19. doi: 10.1089/apc.2011.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang K, Hirode G, Singal AK, Sundaram V, & Wong RJ (2020). Alcoholic liver disease epidemiology in the United States. The American Journal of Gastroenterology, 115(1), 96–104. 10.14309/ajg.0000000000000380 [DOI] [PubMed] [Google Scholar]
- Dawson-Rose C, Cuca YP, Webel AR, Solís Báez SS, Holzemer WL, Rivero-Méndez M, … Lindgren T (2016). Building Trust and Relationships Between Patients and Providers: An Essential Complement to Health Literacy in HIV Care. Journal of the Association of Nurses in AIDS Care, 27(5), 574–584. doi: 10.1016/j.jana.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Dios MA, Stanton CA, Cano MA, Lloyd-Richardson E, & Niaura R (2016). The Influence of Social Support on Smoking Cessation Treatment Adherence Among HIV+ Smokers. Nicotine Tob Res, 18(5), 1126–1133. doi: 10.1093/ntr/ntv144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martel C, Plummer M, Vignat J, & Franceschi S (2017). Worldwide burden of cancer attributable to HPV by site, country and HPV type. International Journal of Cancer, 141, 664–670. 10.1002/ijc.30716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanathan AS, Anderson DJC, Cottrell ML, Burgunder EM, Saunders AC, & Kashuba ADM (2019). Contemporary Drug–Drug Interactions in HIV Treatment. Clinical Pharmacology & Therapeutics, 105(6), 1362–1377. doi: 10.1002/cpt.1393 [DOI] [PubMed] [Google Scholar]
- Diana NE, & Naicker S (2016). Update on current management of chronic kidney disease in patients with HIV infection. International journal of nephrology and renovascular disease, 9, 223. DOI: 10.2147/IJNRD.S93887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore GJ, Soriano V, Rockstroh J, Kupfer B, Tedaldi E, Peters L, Neuhaus J, Puoti M, Klein MB, Mocroft A, Clotet B, Lundgren JD, & SMART INSIGHT study group (2010). Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS, 24(6), 857–865. 10.1097/QAD.0b013e328334bddb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duko B, Ayalew M, & Ayano G (2019). The prevalence of alcohol use disorders among people living with HIV/AIDS: A systematic review and meta-analysis. Substance Abuse: Treatment, Prevention, and Policy, 14, 52. 10.1186/s13011-019-0240-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehren K, Hertenstein C, Kummerle T, Vehreschild JJ, Fischer J, Gillor D, … Fatkenheuer G (2014). Causes of death in HIV-infected patients from the Cologne-Bonn cohort. Infection, 42(1), 135–140. doi: 10.1007/s15010-013-0535-7 [DOI] [PubMed] [Google Scholar]
- Ekrikpo UE, Kengne AP, Bello AK, Effa EE, Noubiap JJ, Salako BL, … Okpechi IG (2018). Chronic kidney disease in the global adult HIV-infected population: a systematic review and meta-analysis. PLOS ONE, 13(4), e0195443. DOI: 10.1371/journal.pone.0195443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, & Kanwal F (2014). Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology, 60(5), 1767–1775. 10.1002/hep.27222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels EA, Yanik EL, Wheeler W, Gill MJ, Shiels MS, Dubrow R, … Design of the International Epidemiologic Databases to Evaluate, A. (2017). Cancer-Attributable Mortality Among People With Treated Human Immunodeficiency Virus Infection in North America. Clin Infect Dis, 65(4), 636–643. doi: 10.1093/cid/cix392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerging Risk Factors Collaboration. (2010). Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. The Lancet, 375(9733), 2215–2222. DOI: 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, … Post WS (2019). Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation, 140(2), e98–e124. doi: 10.1161/CIR.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferketich AK, Diaz P, Browning KK, Lu B, Koletar SL, Reynolds NR, & Wewers ME (2013). Safety of varenicline among smokers enrolled in the lung HIV study. Nicotine Tob Res, 15(1), 247–254. doi: 10.1093/ntr/nts121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnell DS, Nowzari S, Reimann B, Fischer L, Pace E, & Goplerud E (2014). Screening, brief intervention, and referral to treatment (SBIRT) as an integral part of nursing practice. Substance Abuse, 35(2), 114–118. 10.1080/08897077.2014.888384 [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén C, Baker T, & al. e. (2008). Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service. [Google Scholar]
- Fleming J, Berry SA, Moore RD, Nijhawan A, Somboonwit C, Cheever L, & Gebo KA (2019). U.S. Hospitalization rates and reasons stratified by age among persons with HIV 2014–15. AIDS Care, 1–10. doi: 10.1080/09540121.2019.1698705 [DOI] [PubMed] [Google Scholar]
- Fox CS, Golden SH, Anderson C, Bray GA, Burke LE, Boer I. H. d., … Vafiadis DK (2015). Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence. Circulation, 132(8), 691–718. doi: 10.1161/CIR.0000000000000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013; 173:614–622. doi: 10.1001/jamainternmed.2013.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EE, & Duffus WA (2016). Chronic health conditions in Medicare beneficiaries 65 years and older with HIV infection. AIDS (London, England), 30(16), 2529. DOI: 10.1097/QAD.0000000000001215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Retamero R, & Galesic M (2010). Who proficts from visual aids: Overcoming challenges in people’s understanding of risks. Social Science & Medicine, 70(7), 1019–1025. doi: 10.1016/j.socscimed.2009.11.031 [DOI] [PubMed] [Google Scholar]
- Gianotti N, Visco F, Galli L, Barda B, Piatti P, Salpietro S, … Gallotta G (2011). Detecting impaired glucose tolerance or type 2 diabetes mellitus by means of an oral glucose tolerance test in HIV‐infected patients. HIV Medicine, 12(2), 109–117. DOI: 10.1111/j.1468-1293.2010.00860.x. [DOI] [PubMed] [Google Scholar]
- Gillespie BW, Morgenstern H, Hedgeman E, Tilea A, Scholz N, Shearon T, … Plantinga L (2015). Nephrology care prior to end-stage renal disease and outcomes among new ESRD patients in the USA. Clinical kidney journal, 8(6), 772–780. DOI: 10.1093/ckj/sfv103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjærde LI, Shepherd L, Jablonowska E, Lazzarin A, Rougemont M, Darling K, Battegay M, Braun D, Martel-Laferriere V, Lundgren JD, Rockstroh JK, Gill J, Rauch A, Mocroft A, Klein MB, & Peters L (2016). Trends in incidences and risk factors for hepatocellular carcinoma and other liver events in HIV and hepatitis C virus-coinfected individuals from 2001 to 2014: A multicohort study. Clinical Infectious Diseases, 63(6), 821–829. 10.1093/cid/ciw380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, & Younossi ZM (2017). Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine, 96(9). 10.1097/MD.0000000000005904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, & Koziel MJ (2001). Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: A meta-analysis. Clinical Infectious Diseases, 33(4), 562–569. 10.1086/321909 [DOI] [PubMed] [Google Scholar]
