Summary
Hepatic stellate cells (HSCs) are located in the space of Disse, between liver sinusoidal endothelia cells (LSECs) and hepatocytes. They have surprised and excited hepatologists for their biological characteristics. Under physiological quiescent conditions, HSCs are the major vitamin A-storing cells of the liver, playing crucial roles in the liver development, regeneration, and tissue homeostasis. Upon injury-induced activation, HSCs convert to a pro-fibrotic state, producing the excessive extracellular matrix (ECM) and promoting angiogenesis in the liver fibrogenesis. Activated HSCs significantly contribute to liver fibrosis progression and inactivated HSCs are key to liver fibrosis regression. In this review, we summarize the comprehensive understanding of HSCs features, including their roles in normal liver and liver fibrosis in hopes of advancing the development of emerging diagnosis and treatment for hepatic fibrosis.
Keywords: HSCs, Quiescent HSCs, Activated HSCs, Liver fibrosis, Normal liver
Introduction
As a metabolic organ, the liver is known for its regenerative capacity, and plays an important role in the body such as synthesizing, breaking down, filtering, storing and producing bile (Grunsven 2017). The liver consists of parenchymal cells (hepatocytes) and non-parenchymal cells (NPCs) such as HSCs, LSECs, biliary epithelial cells, Kupffer cells, natural killer cells, B cells and macrophages (Mazza et al. 2017). Hepatic fibrosis is a dynamic process and results from chronic liver injury of different etiologies, containing chronic viral infection (e.g. HBC, HCV), alcoholic liver disease (ALD), fatty liver disease, non-alcoholic fatty liver disease (NAFLD)/nonalcoholic steatohepatitis (NASH) and other conditions (Sherman 2018). Hepatic fibrosis is considered as a reversible pathological process, characterized by excessive accumulation of ECM (Schildberg et al. 2015). HSCs play a vital role in the fibrogenesis, differentiating into myofibroblasts leading to hepatic fibrosis after activated (Friedman 2008b).
HSCs located in the perisinusoidal space of Disse are the main ECM-producers of the liver, and significantly contribute to its roles by interaction with adjacent cells (Lee et al. 2015). In a healthy liver, quiescent HSCs represent 15 % of the total number of liver cells and preserve retinoid storage (Seki 2015). Upon liver injury, HSCs receive signals and become activated, transdifferentiating into ECM-producing myofibroblast-like cells, activated HSCs are proliferative and fibrogenic phenotype (Fig. 1). This review focuses on the understanding of HSCs to grasp the characteristics of quiescent HSCs and activated HSCs, directing therapeutic targeting of anti-fibrotic strategy.
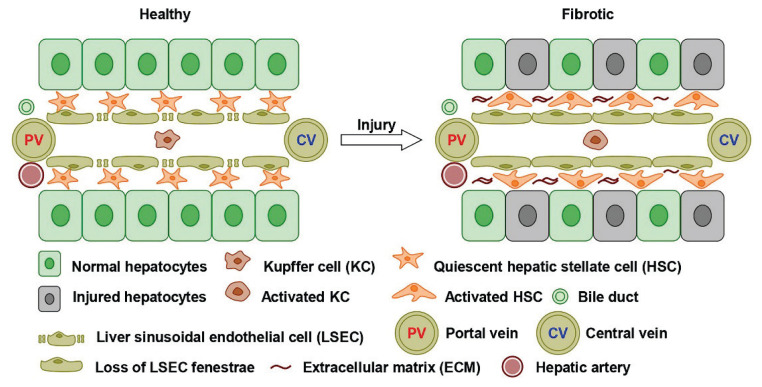
Fig. 1.
Anatomical position of HSCs and liver sinusoids to injury. HSCs are located in the space of Disse, between LSECs and hepatocytes. Under liver injury, HSCs convert to a pro-fibrotic state, producing the excessive ECM and promoting the liver fibrogenesis.
Embryonic origin and ultrastructure of HSCs
In 1876, the HSC was found by Kupffer using gold chloride method (Doherty 2016). As a vitamin A-storing mesenchymal cell type, HSC is crucial to liver regeneration and fibrosis (Koyama et al. 2017). The embryonic origin of HSCs is unclear because they express marker genes of the endoderm and mesodermal (Friedman 2008a, Marrone et al. 2016). In the view of an endoderm origin, HSCs express CD34 and cytokeratin-7/8, which are typically localized to the endodermal during liver development (Eggert et al. 2017). In the view of a mesodermal origin, lineage tracing of the mesoderm transcriptional factor Foxf1 showed that HSCs develop from the septum transversum mesenchyme, suggestive of a mesodermal origin (Yanguas et al. 2016). Meanwhile, bone marrow- and neural-derived cells are also thought to contribute to both quiescent and activated HSCs (Zoubek et al. 2017).
