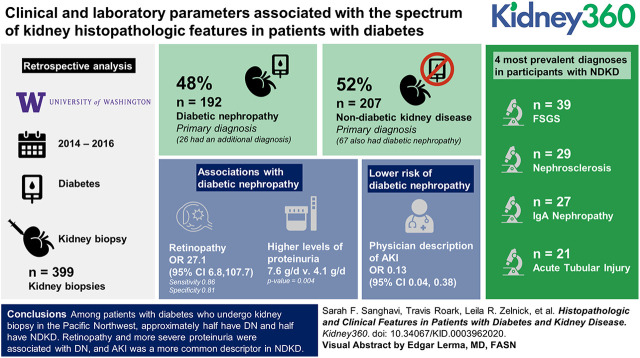
Visual Abstract
Keywords: diabetes and the kidney, diabetes mellitus, diabetic kidney disease, diabetic retinopathy, focal segmental glomerulosclerosis, histopathology, proteinuria
Abstract
Background
The discovery of nondiabetic kidney disease (NDKD) in an individual patient with diabetes may have significant treatment implications. Extensive histopathologic data in this population are lacking, but they may provide insights into the complex pathogenesis of diabetic nephropathy (DN) and reveal specific phenotypes for the development of targeted therapies. This study seeks to elucidate the clinical and laboratory parameters associated with the spectrum of kidney histopathologic features in patients with diabetes.
Methods
This study is a retrospective analysis of 399 kidney biopsies assessed from 2014 to 2016 at the University of Washington among patients with diabetes. More comprehensive clinical data were evaluated in a subset of 79 participants.
Results
Of the 399 biopsies reviewed, 192 (48%) had a primary diagnosis of DN (including 26 with an additional diagnosis), and 207 (52%) had a primary diagnosis of NDKD (including 67 who also had DN). Retinopathy (sensitivity: 0.86; specificity: 0.81; OR, 27.1; 95% CI, 6.8 to 107.7) and higher levels of proteinuria (7.6 versus 4.1 g/d; P=0.004) were associated with DN, whereas a physician description of AKI was associated with a lower risk of DN (OR, 0.13; 95% CI, 0.04 to 0.38). The four most prevalent diagnoses in participants with NDKD were FSGS in 39, nephrosclerosis in 29, IgA nephropathy in 27, and acute tubular injury in 21.
Conclusions
Among patients with diabetes who undergo kidney biopsy in the Pacific Northwest, approximately half have DN, and half have NDKD. Retinopathy and more severe proteinuria were associated with DN, and AKI was a more common descriptor in NDKD.
Podcast
This article contains a podcast at https://www.asnonline.org/media/podcast/K360/2020_11_25_KID0003962020.mp3
Introduction
CKD affects about one-quarter of people with diabetes in the United States (1), many of whom receive a diagnosis of diabetic kidney disease on clinical grounds. However, from an epidemiologic perspective, the risk of a reduced eGFR attributable to diabetes is about 50% (2). Determining which clinical signs are “atypical” for diabetic nephropathy (DN), defined as typical histopathologic findings on kidney biopsy, can be challenging. Proposed indications for kidney biopsy in this population include nephrotic-range proteinuria, hematuria, and a sudden decline in kidney function, but these have not been rigorously evaluated. A recent meta-analysis of 48 studies of kidney biopsies in patients with diabetes revealed significant heterogeneity in the prevalence of nondiabetic kidney disease (NDKD), ranging from 3% to 83% (3). Further, previous studies have not attempted to diagnose the primary disease process in patients with features of both DN and NDKD, although this information is paramount to clinical decision making. Because DN is a heterogenous disease, histopathologic patterns may identify specific phenotypes for future clinical studies, as is the case for histologic variants of FSGS (4). We, therefore, sought to characterize the clinical signs, primary diagnosis, and histopathologic characteristics of a large cohort of patients with diabetes who underwent kidney biopsy in the Pacific Northwest.
Materials and Methods
Patient Population
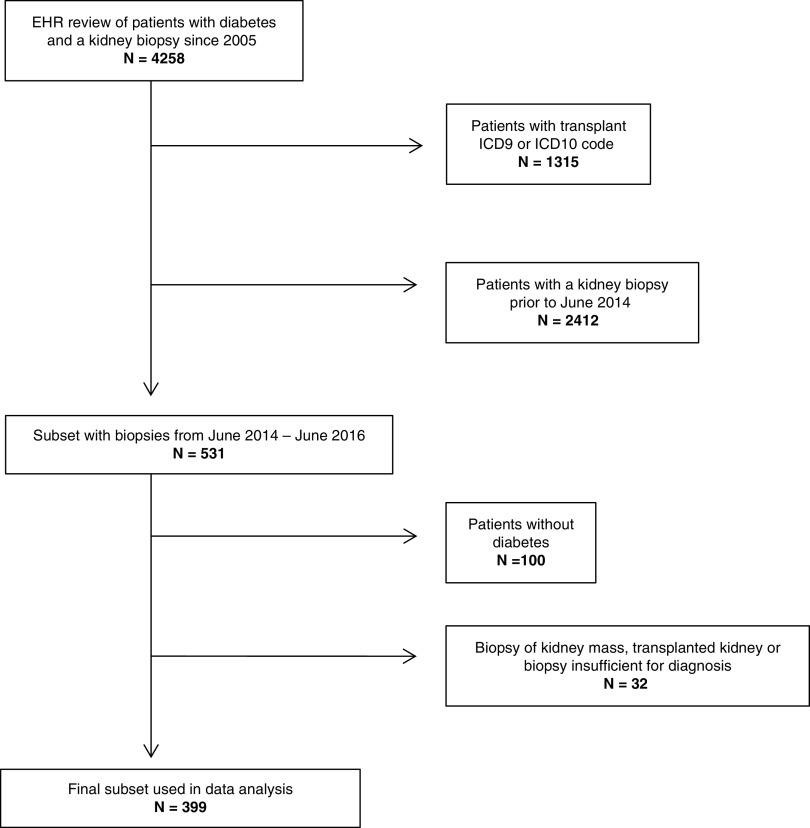
We obtained institutional review board approval for access to patient records. We included adults with diabetes who underwent a native kidney biopsy read at the University of Washington (UW), a regional referral center for the Pacific Northwest, from 2014 to 2016. We identified potential participants using International Classification of Diseases (ICD) 9/ICD10 codes for diabetes and a search of kidney biopsy pathology reports using natural language processing (searching for the terms “diabetes” and “diabetic”). We initially identified 4258 potentially qualifying patients biopsied from 2005 to 2016, of which 1315 were excluded for concurrent ICD9/ICD10 codes for kidney transplant. To reflect the most contemporary biopsy practices, patients were then restricted to those biopsied during the time period between June 2014 and June 2016. The resulting 531 biopsy reports were manually reviewed, leading to the exclusion of 100 patients who did not actually have diabetes and 32 patients who had a biopsy of a kidney mass, a kidney transplant, or insufficient quality for diagnosis. The final dataset comprised 399 participants for analysis (Figure 1).