- Gregg EW, Sattar N, & Ali MK (2016). The changing face of diabetes complications. The lancet Diabetes & endocrinology, 4(6), 537–547. DOI: 10.1016/S2213-8587(16)30010-9 [DOI] [PubMed] [Google Scholar]
- Gritz ER, Danysh HE, Fletcher FE, Tami-Maury I, Fingeret MC, King RM, … Vidrine DJ (2013). Long-term outcomes of a cell phone-delivered intervention for smokers living with HIV/AIDS. Clin Infect Dis, 57(4), 608–615. doi: 10.1093/cid/cit349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group, T. D. C. o. A. E. o. A.-H. D. D. S. (2003). Combination Antiretroviral Therapy and the Risk of Myocardial Infarction. New England Journal of Medicine, 349(21), 1993–2003. doi: 10.1056/NEJMoa030218 [DOI] [PubMed] [Google Scholar]
- (Data Collection on Adverse Events of Anti-HIV Drugs [DAD] Study Group. (2007). Class of Antiretroviral Drugs and the Risk of Myocardial Infarction. New England Journal of Medicine, 356(17), 1723–1735. doi: 10.1056/NEJMoa062744 [DOI] [PubMed] [Google Scholar]
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, … Yeboah J (2019). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 139(25), e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gucciardi E, Vahabi M, Norris N, Del Monte JP, & Farnum C (2014). The intersection between food insecurity and diabetes: a review. Current nutrition reports, 3(4), 324–332. DOI: 10.1007/s13668-014-0104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeman RR, Murphy KA, Karbeah JM, & Kozhimannil KB (2018). Naming Institutionalized Racism in the Public Health Literature: A Systematic Literature Review. Public Health Reports, 133(3), 240–249. doi: 10.1177/0033354918760574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CJP, Burford TI, Cage BN, Nelson TM, Shearon S, Thompson A, & Green S (2011). Multiple pathways linking racism to health outcomes. Du Bois review: social science research on race, 8(1), 143. doi: 10.1017/S1742058X11000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BB, Nordell AD, Okulicz JF, Palfreeman A, Horban A, Kedem E, … Groups E (2018). Inflammation-Related Morbidity and Mortality Among HIV-Positive Adults: How Extensive Is It? Journal of acquired immune deficiency syndromes (1999), 77(1), 1–7. doi: 10.1097/QAI.0000000000001554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Dombrowski JC, Crane HM, Eron JJ, Geng EH, Christopher Mathews W, … Donovan DM (2017). Prevalence and Predictors of Substance Use Disorders Among HIV Care Enrollees in the United States. AIDS Behav, 21(4), 1138–1148. doi: 10.1007/s10461-016-1584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleberg M, Gerstoft J, Afzal S, Kronborg G, Larsen CS, Pedersen C, … Obel N (2014). Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV. AIDS, 28(10), 1499–1508. doi: 10.1097/QAD.0000000000000283 [DOI] [PubMed] [Google Scholar]
- Helleberg M, May MT, Ingle SM, Dabis F, Reiss P, Fatkenheuer G, … Obel N (2015). Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS, 29(2), 221–229. doi: 10.1097/QAD.0000000000000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Ramírez RU, Shiels MS, Dubrow R, & Engels EA (2017). Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. The Lancet HIV, 4(11), e495–e504. doi: 10.1016/S2352-3018(17)30125-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrin M, Tate JP, Akgün KM, Butt AA, Crothers K, Freiberg MS, … Justice AC (2016). Weight Gain and Incident Diabetes Among HIV-Infected Veterans Initiating Antiretroviral Therapy Compared With Uninfected Individuals. Journal of acquired immune deficiency syndromes (1999), 73(2), 228–236. doi: 10.1097/QAI.0000000000001071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmeister MG, Rosenthal EM, Barker LK, Rosenberg ES, Barranco MA, Hall EW, Edlin BR, Mermin J, Ward JW, & Ryerson AB (2019). Estimating prevalence of hepatitis C virus infection in the United States, 2013–2016. Hepatology, 69(3), 1020–1031. 10.1002/hep.30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner MJ, Shiels MS, Pfeiffer RM, & Engels EA (2020). Deaths attributable to cancer in the United States HIV population during 2001–2015. Clin Infect Dis. doi: 10.1093/cid/ciaa1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert A, Hooker CM, Keruly JC, Brown T, Horton K, Fishman E, … Brock MV (2014). Prospective CT screening for lung cancer in a high-risk population: HIV-positive smokers. J Thorac Oncol, 9(6), 752–759. doi: 10.1097/JTO.0000000000000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humfleet GL, Hall SM, Delucchi KL, & Dilley JW (2013). A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine Tob Res, 15(8), 1436–1445. doi: 10.1093/ntr/ntt005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ (2006). Risk evaluation of viral load elevation and associated liver disease/cancer-in HBV (the REVEAL-HBV) study group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology, 130(3), 678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Ingersoll KS, Cropsey KL, & Heckman CJ (2009). A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS Behav, 13(3), 545–554. doi: 10.1007/s10461-007-9334-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou GN, Green PK, Beste LA, Mun EJ, Kerr KF, & Berry K (2018). Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. Journal of Hepatology, 69(5), 1088–1098. 10.1016/j.jhep.2018.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islami F, Ferlay J, Lortet-Tieulent J, Bray F, & Jemal A (2017). International trends in anal cancer incidence rates. International Journal of Epidemiology, 46(3), 924–938. 10.1093/ije/dyw276 [DOI] [PubMed] [Google Scholar]
- JACC. (2020). ASCVD Risk Estimator Plus. Retrieved from http://tools.acc.org/ASCVD-Risk-Estimator-Plus/#!/calculate/estimate/