HSC is one of the non-parenchymal cells in the liver, also known as fat-storing cell, Ito cells, lipocyte, interstitial cell, perisinusoidal cell or vitamin A-storing cell (Schumacher et al. 2016). HSCs are located in the space of Disse, communicating with the neighboring cell types such as hepatocytes, LSECs, biliary epithelial cells, hepatic progenitor cells, Kupffer cells and B cells via soluble mediators or cytokines (Thompson et al. 2015). HSCs in normal liver have irregular star-shaped cell bodies with round or oval nuclei (Koyama et al. 2016). In quiescent state, they do not or rarely express α-smooth muscle actin (α-SMA), with low proliferative activity and low capacity of collagen synthesis (Huang et al. 2017). Their perikaryons lie in recesses between hepatocytes and LSECs (Bansal 2016). They have small Golgi complex, rough endoplasmic reticulum (rER) and dendritic cytoplasmic protuberances (Kitano et al. 2016).
Activation and deactivation of HSCs
HSCs are quiescent in normal liver, the functions of quiescent HSCs mainly include vitamin A-metabolizing and -storing, fat-storing, synthesis of matrix metallo proteinase (MMP) and tissue inhibitor of metallo proteinase (TIMP-1), expression of cytokines and receptors, and regulation of liver sinusoidal blood (Tsuchida et al. 2017). HSCs are activated when the liver is damaged by inflammation or mechanical stimulation, its features contain expression of α-SMA, secretion of ECM and so on (Shang et al. 2018). The mechanism of HSCs activation is complex, including cellular events, molecular dysregulation, and other complex factors (Seki 2015). As the predecessors of proliferative myofibroblasts, activated HSCs spark efforts to understand their phenotypes transdifferentiation and how they contribute to liver fibrosis (Jung et al. 2017). Quiescent HSC is identified in a nonproliferative state, with a distinctive feature of cytoplasmic lipid droplets (Hellerbrand 2013). Upon liver injury, HSCs become activated, transdifferentiating to myofibroblasts (Preziosi et al. 2017). These activated HSCs are proliferative, contractile, and characterized by excessive ECM production (Zhang et al. 2016). Other resident liver cells promote or inhibit HSCs activation via released or secreted factors (Fig. 2). The sequence of HSC activation featuring initiation followed by perpetuation (Hsieh et al. 2015). Initiation is characterized by events rendering the HSCs responsive to many extracellular signals (Seo et al. 2016). Development of a contractile and fibrogenic phenotype, modulation of growth factor signaling, as well as are initiation phase features (Zhou et al. 2014). Perpetuation characterizes amplification of the activated phenotype (Elpek 2014). The perpetuation phase results in fibrogenesis through matrix production, contractility, proinflammatory signaling and enhanced HSC proliferation (Tsuchida et al. 2017).
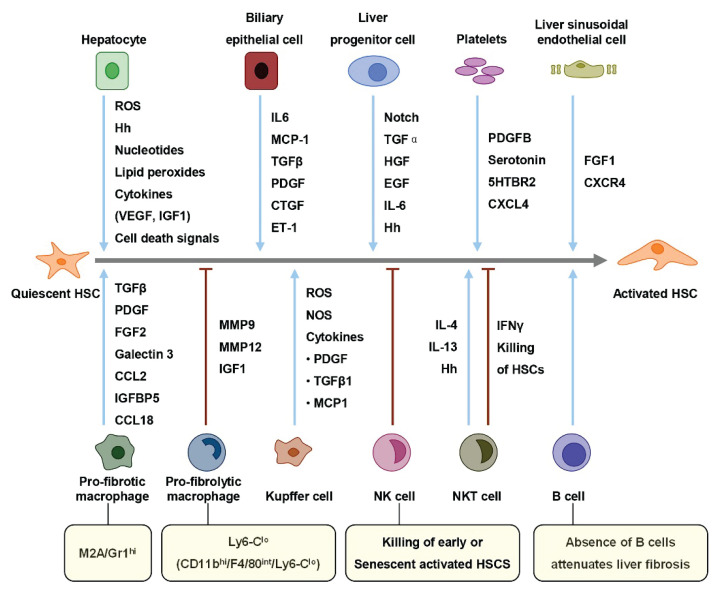
Fig. 2.
Liver resident cells modulators of HSCs activation. HSCs activation is promoted or suppressed by multiple cell types. Liver resident cells promote (green lines) or suppress (red lines) quiescent HSCs to activated HSCs. ROS, reactive oxygen species; Hh, hedgehog; VEGF, vascular endothelial growth factor; IGF1, insulin-like growth factor 1; IL6, inter leukin 6; MCP-1, monocyte chemoattractant protein-1; TGFβ, transforming growth factor β; PDGF, platelet-derived growth factor; CTGF, connective tissue growth factor; ET-1, endothelin-1; HGF, hepato-cyte growth factor; EGF, epidermal growth factor; FGF1, fibroblast growth factor 1; IFNγ, interferon-γ; NOS, nitric oxide synthase; IGFBP5, insulin-like growth factor binding protein 5; NKT, natural killer T; NK, natural killer.
There are three ways of activated HSCs elimination: reversion, senescence and apoptosis (Friedman 2008b). The reversion of activated HSCs to inactivated state has shown a vital role in hepatic fibrosis regression, suggesting the expression of fibrogenic genes relative to activated HSCs was reduced. HSC senescence also plays a crucial role in liver fibrosis recovery, senescent HSCs represented reduced ECM component production (DeRossi et al. 2019). In the liver, HSC apoptosis has been reported during hepatic fibrosis reversion through two approaches: mitochondrial dependence and death receptors (Tao et al. 2019). The mechanisms of HSCs activation and deactivation could provide antifibrotic targeted therapeutic strategies (Wang et al. 2020).