Figure 1.
Consort diagram. ICD, International Classication of Diseases.
Data Acquisition
Clinical information, pathologic diagnosis, and histologic findings were manually extracted from pathology reports and entered into a customized RedCap database. Laboratory data and demographic information were acquired through data extraction from electronic medical records using ICD9/ICD10 codes. The algorithm was designed to provide the data closest in time to when the biopsy was performed. Of the 399 participants, 79 were seen by a nephrologist within the UW health care system. In this UW subset, we were able to verify pathology report data and acquire additional clinical and laboratory information, including the presence or absence of a chart history of retinopathy and hypertension, diabetes type (1 or 2), duration of diabetes, and the use of inhibitors of the renin-angiotensin-aldosterone system or immunosuppression. Abnormal serology was defined as a clinical history of positive antinuclear antibody, ANCA, antiglomerular basement membrane (anti-GBM) antibody, or low complement levels. Proteinuria was reported on spot specimens as grams of protein per gram of creatinine. We considered spot urine specimens to be equivalent to a 24-hour urine collection. The presence or absence of AKI was ascertained by chart review of nephrology notes.
Classification Scheme
Biopsies were categorized into two groups, DN and NDKD, with cases that included findings of both classified by the primary diagnosis for analysis. We chose this approach because the primary diagnosis is the most likely to direct treatment decisions and is, therefore, the most clinically relevant. The criteria for a diagnosis of DN required the following three pathologic findings: (1) GBM thickening, (2) mesangial expansion, and (3) arteriolar hyalinosis (5). We also classified several histologic findings as concomitant findings to DN rather than a separate diagnosis: (1) acute tubular injury if noted secondary to DN, (2) arteriosclerosis, (3) patchy eosinophilic infiltrate if noted secondary to DN, (4) global glomerulosclerosis, (5) FSGS if not primary, (6) tubular atrophy, (7) interstitial fibrosis, (8) interstitial inflammation, (9) Kimmelstiel–Wilson nodules, (10) microaneurysms, (11) mesangiolysis, and (12) podocyte foot process effacement if not a primary podocytopathy (5). Two reviewers independently reviewed the pathologic diagnoses to ensure proper classification, and any discordance was resolved through discussion with a renal pathologist. FSGS was classified as the primary diagnosis (NDKD) if the biopsy showed extensive foot process effacement but no evidence of advanced DN. It was classified as likely secondary to DN for any case with advanced DN or at least moderate DN with subnephrotic-range proteinuria and segmental foot process effacement (6). All of the cases of FSGS were reviewed by a renal pathologist.
Statistical Analyses
Distributions of each biopsy characteristic were summarized using descriptive statistics, such as the mean and SD for continuous variables or number and percent of nonmissing responses for categorical variables. For continuous variables, we compared means of those with DN and those with NDKD via a two-sample t test assuming unequal variances; for categorical variables, we compared the distributions between these two groups using a chi-squared test. To evaluate the association of key clinical covariates and biopsy findings in the UW subset, we used logistic regression to estimate odds ratios (ORs) and associated 95% confidence intervals (95% CIs) and P values; we also calculated the sensitivity, specificity, positive predictive value, and negative predictive value of the clinical covariate for DN. All analyses were performed with R version 3.6.2 computing environment (R Foundation for Statistical Computing).
Results
Patient Characteristics
The study cohort included 399 participants: 230 men and 169 women. The mean age of our participants was 58 years, and 55% were White. Average serum creatinine was 3 mg/dl (SD 2.4 mg/dl), and eGFR was 36±28 ml/min per 1.73 m2. Proteinuria was quantified at 6.2 (SD 5) g/d, with nephrotic-range proteinuria occurring in about half of participants. Average hemoglobin A1c was 7.9%. Our subset of 79 participants with more comprehensive clinical information available (UW subset) was similar to the overall cohort (Table 1). Data on retinopathy were available on 61 of the 79 participants, and only seven participants had a diagnosis of type 1 diabetes. Sixty-eight percent of participants in the subset were prescribed medications that inhibited that the renin-angiotensin-aldosterone axis (Supplemental Table 1).
Table 1.