- Kagaruki GB, Mayige MT, Ngadaya ES, Kimaro GD, Kalinga AK, Kilale AM, … Mfinanga SG (2014). Magnitude and risk factors of non-communicable diseases among people living with HIV in Tanzania: a cross sectional study from Mbeya and Dar es Salaam regions. BMC public health, 14(1), 904. DOI: 10.1186/1471-2458-14-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra S, Kalra B, Agrawal N, & Unnikrishnan A (2011). Understanding diabetes in patients with HIV/AIDS. Diabetology & metabolic syndrome, 3(1), 2. DOI: 10.1186/1758-5996-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly H, Chikandiwa A, Alemany Vilches L, Palefsky JM, de Sanjose S, & Mayaud P (2020). Association of antiretroviral therapy with anal high-risk human papillomavirus, anal intraepithelial neoplasia, and anal cancer in people living with HIV: a systematic review and meta-analysis. The Lancet HIV, 7(4), e262–e278. 10.1016/S2352-3018(19)30434-5 [DOI] [PubMed] [Google Scholar]
- Kim AY, Onofrey S, & Church DR (2013). An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. Journal of Infectious Diseases, 207(Suppl 1), S1–S6. 10.1093/infdis/jis927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Westfall AO, Chamot E, Willig AL, Mugavero MJ, Ritchie C, … Saag MS (2012). Multimorbidity patterns in HIV-infected patients: the role of obesity in chronic disease clustering. J Acquir Immune Defic Syndr, 61(5), 600. DOI: 10.1097/QAI.0b013e31827303d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PS, Woods C, Dutcher L, Georgoff P, Rosenberg A, Mican JAM, … Hadigan C (2011). Increased prevalence of albuminuria in HIV-infected adults with diabetes. PLOS ONE, 6(9), e24610. DOI: 10.1371/journal.pone.0024610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DB, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, … Silverberg MJ (2015). Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis, 60(8), 1278–1280. doi: 10.1093/cid/civ014 [DOI] [PubMed] [Google Scholar]
- Kowalkowski MA, Day RS, Du XL, Chan W, & Chiao EY (2014). Cumulative HIV viremia and non-AIDS-defining malignancies among a sample of HIV-infected male veterans. Journal of Acquired Immune Deficiency Syndromes, 67(2), 204–211. 10.1097/QAI.0000000000000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JR, Kowalkowski MA, Duan Z, & Chiao EY (2015). The effect of HIV viral control on the incidence of hepatocellular carcinoma in veterans with hepatitis C and HIV coinfection. Journal of Acquired Immune Deficiency Syndromes, 68(4), 456–462. 10.1097/QAI.0000000000000494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labaki WW, & Rosenberg SR (2020). Chronic Obstructive Pulmonary Disease. Ann Intern Med, 173(3), ITC17–ITC32. doi: 10.7326/AITC202008040 [DOI] [PubMed] [Google Scholar]
- Lake JE (2017). The Fat of the Matter: Obesity and Visceral Adiposity in Treated HIV Infection. Current HIV/AIDS Reports, 14(6), 211–219. doi: 10.1007/s11904-017-0368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen HK, Hædersdal M, Thomsen LT, Hertzum-Larsen R, Lok TT, Bonde J, Sørensen SS, Hansen JM, Palefsky JM, & Kjær SK (2020). Risk of anal high-grade squamous intraepithelial lesions among renal transplant recipients compared with immunocompetent controls. Clinical Infectious Diseases. 10.1093/cid/ciaa781 [DOI] [PubMed] [Google Scholar]
- Lalloo UG, Pillay S, Mngqibisa R, Abdool-Gaffar S, & Ambaram A (2016). HIV and COPD: a conspiracy of risk factors. Respirology, 21(7), 1166–1172. doi: 10.1111/resp.12806 [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, & Yskes R (2016). Smoking Cessation for People Living With HIV/AIDS: A Literature Review and Synthesis. Nicotine Tob Res, 18(12), 2177–2184. doi: 10.1093/ntr/ntw126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds IL (2016). Anal cancer and intraepithelial neoplasia screening: A review. World Journal of Gastrointestinal Surgery, 8(1), 41. 10.4240/wjgs.v8.i1.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leumi S, Bigna JJ, Amougou MA, Ngouo A, Nyaga UF, & Noubiap JJ (2020). Global burden of hepatitis B infection in people living with human immunodeficiency virus: A systematic review and meta-analysis. Clinical Infectious Diseases, ciz1170. 10.1093/cid/ciz1170 [DOI] [PubMed] [Google Scholar]
- Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, Wood K, Holmberg SD, Brooks JT; HIV Outpatient Study (HOPS) Investigators. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study.Clin Infect Dis. 2010; 51:435–447. doi: 10.1086/655 [DOI] [PubMed] [Google Scholar]
- Liverpool, U. o. (2020). HIV Drug Interactions. Retrieved from https://www.hiv-druginteractions.org/
- Lloyd-Jones DM, Huffman MD, Karmali KN, Sanghavi DM, Wright JS, Pelser C, … Goff DC (2017). Estimating Longitudinal Risks and Benefits From Cardiovascular Preventive Therapies Among Medicare Patients. The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A Special Report From the American Heart Association and American College of Cardiology, 69(12), 1617–1636. doi: 10.1016/j.jacc.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Richardson EE, Stanton CA, Papandonatos GD, Shadel WG, Stein M, Tashima K, … Niaura R (2009). Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction, 104(11), 1891–1900. doi: 10.1111/j.1360-0443.2009.02623.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker KJ, Rönn MM, Brock PM, Brisson M, Drolet M, Mayaud P, & Boily MC (2018). Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. Journal of the International AIDS Society, 21(6), e25110. 10.1002/jia2.25110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba R, Lim JK, Patton H, & El-Serag HB (2020). AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: Expert review. Gastroenterology, 158(6), 1822–1830. 10.1053/j.gastro.2019.12.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Ross MJ, Stock PG, Shlipak MG, Wyatt CM, Gupta SK, … Bruggeman LA (2014). Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical Infectious Diseases, 59(9), e96–e138. DOI: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey MR, Im GY, Mellinger JL, Szabo G, & Crabb DW (2020). Introducing the 2019 American Association for the Study of Liver Diseases guidance on alcohol-associated liver disease. Liver Transplantation, 26(1), 14–16. 10.1002/lt.25600 [DOI] [PubMed] [Google Scholar]