HSCs in normal liver
A fundamental understanding of the feature and function of HSCs in normal liver is essential to explore their role in liver diseases. Researches of HSCs in normal liver have showed that they play a vital role during liver development, regeneration, vitamin A storage and other processes (Fig. 3).
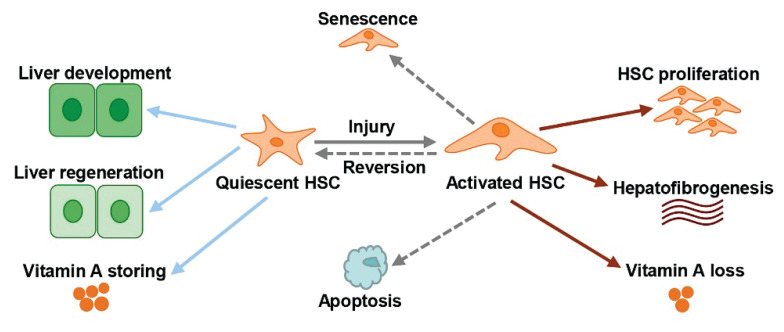
Fig. 3.
Functions, features and phenotypes of HSC in normal and liver fibrosis, and activated HSC clearance approaches. Quiescent HSC in normal liver plays a vital role during liver development, regeneration, and vitamin A storage. Activated HSC is related with liver fibrosis. There are three activated HSCs clearance approaches: apoptosis, senescence, and reversion.
HSCs in liver development
Throughout the liver development, HSCs are closely related to hematopoietic cells, liver progenitor cells, biliary epithelial cells, LSECs, hepatocytes and Kupffer cells (Lee et al. 2015). It suggests that HSCs may contribute to hepatic organogenesis via regulating the growth, differentiation, or morphogenesis of these cells (Arab et al. 2020). As is well-known, the liver is the primary site of hematopoiesis during mammalian embryogenesis. HSCs are involved in fetal liver hematopoiesis through the secretions of Hlx and CXCL12 (Friedman 2008a, Yin et al. 2013). HSCs also can promote the progenitor cells maturation through its markers (α-SMA, desmin and vimentin) (Arriola et al. 2019). Quiescent HSCs express mesenchymal morphogenic proteins pleiotrophin and epimorphin, which are contribute to liver regeneration after partial hepatectomy (Chen et al. 2020).
HSCs may also be critical to the morphogenesis of intrahepatic bile ducts via interacting with biliary epithelial cells (Chen et al. 2020). Fetal HSCs secrete hepatocyte growth factor (HGF), this growth factor has significant effects on the development of biliary epithelial cells during hepatic organogenesis (Das et al. 2020). In the injured liver, HSCs expressed hepatocyte and epithelial cell markers. The role of these cells in biliary epithelial cells differentiation is not quite clear, which may be mediated by cell-cell contacts (Dong et al. 2020). In both fetal and adult livers, HSCs and LSECs have been proposed to share a common precursor because of their location proximity and coexpression of angiogenic factors, their communication by means of paracrine signaling (Hoffmann et al. 2020). During angiogenesis, HSCs are thought to have the same effect on the vascular tube maturation and integrity with LSECs when the liver responds to injury, understanding their interactions during growth is vital for liver injury recovery (Kong et al. 2020).
HSCs in liver regeneration
A deep understanding of the relationship between HSCs and liver regeneration has important significances for stimulating liver recovery following partial hepatectomy (PH) (Forbes et al. 2016). Activated HSCs have involved in liver regeneration through producing hepatocyte proliferation factors, angiogenic factors and remodeled ECM, these cytokines and chemokines directly promote the proliferation of hepatocytes, and act indirectly through LSECs or Kupffer cells to stimulate regeneration (Li et al. 2020). Activated HSCs-derived HGF can promote hepatocytes proliferation during liver injury. However, HSCs also can inhibit the hepatocyte proliferation, HSCs-derived transforming growth factor-β (TGF-β) is the antiproliferative factor, and even induces apoptosis (Yin et al. 2013). Therefore, liver regeneration is a complex process, HSCs modulate the process both the initiation and termination.
Quiescent HSCs are critical to the liver regenerative response in injured liver, they contribute to hepatocellular development following injury, reducing hepatic fibrosis and diminishing the expression of α-SMA (Friedman 2008a). At the same time, activated HSCs can increase liver cell injury and even apoptosis. The role of HSCs in liver injury and repair is unclear because they have quiescence and activation phenotypes (Li et al. 2020). An advanced model in vivo and in vitro is necessary to clarify the function of these enigmatic cells.
