Introduction
Kidney failure, leading to ESKD, is invariably an irreversible condition that, for survival, typically requires RRT—either in the form of dialysis or a kidney transplant. ESKD is associated with high mortality and morbidity, lower quality of life, and high resource utilization and cost, thereby posing a substantial burden primarily on patients, but also on families and society at large. The focus of this mini-review is to summarize the state of dialysis management and changes in financing of dialysis in the United States over the last decade. In addition we briefly highlight the recently announced federal executive order, “Advancing American Kidney Health,” that promises to raise awareness and improve kidney disease management in the United States.
Epidemiology of Patients on Dialysis
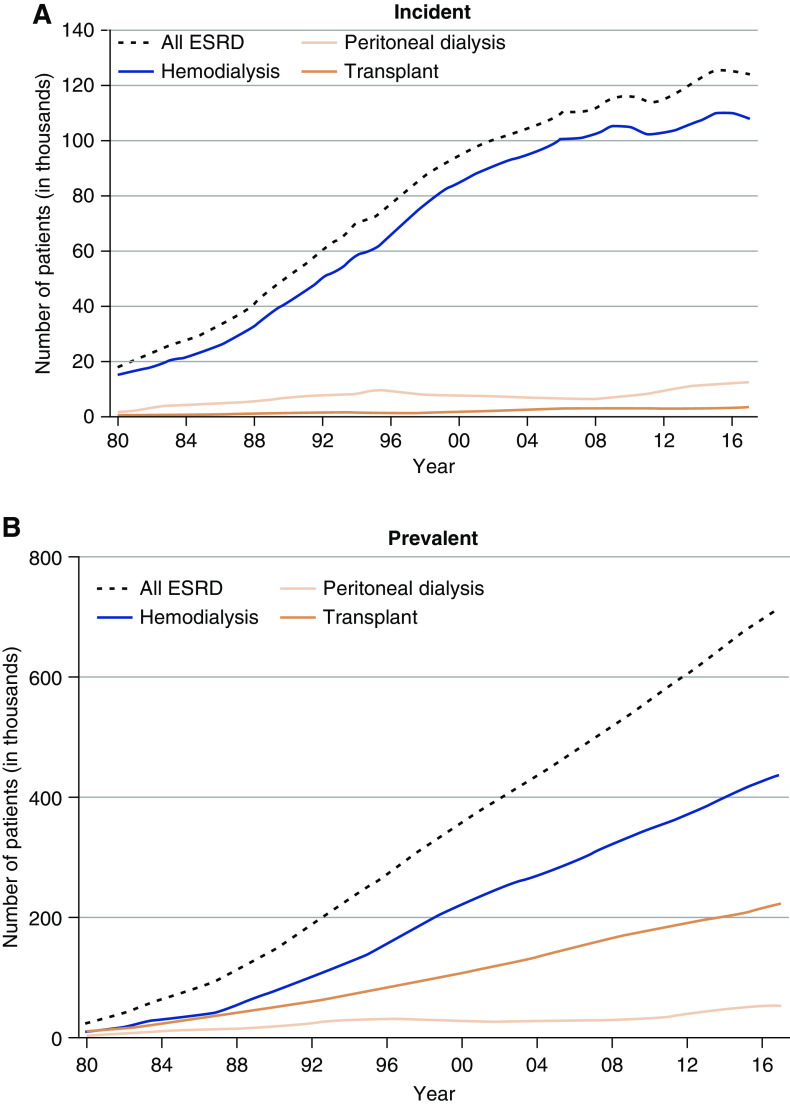
Based on a survey of 79 countries (1), the United States has the second highest incidence of treated ESKD, which is attributed to age, race, genetic predisposition, and a high prevalence of risk factors such as obesity, diabetes, and hypertension (2). Primordial risk factors—such as inequities related to race, income, education, food security, recreational and routine physical activity, access to healthy food options, healthcare, and environmental factors—likely play a major role to in the predisposition to disease and its complications; kidney disease is no exception (3). There were 746,557 prevalent patients with ESKD in the United States at the end of 2017, 124,500 of which were new cases of ESKD, translating to a crude incidence rate of 370.2 per million per year (Figure 1). The adjusted ESKD incidence rate has stabilized, however, and has begun to decline slightly in recent years. About 30% of patients with prevalent ESKD and 3% of patients with incident ESKD had a functioning kidney transplant (4). For quite some time now, diabetes and hypertension have remained the leading assigned primary causes of ESKD in the United States (Table 1) (4).