Characteristics assessed by pathology report of 399 participants with diabetes who underwent kidney biopsy in the Pacific Northwest, including a subset of 79 with more extensive clinical data charted
| Sample Population | Cohort of N=399 Patients | Subset of N=79 Patients | ||
| No. with Data | Mean (SD) or N (%)a | No. with Data | Mean (SD) or N (%)a | |
| Demographics | ||||
| Age, yr, mean (SD) | 398 | 57.6 (13.1) | 78 | 53.6 (12.4) |
| Men | 398 | 230 (58) | 78 | 53 (68) |
| Race/ethnicity | ||||
| White | 130 | 71 (55) | 70 | 38 (54) |
| Black | 130 | 18 (14) | 70 | 10 (14) |
| Asian | 130 | 23 (18) | 70 | 10 (14) |
| Native American/Pacific Islander | 130 | 8 (6) | 70 | 5 (7) |
| Hispanic | 130 | 10 (8) | 70 | 7 (10) |
| Medical history | ||||
| AKI | 399 | 98 (25) | 79 | 26 (33) |
| Rapid decline in eGFR | 399 | 91 (23) | 79 | 23 (29) |
| SLE | 399 | 7 (2) | 79 | 4 (5) |
| Organ transplant | 399 | 12 (3) | 79 | 6 (8) |
| Diagnostic results | ||||
| Proteinuria | 399 | 312 (78) | 79 | 59 (75) |
| Nephrotic range | 399 | 196 (49) | 79 | 41 (52) |
| Hematuria | 399 | 80 (20) | 79 | 20 (25) |
| Abnormal serology | 399 | 78 (20) | 79 | 18 (23) |
| Monoclonal gammopathy | 399 | 33 (8) | 79 | 11 (14) |
| HCV antibody | 399 | 33 (8) | 79 | 16 (20) |
| Laboratory values | ||||
| Serum creatinine, mg/dl | 302 | 3.0 (2.5) | 77 | 2.9 (2.1) |
| eGFR, ml/min per 1.73 m2 | 301 | 35.7 (28.2) | 76 | 38.7 (29.3) |
| Proteinuria, g/da | 223 | 6.2 (5.1) | 67 | 5.7 (5.0) |
| HbA1c, % | 72 | 7.9 (2.5) | 59 | 7.8 (2.2) |
| Biopsy characteristics | ||||
| Biopsy size (LM) | 395 | 1.3 (0.4) | 79 | 1.2 (0.4) |
| No. of glomeruli | 399 | 18.7 (10.4) | 79 | 20.2 (11.0) |
HCV, Hepatitis C virus; HbA1c, hemoglobin A1c; LM, light microscopy.
Proteinuria from spot specimens was assumed to be equivalent to grams per day.
Characteristics by Diagnosis
In the entire study cohort, 192 (48%) had a primary diagnosis of DN, and 207 (52%) had a primary diagnosis of NDKD. Of participants with a primary diagnosis of DN, 26 had a second diagnosis of NDKD. Sixty-seven participants with NDKD had a second diagnosis of DN. Overall, 93 (23%) of the total cohort had evidence of both NDKD and DN. Nephrotic-range proteinuria was significantly more prevalent in participants with DN (60%) than NDKD (39%). Mean proteinuria was also higher at 7.5 g/d in DN versus 4.9 g/d in NDKD. Abnormal serologies (ANCA, antinuclear antibody, and low complement) and hematuria were observed statistically significantly more often in participants with NDKD. The presence of a monoclonal gammopathy was not statistically different between groups (Table 2).
Table 2.
Total cohort characteristics by primary diagnosis of diabetic nephropathy or nondiabetic kidney disease (N=399)
| Sample Population | Diabetic Nephropathy, Mean (SD) or N (%)a | Nondiabetic Kidney Disease, Mean (SD) or N (%)a | P Value |
| No. | 192 | 207 | |
| Demographics | |||
| Age, yr, mean (SD) | 55.8 (13.0) | 59.3 (13.0) | 0.007 |
| Men | 111 (58) | 119 (58) | 0.99 |
| Race/ethnicity | |||
| White | 29 (50) | 42 (58) | 0.36 |
| Black | 7 (12) | 11 (15) | |
| Asian | 15 (26) | 8 (11) | |
| Native American/Pacific Islander | 3 (5) | 5 (7) | |
| Hispanic | 4 (7) | 6 (8) | |
| Diagnostic results | |||
| Proteinuria | 158 (82) | 154 (74) | 0.07 |
| Nephrotic range | 116 (60) | 80 (39) | <0.001 |
| Hematuria | 25 (13) | 55 (27) | 0.001 |
| Abnormal serology | 26 (14) | 52 (25) | 0.005 |
| Monoclonal gammopathy | 17 (8) | 16 (8) | 0.82 |
| HCV antibody | 16 (8) | 17 (8) | 0.99 |
| Laboratory values | |||
| Serum creatinine, mg/dl | 2.8 (2.4) | 3.2 (2.5) | 0.20 |
| eGFR, ml/min per 1.73 m2 | 36.2 (26.3) | 35.3 (29.8) | 0.79 |
| Proteinuria, g/da | 7.5 (5.1) | 4.9 (4.8) | <0.001 |
| HbA1c, % | 8.3 (2.7) | 7.6 (2.2) | 0.22 |
P values are from the t test assuming unequal variances or the chi-squared test, as appropriate. HCV, Hepatitis C virus; HbA1c, hemoglobin A1c.
Proteinuria from spot specimens was assumed to be equivalent to grams per day.
Our UW subset of 79 participants provided more detailed clinical information verified by chart review. The sensitivity and specificity of a chart history of retinopathy for primary DN were 0.86 and 0.81, respectively, with an OR for DN of 27.1 (95% CI, 6.82 to 107.77). “AKI” was referenced less frequently (OR, 0.1; 95% CI, 0.04 to 0.38) in participants with DN. Nephrotic-range proteinuria was common in both groups, and proteinuria <3.5 g/d was not significantly associated with DN (OR, 0.5; 95% CI, 0.10 to 2.86). The presence of hematuria did not aid in differentiation of NDKD from DN (OR, 0.5; 95% CI, 0.16 to 1.43) when its presence or absence was confirmed by chart review (Table 3).
Table 3.
