Abstract
Background
Acute kidney injury (AKI) is a common complication of cardiac surgery. An intra-operative monitor of kidney perfusion is needed to identify patients at risk for AKI. We created a noninvasive urinary oximeter that provides continuous measurements of urinary oxygen partial pressure (PuO2) and instantaneous urine flow. We hypothesized that intra-operative PuO2 measurements are feasible with this prototype device and that low PuO2 during cardiac surgery is associated with the subsequent development of AKI.
Methods
This was a prospective observational pilot study. Continuous PuO2 and instantaneous urine flow were measured in 91 patients undergoing cardiac surgery using a novel device placed between the urinary catheter and collecting bag. Data were collected throughout the surgery and for 24 hours post-operatively. Clinicians were blinded to the intra-operative PuO2 and instantaneous flow data. Patients were then followed post-operatively and the incidence of AKI was compared to PuO2 measurements.
Results
Intra-operative PuO2 measurements were feasible in 86/91 (95%) of patients. When PuO2 data was filtered for valid urine flows > 0.5ml/kg/h, then 70/86 (81%) and 77/86 (90%) of patients in the cardiopulmonary bypass (CPB) and post-CPB periods, respectively were included in the analysis. Mean PuO2 in the post-CPB period was significantly lower in patients who subsequently developed AKI than in those that did not (mean difference 6 mmHg 95% CI 0,11; p=0.038). In a multivariable analysis, mean PuO2 during the post-CPB period remained an independent risk factor for AKI (relative risk 0.82 95% CI 0.71, 0.95; p=0.009 for every 10mmHg increase in mean PuO2).
Conclusions
Low urinary oxygen partial pressures after CPB may be associated with the subsequent development of AKI after cardiac surgery.
Summary Statement:
A novel device that measures urinary oxygen partial pressure was evaluated in patients undergoing cardiac surgery. Low urine oxygen partial pressure was associated with post-operative acute kidney injury.
Introduction
Acute kidney injury (AKI) is a common complication of cardiac surgery with an incidence of 19% - 42% and renal replacement therapy is required in 1–3% of patients.1–5 AKI after cardiac surgery has been associated with significant increases in mortality, intensive care unit (ICU) length of stay, and hospital costs.3,6
The Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines define AKI as an elevation in serum creatinine >0.3 mg/dl above baseline or prolonged oliguria (>6 hours).7 Both of these criteria, however, take several hours to days to become diagnostic. It is this delay in diagnosis that has limited the development of effective mitigation strategies for cardiac surgery associated AKI. More recently, serum and urinary biomarkers of renal injury have been developed for earlier detection of AKI.8–10 While there is evidence to suggest that some of these biomarkers may predict AKI after cardiopulmonary bypass (CPB), bioassays can only be performed intermittently, require time for processing, and can be expensive. As such, a real-time intra-operative monitor for renal injury risk may be warranted.
The pathophysiology of AKI after cardiac surgery is likely multifactorial, but decreased oxygen delivery and renal hypoxia are thought to be important factors.11,12 The region of the kidney that is most susceptible to hypoxic injury is the outer medulla because of the high metabolic rate of the thick ascending limbs of renal tubules in that area and relatively low tissue perfusion compared with the renal cortex. The vasa recta are a network of post-glomerular peritubular capillaries within the medulla that lie in close proximity to the urinary collecting ducts. Thus, when urine is first excreted, its oxygen partial pressure (PuO2) is similar to that of the renal medulla.13,14 As such, continuous PuO2 measurements might be used as a real-time monitor of renal hypoxia and injury risk. PuO2 in the bladder has been shown to decrease in the setting of sepsis, reduced renal blood flow, and decreased cardiac output.15,16 In humans, PuO2 measured either in the bladder or with a polarographic electrode placed in the urinary catheter has been shown to be predictive of post-operative AKI in cardiac surgery patients.17,18 We have created a prototype investigational urinary oximeter that can be placed between the urinary catheter and collection bag to monitor continuous PuO2 measurements (Figure 1). As urine passes through the device, PuO2, temperature, and instantaneous flow rate (Uflow) are measured. The novelty of our approach is that it is noninvasive (as opposed to within the bladder), provides continuous flow measurements that are used to determine the accuracy of PuO2 measurements, is capable of providing second-to-second data, and does not need to be continually calibrated. This was an observational pilot study to test the feasibility of intra-operative PuO2 measurements using this novel device. We hypothesized that intra-operative PuO2 measurements are feasible and that low PuO2 is associated with AKI based on the above KDIGO criteria. Our secondary hypothesis was that low PuO2 is associated with increased ventilator time, ICU and hospital length of stay.
Figure 1:
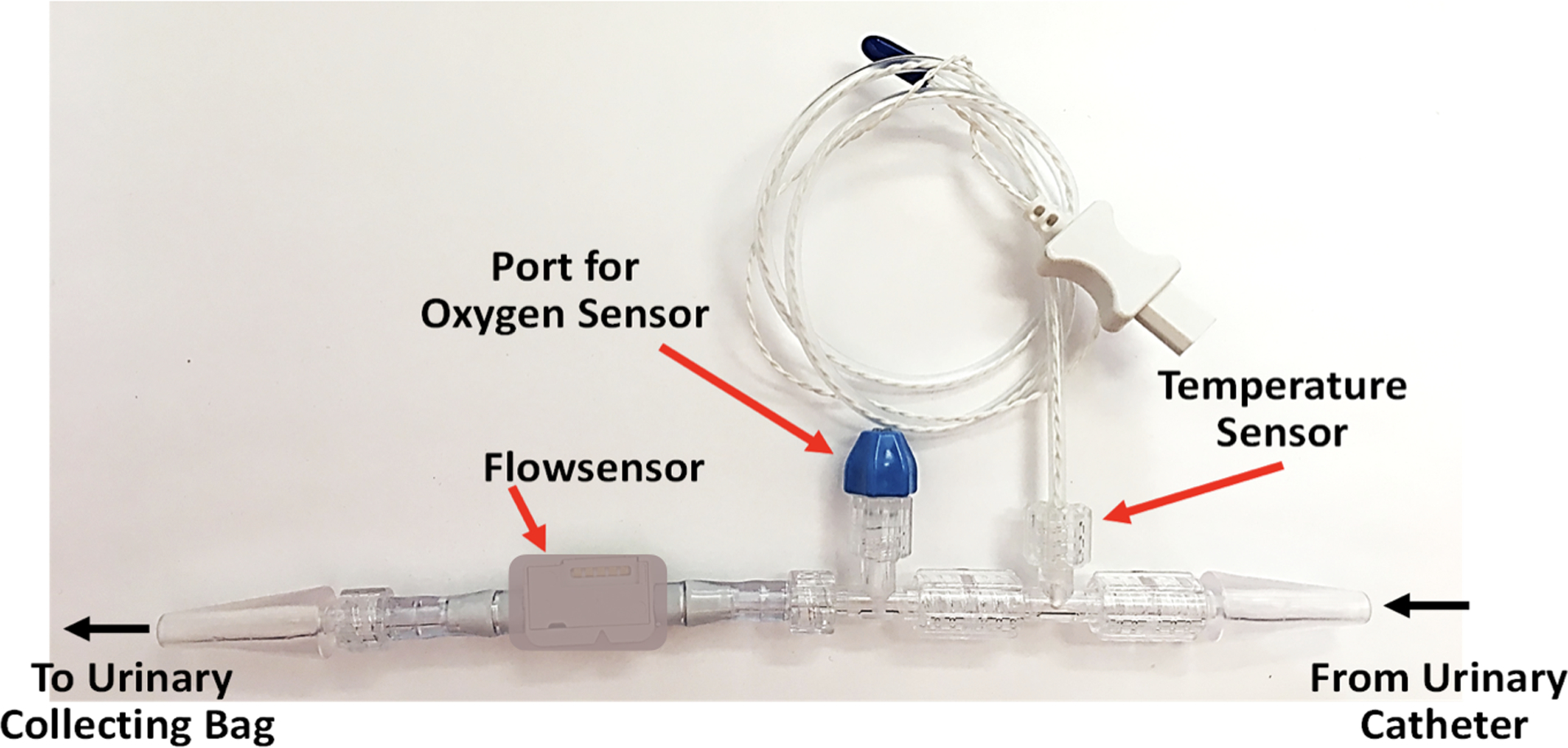
Urinary oximeter used in this study, including flow sensor, oxygen sensor port, and temperature sensor as well as the direction of urine flow from the urinary catheter, through the device, to the collecting bag.