Figure 1.
Recent stabilization of trends in incidence rate, but continually rising prevalence of ESKD in the United States population, 1980–2017. (A) Incident patients. (B) Prevalent patients. Persons with “uncertain dialysis” were included in the “all ESKD” total, but are not represented separately.
Table 1.
Clinical dialysis practice and its financing in the United States
| Measures | Statistics | Reference |
| Epidemiology of patients on dialysis | ||
| Total patients with ESKD in the United States in 2017 | Incident patients: 124,500 (370.2 per million per year) | 2019 USRDS ADR (4) |
| Prevalent patients: 746,557 (2203.5 per million per year) | ||
| Number of patients on dialysis (per million people in the general population), total (HD versus PD, 2017) | Incident patients: 359.3 (232.0 versus 37.4) | 2019 USRDS ADR (4) |
| Prevalent patients: 1545.8 (1381.6 versus 155.6) | ||
| Percent of patients with ESKD on home dialysis (2017) | Incident patients: 11% | 2019 USRDS ADR (4) |
| Prevalent patients: 8% | ||
| Primary cause of ESKD in incident patients on dialysis, total (HD versus PD, 2017) | Diabetes: 47% (48% versus 44%) | 2019 USRDS ADR (4) |
| Hypertension: 29% (30% versus 28%) | ||
| GN: 7% (6% versus 13%) | ||
| Cystic kidney: 3% (2% versus 6%) | ||
| Other/Unknown: 14% (14% versus 8%) | ||
| Standardized mortality rate, total (HD versus PD versus transplant, 2017) | 134 (167 versus 156 versus 29) per 1000 patient-yr | 2019 USRDS ADR (4) |
| Hospitalization, HD versus PD, 2016 | 1.7 versus 1.7 per patient-yr | 2018 USRDS ADR (1) |
| Proportion of readmission within 30 d of discharge, HD versus PD, 2016 | 38% versus 37% | 2018 USRDS ADR (1) |
| Emergency department visit, HD versus PD, 2016 | 3.0 versus 2.3 per patient-yr | 2018 USRDS ADR (1) |
| Observation stay, HD versus PD, 2016 | 0.4 versus 0.2 per patient-yr | 2018 USRDS ADR (1) |
| Pre-ESKD care | ||
| Proportion of receiving pre-ESKD nephrology care | >12 mo: 33% | 2019 USRDS ADR (4) |
| 6–12 mo: 20% | ||
| 0–6 mo: 14% | ||
| None: 19% | ||
| Unknown/missing: 14% | ||
| Clinical characteristics by pre-ESKD nephrology care, >12 mo versus none | Dietary care: 13% versus 0.3% | 2019 USRDS ADR (4) |
| Erythropoiesis-stimulating agent use: 22% versus 2% | ||
| AV fistula: 24% versus 2% | ||
| Central venous catheter: 52% versus 93% | ||
| eGFR <5 ml/min per 1.73 m2: 12% versus 20% | ||
| Vascular access in patients on HD (2018) | AV fistula: 63% | 2019 USRDS ADR (4) |
| AV graft: 18% | ||
| Central venous catheter 20% | ||
| Provision of care | ||
| Average FTE | Physician FTEs: 0.05±0.47 | Shreay et al. (5) |
| Nursing FTEs: 4.24±3.59 | ||
| Technician FTEs: 6.01±4.68 | ||
| Other clinical FTEs: 0.70±1.81 | ||
| Average length of a dialysis session | 216.5±26.1 min | Flythe et al. (6) |
