Visual Abstract
Keywords: clinical nephrology, IgA glomerulonephritis, IgA nephropathy, prognosis, tonsillectomy
Abstract
Background
Tonsillectomy may treat IgA nephropathy (IgAN) by reducing the levels of galactose-deficient IgA1. Therefore, we aimed to analyze the long-term effects of tonsillectomy on patients with IgAN, as an initial treatment and as a treatment at any time in their lives.
Methods
In this retrospective cohort analysis, 1147 patients with IgAN were grouped according to whether they had undergone tonsillectomy at any time, >1 year after renal biopsy (study 1), or within 1 year after renal biopsy (study 2). The patients were propensity-score matched or divided into four groups according to their proteinuria and renal function. The 20-year renal survival rates were evaluated until serum creatinine levels doubled (primary end point) and ESKD occurred (secondary end point).
Results
Patients in both studies had similar background characteristics after propensity score matching. In study 1, the renal survival rates for the primary and secondary end points were significantly higher for patients who underwent tonsillectomy at any time or >1 year after renal biopsy compared with those who did not. In study 2, the renal survival rates for the primary and secondary end points were significantly higher for patients who underwent tonsillectomy soon after renal biopsy compared with those who did not (primary end point, 98% versus 69%, P=0.001; secondary end point, 100% versus 75%, P=0.0001). A stratified analysis showed that significant treatment efficacy was observed for patients with proteinuria >1.0 g/d. Multivariate Cox regression analyses showed that tonsillectomy was associated with disease progression (hazard ratio, 0.27; P=0.04). Complications associated with tonsillectomy occurred in 8% of patients.
Conclusions
Among patients with IgAN, tonsillectomy at any time of life, or soon after renal biopsy, prevents disease progression, and the procedure is relatively safe.
Introduction
Worldwide, IgA nephropathy (IgAN) is the most common GN. IgAN is characterized by mesangial IgA deposits in the glomeruli, the proliferation of mesangial cells, an abundance of mesangial matrix (observed using light microscopy), and the presence of electron-dense deposits in the mesangial area (observed using electron microscopy).
The most important molecule in the pathogenesis of IgAN is a subtype of IgA1 that has galactose-deficient O-glycans (Gd-IgA1) in the hinge region. It is produced by plasma cells in response to class-switching B cells in the mucosa, circulates with autoantibodies against Gd-IgA1 in the serum, and combines with cluster of differentiation 89 and transferrin receptors in the mesangial area to form IgA1 deposits (1–3). During the production of Gd-IgA1, toll-like receptor-9 (TLR-9) is overexpressed in the tonsils after upper respiratory mucosal infections, including tonsillitis, and activation of TLR-9 produces a proliferation-inducing ligand (APRIL) in the tonsillar B cells and a B cell–activating factor (BAFF) from the TNF super family. APRIL and BAFF activate B cells, in the absence of a T cell–dependent pathway, to generate antibody-producing plasma cells that produce Gd-IgA1 (1–3). Tonsillectomy appears to impede the first step in the pathway that produces Gd-IgA1. Indeed, stimulation of the tonsillar mononuclear cells by unmethylated deoxycytidyl-deoxyguanosine oligodeoxynucleotides (CpG-ODN) provokes innate immune responses via TLR-9, thereby inducing the overexpression of APRIL (4), BAFF, IFN-γ, and IgA (5) in patients with IgAN, but not in tonsillar mononuclear cells in patients who do not have IgAN. APRIL expression in the germinal centers of B cells in tonsils is significantly higher in patients with IgAN compared with that in patients with chronic tonsillitis, and it is correlated with the expression of TLR-9 in whole tonsils and tonsillar B cells (6). Furthermore, CpG-ODN increases the expression of APRIL receptors, transmembrane activators, calcium modulators, cyclophilin ligand interactors, and B cell maturation antigens in tonsils (4,6).
The serum Gd-IgA1 levels and the mesangial deposition of Gd-IgA1 are significantly higher in patients with IgAN compared with those in patients with other forms of chronic GN (7), and they are associated with the histologic grades determined by the Japanese classification of IgAN (8). The serum Gd-IgA1 and IgA/IgG immunocomplex levels are correlated with hematuria and proteinuria (9), and mesangial Gd-IgA1 deposits are correlated with segmental sclerosis (7). Moreover, compared with patients with IgAN without depleted serum Gd-IgA1 levels, patients with IgAN whose serum Gd-IgA1 levels are depleted after tonsillectomy show significantly higher levels of tonsillar TLR-9 expression and greater improvements in hematuria (10); additionally, larger reductions in the Gd-IgA1 and IgA/IgG immunocomplex levels after tonsillectomy are correlated with greater improvements in hematuria (9). Taken together, these findings are indicative of the clinical relevance of tonsillectomy for patients with IgAN. Therefore, we aimed to analyze the effects of tonsillectomy on patients with IgAN. Patients were divided into two groups according to whether or not they had undergone tonsillectomy at any time in their lives, >1 year after renal biopsy (study 1), or within 1 year after renal biopsy (study 2).
Materials and Methods
Study Population and Design
Patients with primary IgAN who were diagnosed on the basis of the results of renal biopsies, performed at Tokyo Women’s Medical University between 1974 and 2015, were eligible to participate in this study (n=1147). None of the patients had been diagnosed with systemic diseases, including SLE, liver cirrhosis, or IgA vasculitis with nephritis. Of these 1147 patients, 127 who were observed for <1 year after renal biopsy were excluded because they did not progress to ESKD. Therefore, 1020 patients were assigned to two groups according to whether or not they had undergone tonsillectomy at any time in their lives: 282 patients, including nine patients who underwent tonsillectomy before renal biopsy, had undergone a tonsillectomy; 211 patients underwent tonsillectomy within 1 year after renal biopsy; 62 patients underwent tonsillectomy >1 year after renal biopsy (median, 5.5 years after renal biopsy; interquartile range, 3.25–9 years after renal biopsy); and 738 patients had not undergone tonsillectomy. After propensity score matching, each group comprised 169 patients. Renal survival rates of the groups were compared to determine the effects of tonsillectomy performed at any time during a patient’s life (study 1a). Of these 1020 patients, 994 patients with available eGFR and urinary protein excretion (U-Prot) data at the time of renal biopsy were divided into four groups (study 1b). The renal survival rates of patients who did and did not undergo tonsillectomy were analyzed: 109 patients had an eGFR <60 ml/min per 1.73 m2 and U-Prot <1.0 g/d, 150 patients had an eGFR <60 ml/min per 1.73 m2 and U-Prot ≥1.0 g/d, 512 patients had an eGFR ≥60 ml/min per 1.73 m2 and U-Prot <1.0 g/d, and 223 patients had an eGFR ≥60 ml/min per 1.73 m2 and U-Prot ≥1.0 g/d. Of the 62 patients who underwent tonsillectomy >1 year after renal biopsy and the 738 patients who had not undergone tonsillectomy before propensity matching, 57 patients from each group were also compared after propensity score matching (study 1c). Subsequently, according to the Oxford classification, we excluded 149 patients with fewer than eight glomeruli at the time of the renal biopsy from the cohort of 1020 patients (11–13). Of the 871 patients, 192 had undergone tonsillectomy within 1 year after renal biopsy as initial treatment, and 679 patients had not undergone tonsillectomy. Propensity score matching was used to account for differences in clinical and histologic data. We analyzed the effects of tonsillectomy as the initial treatment by comparing groups comprising 115 patients (study 2a). Of these 871 patients, 854 patients with available eGFR and U-Prot data at the time of the renal biopsy were divided into four groups (study 2b). Renal survival rates of patients who did not did not undergo tonsillectomy were analyzed: 95 patients had an eGFR <60 ml/min per 1.73 m2 and U-Prot <1.0 g/d, 127 patients had an eGFR <60 ml/min per 1.73 m2 and U-Prot ≥1.0 g/d, 440 patients had an eGFR ≥60 ml/min per 1.73 m2 and U-Prot <1.0 g/d, and 192 patients had an eGFR ≥60 ml/min per 1.73 m2 and U-Prot ≥1.0 g/d. Renal survival rates of the groups were analyzed and independent risk factors for progression were identified (Figure 1). The primary end point was a doubling of serum creatinine (Cr) level compared with baseline. The secondary end point was progression to ESKD. We also analyzed the adverse effects for patients with IgAN of tonsillectomy performed from 2006 to 2015.
Figure 1.