Crosstabulations: Association of key clinical predictors assessed by chart review and biopsy findings in the subcohort (N=79)
| Variable | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Odds Ratio (95% Confidence Interval) | P Value |
| Retinopathy | 0.86 | 0.81 | 0.81 | 0.87 | 27.1 (6.82 to 107.77) | <0.001 |
| AKI | 0.17 | 0.39 | 0.18 | 0.37 | 0.1 (0.04 to 0.38) | <0.001 |
| Rapid decline of eGFR | 0.34 | 0.98 | 0.92 | 0.65 | 22.4 (2.75 to 183.09) | 0.004 |
| Type 1 DM | 0.09 | 0.91 | 0.43 | 0.56 | 0.9 (0.20 to 4.48) | 0.94 |
| Type 2 DM | 0.83 | 0.20 | 0.45 | 0.60 | 1.3 (0.39 to 3.90) | 0.71 |
| Duration of DM <10 yr | 0.41 | 0.46 | 0.44 | 0.43 | 0.6 (0.21 to 1.75) | 0.36 |
| eGFR<30 ml/min per 1.73 m2 | 0.68 | 0.25 | 0.50 | 0.42 | 0.7 (0.18 to 2.77) | 0.63 |
| eGFR (continuous), per 15-ml/min per 1.73 m2 increment | 1.0 (0.74 to 1.46) | 0.84 | ||||
| Urine protein <3.5 g/g | 0.14 | 0.76 | 0.43 | 0.42 | 0.5 (0.10 to 2.86) | 0.47 |
| Urine protein (continuous), per 1-g/g increment | 1.1 (0.97 to 1.28) | 0.14 | ||||
| Abnormal serology | 0.23 | 0.59 | 0.31 | 0.49 | 0.4 (0.16 to 1.15) | 0.09 |
| Hematuria (any) | 0.45 | 0.36 | 0.32 | 0.50 | 0.5 (0.16 to 1.43) | 0.19 |
| HbA1c<8.0% | 0.57 | 0.35 | 0.44 | 0.48 | 0.7 (0.26 to 2.10) | 0.56 |
| HbA1c (continuous), per 1.0% increment | 1.0 (0.81 to 1.29) | 0.83 |
Crosstabulation analysis of the subgroup of 79 patients looking at sensitivity, specificity, positive predictive value, negative predictive value, odds ratio, and P values for diabetic nephropathy. DM, diabetes mellitus; HbA1c, hemoglobin A1c.
Histopathologic Findings
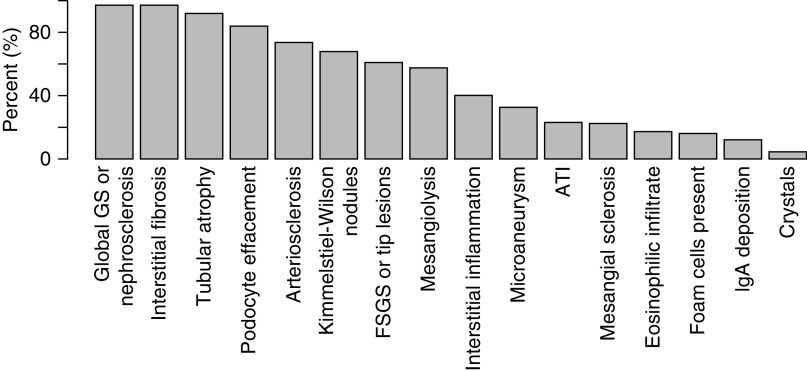
Among the study cohort, 192 participants had a primary diagnosis of DN. Glomerular involvement, such as global glomerulosclerosis, FSGS, and podocyte foot process effacement, was a concomitant finding to DN in 97%, 61%, and 84% of participants, respectively. The classic finding of Kimmelstiel–Wilson lesions was noted in 68%. Other histologic features included acute tubular injury in 23% and eosinophilic infiltrate in 17% (Figure 2). FSGS and acute tubular injury (as nonprimary diagnoses) were more likely to be found in participants with DN than those with a primary diagnosis of NDKD.
Figure 2.
Histologic findings in patients with a primary diagnosis of diabetic nephropathy. ATI, acute tubular injury; GS, glomerulosclerosis.
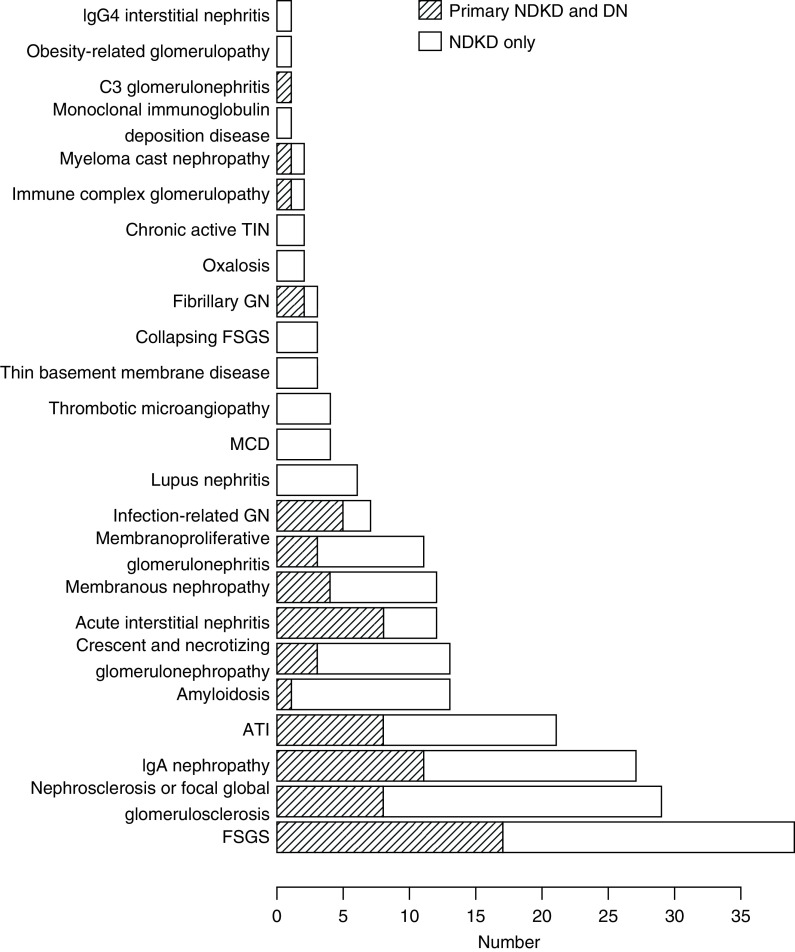
Twenty-four different diagnoses were identified in the 207 participants with primary NDKD. The four leading diagnoses were FSGS in 39, nephrosclerosis in 29, IgA nephropathy in 27, and acute tubular injury in 21 (Figure 3). Thirty-nine percent of this group had diagnoses with treatment options beyond standard care for diabetic kidney disease. These comprised membranoproliferative GN, membranous nephropathy, crescentic GN, amyloidosis, cast nephropathy, monoclonal Ig deposition disease, fibrillary GN, minimal change disease, C3 GN, acute interstitial nephritis, collapsing FSGS, and lupus nephritis (Supplemental Table 2). The proportions of participants with nephrosclerosis and FSGS were similar among all races/ethnicities (Supplemental Table 3).