| Frequency of being seen by nephrologist during dialysis sessions | <4 times per mo: 32% | Kawaguchi et al. (7) |
| 4 times per mo: 51% | ||
| >4 times per mo: 17% | ||
| Type of dialysis units in 2020 | Profit: 89% | US Centers for Medicare and Medicaid Services (8) |
| Nonprofit:11% | ||
| Distribution of patients by unit affiliation in 2011 (proportion) | Hospital based: 9% (13%) | 2013 USRDS ADR (9) |
| Large dialysis organizations: 63% (66%) | ||
| Independent units: 14% (13%) | ||
| Small dialysis organizations: 14% (12%) | ||
| Financing of dialysis | ||
| Percent of insurance coverage | All patients with ESKD are eligible to Medicare coverage | Rettig (10) |
| Government financing versus private insurance financing: | Dominant payer: Medicare, as a federal health insurance program | National Institute of Diabetes and Digestive and Kidney Diseases (11) |
| Other payers: Medicaid, Veterans Affairs, private insurers, and other assistance programs | ||
| Regulations in dialysis financing | January 2011: Implementation of the ESRD Prospective Payment System | |
| January 2012: Implementation of ESRD Quality Incentive Program | ||
| October 2015: Implementation of Dialysis Facility Compare program (star ratings) | ||
| October 2015: Implementation of the Comprehensive ESRD Care Model by the creation of ESRD Seamless Care Organizations | ||
| July 2019: Launch of the federal executive order Advancing American Kidney Health | ||
| Reimbursement per dialysis session in USD | Base rate: $239.33 | 2020 CMS Prospective Payment System (12) |
| Medicare expenditure | Total: $46.6 billion | 2019 USRDS ADR (4) |
| Medicare fee-for-service plan: $35.9 billion | ||
| Medicare advantage plan: $10.7 billion | ||
| Medicare per person per year spending in 2017 | HD: $91.795 | 2019 USRDS ADR (4) |
| PD: $78,159 | ||
| Transplant: $35,817 | ||
USRDS, United States Renal Data System; ADR, Annual Data Report; HD, hemodialysis; PD, peritoneal dialysis; AV, arteriovenous; FTE, full-time employment; CMS, Centers for Medicare and Medicaid Services.
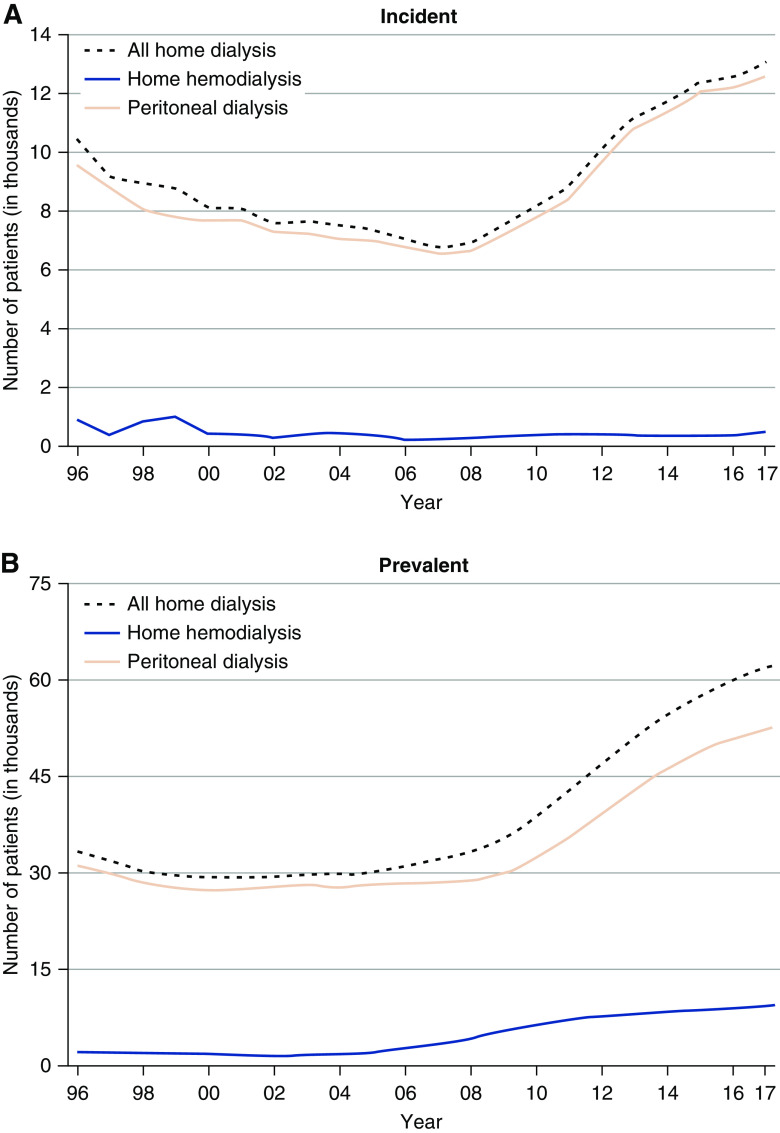
Thrice-weekly hemodialysis (HD) is the predominant form of RRT. In 2017, a total of 1545.8 per million population were receiving dialysis treatment (Table 1) (4). There has been an increase in home dialysis since 2008, predominantly in the form of peritoneal dialysis (PD) (Figure 2) (4). Patients receiving PD are on average younger, more likely to be White, non-Hispanic, and/or with GN or cystic kidney disease as the assigned primary cause of ESKD, compared with those on HD (4).
Figure 2.
Rising uptake of home dialysis therapies in the United States population (1996–2017) is predominantly accounted for by peritoneal dialysis. (A) Incident patients. (B) Prevalent patients.