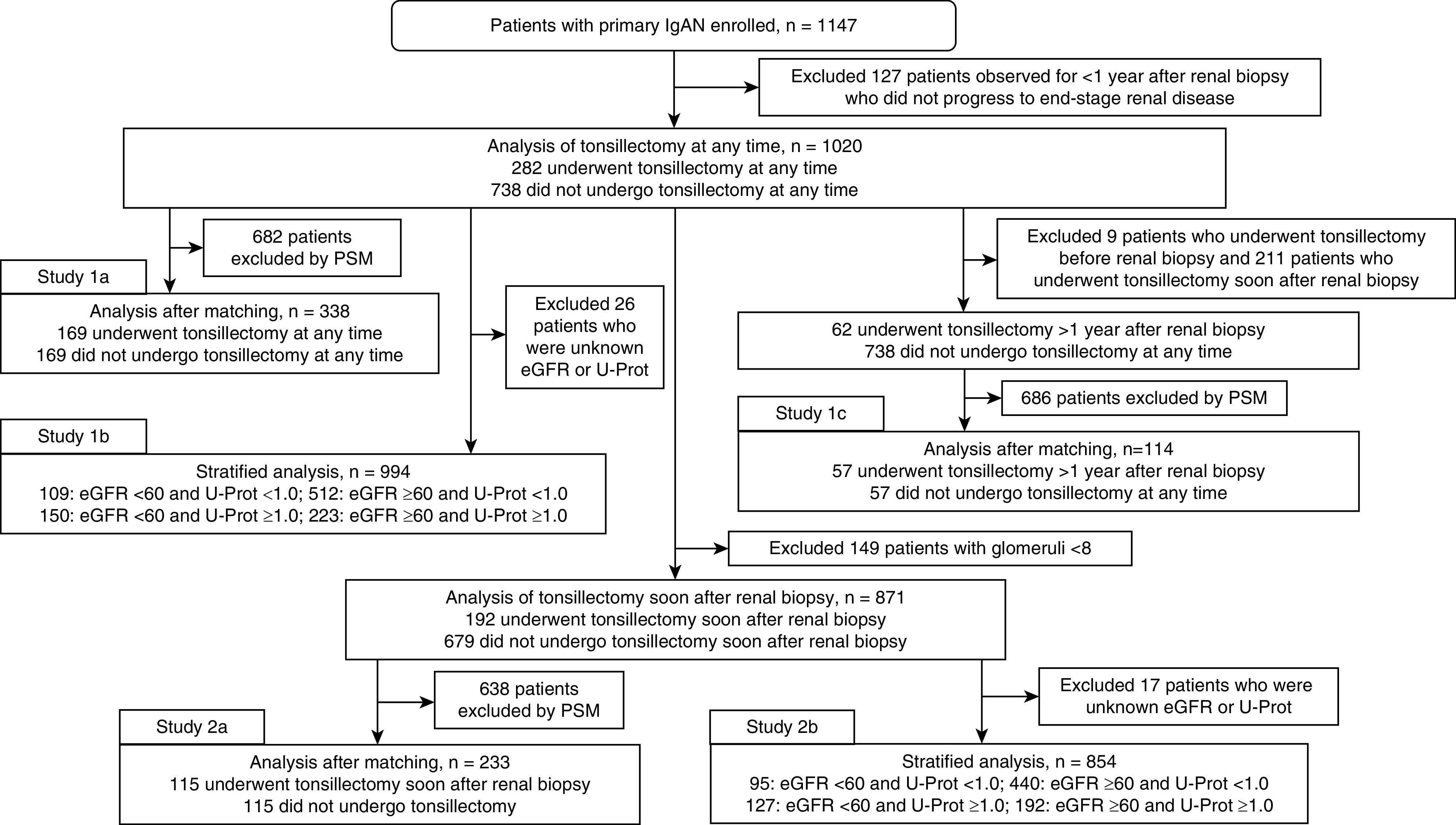
Algorithm of the study design. IgAN, IgA nephropathy; PSM, propensity score matching; U-Prot, urinary protein excretion in g/d.
This retrospective, cohort study was conducted in accordance with the principles of the Declaration of Helsinki, and it was approved by the medical ethics committee at Tokyo Women’s Medical University (reference number 5104). Written informed consent to perform renal biopsies was obtained from all patients, and opt-out information for this study was available on the home page of the website of our institution.
IgAN Diagnosis and Histologic Evaluations of Renal Biopsy Specimens
All renal biopsy specimens were stained with hematoxylin and eosin, Periodic acid–Schiff, silver methenamine, and Masson trichrome; specimens were evaluated using light microscopy. Routine immunohistochemical analyses consisted of immunofluorescence staining for IgG, IgA, IgM, complement 3 (C3), C4, C1q, fibrinogen, and fibronectin. A diagnosis of IgAN was made on the basis of mesangial proliferative changes identified using light microscopy, mesangial IgA and C3 deposits detected using immunohistochemistry, and mesangial electron-dense deposits identified using electron microscopy.
The Oxford classification (11–13) was used to grade the histologic findings; therefore, mesangial hypercellularity (M) was M0 if >50% of the glomeruli had fewer than three cells per mesangial area, or M1 if >50% of glomeruli had more than three cells per mesangial area. Segmental glomerulosclerosis (S) was graded as absent (S0) or present (S1). Endocapillary hypercellularity (E) was graded as absent (E0) or present (E1). The tubular atrophy/interstitial fibrosis ratio (T) in the interstitium was graded as follows: T0, 0%–25%; T1, 26%–50%; or T2, >50%. The crescent (C) score was based on the ratio of the number of glomeruli with cellular and fibrocellular crescents and graded as follows: C0, 0%; C1, 0%–25%; or C2, ≥25%.
Clinical and Laboratory Data
Clinical data, including sex, age, body mass index, systolic BP (SBP), diastolic BP (DBP), and mean arterial pressure (MAP), and the follow-up duration were evaluated. Baseline laboratory data, including serum total protein (TP), Cr, uric acid (UA), total cholesterol, triglyceride (TG) levels, eGFR, U-Prot, and urinary red blood cell (U-RBC) levels were analyzed at the time of the renal biopsy. Renin-angiotensin system inhibitors (RASI) and immunosuppressants, including corticosteroid therapy, were evaluated as initial treatments. Those treatments were strongly affected by the historical backgrounds and characteristics of the patients. We started using RASI to treat IgAN patients with hypertension in the late 1990s. From the early 1990s to early 2000s, we used oral steroid therapy that began with daily 0.5–0.8 mg/kg body wt prednisolone which was gradually tapered over the course of 2 years for patients with IgAN who had a higher amount of proteinuria and/or active histologic findings. From the latter half of the 2000s, we used tonsillectomy combined with intermittent steroid pulse therapy every 2 months during a 6-month period for these patients. It was difficult to adjust for the differences in these treatments on the basis of the year of diagnosis; therefore, study 1 included treatment performed at any time. The eGFR was calculated using the following isotope dilution–, mass spectrometry–traceable Modification of Diet in Renal Disease study equation modified for Japanese individuals: eGFR=194×Cr−1.094×age−0.287×0.739 (if female) (14). The time to progression to ESKD, which was defined as requiring dialysis or renal transplantation, was evaluated as an end point, and the risk factors associated with progression to ESKD were evaluated.
Statistical Analyses
The normally distributed data are expressed as means and SDs, and they were compared using unpaired t tests. The skewed data are expressed as medians and interquartile ranges, and they were compared using the Mann–Whitney U test. The cumulative renal survival rates until the study end points were reached were calculated using the Kaplan–Meier method and compared using the log-rank test. The chi-squared test was used to compare categoric variables. Propensity score matching was used to account for differences in the clinical and histologic data of the groups, and it involved 1:1 nearest-neighbor matching. Univariate and multivariate Cox regression analyses were used to evaluate the risk of progression to ESKD, and the hazard ratios (HRs) and 95% confidence intervals (95% CIs) were estimated. Data were analyzed using JMP Pro 13.0.0 (SAS Institute Inc., Cary, NC), and P<0.05 was considered statistically significant.
Results
Characteristics and Survival Analyses of Patients with IgAN Who Did or Did Not Undergo Tonsillectomy at Any Time
Table 1 present the baseline data of all 1020 patients with IgAN, including a comparison of the patients who did and did not undergo tonsillectomy at any time in their lives. Patients differed significantly regarding age; SBP; DBP; MAP; year of diagnosis; follow-up duration; timing of tonsillectomy; and TP, Cr, UA, TG, U-Prot, eGFR, and U-RBC levels. The use of steroid therapy was significantly more frequent among the patients with IgAN who underwent tonsillectomy compared with patients who did not undergo tonsillectomy. After propensity score matching for MAP; follow-up duration; immunosuppressant use; and TP, eGFR, UA, TG, U-Prot, and U-RBC levels, the groups had similar clinical data, laboratory findings, and treatment. It was difficult to adjust for the year of diagnosis because tonsillectomy was mainly used after 2005 at our institution.
Table 1.
Baseline characteristics of patients who did or did not undergo tonsillectomy at any time
| Baseline Characteristics | All Patients (n=1020) | Baseline Data | After Propensity Score Matching | ||||
| No Tonsillectomy (n=738) | Tonsillectomy (n=282) | P Value | No Tonsillectomy (n=169) | Tonsillectomy (n=169) | P Value | ||
| Clinical findings | |||||||
| Age (yr; median and IQR) | 31.0 (24.0–41.0) | 31.0 (24.0–43.0) | 30.0 (24.0–43.0) | 0.03 | 30.0 (24.5–43.0) | 30.0 (24.5–38.5) | 0.41 |
| Sex (male/female) | 418/602 | 310/428 | 108/174 | 0.28 | 61/108 | 66/103 | 0.57 |
| BMI (kg/m2; median and IQR) | 21.3 (19.6–23.5) | 21.4 (19.7–23.5) | 20.1 (19.2–23.5) | 0.13 | 21.2 (20.0–23.5) | 21.3 (19.2–24.0) | 0.72 |
| SBP (mm Hg; median and IQR) | 120.0 (110.0–134.0) | 120.5 (111.0–134.0) | 117.0 (107.0–125.0) | <0.001 | 118.0 (108.5–128.0) | 119.0 (109.0–128.0) | 0.65 |
| DBP (mm Hg; median and IQR) | 74.0 (66.0–83.0) | 76.0 (67.0–84.0) | 71.0 (64.0–80.0) | 0.0001 | 74.0 (66.0–80.0) | 73.0 (66.0–82.5) | 0.76 |
| MAP (mm Hg; median and IQR) | 89.8 (81.3–99.0) | 91.3 (82.7–100.5) | 85.7 (80.0–95.3) | <0.001 | 89.3 (80.8–97.2) | 88.3 (81.7–97.2) | 0.79 |
| Distribution of year of diagnosis (before 1989/1990–1999/2000 –2009/after 2010) | 232/276/230/192 | 232/256/207/51 | 8/20/113/141 | <0.001 | 19/49/81/20 | 5/19/80/65 | <0.001 |
| Duration of follow-up (yr; median and IQR) | 8.0 (4.0–15.0) | 9.5 (4.0–17.0) | 6.8 (4.0–10.0) | <0.001 | 7.5 (4.0–13.0) | 8.0 (4.8–11.3) | 0.68 |
| Timing of tonsillectomy (none/before/early/later) | 738/9/211/62 | 738/−/−/− | −/9/211/62 | — | 169/−/−/− | −7/115/47 | — |
| Laboratory findings | |||||||
| TP (g/dl; median and IQR) | 6.8 (6.3–7.2) | 6.7 (6.3–7.2) | 6.9 (6.–7.3) | 0.0001 | 6.8 (6.3–7.1) | 6.8 (6.5–7.2) | 0.21 |
| Cr (mg/dl; median and IQR) | 0.79 (0.67–1.00) | 0.82 (0.69–1.08) | 0.75 (0.65–0.90) | <0.001 | 0.75 (0.63–0.99) | 0.78 (0.65–0.94) | 0.91 |
| eGFR (ml/min per 1.73 m2; median and IQR) | 76.5 (58.9–94.7) | 73.7 (56.8–94.0) | 80.3 (65.0–96.0) | 0.0005 | 79.8 (58.5–96.3) | 77.6 (63.8–96.7) | 0.95 |
| UA (mg/dl; median and IQR) | 5.5 (4.5–6.7) | 5.6 (4.6–6.9) | 5.3 (4.3–6.4) | 0.0005 | 5.4 (4.5–6.7) | 5.4 (4.4–6.5) | 0.55 |
| T-Cho (mg/dl; median and IQR) | 193.0 (168.7–224.5) | 195.0 (170.0–226.0) | 189.0 (166.0–223.0) | 0.15 | 196.5 (174.0–228.3) | 195.6 (168.0–227.0) | 0.61 |
| TG (mg/dl; median and IQR) | 100.0 (73.0–146.5) | 105.0 (74.0–158.0) | 91.0 (68.0–119.0) | 0.0002 | 97.0 (73.0–139.0) | 97.0 (74.5–141.5) | 0.94 |
| U-Prot (g/d; median and IQR) | 0.69 (0.3–1.4) | 0.76 (0.33–1.59) | 0.55 (0.24–1.07) | <0.001 | 0.80 (0.44–1.39) | 0.72 (0.3–1.38) | 0.46 |
| U-RBC (<5, 5, 25, 26, 49, 50, 99, ≤100 counts/HPF) | 111, 409, 170, 139, 185 | 88, 282, 119, 91, 153 | 23, 127, 51, 48, 32 | 0.0009 | 20, 71, 33, 20, 25 | 16, 68, 32, 28, 25 | 0.77 |
| Treatments, n | |||||||
| Immunosuppressants (−/+ [early/later]) | 501/519 (480/39) | 470/268 (248/20) | 31/251 (232/19) | <0.001 | 27/142 (133/9) | 27/142 (127/15) | >0.99 |
| RAS inhibitors (−/+ [before or early/later]) | 509/511 (325/185) | 368/370 (228/142) | 141/141 (98/43) | 0.97 | 76/93 (67/26) | 71/98 (70/28) | 0.58 |
BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; MAP, mean arterial pressure; TP, serum total protein; Cr, serum creatinine; UA, serum uric acid; T-Cho, serum total cholesterol; TG, triglyceride; U-Prot, urinary protein excretion; U-RBC, urinary red blood cells; HPF, high-power field; RAS, renin-angiotensin system; IQR, interquartile ranges.