Vitamin A storage and metabolism
In normal liver, HSCs present a quiescent state, which are characterized by vitamin A in their cytoplasmic droplets in the form of retinyl esters. There are two types of vitamin A droplets: type I and type II (Friedman 2008a). Under physiological conditions, the vast majority of vitamin A in the liver is stored in HSCs, showing a heterogeneous pattern. Under liver injury, HSCs become activated and lose these characteristic droplets (Lv et al. 2020). The form of vitamin released outside the activated HSCs is retinol, indicating that there is intracellular hydrolysis of esters, rather than export. Several nuclear retinoid receptors secreted by HSCs mediate vitamin A metabolism in the liver, such as retinol-binding protein (RBP), lecithin retinol acetyl transferase (LRAT) and peroxisome proliferator-activated receptors (PPARs). The roles of vitamin A storage and metabolism in the HSCs of liver merit further investigation (Miyazoe et al. 2020).
HSCs in liver fibrosis
The facilitation of liver development and regeneration may be feasible in normal liver, HSCs can also be related with liver diseases, especially liver fibrosis. The overwhelming majority of hepatic fibrosis result in the hepatic carcinoma, the relationship between HSCs and liver fibrosis has been recognized (Nagasaki et al. 2020). Studies suggest that activated HSCs can be involved in hepatic fibrogenesis due to the production of extracellular matrix accumulation. Phenotypic transformation from quiescent HSCs to activated HSCs contributes to many forms of liver fibrosis, at least there are three activated HSCs clearance approaches: apoptosis, senescence, and reversion (Tao et al. 2020). Each clearance way of activated HSCs contributes to a decrease in the number of this ECM-producing cell type, resulting in the regression of hepatic fibrosis. Targeting HSCs provide anti-fibrotic strategies for liver fibrosis therapies. Therefore, the comprehensive understanding of HSCs is necessary to promote the resolution of liver fibrosis (Wang et al. 2020).
ECM-producing cell
Liver fibrosis is caused by chronic liver injury and considered as ECM excess. The unbalance between synthesis and degradation of ECM results in hepatic fibrogenesis, HSCs are the central regulators of liver fibrosis (Wang et al. 2020). In normal liver, HSCs have key roles in vitamin A storage and metabolism. Under liver injury, quiescent HSCs are activated via the mediation of ROS, MCP-1, HGF, CXCR4, CCL2, and activated HSCs are the major ECM producer (Yang et al. 2020). The activated phenotype is characterized as proliferation, contractility, fibrogenesis and retinoid loss, promoting the accumulation of ECM by increasing the expression of collagen type I and decreasing the expression of MMP (Zhang et al. 2020).
Angiogenesis in liver fibrogenesis
Angiogenesis has a key role in liver fibrogenesis and progression. HSCs also mediate angiogenesis through the stimulation of vascular endothelial growth factor (VEGF), which is pro-angiogenic factor. The overexpression of VEGF is associated with liver fibrosis grade and portal hypertension, indicating the important role of pro-angiogenic factor VEGF in liver fibrogenesis (Zou et al. 2019). Activated HSCs are considered to secrete pro-angiogenic factors such as VEGF, IGF1, TGF-β and EGF, and express angiogenic growth factor receptors (Chen et al. 2020, Moon et al. 2019). In liver fibrosis, these factors contribute to pro-angiogenic microenvironment, and their upregulation induces liver fibrogenesis.
Conclusion
As summarized in this review, the HSCs play a critical role in liver development, liver regeneration and hepatic fibrogenesis. Vital regulators of liver fibrosis have been characterized, and HSCs have been identified as potential therapeutic targets for hepatic fibrosis (Dong et al. 2020, Zhang et al. 2019). Concerted efforts to explore the mechanisms that HSCs activation and clearance will enhance the efficiency of targeting these cells in liver fibrosis. The application of targeting HSCs in clinical trials requires further investigation, the function of HSCs and their regulation mechanisms in other tissue are unclear (Zhai et al. 2019). Furthermore, advanced models are necessary for in vivo and in vitro studies of HSCs in the liver, to probe the regulation of phenotypic reversion from activation to quiescence.
Although the advanced understanding of the phenotype reversion of the activated HSCs has been made remarkable progress, treatment options for therapeutic strategy for the control of liver fibrogenesis are still severely limited (Yuan et al. 2019). It is urgent to establish the specific, effective, and safe anti-fibrotic therapeutic strategy, either by apoptosis, senescence, HSC phenotypic reversion, or inhibition of the HSC-mediated angiogenesis. Recently, mounting evidence has demonstrated that HSCs are critical modulators of liver fibrogenesis via the reversal of their phenotype (Wei et al. 2019). In the near future, nanotechnology could be very promising in the innovative therapeutic approaches of liver fibrosis via targeting HSCs (Li et al. 2020, Tao et al. 2019). For example, targeted therapy reverses hepatic fibrosis via exosome mediated delivery system.
In conclusion, this study has offered significant insights regarding HSCs in the liver, providing prospects for emerging target therapies in patients with liver fibrosis.
Acknowledgements
This study was supported by 2020SF-067.