Materials and Methods
This was a prospective observational study approved by the Institutional Review Board of the University of Utah and was registered in ClinicalTrials.gov (NCT03335865). Patients were enrolled from February 2018 to November 2019. We enrolled a convenience sample of adult cardiac surgery patients undergoing procedures that required CPB and in whom a urinary catheter was placed during surgery. Eligible patients were screened one week ahead of time according research staff and device availability. The patients during these weekly screenings who had the “highest-risk” for post-operative AKI based on Cleveland Risk Score were then approached for consent.19 Exclusion criteria included pre-operative end-stage kidney disease requiring dialysis, emergency surgery, pre-operative extracorporeal membrane oxygenation, or patient refusal. When multiple surgeries occurred in a single patient, analysis was restricted to the first surgery.
After written informed consent, the patient was brought to the operating room, monitors were placed, and anesthesia was induced at the discretion of the attending anesthesiologist. Typically, a combination of midazolam, fentanyl, propofol, and ketamine were used for induction with isoflurane and bolus fentanyl for maintenance of anesthesia. Patients received mannitol at the discretion of the attending anesthesiologist for volume management or to improve hematocrit on CPB. No other diuretic was used intra-operatively. Following induction of anesthesia, the urinary oximeter was connected between the urinary catheter and the collecting bag, and then the urinary catheter was placed in a standard sterile fashion. In the operating room, the urinary oximeter was secured to the surgical bed to prevent kinking. Continuous PuO2 and Uflow were recorded from the patient for the entire operative period as well as post-operatively in the ICU for 24 hours after the end of CPB.
Mean arterial blood pressure (MAP) was continuously measured using either a radial or femoral arterial catheter. Continuous cardiac output monitoring via a pulmonary artery catheter was indexed to body surface area and recorded as cardiac index. Cerebral oximetry was measured intra-operatively using near-infrared spectroscopy. CPB was initiated after arterial and venous cannulation. Upon completion of the surgical procedure, patients were weaned from CPB. Epinephrine and milrinone infusions were used for inotropic support. Vasopressin and norepinephrine infusions were used as vasopressors. Arterial blood gases were obtained intra-operatively as determined by the anesthesia team and the perfusionist. After chest closure, patients were transferred to the ICU with either propofol or dexmedetomidine infusions for sedation as needed. In the ICU, PuO2 and Uflow were measured continuously until the device was removed (no later than 24 hours after termination of CPB). All clinicians including the anesthesiologists, surgeons, perfusionists, intensivists, and ICU nursing staff caring for the patients were blinded to the continuous PuO2 and Uflow measurements. In the ICU, serum creatinine was measured upon admission and then at least daily along with other clinically indicated blood work. These data were collected until discharge from the ICU or post-operative day 7, which ever came first. Urine output was measured hourly by the nursing staff and recorded in the medical record until the urinary catheter was removed.
Urinary Oximeter Calibration and Filtering
The device measured PuO2 with an optical oxygen sensor that uses dynamic luminescence quenching (PreSens Precision Sensing GmbH, Germany). In this sensor an indicator dye immobilized on a small polymer disc is interrogated via an optical fiber. The indicator dye’s luminescence is highly specific to oxygen and both its intensity and its luminescent decay time are affected by oxygen partial pressure through the Stern-Volmer relationship. It is necessary to correct the measurement for temperature, as luminescent intensity and decay time are temperature-dependent.20 In contrast to polarographic oxygen sensors which rely on electrochemical principles to measure oxygen, optical luminescent sensors do not consume oxygen and can be operated without calibration over many days. In addition, the urinary oximeter contained a thermal based flow sensor (Sensirion AG, Switzerland) and a standard temperature sensor. The resolution of the oxygen sensor (± 0.2–0.3 mmHg) is within the range of oxygen partial pressure measurements obtained in this study.
Each sensor (oxygen partial pressure, flow, temperature) provided data once a second (1 Hz). The flow and temperature data were directly sampled into a tablet computer. The luminescence measurements were made via optical fiber and stored, also once a second, in a dedicated device (PreSens Precision Sensing GmbH, Germany) from where it was later downloaded for processing and analysis.
With this novel urinary oximeter, each sensor was calibrated individually. Sensors were sterilized prior to use and the effect of the sterilization process on sensor calibration is unknown. Sensors were therefore calibrated after removal from the patient, using a simulated urine solution. The optical signal and temperature were recorded at 0% and 13% oxygen concentrations at room and elevated temperatures. Using these data and multivariable linear regression, calibration constants were calculated for each sensor. Temperature measured by the device and average calibration constants were then used in the calculation of PuO2 from the luminescence measurements.
In addition to measuring the flow rate the flow sensor detected and flagged two different conditions of measurement inaccuracy: 1. if there was air-in-line or 2. if an excessive flow event occurred (defined as when the flow rate exceeded 1000 ml/h, the upper limit of the optimal range of the flow sensor). Air and/or excessive flow events were very brief and often occurred immediately after urinary catheter placement or during patient position changes.
Data points that represented negative flow rate (backflow) were flagged as invalid and triggered an algorithm that tracked the volume of negative flow. Then, when the flow moved in the positive direction again, the algorithm continued to flag data points as invalid until the urine that had flowed backwards was past the sensor. In the majority of patients, these errors represented <4% of the data.
Prior work in animals suggested that the accuracy of PuO2 measurements made distal to the renal pelvis depends on urinary flow rate.21,22 Because this novel urinary oximeter had not yet been tested in humans, we did not know the specific urinary flow rate below which our urinary oxygen measurements would become inaccurate. We chose to filter the oxygen data to measure only during urinary flows of greater than 0.5 ml/kg/h as this is a widely accepted threshold for oliguria and flows below this threshold should already identify the patient as at risk for AKI. After the data were filtered for low flow, if 30% or more of the data points were valid for a specific patient and time period (CPB or post-CPB), then an average PuO2 was calculated for that patient and time period. The mean and standard deviations of PuO2 reported in the results section are group means and group standard deviations calculated from the average PuO2 values of individual patients across the two time periods. The filtering criteria of 0.5ml/kg/h was decided on prior to the analysis and no other thresholds were analyzed.
Outcomes
The primary outcome measure for this study was the incidence of AKI as defined by the KDIGO guidelines, specifically either an increase in serum creatinine by greater than 0.3 mg/dl from baseline within 48 hours, an increase in serum creatinine >1.5 times baseline within 7 days, or urine output <0.5 ml/kg/h for more than 6 hours within the first 48 hours.7 All of these time periods were measured beginning at ICU admission. Severe AKI was defined by the KDIGO stages 2 or 3, specifically as an increase in serum creatinine >2.0–2.9 times baseline (stage 2) or an increase in serum creatinine >3.0 times baseline, >4mg/dl, or initiation of renal replacement therapy (stage 3).7 A combined outcome of death or persistently elevated serum creatinine (> 0.3mg/dl from baseline) at discharge was also determined. Other secondary outcome measures were ventilator time, ICU length of stay, and hospital length of stay.
Statistical Analysis
As the relationship between PuO2 measurements made with this novel device and the subsequent development of AKI had not been previously studied, we took an exploratory approach to the analysis. The data analysis and statistical plan were written after the data were accessed and then additional analyses were done at the request of peer reviewers. This is the primary analysis of these data.
Patient characteristics were compared between AKI and non-AKI groups using either a two-sample chi square test or Fisher’s exact test, as appropriate, for categorical variables. For continuous variables, we used an independent samples t-test or Wilcoxon rank sum test, as appropriate. Histograms were used to identify skewed data. Data were presented as means with either standard deviation or 95% confidence interval (CI) when an independent samples t-test was used or median with interquartile range when the Wilcoxon rank sum test was used. Pearson correlation was used to check for collinearity between PuO2 and other variables. To compare serum creatinine between the AKI and non-AKI groups for the 9 perioperative time points, we used independent sample t-tests and Hommel’s multiple comparison adjustment.23 Two-tailed testing was used for all comparisons. STATA version 15.1 (StataCorp LLC, College Station Texas) was used for the analysis.