Before propensity score matching, the renal survival rates until progression to the primary end point (Figure 2A) and secondary end point (Figure 2B) were significantly higher for patients with IgAN who underwent tonsillectomy compared with those patients who did not undergo tonsillectomy (primary end point, 85% versus 62%, P<0.001; secondary end point, 98% versus 69%, P<0.001). After propensity score matching (study 1a), the renal survival rates until primary end point (Figure 2C) and secondary end point (Figure 2D) remained significantly higher for the patients with IgAN who underwent tonsillectomy compared with those who did not (primary end point, 86% versus 80%, P=0.0001; secondary end point, 98% versus 76%, P=0.0001). In the stratified analysis (study 1b), the renal survival rates until progression to ESKD for patients who underwent tonsillectomy were similar to those who did not and to patients with U-Prot <1.0 g/d, regardless of eGFR (Figure 3, A and B); however, the renal survival rates of patients who underwent tonsillectomy were significantly higher than those who did not and to patients with U-Prot ≥1.0 g/d, regardless of eGFR (eGFR <60 ml/min per 1.73 m2, 88% versus 33%, P=0.005; eGFR ≥60 ml/min per 1.73 m2, 100% versus 69%, P=0.007) (Figure 3, C and D). From the cohort of all 1020 patients with IgAN, we then compared the patients who underwent tonsillectomy >1 year after renal biopsy with those who did not undergo tonsillectomy (study 1c). Significant differences were observed in age; SBP; DBP; MAP; follow-up duration; Cr, eGFR, and TG levels; and use of immunosuppressants and RASI between the groups. After propensity score matching for age, MAP, follow-up duration, eGFR, UA, U-Prot, and the use of immunosuppressants and RASI, the groups had similar background characteristics (Table 2). Before and after propensity score matching, the renal survival rates until primary and secondary end points were significantly higher for patients with IgAN who underwent tonsillectomy compared with those who did not undergo tonsillectomy (before matching, primary end point, 82% versus 62%, P=0.0007 [Figure 4A]; secondary end point, 97% versus 69%, P=0.0009 [Figure 4B]; after matching, primary end point, 92% versus 69%, P=0.01 [Figure 4C]; secondary end point, 82% versus 62%, P=0.03 [Figure 4D]).
Figure 2.
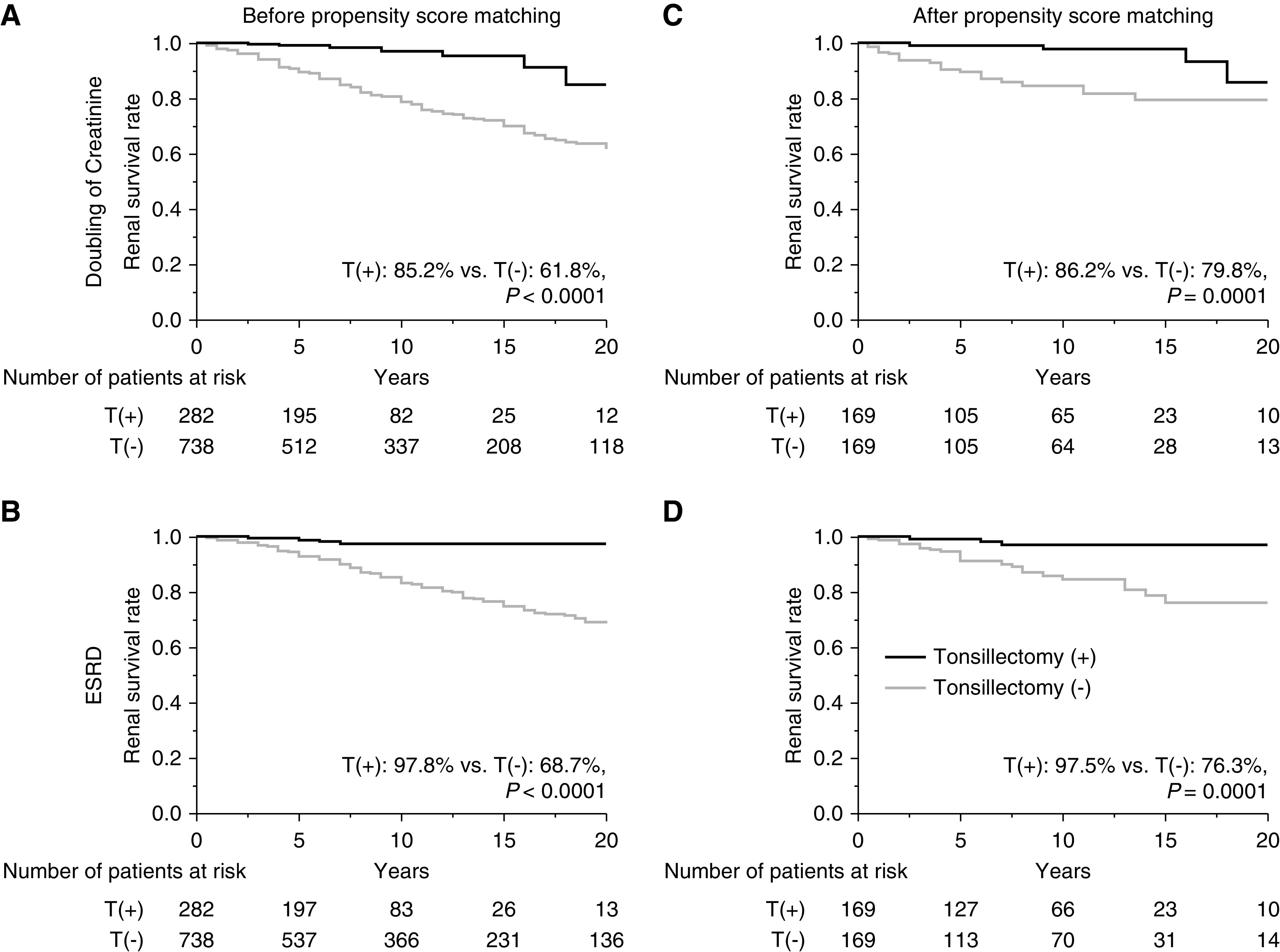
Renal survival rates for patients with IgAN according to whether or not they had undergone tonsillectomy at any time in their lives. Survival rates before propensity score matching (A) until the serum creatinine (Cr) level doubled (T[+], 85%; T[−], 62%; P<0.001) and (B) until ESKD was reached (T[+], 98%; T[−], 69%; P<0.001). Survival rates after propensity score matching (C) until the Cr level doubled (T[+], 86%; T[−], 80%; P=0.0001) and (D) until ESKD was reached (T[+], 98%; T[−], 76%; P=0.0001). T(+), underwent tonsillectomy; T(−), did not undergo tonsillectomy.
Figure 3.