Abbreviations
- ALD
alcoholic liver disease
- ECM
excessive extra-cellular matrix
- HSCs
hepatic stellate cells
- LRAT
lecithin retinol acetyl transferase
- LSECs
liver sinusoidal endothelia cells
- MMP
matrix metallo proteinase
- NAFLD
non-alcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NPCs
non-parenchymal cells
- PH
partial hepatectomy
- PPARs
peroxisome proliferator-activated receptors
- RBP
retinol-binding protein
- rER
rough endoplasmic reticulum
- TGF-β
transforming growth factor-β
- VEGF
vascular endothelial growth factor
- α-SMA
α-smooth muscle actin
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- ARAB JP, CABRERA D, SEHRAWAT TS, JALAN-SAKRIKAR N, VERMA VK, SIMONETTO D, CAO S, YAQOOB U, LEON J, FREIRE M, VARGAS JI, De ASSUNCAO TM, KWON JH, GUO Y, KOSTALLARI E, CAI Q, KISSELEVA T, OH Y, ARRESE M, HUEBERT RC, SHAH VH. Hepatic stellate cell activation promotes alcohol-induced steatohepatitis through Igfbp3 and SerpinA12. J Hepatol. 2020;73:149–160. doi: 10.1016/j.jhep.2020.02.005. https://doi:10.1016/j.jhep.2020.02.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRIOLA BENITEZ PC, PESCE VIGLIETTI AI, GOMES MTR, OLIVEIRA SC, QUARLERI JF, GIAMBARTOLOMEI GH, DELPINO MV. Brucella abortus Infection Elicited Hepatic Stellate Cell-Mediated Fibrosis Through Inflammasome-Dependent IL-1beta Production. Front Immunol. 2019;10:3036. doi: 10.3389/fimmu.2019.03036. https://doi:10.3389/fimmu.2019.03036 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANSAL MB. Hepatic stellate cells: fibrogenic, regenerative or both? Heterogeneity and context are key. Hepatol Int. 2016;10:902–908. doi: 10.1007/s12072-016-9758-x. https://doi:10.1007/s12072-016-9758-x . [DOI] [PubMed] [Google Scholar]
- CHEN H, GAN Q, YANG C, PENG X, QIN J, QIU S, JIANG Y, TU S, HE Y, LI S, YANG H, TAO L, PENG Y. Correction to: A novel role of glutathione S-transferase A3 in inhibiting hepatic stellate cell activation and rat hepatic fibrosis. J Transl Med. 2020;18:182. doi: 10.1186/s12967-020-02346-4. https://doi:10.1186/s12967-020-02346-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN W, YAN X, YANG A, XU A, HUANG T, YOU H. miRNA-150-5p promotes hepatic stellate cell proliferation and sensitizes hepatocyte apoptosis during liver fibrosis. Epigenomics. 2020;12:53–67. doi: 10.2217/epi-2019-0104. https://doi:10.2217/epi-2019-0104 . [DOI] [PubMed] [Google Scholar]
- DAS D, FAYAZZADEH E, LI X, KOIRALA N, WADERA A, LANG M, ZERNIC M, PANICK C, NESBITT P, McLENNAN G. Quiescent hepatic stellate cells induce toxicity and sensitivity to doxorubicin in cancer cells through a caspase-independent cell death pathway: Central role of apoptosis-inducing factor. J Cell Physiol. 2020;235:6167–6182. doi: 10.1002/jcp.29545. https://doi:10.1002/jcp.29545 . [DOI] [PubMed] [Google Scholar]
- DEROSSI C, BAMBINO K, MORRISON J, SAKARIN I, VILLACORTA-MARTIN C, ZHANG C, ELLIS JL, FIEL MI, YBANEZ M, LEE YA, HUANG KL, YIN C, SAKAGUCHI TF, FRIEDMAN SL, VILLANUEVA A, CHU J. Mannose Phosphate Isomerase and Mannose Regulate Hepatic Stellate Cell Activation and Fibrosis in Zebrafish and Humans. Hepatology. 2019;70:2107–2122. doi: 10.1002/hep.30677. https://doi:10.1002/hep.30677 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOHERTY DG. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J Autoimmun. 2016;66:60–75. doi: 10.1016/j.jaut.2015.08.020. https://doi:10.1016/j.jaut.2015.08.020 . [DOI] [PubMed] [Google Scholar]
- DONG L, PU Y, CHEN X, QI X, ZHANG L, XU L, LI W, MA Y, ZHOU S, ZHU J, LI Y, WANG X, SU C. hUCMSC-extracellular vesicles downregulated hepatic stellate cell activation and reduced liver injury in S. japonicum-infected mice, Stem Cell Res Ther. 2020;11:21. doi: 10.1186/s13287-019-1539-8. https://doi:10.1186/s13287-019-1539-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGGERT T, GRETEN TF. Tumor regulation of the tissue environment in the liver. Pharmacol Ther. 2017;173:47–57. doi: 10.1016/j.pharmthera.2017.02.005. https://doi:10.1016/j.pharmthera.2017.02.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELPEK GO. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J Gastroenterol. 2014;20:7260–7276. doi: 10.3748/wjg.v20.i23.7260. https://doi:10.3748/wjg.v20.i23.7260 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORBES SJ, NEWSOME PN. Liver regeneration - mechanisms and models to clinical application. Nat Rev Gastroenterol Hepatol. 2016;13:473–485. doi: 10.1038/nrgastro.2016.97. https://doi:10.1038/nrgastro.2016.97 . [DOI] [PubMed] [Google Scholar]