The intra-operative period was divided into two time periods: CPB and post-CPB. The CPB period was from the start of the first CPB run to the end of the last CPB run if there were multiple periods of CPB. The post-CPB was the time period between the end of CPB and the end of surgery.
As there is currently no well-established cut-off for mean PuO2 measured that distally in a urinary catheter, we used an exploratory approach to identify a meaningful cut-off for AKI. Receiver operating characteristic curve analysis was used to determine the range of cut-offs for PuO2 that best predicted AKI using the KDIGO criteria (creatinine or oliguria), AKI by creatinine only criteria, AKI by oliguria criteria, severe AKI by creatinine (KDIGO stage 2/3 only), and a composite outcome of either death or persistently elevated serum creatinine at discharge (>0.3 mg/dl increase from baseline).7 Cut-off values were then varied by 1 mmHg until a single cut-off was identified that best predicted all outcomes. Risk ratios for the binary AKI outcome were estimated using a univariable binary Poisson regression model with a robust standard error.24 Multivariable binary Poisson regression models with robust standard errors were created to control for potential confounders. Beginning with all peri-operative variables with p<0.20 in the univariable analysis, variables were then eliminated in an interactive backward elimination fashion until all remaining variables had a p<0.05. To assess the stability of the model, the bootstrap inclusion fraction was computed for each predictor variable.25,26 Predictors with bootstrap inclusion fractions >50%, indicating the variable remained significant in the final model in >50% of the resamples, were determined to be reliable and not due to overfitting.
Sample Size Calculation
An a priori sample size calculation was done using the means and standard deviations from a similar study measuring PuO2 in the bladder during cardiac surgery.27 Assuming an AKI incidence of 40%, 89 total patients were needed to detect a difference in the mean PuO2 at a power of 80% using two-sided significance level of 0.05. As this was the first time this urinary oximeter was to be used in the operating room, we did not know how feasible the measurements would be or how often the device would malfunction. We also only consented patients at least one day before surgery. We did not know ahead of time how often surgeries would be canceled or rescheduled. We therefore planned to enroll up to 200 patients in hopes of achieving successful monitoring in 100 patients.
Results
Feasibility of Urinary Oximeter Measurements
Figure 2 describes the study profile. Ninety-one patients had a urinary oximeter placed. In 5 of these patients the device malfunctioned in the operating room and they were excluded from the study. The first of these was the very first patient enrolled. In this patient urine leaked from between the joints of the urinary oximeter. The study was halted and we discovered that the sterilization process had caused the plastic of the device to shrink. After this, we sealed the joints with EP30Med biocompatible glue (Master Bond, Hackensack NJ) and the problem did not occur again. In 3 patients either the flow sensor malfunctioned or was not connected properly. Without flow data, the PuO2 measurements could not be filtered for low flows. In one further patient, the oxygen sensor malfunctioned. This left 86/91 (95%) patients with intra-operative PuO2 and urinary flow measurements.
Figure 2:
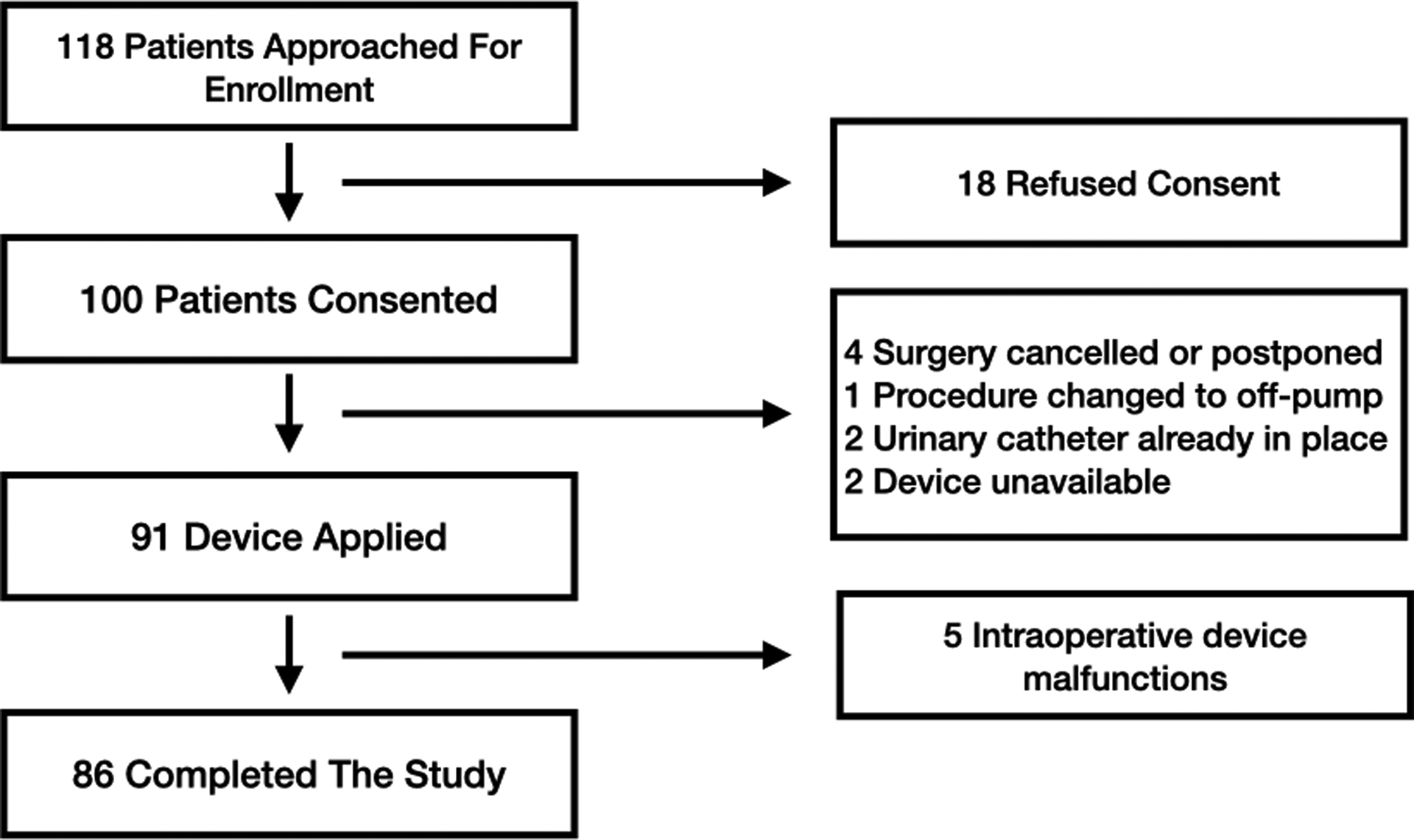
Patient enrollment profile.
In the CPB period 70/86 (81%) patients [41/53 (77%) with AKI and 29/33 (88%) without] had valid urine flows >0.5 ml/kg/h for >30% of the time period and were included in the analysis. The median (interquartile range) percentage of valid data for each patient analyzed in the CPB period was 75% (58–84). In the post-CPB period 77/86 (90%) patients [47/53 (89%) with AKI and 30/33 (91%) without] had valid urine flows >0.5 ml/kg/h for >30% of the time period and were included in the analysis. The median (interquartile range) percentage of valid data for each patient analyzed in the post-CPB period was 78% (57–92). In the ICU period, only 32/86 (37%) patients [13/53 (25%) with AKI and 19/33 (58%) without] had valid urine flows >0.5 ml/kg/h for >30% of the time period. The median (interquartile range) percentage of valid data for each patient analyzed in the post-CPB period was 40% (34–47). The ICU time period, therefore was excluded from the PuO2 analysis because there were too few patients with adequate urine flow to make a meaningful comparison.