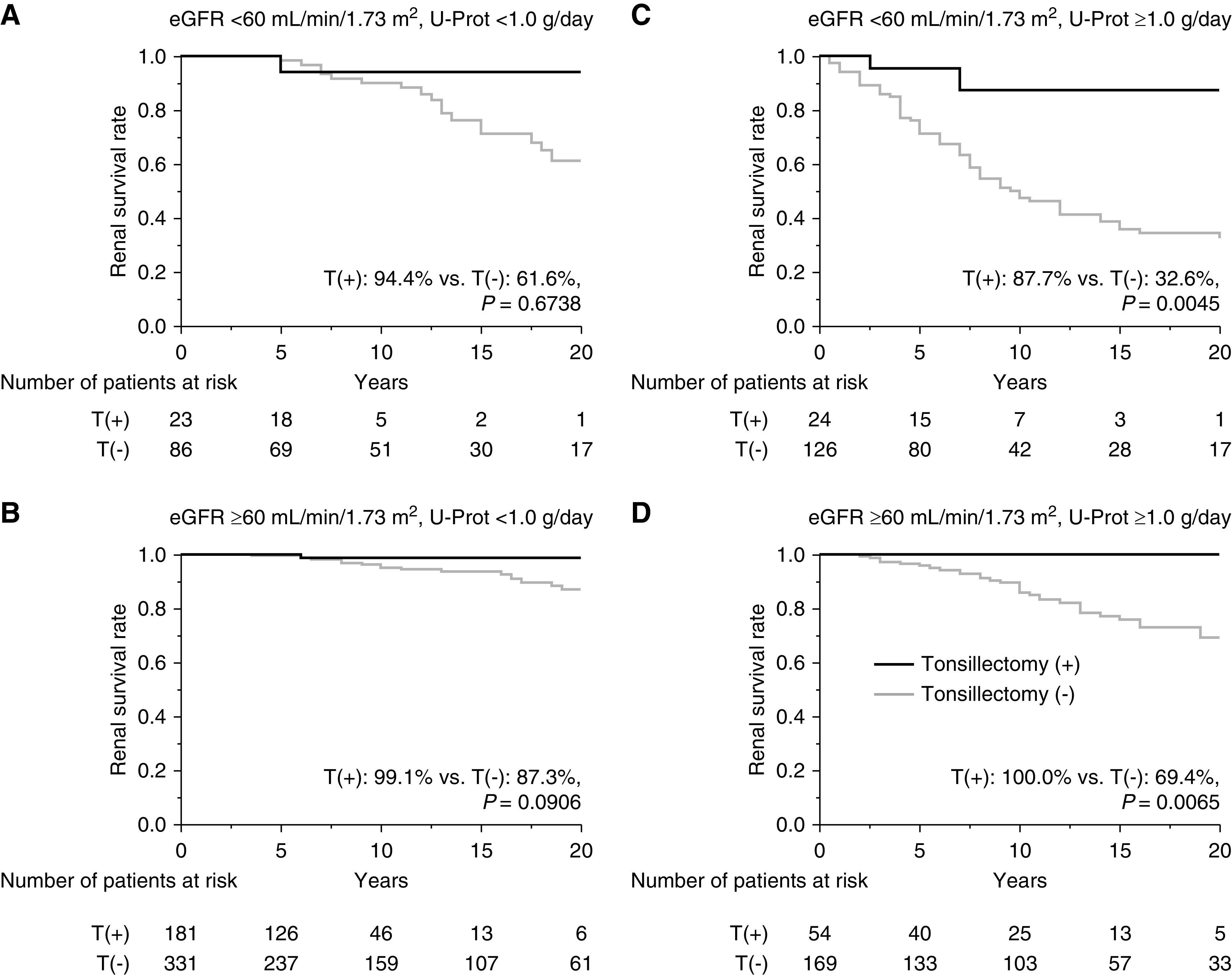
Stratified analysis on the basis of eGFR and U-Prot to determine renal survival rates of patients with IgAN treated with or without tonsillectomy at any time. Survival rates until ESKD was reached (A) for patients with eGFR <60 ml/min per 1.73 m2 and U-Prot <1.0 g/d (T[+], 94%; T[−], 62%; P=0.67), (B) for patients with eGFR ≥60 ml/min per 1.73 m2 and U-Prot <1.0 g/d (T[+], 99%; T[−], 87%; P=0.09), (C) for patients with eGFR <60 ml/min per 1.73 m2 and U-Prot ≥1.0 g/d (T[+], 88%; T[−], 33%; P=0.005), and (D) for patients with eGFR ≥60 ml/min per 1.73 m2 and U-Prot ≥1.0 g/d (T[+], 100%; T[−], 69%; P=0.007). T(+), underwent tonsillectomy; T(−), did not undergo tonsillectomy.
Table 2.
Baseline characteristics of those who did or did not undergo tonsillectomy >1 yr after renal biopsy
| Baseline Characteristics | All Patients (n=800) | Baseline Data | After Propensity Score Matching | ||||
| No Tonsillectomy (n=738) | Tonsillectomy (n=62) | P Value | No Tonsillectomy (n=57) | Tonsillectomy (n=57) | P Value | ||
| Clinical findings | |||||||
| Age (yr; median and IQR) | 31.0 (25.0–42.0) | 31.0 (24.0–43.0) | 27.0 (22.8–34.3) | 0.01 | 27.0 (22.0–35.0) | 27.0 (22.0–340) | 0.80 |
| Sex (male/female) | 332/468 | 310/428 | 22/40 | 0.31 | 20/37 | 21/36 | 0.85 |
| BMI (kg/m2; median and IQR) | 21.4 (19.7–23.5) | 21.4 (19.7–23.5) | 21.1 (19.1–23.0) | 0.32 | 20.7 (18.9–22.0) | 21.1 (19.2–23.0) | 0.28 |
| SBP (mm Hg; median and IQR) | 121.0 (111.3–136.0) | 120.5 (111.0–134.0) | 117.0 (106.0–124.0) | 0.002 | 113.0 (105.5–124.5) | 116.5 (106.0–124.0) | 0.53 |
| DBP (mm Hg; median and IQR) | 76.0 (68.0–85.0) | 76.0 (67.0–84.0) | 70.0 (64.5–78.0) | 0.007 | 71.0 (62.5–80.0) | 70.0 (64.3–78.0) | 0.87 |
| MAP (mm Hg; median and IQR) | 92.0 (83.3–100.7) | 91.3 (82.7–100.5) | 85.3 (80.7–92.7) | 0.003 | 84.0 (79.3–93.0) | 85.8 (80.7–92.7) | 0.75 |
| Duration of follow-up (yr; median and IQR) | 10.0 (5.0–16.9) | 9.5 (4.0–17.0) | 12.5 (10–16.7) | 0.0006 | 14.5 (6.75–19.5) | 12.5 (10.0–16.5) | 0.68 |
| Laboratory findings | |||||||
| TP (g/dl; median and IQR) | 6.6 (6.1–7.1) | 6.7 (6.3–7.2) | 6.8 (6.3–7.2) | 0.75 | 6.6 (6.2–7.1) | 6.8 (6.3–7.3) | 0.18 |
| Cr (mg/dl; median and IQR) | 0.83 (0.69–1.08) | 0.82 (0.69–1.08) | 0.75 (0.65–0.88) | 0.004 | 0.74 (0.62–0.99) | 0.76 (0.65–0.88) | 0.65 |
| eGFR (ml/min per 1.73 m2; median and IQR) | 73.5 (56.3–91.6) | 73.7 (56.8–94.0) | 84.5 (66.5–100.3) | 0.001 | 85.6 (61.8–104.2) | 87.0 (65.9–101.0) | 0.60 |
| UA (mg/dl; median and IQR) | 5.8 (4.8–6.9) | 5.6 (4.6–6.9) | 5.3 (66.5–100.3) | 0.07 | 5.4 (4.4–6.7) | 5.3 (4.2–6.6) | 0.51 |
| T-Cho (mg/dl; median and IQR) | 192.5 (171.0–227.0) | 195.0 (170.0–226.0) | 177.0 (162.5–221.1) | 0.06 | 196.0 (169.8–228.5) | 176.5 (162.3–221.6) | 0.08 |
| TG (mg/dl; median and IQR) | 105.0 (72.0–153.0) | 105.0 (74.0–158.0) | 85.0 (60.5–121.0) | 0.02 | 109.0 (74.0–145.0) | 83.5 (59.8–120.5) | 0.07 |
| U-Prot (g/d; median and IQR) | 0.92 (0.48–1.87) | 0.76 (0.33–1.59) | 0.64 (0.30–1.10) | 0.10 | 0.70 (0.34–1.59) | 0.68 (0.3–1.07) | 0.19 |
| U-RBC (<5, 5–25, 26–49, 50–99, ≤100 counts/HPF) | 93, 305, 130, 106, 161 | 88, 282, 119, 91, 153 | 5, 23, 11, 15, 8 | 0.15 | 5, 18, 12, 8, 14 | 5, 21, 9, 15, 7 | 0.37 |
| Treatments, n | |||||||
| Immunosuppressants (−/+) | 482/318 | 470/268 | 12/50 | <0.001 | 10/47 | 11/46 | 0.81 |
| RAS inhibitors (−/+ ) | 387/413 | 368/370 | 19/43 | 0.003 | 18/39 | 19/38 | 0.84 |
BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; MAP, mean arterial pressure; TP, serum total protein; Cr, serum creatinine; UA, serum uric acid; T-Cho, serum total cholesterol; TG, triglyceride; U-Prot, urinary protein excretion; U-RBC, urinary red blood cells; HPF, high-power field; RAS, renin-angiotensin system; IQR, interquartile ranges.
Figure 4.