Kidney disease ranked as the ninth leading cause of death in the United States, although the mortality rates among those with ESKD, similar to the general population, have steadily decreased from 2001 through 2017 (Table 1) (4,13). Although this trend could be attributed to increased pre-ESKD care, improved quality of care on dialysis, and better management of comorbid conditions (14), it may also largely be a reflection of the improved life expectancy in the general population. Cardiovascular causes comprised 54% of known causes of death among patients on dialysis (1). Patients on dialysis remain at high risk of hospitalization, although—like mortality—rates have declined over time from 2.0 to 1.7 per patient per year (2007–2016) (1). Accompanying a decline in hospitalization rates, an increase in emergency-room and observation-care stays has been observed (1). Readmission within 30 days of discharge occurs at a rate of 38% and 37% in patients on HD and those on PD, respectively. Dialysis facility readmission rates are being tracked as a quality measure by the Centers for Medicaid and Medicare (CMS) (8). Not surprisingly, the leading causes of hospitalization in patients on dialysis are cardiovascular events and infections (1).
Pre-ESKD Care
Early nephrology referral has been associated with lower risk of morbidity and mortality in patients with ESKD (15). By 2017, about 33% of US patients had been seen by nephrologists at least 12 months ahead of initiation of dialysis, whereas there was no evidence of having been seen by nephrologists before declaration of ESKD in about 19% of patients (Table 1) (4). Patients with earlier nephrology referral had better biochemical and care quality parameters (e.g., dietary care) than those without nephrology visits (Table 1) (4).
Major improvements in vascular access practices, particularly at dialysis start, are urgently needed. In 2017, only 17% of patients with incident ESKD received HD through autogenous arteriovenous (AV) fistula, the preferred mode of vascular access for HD (4), with 80% of incident patients being documented as initiating HD using a central venous catheter (CVC), an access type fraught with higher risk of infection and death (4). In 2018, 63% of prevalent patients on dialysis used an AV fistula, whereas 18% and 20% of patients were on AV graft and central venous catheter, respectively (Table 1) (4). There are many possible contributors to this suboptimal vascular access practice pattern, including barriers to timely access to care and care coordination; suboptimal AV fistula maturation as a major impediment; and inadequate supply of providers with the necessary training, expertise, and commitment to creating high-quality AV fistulas (16,17).