Incidence of AKI
Of the 86 patients who completed the study, 53 (62%) developed AKI. Twenty-one of those patients met AKI criteria by creatinine elevation only and of these 10 patients had severe AKI (KDIGO stages 2/3). Five patients (5.9%) required renal replacement therapy. One patient died prior to discharge and 10 patients had persistently elevated serum creatinine at discharge. Thirty-two patients met AKI criteria by oliguria only. Table 1 compares the pre-operative clinical characteristics and risk factors of the patients who subsequently developed AKI (defined by KDIGO criteria of creatinine elevation or oliguria) and those who did not. In univariable analyses, body mass index and pre-operative insulin dependent diabetes mellitus were associated with post-operative AKI. Table 2 compares the intra-operative hemodynamic and management data for patients who subsequently developed AKI and those who did not.
Table 1:
Demographics and Pre-operative Risk Factors. The diagnosis of AKI was based on the KDIGO criteria.7
| No AKI (n=33) |
AKI (n=53) |
P value | |
|---|---|---|---|
| Age (years), mean ± SD | 62 ± 16 | 64 ± 12 | 0.628 |
| Female, n (%) | 10 (30) | 18 (34) | 0.725 |
| Body Mass Index (kg/m2), mean ± SD | 26 ± 5 | 30 ± 6 | 0.006 |
| Type of Surgery | |||
| Isolated CABG, n (%) | 8 (24) | 16 (30) | 0.550 |
| Single Valve, n (%) | 7 (21) | 13 (25) | 0.723 |
| Single Valve + CABG, n (%) | 3 (9) | 8 (15) | 0.418 |
| >1 Valve, n (%) | 4 (12) | 6 (11) | 0.910 |
| Left Ventricular Assist Device, n (%) | 4 (12) | 4 (8) | 0.476 |
| Other, n (%)* | 8 (24) | 8 (15) | 0.289 |
| Risk Factors/Comorbidities | |||
| New York Heart Associate Class >II, n (%) | 10 (30) | 18 (34) | 0.725 |
| Left Ventricular Ejection Fraction <35%, n (%) | 3 (9) | 7 (13) | 0.562 |
| Pre-operative Intra-aortic Balloon Pump, n (%) | 0 (0) | 1 (2) | >0.999 |
| COPD, n (%) | 5 (15) | 3 (6) | 0.251 |
| Insulin Dependent Diabetes, n (%) | 2 (6) | 15 (28) | 0.012 |
| Redo sternotomy, n (%) | 8 (24) | 8 (15) | 0.289 |
| Baseline Creatinine (mg/dl), mean ±SD | 1.11 ± 0.51 | 1.14 ± 0.31 | 0.700 |
| Glomerular Filtration Rate (ml/min/1.73m2), mean ± SD | 75 ± 26 | 67 ± 20 | 0.109 |
| Cleveland Risk Score, mean ± SD | 4 ± 2 | 4 ± 2 | 0.867 |
| Euroscore (%), median (Interquartile range) | 3 (2–7) | 3 (2–5) | 0.461 |
Categorical variables compared with chi-square test or Fisher’s exact test and continuous variables compared with an independent sample t-test or Wilcoxon rank sum test.
Other= septal myectomy, aortic procedures, and pulmonary endarterectomies.
CABG = coronary artery bypass grafting; COPD = chronic obstructive pulmonary disease; SD = standard deviation; There were no missing data in this table.
Table 2:
Intraoperative Hemodynamic Variables and Risk Factors. The diagnosis of AKI was based on the KDIGO criteria.7
| No AKI (n=33) |
AKI (n=53) |
Mean Difference (95% CI) |
P value* | |
|---|---|---|---|---|
| Post-CPB | 43 ± 13 n=3 (9%) missing |
37 ± 11 n=6 (11%) missing |
6 (0,11) | 0.038 |
| Post-CPB | 1.8 ± .9 | 1.5 ± 1.3 | .3 (−.3,.8) | 0.329 |
| Post-CPB | 68 ± 5 n=0 missing |
68 ± 7 n=1 (2%) missing |
0 (−3,3) | 0.917 |
| Post-CPB | 72 ± 6 n=8 (24%) missing |
71 ± 7 n=15 (28%) missing |
1 (−2,4) |
0.586 |
| Post-CPB | 2.6 ± .4 n=16 (48%) missing |
2.4 ± .4 n=39 (74%) missing |
.2 (−.1,.5) | 0.146 |
| Post-CPB | 9.7 ± 1.5 | 9.3 ± 1.5 | .3 (−.4,1.0) | 0.359 |
| Given Mannitol, n (%) | 26 (79) | 33 (62) | 17 (−3,36) | 0.108 |
| Crystalloid (L), mean ± SD | 2.1 ± .8 n=2 (6%) missing |
2.0 ± .9 n=1 (2%) missing |
.1 (−.3,.5) | 0.559 |
| Received Red Blood Cell Transfusion, n (%) | 12 (36) | 19 (36) | 0 (−20,21) | 0.961 |
| Received Fresh Frozen Plasma Transfusion, n (%) | 11 (33) | 24 (45) | −12 (−33,9) | 0.273 |
| Received Platelet transfusion, n (%) | 10 (30) | 14 (26) | 3 (−16,24) | 0.696 |
| CPB Time (minutes), mean ± SD | 167 ± 70 | 167 ± 70 | 0 (−31,31) | 0.980 |
Comparisons were made using either a two-sample chi square test or Fisher’s exact test, as appropriate for categorical variables and an independent samples t-test or Wilcoxon rank sum test as appropriate for continuous variables.
CPB = cardiopulmonary bypass. SD = standard deviation. 95% CI = 95% confidence interval. The mean and standard deviations of PuO2 reported in the results section are group means and group standard deviations calculated from the average PuO2 values of individual patients during each time period.
Urine Oxygen Data
Figure 3 provides an example of the intra-operative record of a 50-year-old male patient who subsequently developed AKI. This patient had a period of significant hypoxemia and hypotension immediately after weaning from CPB that only resolved after initiating inhaled pulmonary vasodilators. During this period, there was also a decrease in both cerebral oximetry and PuO2.
Figure 3:
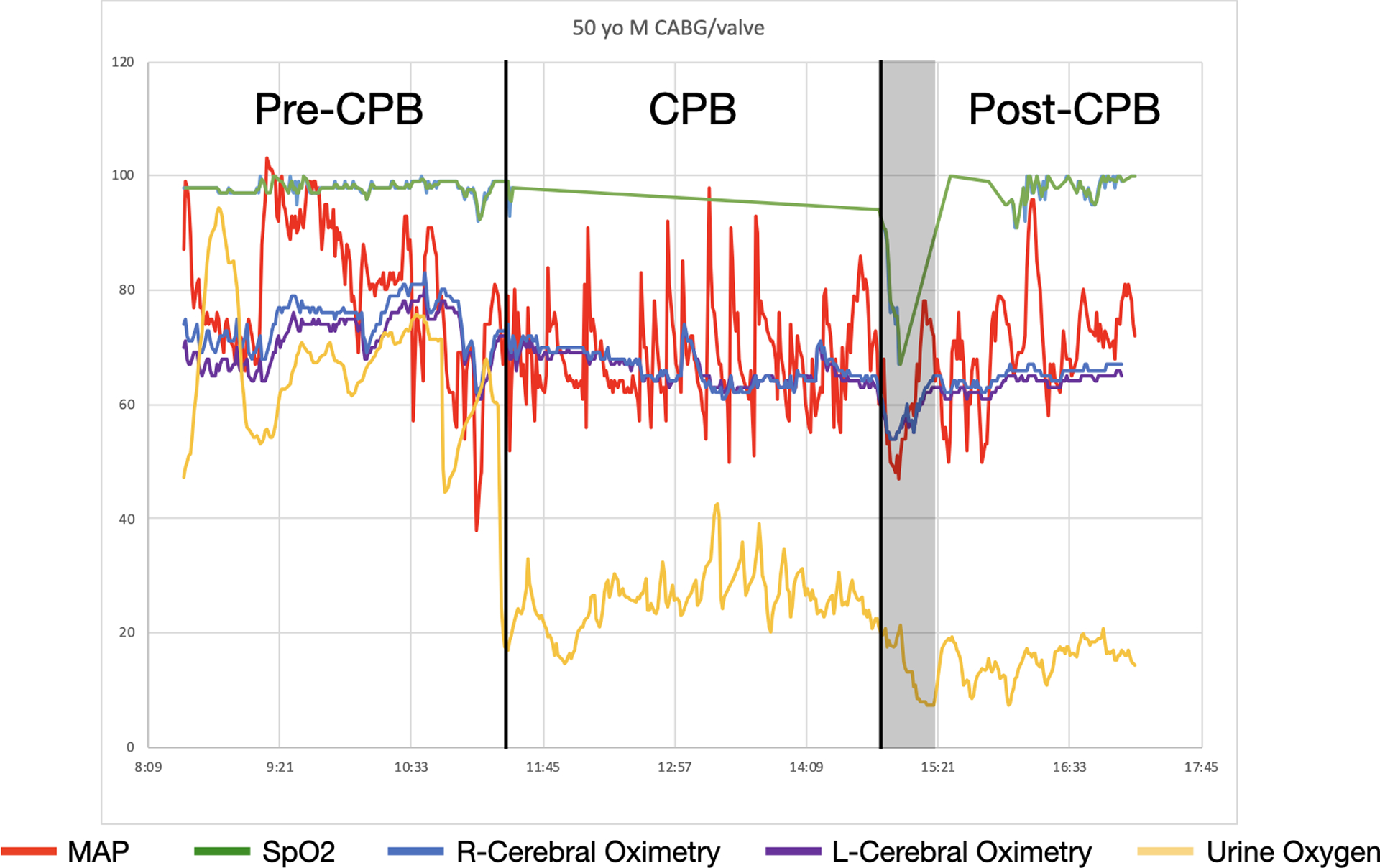
An example of the intra-operative urinary oxygen partial pressure, mean arterial pressure (MAP), pulse oximetry (SpO2), and cerebral oximetry tracings from a patient who subsequently developed AKI. The black lines indicate the start and end of CPB. The grey box highlights a time period of both hypotension and hypoxemia after bypass. During this period there was also a decrease in both cerebral oximetry and urine oxygen. MAP = red; SpO2 = green; Right cerebral oximetry = blue; Left cerebral oximetry = purple; Urine Oxygen Partial Pressure = yellow.