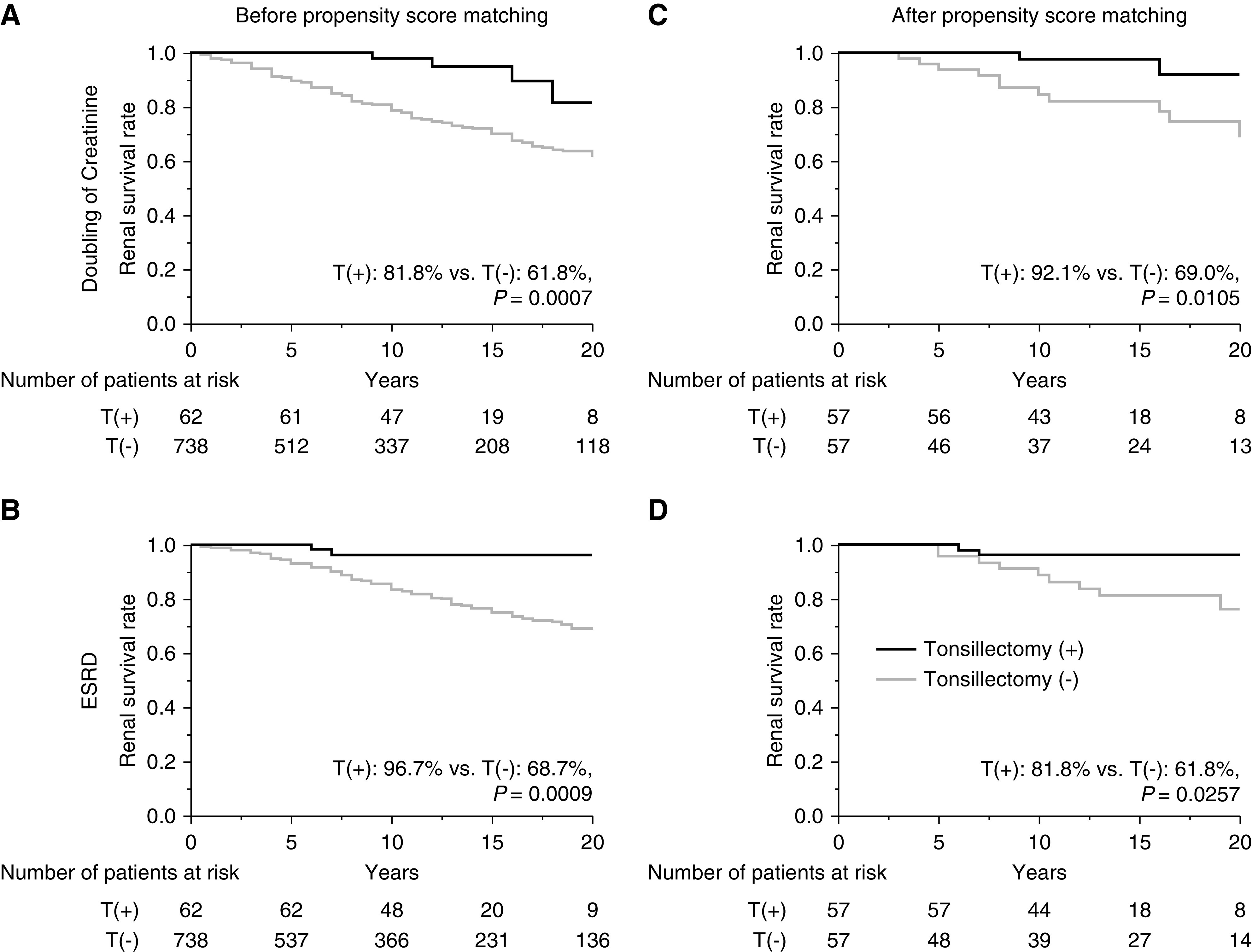
Renal survival rates for patients with IgAN according to whether or not they had undergone tonsillectomy >1 year after renal biopsy. Survival rates before propensity score matching (A) until the Cr level doubled (T[+], 82%; T[−], 62%; P=0.0007) and (B) until ESKD was reached (T[+], 97%; T[−], 69%; P=0.0009). Survival rates after propensity score matching (C) until the Cr level doubled (T[+], 92%; T[−], 69%; P=0.01) and (D) until ESKD was reached (T[+], 82%; T[−], 62%; P=0.03). T(+), underwent tonsillectomy; T(−), did not undergo tonsillectomy.
Characteristics and Survival Analyses of Patients with IgAN Who Did or Did Not Undergo Tonsillectomy within 1 year after Renal Biopsy
Table 3 presents the baseline data of 871 patients with IgAN, including a comparison of the patients who did and did not undergo tonsillectomy within 1 year after renal biopsy. Significant differences were evident regarding age; SBP; DBP; MAP; follow-up duration; TP, Cr, UA, TG, eGFR, U-Prot, and U-RBC levels; and the M, E, T, and C scores from the Oxford classification. Regarding treatment after renal biopsy, immunosuppressant use was significantly higher among patients with IgAN who underwent tonsillectomy. The groups had similar clinical, laboratory, histologic findings, and treatments after propensity score matching for MAP; follow-up duration; TP, eGFR, UA, TG, U-Prot, and U-RBC levels; immunosuppressant use; and M, E, T, and C scores from the Oxford classification.
Table 3.
Baseline characteristics of patients who did or did not undergo tonsillectomy within 1 yr after renal biopsy
| Baseline Characteristics | All patients (n=871) | Baseline Data | After Propensity Score Matching | ||||
| No Tonsillectomy (n=679) | Tonsillectomy (n=192) | P Value | No Tonsillectomy (n=115) | Tonsillectomy (n=115) | P Value | ||
| Clinical findings | |||||||
| Age (yr; median and IQR) | 31.0 (24.0–41.0) | 31.0 (24.0–42.0) | 30.5 (24.0–39.0) | 0.41 | 29.0 (23.0–43.0) | 29.0 (23.0–38.0) | 0.45 |
| Sex (male/female) | 356/515 | 281/398 | 75/117 | 0.56 | 61/54 | 70/45 | 0.23 |
| BMI (kg/m2; median and IQR) | 21.3 (19.6–23.5) | 21.4 (19.7–23.5) | 20.9 (19.2–23.8) | 0.18 | 21.3 (19.9–23.2) | 21.3 (19.3–23.9) | 0.76 |
| SBP (mm Hg; median and IQR) | 120.0 (110–132.0) | 120.0 (110.0–134.0) | 116.0 (107.0–125.8) | <0.001 | 116.0 (108.0–127.0) | 120.0 (109.0–128.0) | 0.43 |
| DBP (mm Hg; median and IQR) | 74.0 (66.0–83.0) | 76.0 (67.0–83.0) | 71.0 (64.0–81.0) | 0.003 | 74.0 (65.0–80.0) | 71.0 (63.0–81.0) | 0.38 |
| MAP (mm Hg; median and IQR) | 89.7 (81.3–99.0) | 90.07 (82.3–100.0) | 85.7 (79.8–96.0) | 0.0002 | 88.7 (82.0–96.7) | 86.7 (80.0–96.7) | 0.69 |
| Duration of follow-up (yr; median and IQR) | 8.0 (4.0–15.0) | 10.0 (4.5–17.0) | 5.8 (4.0–180) | <0.001 | 5.5 (3.0–10.5) | 6.5 (4.5–9.0) | 0.42 |
| Laboratory findings | |||||||
| TP (g/dl; median and IQR) | 6.8 (6.3–7.2) | 6.7 (6.3–7.2) | 6.9 (6.6–7.3) | 0.0002 | 6.8 (6.3–7.2) | 6.8 (6.5–7.3) | 0.15 |
| Cr (mg/dl; median and IQR) | 0.79 (0.67–1.00) | 0.81 (0.69–1.06) | 0.75 (0.64–0.92) | 0.01 | 0.78 (0.66–1.00) | 0.76 (0.65–0.88) | 0.24 |
| eGFR (ml/min per 1.73 m2; median and IQR) | 77.0 (60.0–95.6) | 75.1 (58.1–94.8) | 81.6 (67.6–97.5) | 0.11 | 81.5 (61.1–96.7) | 78.8 (66.2–95.5) | 0.95 |
| UA (mg/dl; median and IQR) | 5.5 (4.5–6.7) | 5.6 (4.6–6.8) | 5.2 (4.3–6.4) | 0.003 | 5.7 (4.7–6.9) | 5.4 (4.5–6.5) | 0.12 |
| T-Cho (mg/dl; median and IQR) | 192.0 (168.0–225.0) | 193.0 (168.0–226.0) | 190.0 (169.0–223.2) | 0.80 | 192.5 (170.8–225.3) | 192.0 (166.0–227.0) | 0.89 |
| TG (mg/dl; median and IQR) | 100.0 (73.0–144.0) | 104.0 (74.0–153.0) | 90.5 (68.8–118.5) | 0.005 | 96.0 (73.0–132.0) | 93.0 (75.0–138.0) | 0.95 |
| U-Prot (g/d; median and IQR) | 0.68 (0.3–1.4) | 0.74 (0.31–1.51) | 0.52 (0.23–1.04) | 0.0001 | 0.68 (0.26–1.23) | 0.59 (0.25–1.3) | 0.86 |
| U-RBC (<5, 5–25, 26–49, 50–99, ≤100 counts/HPF) | 85, 365, 140, 119, 157 | 75, 269, 109, 89, 133 | 10, 96, 31, 30, 24 | 0.008 | 12, 49, 24, 12, 18 | 8, 58, 15, 21, 13 | 0.14 |
| Histologic findings, n | |||||||
| M0/M1 | 441/430 | 366/313 | 75/117 | 0.0003 | 45/70 | 53/62 | 0.29 |
| E0/E1 | 479/391 | 390/289 | 90/102 | 0.009 | 51/64 | 49/66 | 0.79 |
| S0/S1 | 243/628 | 180/399 | 63/129 | 0.09 | 24/91 | 33/82 | 0.17 |
| T0/T1/T2 | 631/189/51 | 471/161/47 | 160/28/4 | 0.0004 | 87/22/6 | 89/24/2 | 0.33 |
| C0/C1/C2 | 454/370/46 | 382/279/37 | 82/100/9 | 0.008 | 48/59/8 | 44/65/6 | 0.69 |
| Treatment, n | |||||||
| Immunosuppressants (−/+) | 445/426 | 430/249 | 15/177 | <0.001 | 14/101 | 14/101 | >0.99 |
| RAS inhibitors (−/+) | 578/293 | 452/227 | 126/66 | 0.81 | 70/45 | 72/43 | 0.79 |
BMI, body mass index; SBP, systolic BP; DBP, diastolic BP; MAP, mean arterial pressure; TP, serum total protein; Cr, serum creatinine; UA, serum uric acid; T-Cho, serum total cholesterol; TG, triglyceride; U-Prot, urinary protein excretion; U-RBC, urinary red blood cells; HPF, high-power field; M, mesangial hypercellularity; M0, >50% of glomeruli had fewer than three cells per mesangial area; M1, >50% of glomeruli had more than three cells per mesangial area; E0, endocapillary hypercellularity absent; E1, endocapillary hypercellularity present; S0, segmental sclerosis absent; S1, segmental sclerosis present; T, interstitial fibrosis/tubular atrophy; T0, 0%–25%; T1, 26%–50%; T2, >50%; C0, 0% crescents; C1, 0%–25% crescents; C2, ≥25% crescents; RAS, renin-angiotensin system; IQR, interquartile ranges.