Provision of Care
In the United States, the majority of dialysis services are provided through private, for-profit, freestanding, outpatient dialysis facilities (Table 1) (9). Fresenius and DaVita were the two dominant, large dialysis organizations, owning 60% of all dialysis units, comprising 63% of patients on dialysis in 2011 (9). The supply of nephrologists varies across regions in the United States, where some regions have <62 patients with ESKD per nephrologist compared with regions that have >105 patients with ESKD per nephrologist (18). Nurse practitioners and physician assistants play an important role in provision of dialysis services (5). On average, 68% of facilities had their patients seen by nephrologists at least weekly during their dialysis sessions (7). The estimated average duration of a dialysis session was 216.5±26.1 minutes among those receiving thrice-weekly HD (6).
Financing of Dialysis
Medicare is a national social insurance program (enacted in 1965) that provides health insurance coverage for adults aged ≥65. Medicare has become the predominant payer for dialysis services since the enactment of the Medicare Entitlement Act of 1972, when the coverage was expanded to cover even younger adults, and those with disabilities (including ESKD) (10). Originally, Medicare was on a fee-for-service basis, which provided coverage for hospitalization under Medicare Part A and outpatient services or supplies under Part B. In 2006, with the launch of the Medicare prescription program, patients with ESKD began to receive medication coverage through Medicare Part D. The Medicare Advantage Plan, also known as Part C, is a prospective, capitated-fee coverage provided by Medicare that combines medical services and prescription drug coverage to those who have private health insurance coverage (typically after retirement) among those aged ≥65. Patients with ESKD are not eligible for the Medicare Advantage Plan, unless they had enrolled in such a plan before the onset of ESKD.
Although Medicare remains the dominant payer for the treatment of kidney failure, the remaining costs are paid by Medicaid, the Department of Veterans Affairs, private insurers, and other assistance programs (11). Overall Medicare spending for ESKD reached $46.6 billion in 2017, which is approximately 7% of all Medicare spending for 1% of its covered population (4). Patients’ out-of-pocket costs were an additional $3.5 billion in 2017, 1% more than in 2016 (4). Outpatient services accounted for the largest share of Medicare spending for ESKD (33%), followed by inpatient services (32%), and physician and supplier-billing claims (15%). The fastest growth occurred in spending on outpatient prescriptions ($5.1 billion), a 5% increase from 2016 (4). Medicare spending per person per year was far lower for patients receiving transplants compared with those on dialysis (4).
Federal Regulation and Policy
Like for other medical conditions, CMS has implemented continuous regulations with a view to improving care quality, patient outcomes, and patient experience, but also with an eye on reducing healthcare expenditures for patients with ESKD (Table 1). From 2011, CMS implemented the ESRD Prospective Payment System (PPS) to improve the quality of dialysis care, while reducing costs through financing reforms. Different from the traditional fee-for-service payment model, dialysis facilities receive bundled payment per dialysis treatment from this PPS for their dialysis services. The bundled payment is adjusted based on patient-level and facility-level factors, and covers supplies, equipment, laboratory services, oral medications, injectable drugs, and biologics (12). The ESRD PPS may pay an additional amount for patients who are high cost, based on medical necessity. Meanwhile, the ESRD PPS provides training add-on payments to facilities for patients with home and self-dialysis modalities. The most current ESRD PPS base rate is $239.33 in 2020 (12). Usually, patients pay 20% of the payment and insurance pays the rest (12).
The ESRD Quality Incentive Program (QIP) invokes the value-based purchasing concept to incentivize dialysis facilities to improve healthcare quality (19). The ESRD QIP evaluates facilities’ performance through quality measures, and then links this to part of Medicare-bundled PPS payments. Facilities receive payment penalties of up to 2% when their total performance score does not meet or exceed the minimum requirement set by CMS. The Dialysis Facility Compare program provides patients and their families with a tool for comparing and selecting dialysis facilities based on their quality performance (8). In recent years, star ratings have been applied to individual dialysis facilities, based on core quality measures.