- FRIEDMAN SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008a;88:125–172. doi: 10.1152/physrev.00013.2007. https://doi:10.1152/physrev.00013.2007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDMAN SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008b;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. https://doi:10.1053/j.gastro.2008.03.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLERBRAND C. Hepatic stellate cells--the pericytes in the liver. Pflugers Arch. 2013;465:775–778. doi: 10.1007/s00424-012-1209-5. https://doi:10.1007/s00424-012-1209-5 . [DOI] [PubMed] [Google Scholar]
- HOFFMANN C, DJERIR NEH, DANCKAERT A, FERNANDES J, ROUX P, CHARRUEAU C, LACHAGES AM, CHARLOTTE F, BROCHERIOU I, CLEMENT K, ARON-WISNEWSKY J, FOUFELLE F, RATZIU V, HAINQUE B, BONNEFONT-ROUSSELOT D, BIGEY P, ESCRIOU V. Hepatic stellate cell hypertrophy is associated with metabolic liver fibrosis. Sci Rep. 2020;10:3850. doi: 10.1038/s41598-020-60615-0. https://doi:10.1038/s41598-020-60615-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIEH CC, HUNG CH, LU L, QIAN S. Hepatic immune tolerance induced by hepatic stellate cells. World J Gastroenterol. 2015;21:11887–11892. doi: 10.3748/wjg.v21.i42.11887. https://doi:10.3748/wjg.v21.i42.11887 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG Y, DENG X, LIANG J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp Cell Res. 2017;352:420–426. doi: 10.1016/j.yexcr.2017.02.038. https://doi:10.1016/j.yexcr.2017.02.038 . [DOI] [PubMed] [Google Scholar]
- JUNG YK, YIM HJ. Reversal of liver cirrhosis: current evidence and expectations. Korean J Intern Med. 2017;32:213–228. doi: 10.3904/kjim.2016.268. https://doi:10.3904/kjim.2016.268 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITANO M, BLOOMSTON PM. Hepatic Stellate Cells and microRNAs in Pathogenesis of Liver Fibrosis. J Clin Med. 2016;5 doi: 10.3390/jcm5030038. https://doi:10.3390/jcm5030038 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONG D, CHEN L, HUANG W, ZHANG Z, WANG L, ZHANG F, ZHENG S. Combined therapy with ligustrazine and paeonol mitigates hepatic fibrosis through destroying mitochondrial integrity of stellate cell. Am J Transl Res. 2020;12:1255–1266. [PMC free article] [PubMed] [Google Scholar]
- KOYAMA Y, BRENNER DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55–64. doi: 10.1172/JCI88881. https://doi:10.1172/JCI88881 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOYAMA Y, XU J, LIU X, BRENNER DA. New Developments on the Treatment of Liver Fibrosis. Dig Dis. 2016;34:589–596. doi: 10.1159/000445269. https://doi:10.1159/000445269 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE YA, WALLACE MC, FRIEDMAN SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–841. doi: 10.1136/gutjnl-2014-306842. https://doi:10.1136/gutjnl-2014-306842 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI F, HUANGYANG P, BURROWS M, GUO K, RISCAL R, GODFREY J, LEE KE, LIN N, LEE P, BLAIR IA, KEITH B, LI B, SIMON MC. FBP1 loss disrupts liver metabolism and promotes tumorigenesis through a hepatic stellate cell senescence secretome. Nat Cell Biol. 2020;22:728–739. doi: 10.1038/s41556-020-0511-2. https://doi:10.1038/s41556-020-0511-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Y, ZHANG Y, CHEN T, HUANG Y, ZHANG Y, GENG S, LI X. Role of aldosterone in the activation of primary mice hepatic stellate cell and liver fibrosis via NLRP3 inflammasome. J Gastroenterol Hepatol. 2020;35:1069–1077. doi: 10.1111/jgh.14961. https://doi:10.1111/jgh.14961 . [DOI] [PubMed] [Google Scholar]
- LV F, LI N, KONG M, WU J, FAN Z, MIAO D, XU Y, YE Q, WANG Y. CDKN2a/p16 Antagonizes Hepatic Stellate Cell Activation and Liver Fibrosis by Modulating ROS Levels. Front Cell Dev Biol. 2020;8:176. doi: 10.3389/fcell.2020.00176. https://doi:10.3389/fcell.2020.00176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARRONE G, SHAH VH, GRACIA-SANCHO J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol. 2016;65:608–617. doi: 10.1016/j.jhep.2016.04.018. https://doi:10.1016/j.jhep.2016.04.018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAZZA G, AL-AKKAD W, ROMBOUTS K. Engineering in vitro models of hepatofibrogenesis. Adv Drug Deliv Rev. 2017;121:147–157. doi: 10.1016/j.addr.2017.05.018. https://doi:10.1016/j.addr.2017.05.018 . [DOI] [PubMed] [Google Scholar]