For the 70 patients analyzed in the CPB period, the range of individual mean PuO2 values was 18 – 66 mmHg and the group mean (SD) was 38 mmHg (11). The coefficient of variation was 0.29. During this time period there was no difference in mean PuO2 between patients who subsequently developed AKI and those who did not (mean difference 1 mmHg 95% CI −4,7; p=0.613). For the 77 patients analyzed in the post-CPB period, the range of individual mean PuO2 values was 10 – 74 mmHg and the group mean (SD) was 39 mmHg (12). The coefficient of variation was 0.31. During this time period, however, mean PuO2 was lower in those patients who subsequently developed AKI compared to those who did not (mean difference 6 mmHg 95% CI 0,11; p=0.038). When multivariable analysis was done to adjust for confounders, mean PuO2 in the post-CPB remained significantly associated with AKI. For every 10mmHg increase in post-CPB mean PuO2 there was a 18% reduction in the risk of AKI (RR 0.82 95% CI 0.71,0.95; p=0.009). We did not identify any collinearity in this model (all r values <0.15 and all p values >0.17). Figure 4 shows the timing of PuO2 changes compared to that of serum creatinine elevation over a seven-day time period. Mean PuO2 was significantly lower in AKI patients during the operative period (post-CPB) while serum creatinine did not become significantly elevated until post-operative day 2.
Figure 4:
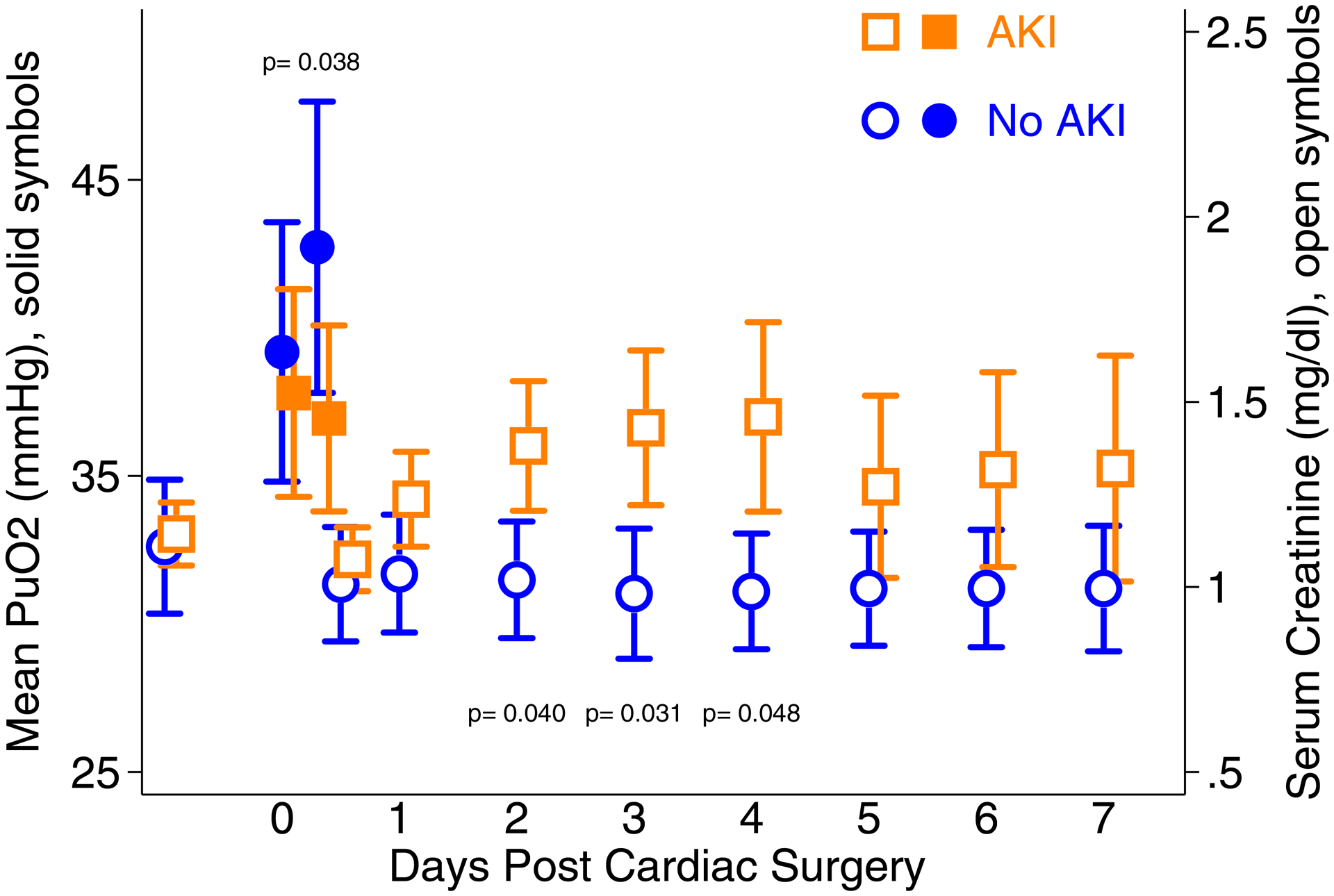
Left axis: the mean urinary oxygen partial pressure (PuO2) with 95% confidence intervals during the two intra-operative time periods (cardiopulmonary bypass and post cardiopulmonary bypass) for the patients who developed AKI (orange solid squares) and those who did not (blue solid circles). Right axis: daily serum creatinine measurements with 95% confidence intervals from baseline until post-operative day 7 for patients who developed AKI (orange open squares) and those who did not (blue open circles). P values from comparisons at specific time points for the AKI and non-AKI groups are reported if < 0.05. P values for serum creatinine were adjusted for multiple comparisons using Hommel’s procedure.