- MacDonald D, Melzer A, Collins G, Avihingsanon A, Crothers K, Ingraham N, … Group, I. S. P. S. (2018). Smoking and accelerated lung function decline in HIV-positive individuals: a secondary analysis of the START Pulmonary Substudy. J Acquir Immune Defic Syndr, 79(3), e85–e92. DOI: 10.1097/QAI.0000000000001797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod J, Franz MJ, Handu D, Gradwell E, Brown C, Evert A, … Robinson M (2017). Academy of Nutrition and Dietetics nutrition practice guideline for type 1 and type 2 diabetes in adults: nutrition intervention evidence reviews and recommendations. Journal of the Academy of Nutrition and Dietetics, 117(10), 1637–1658. DOI: 10.1016/j.jand.2017.03.023 [DOI] [PubMed] [Google Scholar]
- Makinson A, Eymard-Duvernay S, Raffi F, Abgrall S, Bommart S, Zucman D, … Le Moing V (2016). Feasibility and efficacy of early lung cancer diagnosis with chest computed tomography in HIV-infected smokers. AIDS (London, England), 30(4), 573–582. doi: 10.1097/qad.0000000000000943 [DOI] [PubMed] [Google Scholar]
- Makinson A, Hayot M, Eymard-Duvernay S, Ribet C, Raffi F, Pialoux G, … Le Moing V (2018). HIV is associated with airway obstruction: a matched controlled study. AIDS, 32(2), 227–232. doi: 10.1097/qad.0000000000001691 [DOI] [PubMed] [Google Scholar]
- Mamary E, Bahrs D, & Martinez S (2002). Cigarette smoking and the desire to quit among individuals living with HIV. AIDS Patient Care and STDs, 16(1), 39–42. DOI: 10.1089/108729102753429389 [DOI] [PubMed] [Google Scholar]
- Manuel JK, Lum PJ, Hengl NS, & Sorensen JL (2013). Smoking cessation interventions with female smokers living with HIV/AIDS: a randomized pilot study of motivational interviewing. AIDS Care, 25(7), 820–827. doi: 10.1080/09540121.2012.733331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JL, Chao C, Leyden WA, Xu L, Yu J, Horberg MA, … Silverberg MJ (2015). Survival among HIV-infected and HIV-uninfected individuals with common non-AIDS-defining cancers. Cancer Epidemiol Biomarkers Prev, 24(8), 1167–1173. doi: 10.1158/1055-9965.Epi-14-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, & Heimbach JK (2018). Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases, 68(2). 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- Matthews AK, Conrad M, Kuhns L, Vargas M, & King AC (2013). Project Exhale: preliminary evaluation of a tailored smoking cessation treatment for HIV-positive African American smokers. AIDS Patient Care STDS, 27(1), 22–32. doi: 10.1089/apc.2012.0253 [DOI] [PubMed] [Google Scholar]
- Maurice JB, Patel A, Scott AJ, Patel K, Thursz M, & Lemoine M (2017). Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS, 31(11), 1621–1632. 10.1097/QAD.0000000000001504 [DOI] [PubMed] [Google Scholar]
- McGlynn KA, Petrick JL, & El‐Serag HB (2020). Epidemiology of hepatocellular carcinoma. Hepatology. 10.1002/hep.31288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, & Skarbinski J (2015). Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Annals of Internal Medicine, 162(5), 335–344. doi: 10.7326/M14-0954 [DOI] [PubMed] [Google Scholar]
- Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, & Markowitz LE (2019). Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. Morbidity and Mortality Weekly Report, 68(32), 698–702. 10.15585/mmwr.mm6832a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, & Shuter J (2012). A randomized controlled trial of a tailored group smoking cessation intervention for HIV-infected smokers. J Acquir Immune Defic Syndr, 61(2), 208–215. doi: 10.1097/QAI.0b013e3182645679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocroft A, Lundgren JD, Ross M, Law M, Reiss P, Kirk O, … & Moranne O (2015). Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D: A: D study. PLoS Med, 12(3), e1001809. 10.1371/journal.pmed.1001809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya JL, Jankowski CM, O’Brien KK, Webel AR, Oursler KK, Henry BL, … Erlandson KM (2019). Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS (London, England), 33(6), 931–939. doi: 10.1097/QAD.0000000000002137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TR, Mandayam S, & Jamal MM (2004). Alcohol and hepatocellular carcinoma. Gastroenterology, 127(5 Suppl 1), S87–96. 10.1053/j.gastro.2004.09.020 [DOI] [PubMed] [Google Scholar]
- Naggie S, Cooper C, Sagg M, Workowski K, Ruane P, Towner WJ, Marks K, Luetkemeyer A, Baden RP, Sax PE, Gane E, Santana-Bagur J, Stamm LM, Yange JC, German P, Dvory-Sobol H, Ni L, Pang PS, McHutchinson JG, … Sulkowski M (2015). Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. New England Journal of Medicine, 373, 705–713. 10.1056/NEJMoa1501315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Cancer Institute. (2020). Cancer stat facts: Anal cancer. Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/statfacts/html/anus.html [Google Scholar]
- Nelson RA, Levine AM, Bernstein L, Smith DD, & Lai LL (2013). Changing patterns of anal canal carcinoma in the United States. Journal of Clinical Oncology, 31(12), 1569–1575. 10.1200/JCO.2012.45.2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuen BL, Chadban SJ, Demaio AR, Johnson DW, & Perkovic V (2017). Chronic kidney disease and the global NCDs agenda. In: BMJ Specialist Journals. DOI: 10.1136/bmjgh-2017-000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York State Department of Health AIDS Institute. (2020). Screening for anal dysplasia and cancer in patients with HIV. https://www.hivguidelines.org/hiv-care/anal-dysplasia-cancer
- Nguyen I, Kim AS, & Chow FC (2020). Prevention of stroke in people living with HIV. Progress in Cardiovascular Diseases, 63(2), 160–169. doi: 10.1016/j.pcad.2020.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Shadel W, Morrow K, Tashima K, Flanigan T, & Abrams D (2000). Human Immunodeficiency Virus Infection, AIDS, and Smoking Cessation: The Time is Now. Clinical Infectious Diseases, 31, 808–812. DOI: 10.1086/314048 [DOI] [PubMed] [Google Scholar]
- Norton JM, Moxey-Mims MM, Eggers PW, Narva AS, Star RA, Kimmel PL, & Rodgers GP (2016). Social determinants of racial disparities in CKD. Journal of the American Society of Nephrology, 27(9), 2576–2595. DOI: 10.1681/ASN.2016010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nou E, Lo J, & Grinspoon SK (2016). Inflammation, immune activation, and cardiovascular disease in HIV. AIDS (London, England), 30(10), 1495–1509. doi: 10.1097/QAD.0000000000001109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystoriak MA, & Bhatnagar A (2018). Cardiovascular Effects and Benefits of Exercise. Frontiers in cardiovascular medicine, 5, 135. doi: 10.3389/fcvm.2018.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil A, Scovelle AJ, Milner AJ, & Kavanagh A (2018). Gender/Sex as a Social Determinant of Cardiovascular Risk. Circulation, 137(8), 854–864. doi: 10.1161/CIRCULATIONAHA.117.028595 [DOI] [PubMed] [Google Scholar]