Figure 3.
The distribution of diagnoses in participants with a primary diagnosis of nondiabetic kidney disease. Thirteen biopsies had both FSGS and nephrosclerosis and were included in both categories. DN, diabetic nephropathy; MCD, minimal change disease; NDKD, nondiabetic kidney disease; TIN, tubulointerstitial nephritis.
Discussion
In our cohort of 399 participants with diabetes selected for kidney biopsy in the Pacific Northwest, we found that 48% had a primary diagnosis of DN and that 52% had a primary diagnosis of NDKD. Twenty-three percent had evidence of both DN and NDKD. Retinopathy and higher mean proteinuria were associated with a primary diagnosis of DN, whereas a clinical history of AKI was associated with NDKD. Participants with DN as the primary diagnosis frequently had findings of global glomerulosclerosis and FSGS. The leading diagnoses among participants with NDKD were FSGS, nephrosclerosis, IgA nephropathy, and acute tubular injury. Our classification scheme enabled us to determine that 20% of the total cohort carried diagnoses that favored treatment beyond standard therapies for diabetic kidney disease.
Although an association between retinopathy and DN is well known, the strength of this association in our study was nonetheless surprising. Retinopathy was 86% sensitive and 81% specific for DN, with an OR of 27 (95% CI, 6.82 to 107.77). Our magnitude of association was larger than that in a meta-analysis of 26 studies, which found an OR of 5.67 (95% CI, 3.45 to 9.34) (7), but it was similar to the largest contemporary single-center study, which restricted inclusion to patients with a dilated optic fundus examination. In that study, difficult cases were confirmed with eye-ground photography or fundus fluorescence angiography, suggesting that the greater strength of association may lie in more precise ascertainment of the diagnosis of retinopathy (8).
Our finding that more severe proteinuria is more likely to be found in DN rather than NDKD confirms the other two United States studies that evaluated proteinuria (9,10). This observation may be due to the high rate of FSGS in our cohort with DN. Because we did not assess changes in proteinuria over time, these results do not inform decisions in patients with a sudden increase in proteinuria. On the basis of these collective data, progressive severe proteinuria should not be the sole indication to biopsy patients with diabetes, such as has been recommended in the past.
The term “AKI” in the clinical history was associated with NDKD, whereas “rapid decline in eGFR” was associated with DN. Working groups have defined AKI as a rise in creatinine occurring over 7 days or less and a “rapid decline in eGFR” as a drop in eGFR>5 ml/min per year (11,12). The eGFR decline in persons with diabetes and advanced kidney disease with nephrotic-range proteinuria similar to our population may be about 1 ml/min per month, indicating a rapid decline but not an AKI (13). In contrast, of the numerous diagnoses in our NDKD category, many may present clinically with AKI (9,14). In our study, we evaluated the use of these terms by the referring physician. Data that quantify changes in eGFR over time are required to confirm these findings.
Clinical characteristics that did not aid in differentiation between DN and NDKD included hematuria, monoclonal gammopathy, and duration of diabetes. Glomerular hematuria is considered atypical in diabetic kidney disease and has been a criterion for inclusion in studies of NDKD in patients with diabetes (15,16). However, multiple studies have failed to show a correlation between hematuria and NDKD, except in cohorts with a high prevalence of IgA nephropathy (8,17–19). Hematuria was noted more frequently in the clinical history of the pathology report in the NDKD group. However, in our UW subset in which we verified the presence or absence of hematuria, this finding had poor sensitivity and specificity for DN. The reason for this difference may be that other factors suggesting a GN may influence the referring clinician’s clinical history.
We included the presence of a monoclonal gammopathy as a clinical parameter as it is associated with a diverse group of kidney diseases that may cause proteinuria and a decline in eGFR (20). Its presence increases with age and is present in 3% of people over the age of 50 and 5% of people older than 70 (21). Sharma et al. (9) found that a monoclonal gammopathy was associated with NDKD, but only 24% of participants with a positive M spike had related pathologies, such as cast nephropathy or amyloidosis. In our population, a monoclonal gammopathy was noted on the pathology report in 8% of patients and did not aid in differentiation of DN from NDKD. This test may offer greater discriminating power when paired with free light-chain ratios adjusted for eGFR rather than on its own (22). Studies that include negative serum protein electrophoresis results are needed to describe the specificity of this test. In our study, a duration of diabetes of <10 years was not associated with NDKD, which may be because most of our patients had a longer duration. Previous studies have shown that shorter duration of diabetes (<5 years) is associated with NDKD, whereas longer duration (>10 years) is associated with DN (8,9,15,23,24).