Before and after propensity score matching, the renal survival rates until progression to primary and secondary end points were significant higher for patients with IgAN who underwent tonsillectomy compared with those who did not (before matching, primary end point, 98% versus 64%, P<0.001 [Figure 5A]; secondary end point, 99% versus 70%, P<0.001 [Figure 5B]; after matching, primary end point, 98% versus 69%, P=0.001 [Figure 5C]; secondary end point, 100% versus 75%, P=0.0001 [Figure 5D]). In the stratified analysis (study 2b), renal survival rates until progression to ESKD in patients who underwent tonsillectomy were significantly higher than for patients who did not undergo tonsillectomy and for patients with U-Prot ≥1.0 g/d and eGFR <60 ml/min per 1.73 m2 (100% versus 35% over 15 years; P=0.003) (Figure 6C), but the renal survival rates were similar in terms of other patient characteristics (Figure 6, A, B, and D). The multivariate Cox regression analysis of factors that reached significance in the univariate Cox regression analysis showed that lower eGFR, higher U-Prot, and Oxford classification T2 were associated with progression to ESKD, and that tonsillectomy (HR, 0.27; 95% CI, 0.04 to 0.95; P=0.04), oral steroid therapy, and oral steroid therapy with steroid pulse therapy were associated with less disease progression (Table 4).
Figure 5.
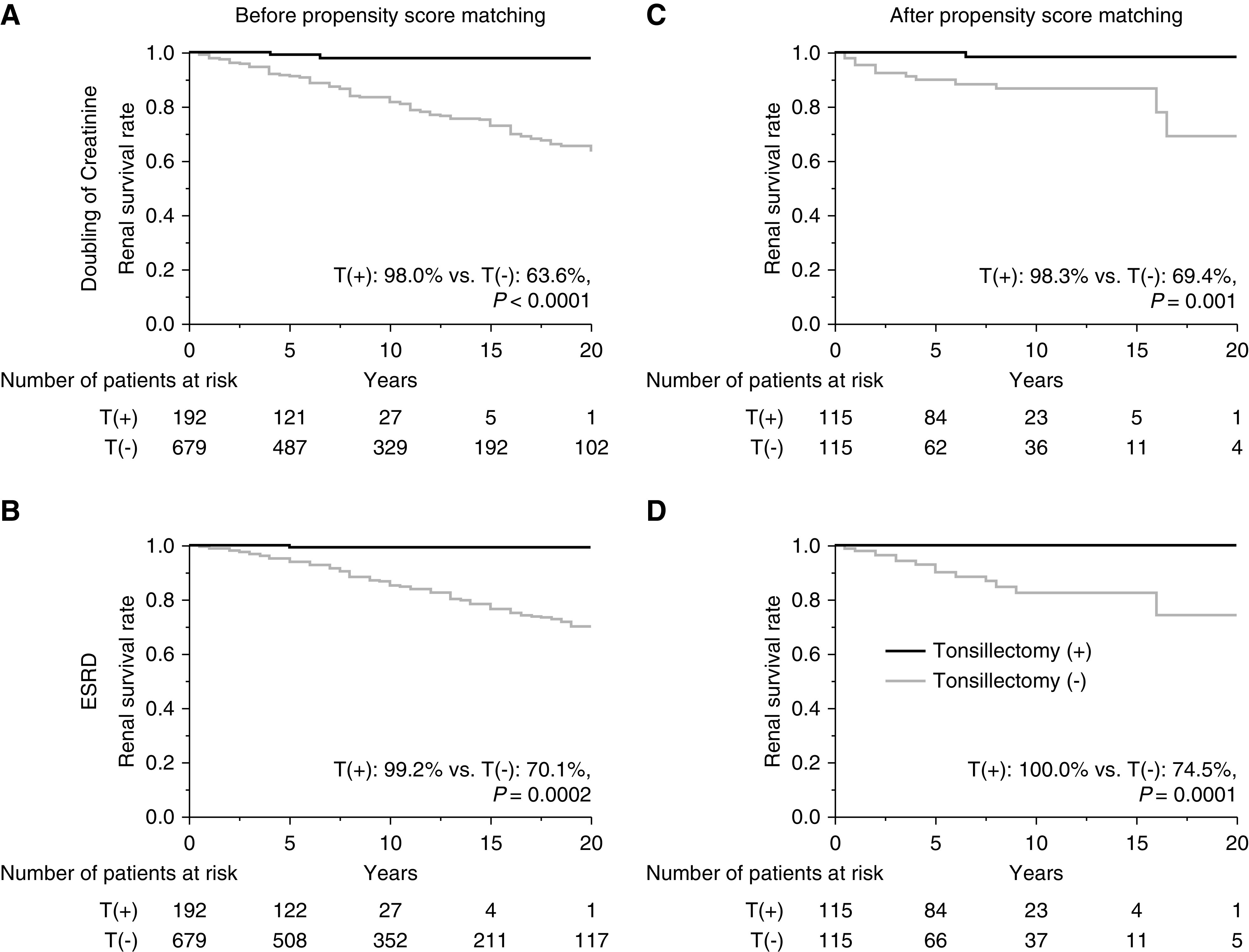
Renal survival rates of patients with IgAN on the basis of whether they had undergone tonsillectomy as the initial treatment. Survival rates before propensity score matching (A) until the Cr level doubled (T[+], 98%; T[−], 64%; P<0.001) and (B) until ESKD was reached (T[+], 99%; T[−], 70%; P=0.0002). Survival rates after propensity score matching (C) until the Cr level doubled (T[+], 98%; T[−], 69%; P=0.001) and (D) until ESKD was reached (T[+], 100%; T[−], 75%; P=0.0001). T(+), underwent tonsillectomy; T(−), did not undergo tonsillectomy.
Figure 6.
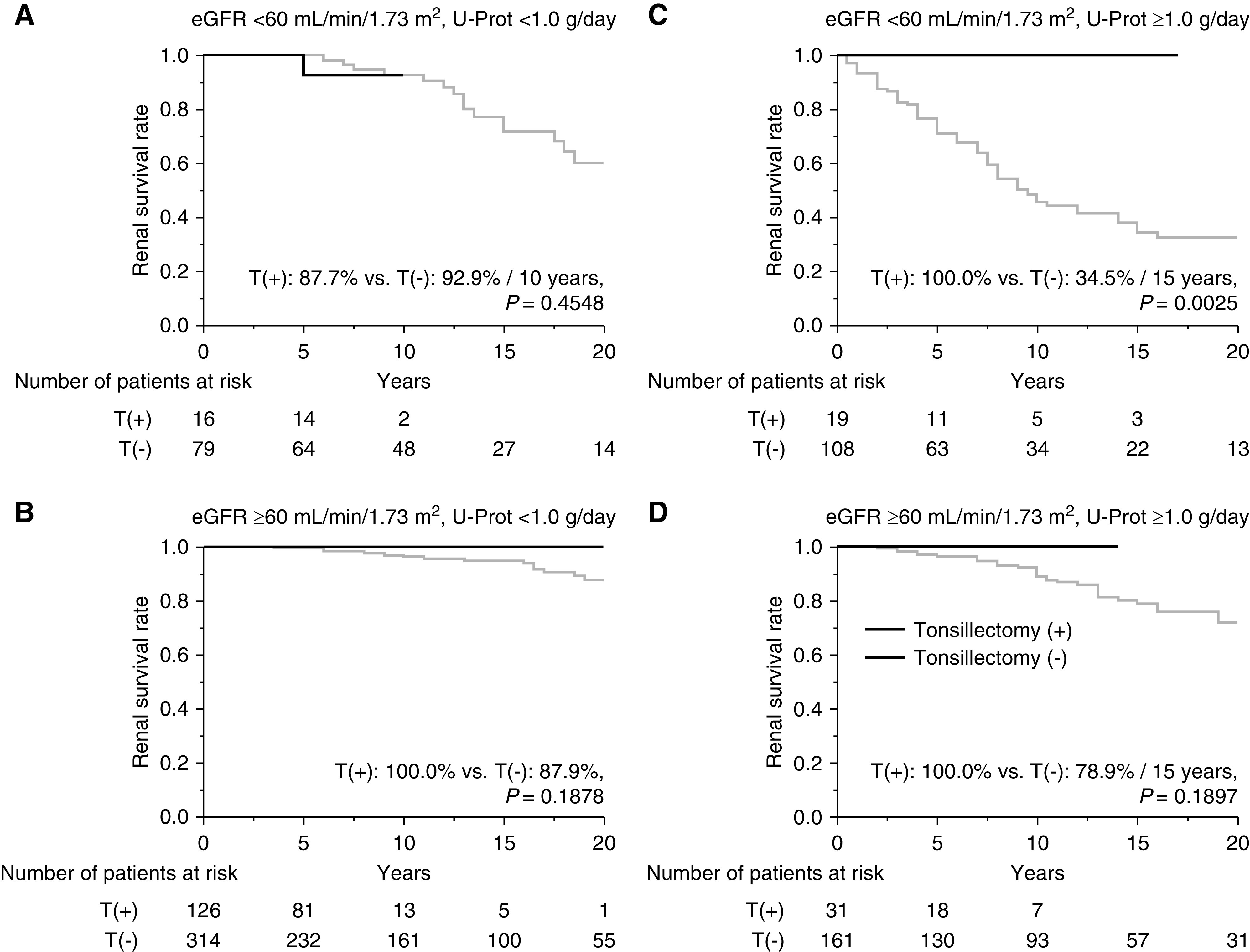
Stratified analysis on the basis of eGFR and U-Prot to determine renal survival rates for patients with IgAN treated with or without tonsillectomy as the initial treatment. Survival rates until ESKD (A) for patients with eGFR <60 ml/min per 1.73 m2 and U-Prot <1.0 g/d (T[+], 88% over 10 years; T[−], 93% over 10 years and 60% over 20 years; P=0.45), (B) for patients with eGFR ≥60 ml/min per 1.73 m2 and U-Prot <1.0 g/d (T[+], 100%; T[−], 88%; P=0.19), (C) for patients with eGFR <60 ml/min per 1.73 m2 and U-Prot ≥1.0 g/d (T[+], 100% over 15 years; T[−], 35% over 15 years and 33% over 20 years; P=0.003), and (D) for patients with eGFR ≥60 ml/min per 1.73 m2 and U-Prot ≥1.0 g/d (T[+], 100% over 15 years; T[−], 79% over 15 years and 72% over 20 years; P=0.19). T(+), underwent tonsillectomy; T(−), did not undergo tonsillectomy.
Table 4.