- Ogurtsova K, da Rocha Fernandes J, Huang Y, Linnenkamp U, Guariguata L, Cho NH, … Makaroff L (2017). IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes research and clinical practice, 128, 40–50. DOI: 10.1016/j.diabres.2017.03.024 [DOI] [PubMed] [Google Scholar]
- Olaiya O, Weiser J, Zhou W, Patel P, & Bradley H (2018). Hypertension Among Persons Living With HIV in Medical Care in the United States-Medical Monitoring Project, 2013–2014. Open forum infectious diseases, 5(3), ofy028–ofy028. doi: 10.1093/ofid/ofy028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson DE, Rhee MK, Herrick K, Ziemer DC, Twombly JG, & Phillips LS (2010). Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes care, 33(10), 2184–2189. DOI: 10.2337/dc10-0433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong WM, Chua SS, & Ng CJ (2014). Barriers and facilitators to self-monitoring of blood glucose in people with type 2 diabetes using insulin: a qualitative study. Patient preference and adherence, 8, 237. DOI: 10.2147/PPA.S57567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn CY, De Groot M, & Wagner JA (2013). Racial and ethnic disparities in diabetes complications in the northeastern United States: the role of socioeconomic status. Journal of the National Medical Association, 105(1), 51–58. DOI: 10.1016/s0027-9684(15)30085-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, & Cioe PA (2015). Tobacco Use, Use Disorders, and Smoking Cessation Interventions in Persons Living With HIV. Curr HIV/AIDS Rep, 12(4), 413–420. doi: 10.1007/s11904-015-0281-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Harrell PT, & Martins SS (2014). Cigarette smoking and drug use among a nationally representative sample of HIV-positive individuals. Am J Addict, 23(6), 582–590. doi: 10.1111/j.1521-0391.2014.12145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Latkin C, Crum RM, Stuart EA, & Knowlton AR (2014). Interest in quitting and lifetime quit attempts among smokers living with HIV infection. Drug Alcohol Depend, 138, 220–224. doi: 10.1016/j.drugalcdep.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel on Opportunistic Infections in Adults and Adolescents with HIV. (2019). Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-opportunistic-infection/343/human-papillomavirus [PubMed]
- Park J, ZuÑiga J, & Garcia A (2019). Diabetes negatively impacts the 10-year survival rates of persons living with HIV (vol 30, pg 991, 2019). INTERNATIONAL JOURNAL OF STD & AIDS. 10.1177/0956462419857005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, & ZuÑiga JA (2018). Chronic kidney disease in persons living with HIV: a systematic review. Journal of the Association of Nurses in AIDS Care, 29(5), 655–666. DOI: 10.1016/j.jana.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Park LS, Tate JP, Sigel K, Brown ST, Crothers K, Gibert C, Goetz MB, Rimland D, Rodriguez-Barradas MC, Bedimo RJ, Justice AC, & Dubrow R (2018). Association of viral suppression with lower AIDS-defining and non–AIDS-defining cancer incidence in HIV-infected veterans: A prospective cohort study. Annals of Internal Medicine, 169(2), 87–96. 10.7326/M16-2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, Ferlay J, Valery PC, Bray F, McGlynn KA (2020). International trends in hepatocellular carcinoma incidence, 1978–2012. International Journal of Cancer, 147(2):317–330. doi: 10.1002/ijc.32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinato DJ, Allara E, Chen TY, Trevisani F, Minguez B, Zoli M, Harris M, Pria AD, Merchante N, Platt H, Jain M, Caturelli E, Kikuchi L, Pineda J, Nelson M, Farinati F, Rapaccini GL, Aytaman A, Yin M, … Bräu N (2019). Influence of HIV infection on the natural history of hepatocellular carcinoma: Results from a global multicohort study. Journal of Clinical Oncology, 37(4), 296–304. 10.1200/JCO.18.00885 [DOI] [PubMed] [Google Scholar]
- Piscaglia F, Svegliati-Baroni G, Barchetti A, Pecorelli A, Marinelli S, Tiribelli C, Bellentani S, Bernardi M, Biselli M, Caraceni P, Domenicali M, Garuti F, Gramenzi A, Lenzi B, Magalotti D, Cescon M, Ravaioli M, Del Poggio P, Olmi S, … Borzio F (2016). Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology, 63(3), 827–838. 10.1002/hep.28368 [DOI] [PubMed] [Google Scholar]
- Pocha C, & Xie C (2019). Hepatocellular carcinoma in alcoholic and non-alcoholic fatty liver disease—one of a kind or two different enemies? Translational Gastroenterology and Hepatology, 4, 72–72. 10.21037/tgh.2019.09.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussing C, Chan C, Pinchoff J, Kersanske L, Bornschlegel K, Balter S, Drobnik A, & Fuld J (2015). HIV and viral hepatitis co-infection in New York City, 2000–2010: prevalence and case characteristics. Epidemiology and Infection, 143(7), 1408–1416. 10.1017/S0950268814002209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkey E, Patel R, & Phillips SP (2018). Trauma-informed care: better care for everyone. Canadian Family Physician, 64(3), 170. https://www.cfp.ca/content/64/3/170.long [PMC free article] [PubMed] [Google Scholar]
- Qaseem A, Barry MJ, Humphrey LL, & Forciea MA (2017). Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Annals of Internal Medicine, 166(4), 279–290. DOI: 10.7326/M16-1860 [DOI] [PubMed] [Google Scholar]
- Raposeiras-Roubín S, Abu-Assi E, & Iñiguez-Romo A (2017). Tobacco, illicit drugs use and risk of cardiovascular disease in patients living with HIV. Current Opinion in HIV and AIDS, 12(6), 523–527. doi: 10.1097/COH.0000000000000407 [DOI] [PubMed] [Google Scholar]
- Reddy KP, Parker RA, Losina E, Baggett TP, Paltiel AD, Rigotti NA, … Walensky RP (2016). Impact of Cigarette Smoking and Smoking Cessation on Life Expectancy Among People With HIV: A US-Based Modeling Study. J Infect Dis, 214(11), 1672–1681. doi: 10.1093/infdis/jiw430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo B, Videla S, Llibre JM, Paredes R, Piñol M, García-Cuyàs F, Ornelas A, Puig J, Parés D, Corral J, Clotet B, & Sirera G (2020). Routine screening of anal cytology in persons with human immunodeficiency virus and the impact on invasive anal cancer: A prospective cohort study. Clinical Infectious Diseases, 71(2), 390–399. [DOI] [PubMed] [Google Scholar]
- Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA (2015). Excess cancers among HIV-infected people in the United States. Journal of the National Cancer Institute, 107(4), dju503. 10.1093/jnci/dju503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JR, Siekas LL, & Kaz AM (2017). Anal intraepithelial neoplasia: A review of diagnosis and management. World Journal of Gastrointestinal Oncology, 9(2), 50–61. 10.4251/wjgo.v9.i2.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockstroh Jürgen K, Lacombe K, Viani RM, Orkin C, Wyles D, Luetkemeyer AF, Soto-Malave R, Flisiak R, Bhagani S, Sherman KE, Shimonova T, Ruane P, Sasadeusz J, Slim J, Zhang Z, Samanta S, Ng TI, Gulati A, Kosloski MP, … Sulkowski M (2018). Efficacy and safety of glecaprevir/pibrentasvir in patients coinfected with hepatitis C virus and human immunodeficiency virus type 1: The EXPEDITION-2 Study. Clinical Infectious Diseases, 67, 1010–1017. 10.1093/cid/ciy220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockstroh Jurgen K, Nelson M, Katlama C, Lalezari J, Mallolas J, Bloch M, Matthews GV, Saag MS, Zamor PJ, Orkin C, Gress J, Klopfer S, Shaughnessy M, Wahl J, Nguyen B-YT, Barr E, Platt HL, Robertson MN, & Sulkowski M (2015). Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV, 2, e319–327. 10.1016/S2352-3018(15)00114-9 [DOI] [PubMed] [Google Scholar]
- Ronit A, Lundgren J, Afzal S, Benfield T, Roen A, Mocroft A, … Nielsen SD (2018). Airflow limitation in people living with HIV and matched uninfected controls. Thorax, 73(5), 431–438. doi: 10.1136/thoraxjnl-2017-211079 [DOI] [PubMed] [Google Scholar]
- Sahasrabuddhe VV, Shiels MS, McGlynn KA, & Engels EA (2012). The risk of hepatocellular carcinoma among individuals with acquired immunodeficiency syndrome in the United States. Cancer, 118(24), 6226–6233. 10.1002/cncr.27694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, Steinau M, Watson M, Wilkinson EJ, Hopenhayn C, Copeland G, Cozen W, Peters ES, Huang Y, Saber MS, Altekruse S, & Goodman MT (2015). US assessment of HPV Types in cancers: Implications for current and 9-valent HPV vaccines. Journal of the National Cancer Institute, 107(6), 86. 10.1093/jnci/djv086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, & Brown TT (2019). Diabetes in People Living with HIV. In Endotext [Internet]: MDText. com, Inc. [Google Scholar]
- Saydah SH, Pavkov ME, Zhang C, Lacher DA, Eberhardt MS, Burrows NR, … Williams DE (2013). Albuminuria prevalence in first morning void compared with previous random urine from adults in the National Health and Nutrition Examination Survey, 2009–2010. Clinical chemistry, 59(4), 675–683. DOI: 10.1373/clinchem.2012.195644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherzer R, & Shlipak MG (2015). Riks factors: Individual assessment of CKD risk in HIV-positive patients. Nature Reviews Nephrology, 11(7), 392–393. DOI: 10.1038/nrneph.2015.75. [DOI] [PubMed] [Google Scholar]
- Schillie S, Harris A, Link-Gelles R, Romero J, Ward J, & Nelson N (2018). Recommendations of the Advisory Committee on Immunization Practices for use of a Hepatitis B vaccine with a novel adjuvant. Morbidity and Mortality Weekly Report, 67(15), 455–458. 10.15585/mmwr.mm6715a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon LAJ, Carey KB, Johnson BT, & Carey MP (2017). Behavioral interventions targeting alcohol use among people living with HIV/AIDS: A systematic review and meta-analysis. AIDS and Behavior, 21(Suppl 2), 126–143. 10.1007/s10461-017-1886-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, & Sherman KE (2019). Fatty liver disease in persons with HIV infection. Topics in Antiviral Medicine, 27(2), 75–82. [PMC free article] [PubMed] [Google Scholar]
- Seligman HK, Davis TC, Schillinger D, & Wolf MS (2010). Food insecurity is associated with hypoglycemia and poor diabetes self-management in a low-income sample with diabetes. Journal of health care for the poor and underserved, 21(4), 1227. DOI: 10.1353/hpu.2010.0921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, … Mills NL (2018). Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV. Circulation, 138(11), 1100–1112. doi: 10.1161/CIRCULATIONAHA.117.033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbuk JE, McManus KA, Kemp Knick T, Canan CE, Flickinger T, & Dillingham R (2019). Disparities in hepatitis C linkage to care in the direct acting antiviral era: Findings from a referral clinic with an embedded nurse navigator model. Frontiers in Public Health, 7, 362. 10.3389/fpubh.2019.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, … Engels EA (2011). Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst, 103(9), 753–762. doi: 10.1093/jnci/djr076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Islam JY, Rosenberg PS, & Engels EA (2018). Projected Cancer Incidence Rates and Burden of Incident Cancer Cases in HIV-Infected Adults in the United States Through 2030. Annals of Internal Medicine, 168(12), 866–873. 10.7326/M17-2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing JZ, Ly KN, Xing J, Teshale EH, & Jiles RB (2020). Prevalence of hepatitis B virus infection among US adults aged 20–59 years with a history of injection drug use: National Health and Nutrition Examination Survey, 2001–2016. Clinical Infectious Diseases, 70(12), 2619–2627. 10.1093/cid/ciz669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuter J, Bernstein SL, & Moadel AB (2012). Cigarette smoking behaviors and beliefs in persons living with HIV/AIDS. Am J Health Behav, 36(1), 75–85. DOI: 10.5993/ajhb.36.1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel K, Makinson A, & Thaler J (2017). Lung cancer in persons with HIV. Curr Opin HIV AIDS, 12(1), 31–38. doi: 10.1097/COH.0000000000000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel K, Wisnivesky J, Crothers K, Gordon K, Brown ST, Rimland D, … Dubrow R (2016). Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV. doi: 10.1016/s2352-3018(16)30215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel K, Wisnivesky J, Gordon K, Dubrow R, Justice A, Brown ST, … Crothers K (2012). HIV as an independent risk factor for incident lung cancer. AIDS, 26(8), 1017–1025. doi: 10.1097/QAD.0b013e328352d1ad [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, Hurley LB, Quesenberry CP, Klein DB. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014; 65:160–166. doi: 10.1097/QAI.000000000000000 [DOI] [PubMed] [Google Scholar]