The histopathologic findings among patients with DN shed light upon the complex pathogenesis of this disease. GBM thickening occurs early in diabetes and can be detected in the absence of microalbuminuria (25). The disease subsequently progresses to mesangial expansion, Kimmelstiel–Wilson lesions, and global glomerulosclerosis (26). We therefore chose GBM thickening and mesangial expansion as two of three criteria in establishing the diagnosis of DN. Notably, 80% of these biopsies had moderate to severe GBM thickening, and >70% showed moderate to severe mesangial expansion, consistent with the long duration of disease in our cohort.
Mesangiolysis may be a stage in the development of capillary aneurysms and mesangial nodules (Kimmelstiel–Wilson nodules), but the exact mechanisms are still unknown. One hypothesis is that capillary loops rupture when they lose their anchoring sites to degenerating mesangial cells, forming capillary aneurysms. Attempts by the mesangial cells to repair this damage may result in reactive nodules (27,28). Kimmelstiel–Wilson nodules and mesangiolysis were seen in the majority of our biopsies with primary DN, and microaneurysms were present in one-third.
Global glomerulosclerosis and FSGS were dominant findings among participants with DN. Experimental studies show that when the glomerular filtration surface area exceeds podocyte foot process coverage, proteinuria and lesions of FSGS develop (29), and loss of nephron mass accelerates this process (30). Therefore, two mechanisms likely contribute to these lesions in patients with diabetes: glomerulomegaly in the early stages and loss of podocytes and nephron mass in later stages (31). The mean proteinuria in our cohort of 6 g/d may reflect the selection of patients with greater proteinuria for kidney biopsy. Our rates of podocyte effacement, FSGS, and global glomerulosclerosis may account for the high levels of proteinuria in our cohort with DN. Biopsies from patients with diabetes and lower levels of proteinuria would be required to test this hypothesis.
The classic inflammation in DN is composed of T lymphocytes and macrophages, but our group has previously shown that eosinophilic infiltrate is prevalent in DN. We confirm these findings in our study in which eosinophilic infiltrate was present in 19% of our participants with DN (32). Our finding that acute tubular injury is more likely to occur with DN than without corroborates previous findings (9) and lends evidence to the argument that tubular injury may be involved in the pathogenesis of DN (33).
In patients with a primary diagnosis of NDKD, FSGS, nephrosclerosis, and IgA nephropathy were the most common lesions. The high prevalence of FSGS in our cohort is similar to that in every previous United States cohort evaluating NDKD in patients with diabetes (9,10,34). By definition, the cases of FSGS in this group were not secondary to DN, but we were unable to ascertain whether these lesions represent primary FSGS or secondary FSGS. Because both hypertension and obesity are common comorbidities in this population and shared risk factors for secondary FSGS, they may have contributed to this pattern of injury (35,36). Similarly, nephrosclerosis increases with age and mean BP and was the second most common finding in our cohort with NDKD. The prevalence of IgA nephropathy among our entire cohort was 8.7% compared with 5.7% in the other large United States study. This result may stem from our higher proportion of Asian participants at 18% compared with 2% (9).
Thirty-nine percent of participants with NDKD and 20% of the entire cohort had a diagnosis with treatment options that could potentially reverse or forestall the progression of kidney disease. This is clearly the most relevant outcome to patients and providers when considering a biopsy. Our cohort includes participants with presentations that raised clinical suspicion for NDKD, but we are unable to retrospectively determine the degree of clinical suspicion. Many of the diagnoses found, such as membranoproliferative GN and minimal change disease, are virtually indistinguishable from DN without invasive testing. Others, such as membranous nephropathy, lupus nephritis, and pauci-immune GN, may be supported by serologic testing. Nevertheless, these numbers can be used in conjunction with the clinical characteristics we identified to inform clinical decision making in pursuing a kidney biopsy in this population.
The strength of this study lies in its size, generalizability, and classification scheme. Because the UW pathology department receives a high volume of biopsies from a regional referral base, we were able to select a narrow time period for analysis reflecting contemporary biopsy practices and medication use. The total cohort and the UW subset were similar in clinical characteristics, lending external validity to this study where much of the detailed clinical information was derived from the subset. Our classification scheme, which adjudicated the primary diagnosis for biopsies with features of both NDKD and DN, enabled us to quantify the effect on clinical decision making. The most significant limitation of this retrospective study is sampling bias as only patients with suspected NDKD were biopsied. In unselected populations with diabetes, the prevalence of NDKD is low (37,38). Our results should, therefore, be interpreted within this context. Additionally, we did not have longitudinal eGFR data to assess whether clinical or histopathologic characteristics were associated with a more aggressive course.
Our study adds to the global body of literature of NDKD in patients with diabetes. With the growing burden of diabetes in the United States (39), clinicians will be increasingly faced with the challenge of selecting patients for whom a kidney biopsy will favorably alter outcomes. Combining our results with those of similar studies suggest that retinopathy and GFR trajectory can guide clinical decision making. Prospective studies in which highly specific testing for diabetic retinopathy is performed at the time of kidney biopsy may reveal the true degree of this association. Additionally, serial measurements of GFR before and after protocol biopsies may demonstrate which histopathologic features portend a more aggressive course. The spectrum of biopsy findings in people with diabetes is as diverse as the population it represents. Large-scale biopsy studies in people with diabetes who are more representative of the overall population are required to determine if this diversity is the norm or a result of sampling bias.
Disclosures
I. de Boer received research support from Abbott and MedTronic, outside the submitted work; consulted for Boehringer-Ingleheim and Ironwood; and received personal fees from Cyclerion Therapeutics, George Clinical, and Goldfinch Bio. All remaining authors have nothing to disclose.
Funding
This work was supported by an unrestricted fund from the Northwest Kidney Centers and National Institute of Diabetes and Digestive and Kidney Disease grant R01DK088762.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0003962020/-/DCSupplemental.