- MIYAZOE Y, MIUMA S, MIYAAKI H, KANDA Y, NAKASHIKI S, SASAKI R, HARAGUCHI M, SHIBATA H, HONDA T, TAURA N, NAKAO K. Extracellular vesicles from senescent hepatic stellate cells promote cell viability of hepatoma cells through increasing EGF secretion from differentiated THP-1 cells. Biomed Rep. 2020;12:163–170. doi: 10.3892/br.2020.1279. https://doi:10.3892/br.2020.1279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOON MY, KIM HJ, KIM MJ, UHM S, PARK JW, SUK KT, PARK JB, KIM DJ, KIM SE. Rap1 regulates hepatic stellate cell migration through the modulation of RhoA activity in response to TGFbeta1. Int J Mol Med. 2019;44:491–502. doi: 10.3892/ijmm.2019.4215. https://doi:10.3892/ijmm.2019.4215 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGASAKI A, SAKAMOTO S, CHEA C, ISHIDA E, FURUSHO H, FUJII M, TAKATA T, MIYAUCHI M. Odontogenic infection by Porphyromonas gingivalis exacerbates fibrosis in NASH via hepatic stellate cell activation. Sci Rep. 2020;10:4134. doi: 10.1038/s41598-020-60904-8. https://doi:10.1038/s41598-020-60904-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREZIOSI ME, MONGA SP. Update on the Mechanisms of Liver Regeneration. Semin Liver Dis. 2017;37:141–151. doi: 10.1055/s-0037-1601351. https://doi:10.1055/s-0037-1601351 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDBERG FA, SHARPE AH, TURLEY SJ. Hepatic immune regulation by stromal cells. Curr Opin Immunol. 2015;32:1–6. doi: 10.1016/j.coi.2014.10.002. https://doi:10.1016/j.coi.2014.10.002 . [DOI] [PubMed] [Google Scholar]
- SCHUMACHER JD, GUO GL. Regulation of Hepatic Stellate Cells and Fibrogenesis by Fibroblast Growth Factors. Biomed Res Int. 2016;2016:8323747. doi: 10.1155/2016/8323747. https://doi:10.1155/2016/8323747 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKI E, BRENNER DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepatobiliary Pancreat Sci. 2015;22:512–518. doi: 10.1002/jhbp.245. https://doi:10.1002/jhbp.245 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKI E, SCHWABE RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–1079. doi: 10.1002/hep.27332. https://doi:10.1002/hep.27332 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEO W, JEONG WI. Hepatic non-parenchymal cells: Master regulators of alcoholic liver disease? World J Gastroenterol. 2016;22:1348–1356. doi: 10.3748/wjg.v22.i4.1348. https://doi:10.3748/wjg.v22.i4.1348 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANG L, HOSSEINI M, LIU X, KISSELEVA T, BRENNER DA. Human hepatic stellate cell isolation and characterization. J Gastroenterol. 2018;53:6–17. doi: 10.1007/s00535-017-1404-4. https://doi:10.1007/s00535-017-1404-4 . [DOI] [PubMed] [Google Scholar]
- SHERMAN MH. Stellate Cells in Tissue Repair, Inflammation, and Cancer. Annu Rev Cell Dev Biol. 2018;34:333–355. doi: 10.1146/annurev-cellbio-100617-062855. https://doi:10.1146/annurev-cellbio-100617-062855 . [DOI] [PubMed] [Google Scholar]
- TAO L, MA W, WU L, XU M, YANG Y, ZHANG W, SHA W, LI H, XU J, FENG R, XUE D, ZHANG J, DOOLEY S, SEKI E, LIU P, LIU C. Glial cell line-derived neurotrophic factor (GDNF) mediates hepatic stellate cell activation via ALK5/Smad signalling. Gut. 2019;68:2214–2227. doi: 10.1136/gutjnl-2018-317872. https://doi:10.1136/gutjnl-2018-317872 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAO L, WU L, ZHANG W, MA WT, YANG GY, ZHANG J, XUE DY, CHEN B, LIU C. Peroxisome proliferator-activated receptor gamma inhibits hepatic stellate cell activation regulated by miR-942 in chronic hepatitis B liver fibrosis. Life Sci. 2020;253:117572. doi: 10.1016/j.lfs.2020.117572. https://doi:10.1016/j.lfs.2020.117572 . [DOI] [PubMed] [Google Scholar]
- THOMPSON AI, CONROY KP, HENDERSON NC. Hepatic stellate cells: central modulators of hepatic carcinogenesis. BMC Gastroenterol. 2015;15:63. doi: 10.1186/s12876-015-0291-5. https://doi:10.1186/s12876-015-0291-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUCHIDA T, FRIEDMAN SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. https://doi:10.1038/nrgastro.2017.38 . [DOI] [PubMed] [Google Scholar]
- Van GRUNSVEN LA. 3D in vitro models of liver fibrosis. Adv Drug Deliv Rev. 2017;121:133–146. doi: 10.1016/j.addr.2017.07.004. https://doi:10.1016/j.addr.2017.07.004 . [DOI] [PubMed] [Google Scholar]
- WANG L, WANG Y, QUAN J. Exosomes derived from natural killer cells inhibit hepatic stellate cell activation and liver fibrosis. Hum Cell. 2020;33:582–589. doi: 10.1007/s13577-020-00371-5. https://doi:10.1007/s13577-020-00371-5 . [DOI] [PubMed] [Google Scholar]