Table 3 shows a sensitivity analysis comparing post-CPB PuO2 to various definitions of AKI. Mean PuO2 during the post-CPB period was associated with the primary definition of AKI (full KDIGO criteria: creatinine or oliguria), but was not associated with oliguria alone, elevated creatinine alone or severe AKI (stage 2/3). Using a threshold approach, however, a cut-off of mean PuO2 <25 mmHg during the post-CPB period was found to be associated with the primary outcome of AKI (full KDIGO criteria: creatinine or oliguria) as well as AKI by creatinine only, severe AKI, and death or persistently elevated creatinine at hospital discharge. These findings remained significant after multivariable analysis was used to adjust for confounders (full KDIGO criteria: relative risk 1.9; 95%CI 1.3,2.8; p=0.001, creatinine elevation only: relative risk 3.1; 95% CI 1.4,6.9; p=0.006, severe AKI: relative risk 4.6; 95% CI 1.4,15.3; p=0.014, death or elevated serum creatinine at discharge: relative risk 6.6; 95% CI 1.6,27.6; p=0.009). We did not identify any collinearity in these models (all r values < 0.17 and all p values > 0.12).
Table 3:
Sensitivity Analysis of Post-CPB Mean Urine Oxygen Partial Pressure Compared to Various Definitions of AKI in 86 Patients
| Number (%) of Patients with AKI |
Unadjusted Relative Risk of AKI for Every 10mmHg Increase in Mean PuO2 (95% CI) | p | Unadjusted Relative Risk of AKI if Mean PuO2 < 25mmHg (95% CI) | p | |
|---|---|---|---|---|---|
| Full KDIGO | 53 (62%) | 0.84 (0.73–0.99) | 0.032 | 1.51 (1.08–2.10) | 0.015 |
| Oliguria Only | 49 (57%) | 0.89 (0.76–1.04) | 0.149 | 1.08 (.61–1.92) | 0.798 |
| Creatinine Only | 21 (24%) | 0.92 (0.65–1.31) | 0.650 | 2.46 (1.06–5.7) | 0.036 |
| KDIGO Stage 2/3 | 10 (12%) | 0.70 (0.43–1.13) | 0.147 | 3.70 (1.18–11.6) | 0.025 |
| Death or Kidney Injury at Discharge | 11 (13%) | 0.81 (0.50–1.31) | 0.390 | 3.23 (1.06–9.9) | 0.039 |
Comparisons were made using univariable binary Poisson regression with a robust standard error. AKI= acute kidney injury; 95% CI = 95% confidence interval. During the post-CPB period there were 9 patients who were excluded because of inadequate PuO2 data after filtering for low or invalid urine flows. AKI= acute kidney injury; 95% CI = 95% confidence interval; PuO2 = urine oxygen partial pressure; Baseline Cr = Baseline serum creatinine; Full KDIGO= creatinine elevation or oliguria based on the KDIGO guidelines; CPB = cardiopulmonary bypass.
Continuous Urinary Flow
Mean continuous urine flow (Uflow) in the CPB and post-CPB was also compared to the primary outcome of AKI (full KDIGO criteria: creatinine or oliguria) but did not differ between groups (Table 2). Only 8 of 86 patients (9.3%) during the CPB period and 9 of 86 patients (10.5%) during the post-CPB period had a mean Uflow <0.5 ml/kg/h. There was no collinearity of between PuO2 and Uflow during either the CPB (r=−0.066; p=0.589) or post-CPB (r=0.056; p=0.627) periods.
Mannitol
Mannitol administration was not associated with a significant difference in mean Uflow during either time period (Uflow for no mannitol vs mannitol given during CPB: 1.9 vs 2.2 ml/kg/h, p = 0.292; post-CPB: 1.7 vs 1.5 ml/kg/h, p=0.548). Mannitol administration was also not associated with a significant difference in PuO2 during either time period (PuO2 for no mannitol vs mannitol given during CPB: 41 vs 37 mmHg, p=0.290; post-CPB: 39 vs 39 mmHg, p=0.949).
Other Secondary Outcomes
The median ventilator time was 14 (interquartile range 10–30) hours. Patients in the upper 25% of ventilator time had a significantly lower mean PuO2 in the post-CPB period (mean difference 7 mmHg 95% CI 1,13; p=0.026). Median ICU and hospital length of stay were 4 (interquartile range 3–7) days and 9 (interquartile range 7–13) days respectively. There was no difference in mean PuO2 for patients in the upper 25% of either ICU or hospital length of stay (mean difference 1 and 0 mmHg; p=0.807 and p=0.954 respectively).
Discussion
Urine oxygen partial pressure has been called “a clinical window on the health of the renal medulla” as numerous studies have demonstrated a strong association with medullary oxygen concentrations.14 In animal models, PuO2 is a sensitive indicator of decreased renal blood flow.15,28 In an ovine model of sepsis, restoration of MAP with norepinephrine improved urine output but resulted in a further reduction of medullary oxygenation and PuO2.16 Kainuma et al. found that cardiac surgery patients with decreased PuO2 after CPB had significantly higher post-operative serum creatinine concentrations.18 More recently, Zhu et al. found that cardiac surgery patients who developed AKI had lower intra-operative PuO2.27 We found that intra-operative PuO2 measurements made with a noninvasive urinary oximeter placed distal to the urinary catheter were feasible and a lower mean PuO2 during the post-CPB period was independently associated with AKI.
Like Kainuma et al. our PuO2 measurements were taken from the urinary catheter but we used luminescence quenching instead of a polarographic electrode, as the latter technology requires more frequent calibration. Zhu et al also used luminescence but their measurements were made within the bladder. In addition, we concomitantly measured urine flow and filtered for low flows, something that was not reported in either of the prior studies. The mean PuO2 values we obtained during post-CPB (37–43mmHg) were lower than the values found by Kainuma et al (65–73mmHg) but higher than those found by Zhu et al. (19–27mmHg). It is possible that measuring PuO2 distal to the bladder results in higher values because of the inadvertent ingress of oxygen into the urinary catheter from the surrounding tissue or atmosphere. Such oxygen ingress would be more pronounced during periods of low urine flow which is why we took care to invalidate oxygen measurements when urine flows were very low. Kainuma et al did not report this type of filtering and that may explain why their oxygen partial pressure values were higher than ours. The oxygen permeability of urinary catheters and the subsequent effect on PuO2 measurements needs further evaluation.
Interestingly, both Kainuma et al. and Zhu et al. found that PuO2 during the post-CPB period was associated with AKI, but PuO2 during CPB was not. This is consistent with our findings and may be related to the difference in hemodynamic conditions that occur during CPB vs immediately after weaning. Recent work suggests that renal oxygen delivery decreases and oxygen extraction increases during CPB and that this impairment of renal oxygen supply/demand is even more pronounced in the post-CPB period.29 During this time, low cardiac index, hemodilution, and the use of vasoactive agents contribute to poor oxygen delivery whilst warm temperatures result in increased oxygen consumption. Thus, more renal hypoxia may occur in the post-CPB period than during CPB when the patient is cool and mechanically supported by the heart and lung machine.
The KDIGO guidelines recommend using serum creatinine elevation and oliguria for the diagnosis of AKI. Both are reflections of glomerular filtration rate, a widely accepted index of renal function.7 Accurate post-operative urine output data, however, are difficult to obtain and many studies of cardiac surgery associated AKI only report serum creatinine changes.27,30–32 Other investigators may forgo urine output criteria for AKI because of the use of diuretics such as mannitol or because of acute shifts in fluid balance that occur in the perioperative period. The prognostic value of oliguria after cardiac surgery is uncertain.33–35 Creatinine criteria appear to be more closely associated with ICU length of stay and short-term mortality, whereas urine output criteria may be more associated with long term mortality.33,34
The severity of AKI after cardiac surgery is known to have a proportional effect on mortality and hospital costs.3,6,36 In one study, severe AKI accounted for up to 94% of hospital costs, 10 times the mortality compared to those without AKI, and 5 times the mortality compared to those with mild AKI.6 We found that mean PuO2 was associated with severe AKI (KDIGO stage 2/3) if a threshold approach was used with a cut-off of 25 mmHg. These results need further validation in larger studies where severe AKI is the primary outcome.
Decreased urinary flow during CPB has previously been described as a risk factor for AKI, though CPB urinary flows appear to be higher than during other perioperative periods.37,38 Hori et al. found that the optimal cut-off to predict post-operative AKI was a urine output of <1.5 ml/kg/h during CPB and that only 5.7% of patients had CPB urine output <0.5 ml/kg/h when 30% of patients developed AKI.38 Similarly, we found that only 9.3% of patients during CPB had a mean Uflow <0.5ml/kg/h whilst 63% of our patients developed AKI. These findings suggest that this well-described cut-off for oliguria may not be useful during CPB.