Independent risk factors associated with disease progression identified by univariate and multivariate Cox regression analysis
| Baseline Data | Univariate Analysis | Multivariate Analysis | ||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Clinical findings | ||||
| Age (per 10-yr increase) | 1.23 (1.06 to 1.38) | 0.005 | 0.88 (0.74 to 1.03) | 0.12 |
| Sex (male versus female) | 1.72 (1.25 to 2.40) | 0.001 | 1.22 (0.86 to 1.72) | 0.27 |
| BMI (per 1-kg/m2 increase) | 1.05 (0.98 to 1.11) | 0.12 | — | — |
| MAP (per 10-mm Hg increase) | 1.63 (1,40 to 1.89) | <0.001 | 1.12 (0.95 to 1.32) | 0.19 |
| Laboratory findings | ||||
| eGFR (per 30-ml/min decrease) | 2.89 (2.30 to 3.65) | <0.001 | 2.41 (1.81 to 3.22) | <0.001 |
| U-Prot (per 0.5-g/d increase) | 1.41 (1.32 to 1.50) | <0.001 | 1.38 (1.27 to 1.50) | <0.001 |
| U-RBC (per 25-HPF increase) | 0.99 (0.99 to 1.10) | 0.92 | — | — |
| Histologic findings | ||||
| M1 (versus M0) | 1.22 (0.88 to 1.69) | 0.24 | — | — |
| E1 (versus E0) | 0.96 (0.69 to 1.34) | 0.76 | — | — |
| S1 (versus S0) | 1.29 (0.89 to 1.94) | 0.19 | — | — |
| T0–T2 (T0) | Reference | Reference | ||
| T1 (versus T0) | 2.40 (1.65 to 3.46) | <0.001 | 1.00 (0.65 to 1.52) | 0.98 |
| T2 (versus T0) | 7.73 (4.94 to 1.18) | <0.001 | 2.43 (1.46 to 4.00) | 0.0008 |
| C0–C2 (C0) | Reference | Reference | ||
| C1 (versus C0) | 1.16 (0.82 to 1.63) | 0.39 | — | — |
| C2 (versus C0) | 1.75 (0.88 to 3.16) | 0.11 | — | — |
| Treatment | ||||
| Tonsillectomy (versus no tonsillectomy) | 0.09 (0.52 to 109) | <0.001 | 0.27 (0.04 to 0.95) | 0.04 |
| Steroid (none) | Reference | Reference | ||
| Oral steroid therapy (versus no steroid) | 0.76 (0.52 to 1.09) | 0.13 | 0.49 (0.32 to 0.72) | 0.0003 |
| Oral steroid therapy and steroid pulse therapy (versis no steroid) | 0.31 (0.13 to 0.66) | 0.002 | 0.27 (0.11 to 0.61) | 0.0008 |
| RASIs (versus no RASIs) | 0.97 (0.68 to 1.36) | 0.85 | — | — |
HR, hazard ratio; CI, confidence intervals; BMI, body mass index; MAP, mean arterial pressure; U-Prot, urinary protein excretion; U-RBC, urinary red blood cells; HPF, high-power field; M, mesangial hypercellularity; M1, >50% of glomeruli had more than three cells per mesangial area; M0, >50% of glomeruli had fewer than three cells per mesangial area; E1, endocapillary hypercellularity present; E0, endocapillary hypercellularity absent; S1, segmental sclerosis present; S0, segmental sclerosis absent; T, interstitial fibrosis/tubular atrophy; T0, 0%–25%; T1, 26%–50%; T2, >50%; C0, 0% crescents; C1, 0%–25% crescents; C2, ≥25% crescents; RASI, renin-angiotensin system inhibitors.
Complications Associated with Tonsillectomy
From 2006 to 2016, 206 patients with IgAN underwent tonsillectomy; there were 80 men and 123 women, with a mean age of 32.4 years. A total of 16 (8%) patients experienced complications. Bleeding after tonsillectomy occurred in 12 (6%) patients. Other complications, namely, dysgeusia, nausea/vomiting, earache, and tongue numbness, each occurred in each patient (0.49%) (Table 5).
Table 5.
Complications associated with tonsillectomy
| Complication | N (%) | Male/Female, n |
| Total adverse effects | 16 (8) | 6/10 |
| Bleeding | 12 (6) | 3/9 |
| Dysgeusia | 1 (0.49) | 1/0 |
| Nausea/vomiting | 1 (0.49) | 0/1 |
| Tooth injury | 0 | 0 |
| Dysphonia | 0 | 0 |
| Focal infection with tonsillectomy | 0 | 0 |
| Others | ||
| Earache | 1 (0.49) | 1/0 |
| Tongue numbness | 1 (0.49) | 1/0 |
Discussion
Our 20-year renal survival analysis indicated that tonsillectomy at any time, >1 year after renal biopsy, and as an initial treatment was associated with a lower increase in Cr level and less progression to ESKD. Our study also indicated that tonsillectomy at any time was efficacious for patients with IgAN who had U-Prot ≥1.0 g/d, regardless of eGFR, and that it was beneficial as an initial treatment for patients with IgAN and a U-Prot ≥1.0 g/d and eGFR <60 ml/min per 1.73 m2. Furthermore, the findings of this study showed that tonsillectomy and oral steroid therapy with or without steroid pulse therapy were associated with less progression to ESKD, and that Oxford classification T2, lower eGFR, and higher U-Prot were associated with progression to ESKD.
Recently, reports from two key studies that analyzed the effects of tonsillectomy on IgAN have been published, namely, the Japanese Nationwide Retrospective Cohort Study in IgAN (JNR-IgAN) (15) and the European Validation of the Oxford Classification of IgAN (VALIGA) study (16). JNR-IgAN investigated patients with IgAN who did and did not undergo tonsillectomy as initial treatment within 1 year after renal biopsy; it was reported that tonsillectomy prevented renal dysfunction for 10 years after propensity score matching, which accounted for between-group differences in clinical data and treatments (15). However, the results of VALIGA, which was a multicenter, retrospective study, did not show any benefits associated with tonsillectomy at any time as a treatment to prevent renal progression or to reduce proteinuria (16). We used this study to validate JNR-IgAN and VALIGA. Our findings were similar to and augmented the findings of JNR-IgAN, because we analyzed renal survival for up to 20 years and used a more rigorous end point (ESKD), the Oxford classification for the pathologic analysis, and tonsillectomy as the initial treatment and as treatment at any time; therefore, the results from VALIGA differed from those of this study and JNR-IgAN.
The pathogenesis underlying IgAN may arise within the mucosa–bone marrow systems in the nasopharynx-associated lymphoid tissue in Asian patients and the gut-associated lymphoid tissue in European patients (17), and this difference may underlie the discrepancies between the results of JNR-IgAN and VALIGA. Previous meta-analyses that were largely performed on the basis of reports from Japan and China have tended to demonstrate the efficacy of tonsillectomy in relation to clinical remission and long-term prognoses (18–20). In contrast, the findings of studies performed in Germany (21) and Italy (16) were included in these meta-analyses, and they did not demonstrate any benefits associated with tonsillectomy. However, only 16 patients underwent tonsillectomy in the study performed in Germany (21). In the VALIGA study, 17 patients underwent tonsillectomy as initial treatment, and 41 patients who underwent tonsillectomy were paired with 41 patients who did not undergo tonsillectomy in the propensity score–adjusted cohort. The findings of two randomized controlled trials that were conducted in Asian countries demonstrated the efficacy of tonsillectomy for clinical remission in China (22), and demonstrated slightly better efficacy of tonsillectomy for remission of proteinuria in Japan (23). A larger, more recent, cohort study performed in Hungary (24) compared 166 patients in a control group with 68 patients who underwent tonsillectomy as initial treatment and 30 patients who underwent tonsillectomy as pretreatment >3 years before renal biopsy. The multivariate analysis performed during that study, which accounted for clinical characteristics and treatment, showed that tonsillectomy could significantly reduce the risk of progression. Therefore, the effectiveness of tonsillectomy as treatment for patients with IgAN remains controversial in Europe.
Because of the pathogenesis of IgAN, it is important to suppress all of the hits that comprise the multihit hypothesis (2,3), from the elevated Gd-IgA1 levels in the lymphoid tissue, which is the first hit, to glomerular inflammation, which is the final hit. Tonsillectomy mainly suppresses increases in Gd-IgA1 levels, which prevents the initiation of IgAN pathogenesis, and steroid pulse therapy suppresses subsequent hits; therefore, together, the synergistic effects of these treatments suppress all hits. The findings of many studies performed in Japan demonstrated the synergistic effects of tonsillectomy and corticosteroid therapy compared with corticosteroid therapy administered alone, and some studies found that these effects are significantly greater for patients with IgAN who have more advanced histologic findings (25,26), more severe proteinuria (27), and worse renal function (28). The stratified analysis of our study (study 1b and study 2b) indicated that tonsillectomy at any time was effective for patients with IgAN who had proteinuria ≥1.0 g/d, and that tonsillectomy as an initial treatment was effective for patients with IgAN who had proteinuria ≥1.0 g/d and eGFR <60 ml/min per 1.73 m2, which were the same results as those found by other reports (27,28). Moreover, an analysis of tonsils from patients with IgAN showed that the extrafollicular areas were enlarged compared with those from patients with chronic tonsillitis, and that steroid pulse therapy could shrink the follicles and the germinal centers (29); therefore, tonsillectomy combined with steroid pulse therapy may play a pivotal role in treating IgAN. Our analysis indicated that tonsillectomy and corticosteroid therapy with or without steroid pulse therapy were associated with less disease progression. Therefore, tonsillectomy combined with corticosteroid therapy may be an effective treatment for IgAN. Moreover, only 8% of the patients in this study experienced complications as a consequence of tonsillectomy; therefore, tonsillectomy appears to be relatively safe.
This study had some limitations. First, this was a single-center, cohort study performed in Japan. Although many studies of tonsillectomy for patients with IgAN in Japan have been reported, the strengths of this study were its large cohort, 20-year analysis of renal survival, use of the Oxford classification for histologic analyses, analysis of tonsillectomy as an initial treatment and as a treatment at any time during a patient’s life, and adjustments for clinical and histologic data and treatments. Of course, other races should be analyzed regarding tonsillectomy, but our study’s strengths could support tonsillectomy for all patients with IgAN. Second, this study was a retrospective analysis. Although the patient data were similar after propensity score matching, it was difficult to completely adjust for background characteristics. This study had a wide enrolment period (since 1974), and treatment and management practices changed during that period. To adjust for background characteristics, during study 1, treatments such as tonsillectomy, immunosuppressants, and RASI were available at any time during the follow-up period. However, it was still difficult to completely adjust for background characteristics because the numbers of diagnoses were significantly different over the years, and tonsillectomy diagnoses have dramatically increased at our institution since 2005. Therefore, large randomized controlled trials should be performed to generate more robust data.