- Singal AK, Bataller R, Ahn J, Kamath PS, & Shah VH (2018). ACG clinical guideline: Alcoholic liver disease. American Journal of Gastroenterology, 113(2), 175–194. 10.1038/ajg.2017.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A, & Feinstein M (2020). Epidemiology, pathophysiology, and prevention of heart failure in people with HIV. Progress in Cardiovascular Diseases, 63(2), 134–141. doi: 10.1016/j.pcad.2020.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slama L, Palella FJ Jr, Abraham AG, Li X, Vigouroux C, Pialoux G, … Margolick JB (2014). Inaccuracy of haemoglobin A1c among HIV-infected men: effects of CD4 cell count, antiretroviral therapies and haematological parameters. Journal of Antimicrobial Chemotherapy, 69(12), 3360–3367. DOI: 10.1093/jac/dku295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, & Wender RC (2019). Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin, 69(3), 184–210. doi: 10.3322/caac.21557 [DOI] [PubMed] [Google Scholar]
- Stanifer JW, Muiru A, Jafar TH, & Patel UD (2016). Chronic kidney disease in low-and middle-income countries. Nephrology Dialysis Transplantation, 31(6), 868–874. DOI: 10.1093/ndt/gfv466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanifer JW, Von Isenburg M, Chertow GM, & Anand S (2018). Chronic kidney disease care models in low-and middle-income countries: a systematic review. BMJ global health, 3(2), e000728. DOI: 10.1136/bmjgh-2018-000728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton CA, Papandonatos GD, Shuter J, Bicki A, Lloyd-Richardson EE, de Dios MA, … Niaura RS (2015). Outcomes of a Tailored Intervention for Cigarette Smoking Cessation Among Latinos Living With HIV/AIDS. Nicotine Tob Res, 17(8), 975–982. doi: 10.1093/ntr/ntv014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starbird LE, Budhathoki C, Han HR, Sulkowski MS, Reynolds NR, & Farley JE (2020). Nurse case management to improve the hepatitis C care continuum in HIV co-infection: Results of a randomized controlled trial. Journal of Viral Hepatitis, 27(4), 376–386. 10.1111/jvh.13241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DB, Gaertner WB, Glasgow SC, Herzig DO, Feingold D, & Steele SR (2018). The American Society of Colon and Rectal Surgeons clinical practice guidelines for anal squamous cell cancers (revised 2018). Diseases of the Colon & Rectum, 61(7), 755–744. doi: 10.1097/DCR.0000000000001114 [DOI] [PubMed] [Google Scholar]
- Stock PG, Barin B, Murphy B, Hanto D, Diego JM, Light J, … Subramanian A (2010). Outcomes of kidney transplantation in HIV-infected recipients. New England Journal of Medicine, 363(21), 2004–2014. DOI: 10.1056/NEJMoa1001197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanya V, McKay HS, Brusca RM, Palella FJ, Kingsley LA, Witt MD, … Haberlen SA (2019). Inflammatory biomarkers and subclinical carotid atherosclerosis in HIV-infected and HIV-uninfected men in the Multicenter AIDS Cohort Study. PLOS ONE, 14(4), e0214735. doi: 10.1371/journal.pone.0214735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczech LA, Hoover DR, Feldman JG, Cohen MH, Gange SJ, Goozé L, … Shi Q (2004). Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clinical Infectious Diseases, 39(8), 1199–1206. DOI: 10.1086/424013 [DOI] [PubMed] [Google Scholar]
- Tabák AG, Herder C, Rathmann W, Brunner EJ, & Kivimäki M (2012). Prediabetes: a high-risk state for diabetes development. The Lancet, 379(9833), 2279–2290. DOI: 10.1016/S0140-6736(12)60283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tami-Maury I, Vidrine DJ, Fletcher FE, Danysh H, Arduino R, & Gritz ER (2013). Poly-tobacco use among HIV-positive smokers: implications for smoking cessation efforts. Nicotine Tob Res, 15(12), 2100–2106. doi: 10.1093/ntr/ntt107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein H-H, Yi Q, Dore GJ, & Krahn MD (2008). Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS, 22(15), 1979–1991. doi: 10.1097/QAD.0b013e32830e6d51 [DOI] [PubMed] [Google Scholar]
- Tingstedt JL, Hove‐Skovsgaard M, Gaardbo J, Ullum H, Nielsen SD, & Gelpi M (2019). The impact of concurrent HIV and type II diabetes on immune maturation, immune regulation and immune activation. Apmis, 127(7), 529–537. DOI: 10.1111/apm.12956 [DOI] [PubMed] [Google Scholar]
- Tong W, Hillman R, Kelleher A, Grulich A, & Carr A (2014). Anal intraepithelial neoplasia and squamous cell carcinoma in HIV-infected adults. HIV Medicine, 15(2), 65–76. 10.1111/hiv.12080 [DOI] [PubMed] [Google Scholar]
- Torgersen J, Kallan MJ, Carbonari DM, Park LS, Mehta RL, D’Addeo K, Tate JP, Lim JK, Goetz MB, Rodriguez-Barradas MC, Gibert CL, Bräu N, Brown ST, Roy JA, Taddei TH, Justice AC, & Lo Re V (2020). HIV RNA, CD4+ percentage, and risk of hepatocellular carcinoma by cirrhosis status. Journal of the National Cancer Institute, 112(7), 747–755. 10.1093/jnci/djz214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007; 92:2506–2512. doi: 10.1210/jc.2006-2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. J Acquir Immune Defic Syndr. 2010; 55:615–619. doi: 10.1097/QAI.0b013e3181f4b752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triant VA, Lyass A, Hurley L., Borowsky LH Ehrbar RQ, Silverberg MJ Massaro JM, & D’Agostino RB,. (2020). Cardiovascular Risk Estimate is Sub-Optimal Across Two HIV Cohorts Abstract Paper presented at the AIDS2020: Virtual. https://natap.org/2020/IAC/IAC_100.htm
- Trullas JC, Cofan F, Tuset M, Ricart MJ, Brunet M, Cervera C, … Moreno A (2011). Renal transplantation in HIV-infected patients: 2010 update. Kidney international, 79(8), 825–842. DOI: 10.1038/ki.2010.545 [DOI] [PubMed] [Google Scholar]
- US Preventive Services Taskforce. (2018). Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults US Preventive Services Task Force recommendation statement. Journal of the American Medical Association (JAMA), 320(18), 1899–1909. 10.1001/jama.2018.16789 [DOI] [PubMed] [Google Scholar]
- Van Greevenbroek M, Schalkwijk C, & Stehouwer C (2013). Obesity-associated low-grade inflammation in type 2 diabetes mellitus: causes and consequences. Neth J Med, 71(4), 174–187. https://pubmed.ncbi.nlm.nih.gov/23723111/ [PubMed] [Google Scholar]
- Vancampfort D, Mugisha J, De Hert M, Probst M, Firth J, Gorczynski P, & Stubbs B (2018). Global physical activity levels among people living with HIV: a systematic review and meta-analysis. Disability and Rehabilitation, 40(4), 388–397. doi: 10.1080/09638288.2016.1260645 [DOI] [PubMed] [Google Scholar]