University of Washington subset characteristics. Download Supplemental Table 1, PDF file, 38 KB (37.4KB, pdf)
Diagnoses among participants with primary nondiabetic kidney disease. Download Supplemental Table 2, PDF file, 38 KB (37.4KB, pdf)
Histopathologic features by race/ethnicity. Download Supplemental Table 3, PDF file, 38 KB (37.4KB, pdf)
Author Contributions
C. Alpers, N. Andeen, I. de Boer, B. Najafian, and R. Pichler conceptualized the study; I. de Boer, B. Najafian, T. Roark, and S. Sanghavi were responsible for data curation; I. de Boer, T. Roark, and S. Sanghavi were responsible for investigation; N. Andeen, I. de Boer, and R. Pichler were responsible for methodology; L. Zelnick was responsible for formal analysis; E. Ayers was responsible for project administration and resources; S. Sanghavi wrote the original draft; and T. Roark, L. Zelnick, N. Andeen, R. Pichler, E. Ayers, C. Alpers, B. Najafian, and I. de Boer reviewed and edited the manuscript.
References
- 1.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH: Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 316: 602–610, 2016. Available at: 10.1001/jama.2016.10924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zelnick LR, Weiss NS, Kestenbaum BR, Robinson-Cohen C, Heagerty PJ, Tuttle K, Hall YN, Hirsch IB, de Boer IH: Diabetes and CKD in the United States population, 2009-2014. Clin J Am Soc Nephrol 12: 1984–1990, 2017. Available at: 10.2215/CJN.03700417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiorentino M, Bolignano D, Tesar V, Pisano A, Biesen WV, Tripepi G, D’Arrigo G, Gesualdo L; ERA-EDTA Immunonephrology Working Group : Renal biopsy in patients with diabetes: A pooled meta-analysis of 48 studies. Nephrol Dial Transplant 32: 97–110, 2017 [DOI] [PubMed] [Google Scholar]
- 4.D’Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA, Cohen AH, Gipson DS, Gassman JJ, Radeva MK, Moxey-Mims MM, Friedman AL, Kaskel FJ, Trachtman H, Alpers CE, Fogo AB, Greene TH, Nast CC: Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol 8: 399–406, 2013. Available at: 10.2215/CJN.06100612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Najafian B, Fogo AB, Lusco MA, Alpers CE: AJKD atlas of renal pathology: Diabetic nephropathy. Am J Kidney Dis 66: e37–e38, 2015. Available at: 10.1053/j.ajkd.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 6.De Vriese AS, Sethi S, Nath KA, Glassock RJ, Fervenza FC: Differentiating primary, genetic, and secondary FSGS in adults: A clinicopathologic approach. J Am Soc Nephrol 29: 759–774, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He F, Xia X, Wu XF, Yu XQ, Huang FX: Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: A meta-analysis. Diabetologia 56: 457–466, 2013. Available at: 10.1007/s00125-012-2796-6 [DOI] [PubMed] [Google Scholar]
- 8.Dong Z, Wang Y, Qiu Q, Zhang X, Zhang L, Wu J, Wei R, Zhu H, Cai G, Sun X, Chen X: Clinical predictors differentiating non-diabetic renal diseases from diabetic nephropathy in a large population of type 2 diabetes patients. Diabetes Res Clin Pract 121: 112–118, 2016. Available at: 10.1016/j.diabres.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 9.Sharma SG, Bomback AS, Radhakrishnan J, Herlitz LC, Stokes MB, Markowitz GS, D’Agati VD: The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol 8: 1718–1724, 2013. Available at: 10.2215/CJN.02510213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pham TT, Sim JJ, Kujubu DA, Liu I-LA, Kumar VA: Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol 27: 322–328, 2007. Available at: 10.1159/000102598 [DOI] [PubMed] [Google Scholar]
- 11.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014. Available at: 10.1056/NEJMra1214243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 63: 713–735, 2014. Available at: 10.1053/j.ajkd.2014.01.416 [DOI] [PubMed] [Google Scholar]
- 13.Kitai Y, Doi Y, Osaki K, Sugioka S, Koshikawa M, Sugawara A: Nephrotic range proteinuria as a strong risk factor for rapid renal function decline during pre-dialysis phase in type 2 diabetic patients with severely impaired renal function. Clin Exp Nephrol 19: 1037–1043, 2015. Available at: 10.1007/s10157-015-1094-2 [DOI] [PubMed] [Google Scholar]
- 14.Soni SS, Gowrishankar S, Kishan AG, Raman A: Non diabetic renal disease in type 2 diabetes mellitus. Nephrology (Carlton) 11: 533–537, 2006. Available at: 10.1111/j.1440-1797.2006.00681.x [DOI] [PubMed] [Google Scholar]
- 15.Wong TYH, Choi PCL, Szeto CC, To KF, Tang NLS, Chan AWH, Li PKT, Lai FM-M: Renal outcome in type 2 diabetic patients with or without coexisting nondiabetic nephropathies. Diabetes Care 25: 900–905, 2002. Available at: 10.2337/diacare.25.5.900 [DOI] [PubMed] [Google Scholar]
- 16.Mazzucco G, Bertani T, Fortunato M, Bernardi M, Leutner M, Boldorini R, Monga G: Different patterns of renal damage in type 2 diabetes mellitus: A multicentric study on 393 biopsies. Am J Kidney Dis 39: 713–720, 2002. Available at: 10.1053/ajkd.2002.31988 [DOI] [PubMed] [Google Scholar]
- 17.Mak SK, Gwi E, Chan KW, Wong PN, Lo KY, Lee KF, Wong AK: Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant 12: 2588–2591, 1997. Available at: 10.1093/ndt/12.12.2588 [DOI] [PubMed] [Google Scholar]
- 18.Lee EY, Chung CH, Choi SO: Non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Yonsei Med J 40: 321–326, 1999. Available at: 10.3349/ymj.1999.40.4.321 [DOI] [PubMed] [Google Scholar]