The CMS Innovation Center implemented the Comprehensive ESRD Care (CEC) Model from October 2015 to December 2020. The CEC Model tests whether the creation of ESRD Seamless Care Organizations (ESCOs)—comprising dialysis clinics, nephrologists, and other providers—can reduce Medicare expenditures while maintaining or improving the quality of care. ESCOs who participate in the model agree to be accountable for care quality and cost of care for patients on dialysis who are aligned to them. Dialysis organizations share in savings regardless of their size, but large dialysis organizations are responsible for shared losses (20). After 2 years (by 2017), the CEC Model reduced Medicare spending by $68 million, or 2%, compared with non-CEC beneficiaries. There was about a 4% reduction in the number of hospitalizations, 8% decrease in catheter use, and 1% increase in outpatient dialysis sessions. However, Medicare experienced an aggregate net loss of $46 million after taking into account shared savings payments made to ESCOs (20). The final results of the evaluation will be available after the program ends in December 2020.
Advancing American Kidney Health
In July 2019, the US Department of Health and Human Services (HHS) launched the federal executive order Advancing American Kidney Health initiative. HHS set three goals to improve kidney health in the United States: (1) reduce the number of Americans developing ESKD; (2) increase the number of new (incident) American patients on home dialysis or transplantation to 80% by 2025; and (3) double the number of organs available for transplantation by 2030 (21). To achieve these ambitious goals, substantial changes will need to occur in the management of kidney disease at multiple levels, e.g., campaigns to raise societal awareness of kidney disease, including the general public, health systems, providers, insurers, and policy makers; earlier diagnosis and optimal management of all stages of the disease; enhancing quality of pre-ESKD care; increasing access to home dialysis options; and reforming the organ procurement and management system (22).
Future Challenges
Kidney failure represents a substantial health and financial burden for the United States. Based on current estimates, the burden of ESKD is projected to continue to grow at least until 2030 (2). The Advancing American Kidney Health initiative has raised hope for those who suffer from kidney disease in this country. For this to be realized, raising societal awareness of kidney disease with increased focus on prevention, through attention to social and environmental determinants of health and enhanced access to high-quality care, should be among the top priorities. Once ESKD is imminent, greater access to home dialysis and kidney transplantation, continually enhancing the quality of patient-centered care, and increasing the supply of donor kidneys is vitally important.
Disclosures
R. Saran has received travel and/or consulting fees from The High Pressure Gas Safety Institute of Japan (KHK), Reata Pharmaceuticals, and Kugai Pharmaceuticals. R. Saran has also received grants, from the US Centers for Disease Control and Prevention, US Patient-Centered Outcomes Research Institute, US Centers for Medicare & Medicaid Services and US National Institutes of Health. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the authors.
Author Contributions
Y. Han wrote the original draft; Y. Han and R. Saran conceptualized the study, were responsible for investigation, and reviewed and edited the manuscript; and R. Saran was responsible for validation.