- Vandenhende MA, Roussillon C, Henard S, Morlat P, Oksenhendler E, Aumaitre H, … Bonnet F (2015). Cancer-Related Causes of Death among HIV-Infected Patients in France in 2010: Evolution since 2000. PLoS One, 10(6), e0129550. doi: 10.1371/journal.pone.0129550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine DJ, Kypriotakis G, Li L, Arduino RC, Fletcher FE, Tami-Maury I, & Gritz ER (2015). Mediators of a smoking cessation intervention for persons living with HIV/AIDS. Drug Alcohol Depend, 147, 76–80. doi: 10.1016/j.drugalcdep.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine DJ, Marks RM, Arduino RC, & Gritz ER (2012). Efficacy of cell phone-delivered smoking cessation counseling for persons living with HIV/AIDS: 3-month outcomes. Nicotine Tob Res, 14(1), 106–110. doi: 10.1093/ntr/ntr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, … Agustí A (2017). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med, 195(5), 557–582. doi: 10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- Wagner JA, Tennen H, Feinn R, & Osborn CY (2015). Self-reported discrimination, diabetes distress, and continuous blood glucose in women with type 2 diabetes. Journal of Immigrant and Minority Health, 17(2), 566–573. DOI: 10.1007/s10903-013-9948-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsemann KM, Goosby BJ, & Farr D (2016). Life course SES and cardiovascular risk: Heterogeneity across race/ethnicity and gender. Social Science & Medicine, 152, 147–155. doi: 10.1016/j.socscimed.2016.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandeler G, Johnson LF, & Egger M (2016). Trends in life expectancy of HIV-positive adults on ART across the globe: comparisons with general population. Curr Opin HIV AIDS, 11(5), 492. DOI: 10.1097/COH.0000000000000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman P, Rubin DS, & Turett G (2017). Review: Anal Intraepithelial Neoplasia in HIV-Infected Men Who Have Sex with Men: Is Screening and Treatment Justified? AIDS Patient Care and STDs, 31(6), 245–253. 10.1089/apc.2017.0063 [DOI] [PubMed] [Google Scholar]
- Webel A, Horvat Davey C, Schexnayder J, Currie J, Al Battashi H, Chang J, & Longenecker CT (2020). Impact of Perceived Cardiovascular Risk on Cardiovascular Disease Prevention Behaviors in People With and Without HIV Infection. JAIDS Journal of Acquired Immune Deficiency Syndromes, 83(5). Retrieved from https://journals.lww.com/jaids/Fulltext/2020/04150/Impact_of_Perceived_Cardiovascular_Risk_on.10.aspx [DOI] [PubMed] [Google Scholar]
- Webel AR, A S, Funderburg N, Kinley B, Longenecker C, Labbato D, … McComsey G (2017). Alcohol and dietary factors associate with gut integrity and inflammation in HIV-infected adults. HIV Medicine, 18(6), 402–411. doi: 10.1111/hiv.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Sabin C, Reiss P, De Wit S, Worm SW, Law M, Dabis F, Monforte ADA, Fontas E, El-Sadr W, Kirk O, Rickenbach M, Phillips A, Ledergerber B, & Lundgren J (2010). HBV or HCV coinfections and risk of myocardial infarction in HIV-infected individuals: the D:A:D Cohort Study. Antiviral Therapy, 15(8), 1077–1086. 10.3851/IMP1681 [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Smith PH, Funk AP, Rabin S, & Shuter J (2017). Sex Differences in Tobacco Use Among Persons Living With HIV/AIDS: A Systematic Review and Meta-Analysis. Journal of acquired immune deficiency syndromes (1999), 74(4), 439–453. doi: 10.1097/QAI.0000000000001279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser SD, Yuan C, Guzman D, Frongillo EA, Riley ED, Bangsberg DR, & Kushel MB (2013). Food insecurity and HIV clinical outcomes in a longitudinal study of urban homeless and marginally housed HIV-infected individuals. AIDS (London, England), 27(18), 2953. DOI: 10.1097/01.aids.0000432538.70088.a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JJ, Sanchez L, Hubbard J, Lo J, Grinspoon SK, & Fitch KV (2019). Diet Quality Is Low and Differs by Sex in People with HIV. The Journal of Nutrition, 149(1), 78–87. doi: 10.1093/jn/nxy241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Lawrence JA, & Davis BA (2019). Racism and Health: Evidence and Needed Research. Annual Review of Public Health, 40(1), 105–125. doi: 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willig A, Wright L, & Galvin TA (2018). Practice Paper of the Academy of Nutrition and Dietetics: Nutrition Intervention and Human Immunodeficiency Virus Infection. Journal of the Academy of Nutrition and Dietetics, 118(3), 486–498. doi: 10.1016/j.jand.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Willig AL, Webel AR, Westfall AO, Levitan EB, Crane HM, Buford TW, … Overton ET (2020). Physical activity trends and metabolic health outcomes in people living with HIV in the US, 2008–2015. Progress in Cardiovascular Diseases, 63(2), 170–177. doi: 10.1016/j.pcad.2020.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Gange SJ, Moore RD, Justice AC, Buchacz K, Abraham AG, … Design. (2017). Multimorbidity Among Persons Living with Human Immunodeficiency Virus in the United States. Clinical Infectious Diseases, 66(8), 1230–1238. doi: 10.1093/cid/cix998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyles D, Bräu N, Kottilil S, Daar ES, Ruane P, Workowski K, Luetkemeyer A, Adeyemi O, Kim AY, Doehle B, Huang KC, Mogalian E, Osinusi A, Mcnally J, Brainard DM, Mchutchison JG, Naggie S, & Sulkowski M (2017). Sofosbuvir and velpatasvir for the treatment of hepatitis C virus in patients coinfected with human immunodeficiency virus type 1: An open-label, phase 3 Study. Clinical Infectious Diseases, 6(1), 6–12. 10.1093/cid/cix260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, & Roberts LR (2019). A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nature Reviews Gastroenterology and Hepatology, 16(10), 589–604. 10.1038/s41575-019-0186-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younossi ZM (2019). Non-alcoholic fatty liver disease – A global public health perspective. Journal of Hepatology, 70(3), 531–544. 10.1016/j.jhep.2018.10.033 [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, & Wymer M (2016). Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology, 64(1), 73–84. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, & Hunt S (2015). Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology, 62(6), 1723–1730. 10.1002/hep.28123 [DOI] [PubMed] [Google Scholar]
- ZuÑiga J, Nguyen ML, & Holstad M (2016). Predictors of dual control of HIV and diabetes. AIDS Care, 28(9), 1124–1127. DOI: 10.1080/09540121.2016.1139667 [DOI] [PubMed] [Google Scholar]



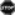 Smoking
Smoking