There is conflicting evidence about the effect of mannitol on cardiac surgery associated AKI.39–41 Although generally used as an osmotic diuretic, we found that the administration of mannitol did not have a statistically significant effect on Uflow. This might have been because of relatively low doses used or because urinary flows after the initiation of CPB are already generally high blunting the effect of mannitol administration. Prior studies have suggested that PuO2 measurements may be affected by the use of diuretics such as furosemide.42 In our study, however, mannitol had no significant effect on PuO2 during either time period.
Limitations
The first limitation of this study was the exploratory approach taken in the analysis. This was the first time the device was tested either in humans or in animals. No established threshold exists for PuO2 and this small pilot study was not powered for diagnostic validation. We theorized that the relationship between PuO2 and the subsequent development of AKI might be more of a threshold response than a linear dose response. Indeed, a mean PuO2 <25 mmHg during the post-CPB period was associated with AKI as defined by the full KDIGO criteria as well as creatinine elevation alone, severe AKI, and a combined outcome of death or persistently elevated creatinine at discharge. The cut-off of 25 mmHg was discovered in an exploratory fashion, however, and should not be adopted for clinical use without proper validation.43,44
The second limitation was a technical limitation in the accuracy of PuO2 in stagnant urine. During low flow, urine in the catheter could be subject to the ingress of oxygen from the surrounding tissue or environment and may poorly reflect the concurrent oxygen environment of the medulla.21 Indeed, we observed that very low flow or no flow states were associated with elevated PuO2 measurements. To account for this limitation, we filtered out PuO2 data during periods of low flow. Because this noninvasive urinary oximeter is so new, we did not know the precise flow below which PuO2 measurements would become inaccurate. We therefore used a clinically relevant flow for filtering (0.5 ml/kg/h). We reasoned that flows below this rate would be considered at risk for AKI regardless of the PuO2. We also arbitrarily chose to include patients from each time period if >30% of their PuO2 data were valid after filtering for these low flows. The actual percentage of valid data, however, was much higher in the majority of patients. Filtering may have limited the feasibility of the device as patients with persistently low urine flows were excluded. This could have led to selection bias. No other filtering criteria were evaluated. Future work should be directed at determining the flow below which PuO2 measurements become inaccurate when using this noninvasive urinary oximeter. This could be done through mathematical modeling, in-vitro studies, or animal models.
Another limitation is that we did not record diuretic use in the ICU. Although rarely used in our ICU in the first 24 hours post-operatively, diuretic use may have confounded our definition of AKI. Finally, although this was a convenience sample, we enrolled patients at high risk for AKI to ensure an adequate incidence of the primary outcome. This could also have contributed to selection bias. Future work should focus on high and low risk patients.
Conclusions
Intra-operative measurements of PuO2 are feasible using a noninvasive device placed distal to the urinary catheter. PuO2 in the post-CPB period was associated with AKI after cardiac surgery. Further research is needed to validate these findings and to elucidate whether PuO2 can be used to trigger interventions to successfully prevent or reduce the severity of cardiac surgery associated AKI.
Acknowledgments:
We would like to acknowledge Ami Stuart, PhD and the University of Utah Department of Anesthesiology Research Council for the support of this project; Julia White, Yuri Kida, Sam Parry, and Cameron Jacobson for their assistance in project management and data collection; the University of Utah Division of Cardiothoracic surgery, and the nurses in the cardiovascular intensive care unit.
Funding Statement:
This work was supported in part by the University of Utah Department of Anesthesiology Research Council, a University Technology Acceleration Grant from the Utah Science, Technology and Research Initiative (USTAR), the University of Utah Population Health Research (PHR) Foundation, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR002538 (formerly 5UL1TR001067-05, 8UL1TR000105 and UL1RR025764), and a career development award from the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR002538 and KL2TR002539. These organizations were not involved in study design, analysis, interpretation, or manuscript creation.
Footnotes
ClinicalTrials.gov Identifier: NCT03335865
Prior Presentations: None
Disclosure Statement: Natalie Silverton, Kai Kuck, Bradley Stringer, Spencer Shumway, and Lars Lofgren are inventors on a patent application for the urine oxygen and flow sensing technology. This prototype is under development for commercial consideration by Natalie Silverton, Kai Kuck, Bradley Stringer and Spencer Shumway but as of yet no commercial activity has occurred. This work was performed under a conflict of interest management plan approved by the University of Utah Conflict of Interest Office. This included disclosure of conflict of interest to patients and collaborators and an independent peer review of the data analysis. The interpretation and reporting of these data are the responsibility of the authors alone and should not be seen as an official policy of or interpretation by the US Government, nor does this report necessarily represent the official views of the National Institutes of Health.
References:
- 1.Reents W, Hilker M, Borgermann J, Albert M, Plotze K, Zacher M, Diegeler A, Boning A: Acute kidney injury after on-pump or off-pump coronary artery bypass grafting in elderly patients. Ann Thorac Surg 2014; 98: 9–14 [DOI] [PubMed] [Google Scholar]
- 2.Gaffney AM, Sladen RN: Acute kidney injury in cardiac surgery. Curr Opin Anaesthesiol 2015; 28: 50–9 [DOI] [PubMed] [Google Scholar]
- 3.Machado MN, Nakazone MA, Maia LN: Prognostic value of acute kidney injury after cardiac surgery according to kidney disease: improving global outcomes definition and staging (KDIGO) criteria. PLoS One 2014; 9: e98028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Englberger L, Suri RM, Li Z, Casey ET, Daly RC, Dearani JA, Schaff HV: Clinical accuracy of RIFLE and Acute Kidney Injury Network (AKIN) criteria for acute kidney injury in patients undergoing cardiac surgery. Crit Care 2011; 15: R16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, Landolfo K: Acute renal failure following cardiac surgery. Nephrol Dial Transplant 1999; 14: 1158–62 [DOI] [PubMed] [Google Scholar]
- 6.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA: Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008; 23: 1970–4 [DOI] [PubMed] [Google Scholar]
- 7.Group KDIGOKAKIW: KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012; 2012: 1–138 [Google Scholar]
- 8.Elmedany SM, Naga SS, Elsharkawy R, Mahrous RS, Elnaggar AI: Novel urinary biomarkers and the early detection of acute kidney injury after open cardiac surgeries. J Crit Care 2017; 40: 171–177 [DOI] [PubMed] [Google Scholar]
- 9.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX, Consortium T-A: Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 2011; 22: 1748–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cummings JJ, Shaw AD, Shi J, Lopez MG, O’Neal JB, Billings FTt: Intraoperative prediction of cardiac surgery-associated acute kidney injury using urinary biomarkers of cell cycle arrest. J Thorac Cardiovasc Surg 2019; 157: 1545–1553 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans RG, Ince C, Joles JA, Smith DW, May CN, O’Connor PM, Gardiner BS: Haemodynamic influences on kidney oxygenation: clinical implications of integrative physiology. Clin Exp Pharmacol Physiol 2013; 40: 106–22 [DOI] [PubMed] [Google Scholar]