In this study of Japanese patients with IgAN, tonsillectomy performed as an initial treatment soon after renal biopsy, or at any time during a patient’s life, was effective for preventing progression to ESKD. Furthermore, its efficacy was significant for patients with IgAN who had proteinuria of >1.0 g/d. Moreover, tonsillectomy was a relatively safe procedure that did not cause major complications among Japanese patients with IgAN.
Disclosures
All authors have nothing to disclose.
Funding
None.
Acknowledgments
We thank Editage (www.editage.jp) for editing the draft of this manuscript for English language.
Dr. Norio Hanafusa reports receiving personal fees from Bayer Japan, Kyowa Kirin, and Nobelpharma, outside the submitted work. This work was presented at the American Society of Nephrology annual meeting of Kidney Week 2020 on October 22 through October 25, with changed embargo policy.
Author Contributions
K. Akiyama, N. Hanafusa, Y. Iwabuchi, K. Karasawa, Y. Miyabe, K. Nitta, S. Ogura, M. Seki, N. Sugiura, T. Takabe, and K. Uchida reviewed and edited the manuscript; K. Akiyama, N. Hanafusa, Y. Iwabuchi, K. Karasawa, Y. Miyabe, S. Ogura, M. Seki, N. Sugiura, and T. Takabe were responsible for validation; K. Akiyama, Y. Iwabuchi, K. Karasawa, Y. Miyabe, and T. Moriyama conceptualized the study; Y. Miyabe, T. Moriyama, and S. Ogura were responsible for data curation; T. Moriyama wrote the original draft and was responsible for formal analysis, funding acquisition, investigation, methodology, project administration, and software; and K. Nitta and K. Uchida provided supervision.
References
- 1.Floege J, Moura IC, Daha MR: New insights into the pathogenesis of IgA nephropathy. Semin Immunopathol 36: 431–442, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, Wyatt RJ, Scolari F, Mestecky J, Gharavi AG, Julian BA: The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki H: Biomarkers for IgA nephropathy on the basis of multi-hit pathogenesis. Clin Exp Nephrol 23: 26–31, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahara M, Nagato T, Nozaki Y, Kumai T, Katada A, Hayashi T, Harabuchi Y: A proliferation-inducing ligand (APRIL) induced hyper-production of IgA from tonsillar mononuclear cells in patients with IgA nephropathy. Cell Immunol 341: 103925, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Goto T, Bandoh N, Yoshizaki T, Nozawa H, Takahara M, Ueda S, Hayashi T, Harabuchi Y: Increase in B-cell-activation factor (BAFF) and IFN-gamma productions by tonsillar mononuclear cells stimulated with deoxycytidyl-deoxyguanosine oligodeoxynucleotides (CpG-ODN) in patients with IgA nephropathy. Clin Immunol 126: 260–269, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Muto M, Manfroi B, Suzuki H, Joh K, Nagai M, Wakai S, Righini C, Maiguma M, Izui S, Tomino Y, Huard B, Suzuki Y: Toll-like receptor 9 stimulation induces aberrant expression of a proliferation-inducing ligand by tonsillar germinal center B cells in IgA nephropathy. J Am Soc Nephrol 28: 1227–1238, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wada Y, Matsumoto K, Suzuki T, Saito T, Kanazawa N, Tachibana S, Iseri K, Sugiyama M, Iyoda M, Shibata T: Clinical significance of serum and mesangial galactose-deficient IgA1 in patients with IgA nephropathy. PLoS One 13: e0206865, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuzawa Y, Yamamoto R, Takahashi K, Katafuchi R, Tomita M, Fujigaki Y, Kitamura H, Goto M, Yasuda T, Sato M, Urushihara M, Kondo S, Kagami S, Yasuda Y, Komatsu H, Takahara M, Harabuchi Y, Kimura K, Matsuo S: Evidence-based clinical practice guidelines for IgA nephropathy 2014. Clin Exp Nephrol 20: 511–535, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki Y, Matsuzaki K, Suzuki H, Okazaki K, Yanagawa H, Ieiri N, Sato M, Sato T, Taguma Y, Matsuoka J, Horikoshi S, Novak J, Hotta O, Tomino Y: Serum levels of galactose-deficient immunoglobulin (Ig) A1 and related immune complex are associated with disease activity of IgA nephropathy. Clin Exp Nephrol 18: 770–777, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakata J, Suzuki Y, Suzuki H, Sato D, Kano T, Yanagawa H, Matsuzaki K, Horikoshi S, Novak J, Tomino Y: Changes in nephritogenic serum galactose-deficient IgA1 in IgA nephropathy following tonsillectomy and steroid therapy. PLoS One 9: e89707, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, Liu ZH, Roberts IS, Yuzawa Y, Zhang H, Feehally J; IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants : Oxford classification of IgA nephropathy 2016: An update from the IgA nephropathy classification working group. Kidney Int 91: 1014–1021, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokonaga H, Hishida A; Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Feehally J, Coppo R, Troyanov S, Bellur SS, Cattran D, Cook T, Roberts IS, Verhave JC, Camilla R, Vergano L, Egido J, Wiecek A, Karkoszka H, Tesar V, Maixnerova D, Ots-Rosenberg M, Quaglia M, Rollino C, Magistroni R, Cusinato S, Cravero R, Peruzzi L, Lundberg S, Gesualdo L, Cancarini G, Feriozzi S, Ferrario F; VALIGA study of ERA-EDTA Immunonephrology Working Group : Tonsillectomy in a European cohort of 1,147 patients with IgA nephropathy. Nephron 132: 15–24, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Hirano K, Matsuzaki K, Yasuda T, Nishikawa M, Yasuda Y, Koike K, Maruyama S, Yokoo T, Matsuo S, Kawamura T, Suzuki Y: Association between tonsillectomy and outcomes in patients with immunoglobulin A nephropathy. JAMA Netw Open 2: e194772, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coppo R: The gut-renal connection in IgA nephropathy. Semin Nephrol 38: 504–512, 2018 [DOI] [PubMed] [Google Scholar]
- 18.You W, Chen J, Wang Y, Chen Y, Wang L, Yongman L: A meta-analysis of the clinical remission rate and long-term efficacy of tonsillectomy in patients with IgA nephropathy. Nephrol Dial Transplant 26: 1923–1931, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Liu LL, Wang LN, Jiang Y, Yao L, Dong LP, Li ZL, Li XL: Tonsillectomy for IgA nephropathy: A meta-analysis. Am J Kidney Dis 65: 80–87, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Duan J, Liu D, Duan G, Liu Z: Long-term efficacy of tonsillectomy as a treatment in patients with IgA nephropathy: A meta-analysis. Int Urol Nephrol 49: 103–112, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Rasche FM, Schwarz A, Keller F: Tonsillectomy does not prevent a progressive course in IgA nephropathy. Clin Nephrol 51: 147–152, 1999 [PubMed] [Google Scholar]
- 22.Yang D, He L, Peng X, Liu H, Peng Y, Yuan S, Liu Y, Chen X, Liu F, Liu C: The efficacy of tonsillectomy on clinical remission and relapse in patients with IgA nephropathy: A randomized controlled trial. Ren Fail 38: 242–248, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Kawamura T, Yoshimura M, Miyazaki Y, Okamoto H, Kimura K, Hirano K, Matsushima M, Utsunomiya Y, Ogura M, Yokoo T, Okonogi H, Ishii T, Hamaguchi A, Ueda H, Furusu A, Horikoshi S, Suzuki Y, Shibata T, Yasuda T, Shirai S, Imasawa T, Kanozawa K, Wada A, Yamaji I, Miura N, Imai H, Kasai K, Soma J, Fujimoto S, Matsuo S, Tomino Y; Special IgA Nephropathy Study Group : A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrol Dial Transplant 29: 1546–1553, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovács T, Vas T, Kövesdy CP, Degrell P, Nagy G, Rékási Z, Wittmann I, Nagy J: Effect of tonsillectomy and its timing on renal outcomes in Caucasian IgA nephropathy patients. Int Urol Nephrol 46: 2175–2182, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Katafuchi R, Kawamura T, Joh K, Hashiguchi A, Hisano S, Shimizu A, Miyazaki Y, Nagata M, Matsuo S; IgA nephropathy Study Group in Japan : Pathological sub-analysis of a multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy versus steroid pulse monotherapy in patients with immunoglobulin A nephropathy. Clin Exp Nephrol 20: 244–252, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto T, Nishino T, Nakata T, Sato Y, Komatsu H, Uramatsu T, Ishimatsu N, Ishida K, Serino R, Otsuji Y, Miyazaki M, Tomo T, Tamura M, Fujimoto S: Impact of tonsillectomy combined with steroid pulse therapy on immunoglobulin A nephropathy depending on histological classification: A multicenter study. Clin Exp Nephrol 20: 50–57, 2016 [DOI] [PubMed] [Google Scholar]
- 27.Hoshino J, Fujii T, Usui J, Fujii T, Ohashi K, Takaichi K, Suzuki S, Ubara Y, Yamagata K: Renal outcome after tonsillectomy plus corticosteroid pulse therapy in patients with immunoglobulin A nephropathy: Results of a multicenter cohort study. Clin Exp Nephrol 20: 618–627, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Kumon S, Moriyama T, Kamiyama T, Karasawa K, Nitta K: The impact of tonsillectomy combined with steroid pulse therapy in patients with advanced IgA nephropathy and impaired renal function. Clin Exp Nephrol 24: 295–306, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Adachi M, Sato M, Miyazaki M, Hotta O, Hozawa K, Sato T, Taguma Y, Katori Y: Steroid pulse therapy transiently destroys the discriminative histological structure of tonsils in IgA nephropathy: Tonsillectomy should be performed before or just after steroid pulse therapy. Auris Nasus Larynx 45: 1206–1213, 2018 [DOI] [PubMed] [Google Scholar]