- 19.Jin Kim Y, Hyung Kim Y, Dae Kim K, Ryun Moon K, Ho Park J, Mi Park B, Ryu H, Eun Choi D, Ryang Na K, Sun Suh K, Wook Lee K, Tai Shin Y: Nondiabetic kidney diseases in type 2 diabetic patients. Kidney Res Clin Pract 32: 115–120, 2013. Available at: 10.1016/j.krcp.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridoux F, Leung N, Hutchison CA, Touchard G, Sethi S, Fermand J-P, Picken MM, Herrera GA, Kastritis E, Merlini G, Roussel M, Fervenza FC, Dispenzieri A, Kyle RA, Nasr SH; International Kidney and Monoclonal Gammopathy Research Group : Diagnosis of monoclonal gammopathy of renal significance. Kidney Int 87: 698–711, 2015. Available at: 10.1038/ki.2014.408 [DOI] [PubMed] [Google Scholar]
- 21.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton JA 3rd: Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 354: 1362–1369, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hutchison CA, Plant T, Drayson M, Cockwell P, Kountouri M, Basnayake K, Harding S, Bradwell AR, Mead G: Serum free light chain measurement aids the diagnosis of myeloma in patients with severe renal failure. BMC Nephrol 9: 11, 2008. Available at: 10.1186/1471-2369-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang TI, Park JT, Kim JK, Kim SJ, Oh HJ, Yoo DE, Han SH, Yoo T-H, Kang S-W: Renal outcomes in patients with type 2 diabetes with or without coexisting non-diabetic renal disease. Diabetes Res Clin Pract 92: 198–204, 2011. Available at: 10.1016/j.diabres.2011.01.017 [DOI] [PubMed] [Google Scholar]
- 24.Tone A, Shikata K, Matsuda M, Usui H, Okada S, Ogawa D, Wada J, Makino H: Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract 69: 237–242, 2005. Available at: 10.1016/j.diabres.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 25.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, Coplon NS, Sun L, Meyer TW: Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest 99: 342–348, 1997. Available at: 10.1172/JCI119163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tervaert TWC, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, Ferrario F, Fogo AB, Haas M, de Heer E, Joh K, Noël LH, Radhakrishnan J, Seshan SV, Bajema IM, Bruijn JA; Renal Pathology Society : Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 21: 556–563, 2010. Available at: 10.1681/ASN.2010010010 [DOI] [PubMed] [Google Scholar]
- 27.Morita T, Churg J: Mesangiolysis. Kidney Int 24: 1–9, 1983. Available at: 10.1038/ki.1983.119 [DOI] [PubMed] [Google Scholar]
- 28.Stout LC, Kumar S, Whorton EB: Focal mesangiolysis and the pathogenesis of the Kimmelstiel-Wilson nodule. Hum Pathol 24: 77–89, 1993. Available at: 10.1016/0046-8177(93)90066-P [DOI] [PubMed] [Google Scholar]
- 29.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC: Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012. Available at: 10.1681/ASN.2012030271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishizono R, Kikuchi M, Wang SQ, Chowdhury M, Nair V, Hartman J, Fukuda A, Wickman L, Hodgin JB, Bitzer M, Naik A, Wiggins J, Kretzler M, Wiggins RC: FSGS as an adaptive response to growth-induced podocyte stress. J Am Soc Nephrol 28: 2931–2945, 2017. Available at: 10.1681/ASN.2017020174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reidy K, Kang HM, Hostetter T, Susztak K: Molecular mechanisms of diabetic kidney disease. J Clin Invest 124: 2333–2340, 2014. Available at: 10.1172/JCI72271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai D-F, Sasaki K, Lin MY, Smith KD, Nicosia RF, Alpers CE, Najafian B: Interstitial eosinophilic aggregates in diabetic nephropathy: Allergy or not? Nephrol Dial Transplant 30: 1370–1376, 2015. Available at: 10.1093/ndt/gfv067 [DOI] [PubMed] [Google Scholar]
- 33.Vaidya VS, Niewczas MA, Ficociello LH, Johnson AC, Collings FB, Warram JH, Krolewski AS, Bonventre JV: Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-D-glucosaminidase. Kidney Int 79: 464–470, 2011. Available at: 10.1038/ki.2010.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nzerue CM, Hewan-Lowe K, Harvey P, Mohammed D, Furlong B, Oster R: Prevalence of non-diabetic renal disease among African-American patients with type II diabetes mellitus. Scand J Urol Nephrol 34: 331–335, 2000. Available at: 10.1080/003655900750048378 [DOI] [PubMed] [Google Scholar]
- 35.Hypertension in Diabetes Study (HDS) : Hypertension in Diabetes Study (HDS). I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens 11: 309–317, 1993. Available at: 10.1097/00004872-199303000-00012 [DOI] [PubMed] [Google Scholar]
- 36.Daousi C, Casson IF, Gill GV, MacFarlane IA, Wilding JPH, Pinkney JH: Prevalence of obesity in type 2 diabetes in secondary care: Association with cardiovascular risk factors. Postgrad Med J 82: 280–284, 2006. Available at: 10.1136/pmj.2005.039032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldherr R, Ilkenhans C, Ritz E: How frequent is glomerulonephritis in diabetes mellitus type II? Clin Nephrol 37: 271–273, 1992 [PubMed] [Google Scholar]
- 38.Fioretto P, Mauer M, Brocco E, Velussi M, Frigato F, Muollo B, Sambataro M, Abaterusso C, Baggio B, Crepaldi G, Nosadini R: Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia 39: 1569–1576, 1996. Available at: 10.1007/s001250050616 [DOI] [PubMed] [Google Scholar]
- 39.Menke A, Casagrande S, Geiss L, Cowie CC: Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 314: 1021–1029, 2015. Available at: 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
University of Washington subset characteristics. Download Supplemental Table 1, PDF file, 38 KB (37.4KB, pdf)
Diagnoses among participants with primary nondiabetic kidney disease. Download Supplemental Table 2, PDF file, 38 KB (37.4KB, pdf)
Histopathologic features by race/ethnicity. Download Supplemental Table 3, PDF file, 38 KB (37.4KB, pdf)