- 12.Ranucci M, Romitti F, Isgro G, Cotza M, Brozzi S, Boncilli A, Ditta A: Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg 2005; 80: 2213–20 [DOI] [PubMed] [Google Scholar]
- 13.Leonhardt KO, Landes RR, McCauley RT: Anatomy and Physiology of Intrarenal Oxygen Tension: Preliminary Study of the Effects of Anesthetics. Anesthesiology 1965; 26: 648–58 [DOI] [PubMed] [Google Scholar]
- 14.Evans RG, Smith JA, Wright C, Gardiner BS, Smith DW, Cochrane AD: Urinary oxygen tension: a clinical window on the health of the renal medulla? Am J Physiol Regul Integr Comp Physiol 2014; 306: R45–50 [DOI] [PubMed] [Google Scholar]
- 15.Kainuma M, Kimura N, Shimada Y: Effect of acute changes in renal arterial blood flow on urine oxygen tension in dogs. Crit Care Med 1990; 18: 309–12 [DOI] [PubMed] [Google Scholar]
- 16.Lankadeva YR, Kosaka J, Evans RG, Bailey SR, Bellomo R, May CN: Intrarenal and urinary oxygenation during norepinephrine resuscitation in ovine septic acute kidney injury. Kidney Int 2016; 90: 100–8 [DOI] [PubMed] [Google Scholar]
- 17.Zhu MZL, Martin A, Cochrane AD, Smith JA, Thrift AG, Harrop GK, Ngo JP, Evans RG: Urinary hypoxia: an intraoperative marker of risk of cardiac surgery-associated acute kidney injury. Nephrol Dial Transplant 2018; 33: 2191–2201 [DOI] [PubMed] [Google Scholar]
- 18.Kainuma M, Yamada M, Miyake T: Continuous urine oxygen tension monitoring in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth 1996; 10: 603–8 [DOI] [PubMed] [Google Scholar]
- 19.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 2005; 16: 162–8 [DOI] [PubMed] [Google Scholar]
- 20.Wang XD, Wolfbeis OS: Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chem Soc Rev 2014; 43: 3666–761 [DOI] [PubMed] [Google Scholar]
- 21.Sgouralis I, Kett MM, Ow CP, Abdelkader A, Layton AT, Gardiner BS, Smith DW, Lankadeva YR, Evans RG: Bladder urine oxygen tension for assessing renal medullary oxygenation in rabbits: experimental and modeling studies. Am J Physiol Regul Integr Comp Physiol 2016; 311: R532–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rennie DW, Reeves RB, Pappenheimer JR: Oxygen pressure in urine and its relation to intrarenal blood flow. Am J Physiol 1958; 195: 120–32 [DOI] [PubMed] [Google Scholar]
- 23.SP W: Adjusted p-values for simultaneous inference. Biometrics 1992; 48: 1005–1013 [Google Scholar]
- 24.Zou G: A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159: 702–6 [DOI] [PubMed] [Google Scholar]
- 25.Sauerbrei W, Schumacher M: A bootstrap resampling procedure for model building: application to the Cox regression model. Stat Med 1992; 11: 2093–109 [DOI] [PubMed] [Google Scholar]
- 26.Royson PSW: Bootstrap Assessment of the Stability of Multivariable Models. Stata J 2009; 9: 547–570 [Google Scholar]
- 27.Zhu MZL, Martin A, Cochrane AD, Smith JA, Thrift AG, Harrop GK, Ngo JP, Evans RG: Urinary hypoxia: an intraoperative marker of risk of cardiac surgery-associated acute kidney injury. Nephrol Dial Transplant 2018 [DOI] [PubMed] [Google Scholar]
- 28.Kitashiro S, Iwasaka T, Sugiura T, Takayama Y, Tamura T, Tamura K, Inada M: Monitoring urine oxygen tension during acute change in cardiac output in dogs. J Appl Physiol (1985) 1995; 79: 202–4 [DOI] [PubMed] [Google Scholar]
- 29.Lannemyr L, Bragadottir G, Krumbholz V, Redfors B, Sellgren J, Ricksten SE: Effects of Cardiopulmonary Bypass on Renal Perfusion, Filtration, and Oxygenation in Patients Undergoing Cardiac Surgery. Anesthesiology 2017; 126: 205–213 [DOI] [PubMed] [Google Scholar]
- 30.Karkouti K, Grocott HP, Hall R, Jessen ME, Kruger C, Lerner AB, MacAdams C, Mazer CD, de Medicis E, Myles P, Ralley F, Rheault MR, Rochon A, Slaughter MS, Sternlicht A, Syed S, Waters T: Interrelationship of preoperative anemia, intraoperative anemia, and red blood cell transfusion as potentially modifiable risk factors for acute kidney injury in cardiac surgery: a historical multicentre cohort study. Can J Anaesth 2015; 62: 377–84 [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen SR, Kandler K, Nielsen RV, Cornelius Jakobsen P, Knudsen NN, Ranucci M, Christian Nilsson J, Ravn HB: Duration of critically low oxygen delivery is associated with acute kidney injury after cardiac surgery. Acta Anaesthesiol Scand 2019; 63: 1290–1297 [DOI] [PubMed] [Google Scholar]
- 32.Mathis MR, Naik BI, Freundlich RE, Shanks AM, Heung M, Kim M, Burns ML, Colquhoun DA, Rangrass G, Janda A, Engoren MC, Saager L, Tremper KK, Kheterpal S, Aziz MF, Coffman T, Durieux ME, Levy WJ, Schonberger RB, Soto R, Wilczak J, Berman MF, Berris J, Biggs DA, Coles P, Craft RM, Cummings KC, Ellis TA 2nd, Fleishut PM, Helsten DL, Jameson LC, van Klei WA, Kooij F, LaGorio J, Lins S, Miller SA, Molina S, Nair B, Paganelli WC, Peterson W, Tom S, Wanderer JP, Wedeven C, Multicenter Perioperative Outcomes Group I: Preoperative Risk and the Association between Hypotension and Postoperative Acute Kidney Injury. Anesthesiology 2020; 132: 461–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petaja L, Vaara S, Liuhanen S, Suojaranta-Ylinen R, Mildh L, Nisula S, Korhonen AM, Kaukonen KM, Salmenpera M, Pettila V: Acute Kidney Injury After Cardiac Surgery by Complete KDIGO Criteria Predicts Increased Mortality. J Cardiothorac Vasc Anesth 2017; 31: 827–836 [DOI] [PubMed] [Google Scholar]
- 34.McIlroy DR, Argenziano M, Farkas D, Umann T, Sladen RN: Incorporating oliguria into the diagnostic criteria for acute kidney injury after on-pump cardiac surgery: impact on incidence and outcomes. J Cardiothorac Vasc Anesth 2013; 27: 1145–52 [DOI] [PubMed] [Google Scholar]
- 35.Howitt SH, Grant SW, Caiado C, Carlson E, Kwon D, Dimarakis I, Malagon I, McCollum C: The KDIGO acute kidney injury guidelines for cardiac surgery patients in critical care: a validation study. BMC Nephrol 2018; 19: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A: Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 2009; 119: 2444–53 [DOI] [PubMed] [Google Scholar]
- 37.Song Y, Kim DW, Kwak YL, Kim BS, Joo HM, Ju JW, Yoo YC: Urine Output During Cardiopulmonary Bypass Predicts Acute Kidney Injury After Cardiac Surgery: A Single-Center Retrospective Analysis. Medicine (Baltimore) 2016; 95: e3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hori D, Katz NM, Fine DM, Ono M, Barodka VM, Lester LC, Yenokyan G, Hogue CW: Defining oliguria during cardiopulmonary bypass and its relationship with cardiac surgery-associated acute kidney injury. Br J Anaesth 2016; 117: 733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisher AR, Jones P, Barlow P, Kennington S, Saville S, Farrimond J, Yacoub M: The influence of mannitol on renal function during and after open-heart surgery. Perfusion 1998; 13: 181–6 [DOI] [PubMed] [Google Scholar]
- 40.Yallop KG, Sheppard SV, Smith DC: The effect of mannitol on renal function following cardio-pulmonary bypass in patients with normal pre-operative creatinine. Anaesthesia 2008; 63: 576–82 [DOI] [PubMed] [Google Scholar]
- 41.Ip-Yam PC, Murphy S, Baines M, Fox MA, Desmond MJ, Innes PA: Renal function and proteinuria after cardiopulmonary bypass: the effects of temperature and mannitol. Anesth Analg 1994; 78: 842–7 [DOI] [PubMed] [Google Scholar]
- 42.Tolley PM, Purcell A, Bolsin SN: Effect of i.v. furosemide on pelvic urinary oxygen tension in humans. Br J Anaesth 1999; 83: 328–9 [DOI] [PubMed] [Google Scholar]
- 43.Royston P, Altman DG, Sauerbrei W: Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006; 25: 127–41 [DOI] [PubMed] [Google Scholar]
- 44.Boucher KM, Slattery ML, Berry TD, Quesenberry C, Anderson K: Statistical methods in epidemiology: a comparison of statistical methods to analyze dose-response and trend analysis in epidemiologic studies. J Clin Epidemiol 1998; 51: 1223–33 [DOI] [PubMed] [Google Scholar]