References
- 1.United States Renal Data System : 2018 USRDS annual data report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018. Available at: https://www.usrds.org/annual-data-report/previous-adrs/ Accessed August 24, 2020 [Google Scholar]
- 2.McCullough KP, Morgenstern H, Saran R, Herman WH, Robinson BM: Projecting ESRD incidence and prevalence in the United States through 2030. J Am Soc Nephrol 30: 127–135, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crews DC, Novick TK: Social determinants of CKD hotspots. Semin Nephrol 39: 256–262, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Renal Data System : 2019 USRDS annual data report: Epidemiology of kidney disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2019. Available at: https://www.usrds.org/annual-data-report/. Accessed August 24, 2020 [Google Scholar]
- 5.Shreay S, Ma M, McCluskey J, Mittelhammer RC, Gitlin M, Stephens JM: Efficiency of U.S. Dialysis centers: An updated examination of facility characteristics that influence production of dialysis treatments. Health Serv Res 49: 838–857, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flythe JE, Curhan GC, Brunelli SM: Shorter length dialysis sessions are associated with increased mortality, independent of body weight. Kidney Int 83: 104–113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawaguchi T, Karaboyas A, Robinson BM, Li Y, Fukuhara S, Bieber BA, Rayner HC, Andreucci VE, Pisoni RL, Port FK, Morgenstern H, Akizawa T, Saran R: Associations of frequency and duration of patient-doctor contact in hemodialysis facilities with mortality. J Am Soc Nephrol 24: 1493–1502, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Centers for Medicare and Medicaid Services: Dialysis Facility Compare. Available at: https://www.medicare.gov/dialysisfacilitycompare/. Accessed March 9, 2020
- 9.United States Renal Data System : 2013 USRDS annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013. Available at: https://www.usrds.org/annual-data-report/previous-adrs/. Accessed August 24, 2020
- 10.Rettig RA: Special treatment--the story of Medicare’s ESRD entitlement. N Engl J Med 364: 596–598, 2011 [DOI] [PubMed] [Google Scholar]
- 11.National Institute of Diabetes and Digestive and Kidney Diseases: Financial help for treatment of kidney failure. Available at: https://www.niddk.nih.gov/health-information/kidney-disease/kidney-failure/financial-help-treatment. Accessed August 5, 2020
- 12.Centers for Medicare and Medicaid Services: CY2020 end-stage renal disease/durable medical equipment final rule (CMS 1713-F). Available at: https://www.cms.gov/newsroom/fact-sheets/cy2020-end-stage-renal-diseasedurable-medical-equipment-final-rule-cms-1713-f. Accessed March 9, 2020
- 13.Murphy SL, Xu J, Kochanek KD, Arias E: Mortality in the United States, 2017. NCHS Data Brief [328]: 1–8, 2018 [PubMed] [Google Scholar]
- 14.Johansen KL: Life expectancy gains for patients with ESRD. Clin J Am Soc Nephrol 13: 11–12, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillespie BW, Morgenstern H, Hedgeman E, Tilea A, Scholz N, Shearon T, Burrows NR, Shahinian VB, Yee J, Plantinga L, Powe NR, McClellan W, Robinson B, Williams DE, Saran R: Nephrology care prior to end-stage renal disease and outcomes among new ESRD patients in the USA. Clin Kidney J 8: 772–780, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shahinian VB, Zhang X, Tilea AM, He K, Schaubel DE, Wu W, Pisoni R, Robinson B, Saran R, Woodside KJ: Surgeon characteristics and dialysis vascular access outcomes in the United States: A retrospective cohort study. Am J Kidney Dis 75: 158–166, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Saran R, Elder SJ, Goodkin DA, Akiba T, Ethier J, Rayner HC, Saito A, Young EW, Gillespie BW, Merion RM, Pisoni RL: Enhanced training in vascular access creation predicts arteriovenous fistula placement and patency in hemodialysis patients: Results from the dialysis outcomes and practice patterns study. Ann Surg 247: 885–891, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Salsberg E, Quigley L, Masselink L, Wu X, Collins A:. The US Nephrology Workforce 2015 Developments and Trends, 2015 [Google Scholar]
- 19.Centers for Medicare and Medicaid Services: Dialysis facilities and the end-stage renal disease quality incentive program (ESRD QIP): Linking quality to payment. Available at: https://www.medicare.gov/dialysisfacilitycompare/#qip/quality-incentive-program. Accessed March 12, 2020
- 20.Centers for Medicare and Medicaid Services: Comprehensive ESRD care model. Available at: https://innovation.cms.gov/initiatives/comprehensive-esrd-care/. Accessed March 9, 2020
- 21.US Department of Health and Human Services: HHS launches President Trump’s ‘Advancing American Kidney Health’ initiative. Available at: https://www.hhs.gov/about/news/2019/07/10/hhs-launches-president-trump-advancing-american-kidney-health-initiative.html. Accessed March 9, 2020
- 22.Knight R: A patient’s perspective on advancing American kidney health initiative. Clin J Am Soc Nephrol 14: 1795–1797, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]