- WANG Q, WEI S, ZHOU H, LI L, ZHOU S, SHI C, SHI Y, QIU J, LU L. MicroRNA-98 Inhibits Hepatic Stellate Cell Activation and Attenuates Liver Fibrosis by Regulating HLF Expression. Front Cell Dev Biol. 2020;8:513. doi: 10.3389/fcell.2020.00513. https://doi:10.3389/fcell.2020.00513 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG ZM, XIA SW, ZHANG T, WANG ZY, YANG X, KAI J, CHENG XD, SHAO JJ, TAN SZ, CHEN AP, WANG SJ, ZHANG F, ZHANG ZL, ZHENG SZ. LncRNA-H19 induces hepatic stellate cell activation via upregulating alcohol dehydrogenase III-mediated retinoic acid signals. Int Immunopharmacol. 2020;84:106470. doi: 10.1016/j.intimp.2020.106470. https://doi:10.1016/j.intimp.2020.106470 . [DOI] [PubMed] [Google Scholar]
- WEI S, WANG Q, ZHOU H, QIU J, LI C, SHI C, ZHOU S, LIU R, LU L. miR-455-3p Alleviates Hepatic Stellate Cell Activation and Liver Fibrosis by Suppressing HSF1 Expression. Mol Ther Nucleic Acids. 2019;16:758–769. doi: 10.1016/j.omtn.2019.05.001. https://doi:10.1016/j.omtn.2019.05.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG L, HAN B, ZHANG M, WANG YH, TAO K, ZHU MX, HE K, ZHANG ZG, HOU S. Activation of BK Channels Prevents Hepatic Stellate Cell Activation and Liver Fibrosis Through the Suppression of TGFbeta1/SMAD3 and JAK/STAT3 Profibrotic Signaling Pathways. Front Pharmacol. 2020;11:165. doi: 10.3389/fphar.2020.00165. https://doi:10.3389/fphar.2020.00165 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANGUAS SC, COGLIATI B, WILLEBRORDS J, MAES M, COLLE I, VAN DEN BOSSCHE B, DE OLIVEIRA C, ANDRAUS W, ALVES VAF, LECLERCQ I, VINKEN M. Experimental models of liver fibrosis. Arch Toxicol. 2016;90:1025–1048. doi: 10.1007/s00204-015-1543-4. https://doi:10.1007/s00204-015-1543-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIN C, EVASON KJ, ASAHINA K, STAINIER DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902–1910. doi: 10.1172/JCI66369. https://doi:10.1172/JCI66369 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN B, CHEN Y, WU Z, ZHANG L, ZHUANG Y, ZHAO X, NIU H, CHENG JC, ZENG Z. Proteomic Profiling of Human Hepatic Stellate Cell Line LX2 Responses to Irradiation and TGF-beta1. J Proteome Res. 2019;18:508–521. doi: 10.1021/acs.jproteome.8b00814. https://doi:10.1021/acs.jproteome.8b00814 . [DOI] [PubMed] [Google Scholar]
- ZHAI X, WANG W, DOU D, MA Y, GANG D, JIANG Z, SHI B, JIN B. A novel technique to prepare a single cell suspension of isolated quiescent human hepatic stellate cells. Sci Rep. 2019;9:12757. doi: 10.1038/s41598-019-49287-7. https://doi:10.1038/s41598-019-49287-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG CY, YUAN WG, HE P, LEI JH, WANG CX. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22:10512–10522. doi: 10.3748/wjg.v22.i48.10512. https://doi:10.3748/wjg.v22.i48.10512 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG R, GAO X, ZUO J, HU B, YANG J, ZHAO J, CHEN J. STMN1 upregulation mediates hepatocellular carcinoma and hepatic stellate cell crosstalk to aggravate cancer by triggering the MET pathway. Cancer Sci. 2020;111:406–417. doi: 10.1111/cas.14262. https://doi:10.1111/cas.14262 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Z, WEN H, WENG J, FENG L, LIU H, HU X, ZENG F. Silencing of EPCAM suppresses hepatic fibrosis and hepatic stellate cell proliferation in mice with alcoholic hepatitis via the PI3K/Akt/mTOR signaling pathway. Cell Cycle. 2019;18:2239–2254. doi: 10.1080/15384101.2019.1642067. https://doi:10.1080/15384101.2019.1642067 . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- ZHOU WC, ZHANG QB, QIAO L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312–7324. doi: 10.3748/wjg.v20.i23.7312. https://doi:10.3748/wjg.v20.i23.7312 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOU GL, ZUO S, LU S, HU RH, LU YY, YANG J, DENG KS, WU YT, MU M, ZHU JJ, ZENG JZ, ZHANG BF, WU X, ZHAO XK, LI HY. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-beta/Smad signaling pathway. World J Gastroenterol. 2019;25:4222–4234. doi: 10.3748/wjg.v25.i30.4222. https://doi:10.3748/wjg.v25.i30.4222 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOUBEK ME, TRAUTWEIN C, STRNAD P. Reversal of liver fibrosis: From fiction to reality. Best Pract Res Clin Gastroenterol. 2017;31:129–141. doi: 10.1016/j.bpg.2017.04.005. https://doi:10.1016/j.bpg.2017.04.005 . [DOI] [PubMed] [Google Scholar]