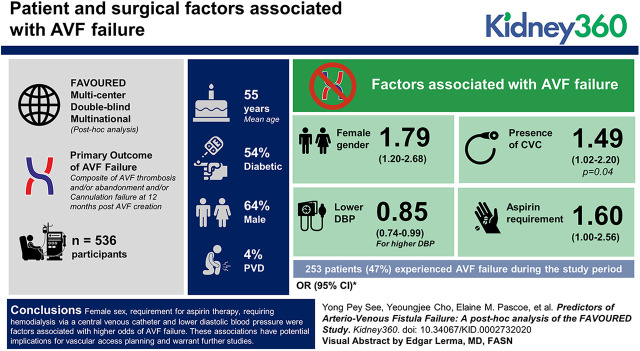
Visual Abstract
Keywords: dialysis, arteriovenous access, arteriovenous fistula, hemodialysis, hemodialysis access, vascular access
Abstract
Background
An autologous arteriovenous fistula (AVF) is the preferred hemodialysis vascular access, but successful creation is hampered by high rates of AVF failure. This study aimed to evaluate patient and surgical factors associated with AVF failure to improve vascular access selection and outcomes.
Methods
This is a post hoc analysis of all participants of FAVOURED, a multicenter, double-blind, multinational, randomized, placebo-controlled trial evaluating the effect of fish oil and/or aspirin in preventing AVF failure in patients receiving hemodialysis. The primary outcome of AVF failure was a composite of fistula thrombosis and/or abandonment and/or cannulation failure at 12 months post-AVF creation, and secondary outcomes included individual outcome components. Patient data (demographics, comorbidities, medications, and laboratory data) and surgical factors (surgical expertise, anesthetic, intraoperative heparin use) were examined using multivariable logistic regression analyses to evaluate associations with AVF failure.
Results
Of 536 participants, 253 patients (47%) experienced AVF failure during the study period. The mean age was 55±14.4 years, 64% were male, 45% were diabetic, and 4% had peripheral vascular disease. Factors associated with AVF failure included female sex (odds ratio [OR], 1.79; 95% confidence interval [CI], 1.20 to 2.68), lower diastolic BP (OR for higher DBP, 0.85; 95% CI, 0.74 to 0.99), presence of central venous catheter (OR, 1.49; 95% CI, 1.02 to 2.20; P=0.04), and aspirin requirement (OR, 1.60; 95% CI, 1.00 to 2.56).
Conclusions
Female sex, requirement for aspirin therapy, requiring hemodialysis via a central venous catheter, and lower diastolic BP were factors associated with higher odds of AVF failure. These associations have potential implications for vascular access planning and warrant further studies.
Introduction
An autologous arteriovenous fistula (AVF) is the vascular access of choice (1–4) for most patients requiring hemodialysis due to improved longevity once successfully established, lower associated mortality (5), and lower health costs (6) compared with an arteriovenous graft or central venous catheter (CVC). However, these long-term benefits are hampered by exceedingly high rates of early AVF failure due to thrombosis and maturation failure, affecting up to 60% of patients (7,8). It is thus not surprising that vascular-access function is one of the most critically important outcomes for patients on hemodialysis and their caregivers (9).
Previous studies have identified delayed nephrology care (10,11), smaller arterial (12,13) and venous (12–16) caliber on sonographic evaluation, and demographic factors, such as older age and female sex (11,15,17–19), to be associated with AVF failure, whereas greater surgical experience and use of regional anesthesia (20–25) were associated with better AVF outcomes. Unfortunately, many of these studies have shown inconsistent and conflicting outcomes, likely driven by differences in study populations, sample size, and methodology, and substantial heterogeneity of outcome definitions (7). Scoring systems incorporating multiple factors have been used to improve predictive scoring for AVF failure; although showing promise (26), they have not been shown to consistently predict vascular access outcomes when applied to different study populations (10). Furthermore, few studies have evaluated potentially modifiable predictors, such as surgical expertise or anesthetic technique, in different study populations, including Australian and New Zealand cohorts (27,28).
This post hoc analysis of the randomized controlled Omega 3 Fatty Acids (Fish Oils) and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED) study conducted in Australia, New Zealand, Malaysia, and the United Kingdom was performed to identify potentially modifiable pre- and perioperative patient and surgical factors associated with AVF failure, defined as a clinically relevant composite outcome of thrombosis, abandonment, and/or failure to cannulate within 1 year of AVF creation in patients requiring hemodialysis.
Materials and Methods
The FAVOURED trial was a multicenter, double-blind, randomized, placebo-controlled trial conducted in 35 hemodialysis centers in Australia, New Zealand, Malaysia, and the United Kingdom investigating the effect of fish oil supplementation or aspirin on preventing AVF failure among patients recruited between August 2008 and February 2014. The trial was registered with the Australia and New Zealand Clinical Trial Registry (ACTRN12607000569404). The original two-by-two factorial design was amended in June 2011 to allow patients who required ongoing aspirin therapy to be randomized to fish oil or matching placebo, but they continued open-label aspirin use when deemed medically required (29). The FAVOURED trial had obtained approval from local human research ethics committees in all participating centers before trial commencement.
Adult patients aged ≥19 years, with stage 4 or 5 CKD, receiving or expected to receive hemodialysis within 12 months, and scheduled for AVF creation, were eligible for the study. Patients with significant bleeding risk or contraindication to use of the study agents were excluded. A detailed description of the study protocol has been published previously (30).
Patient-related factors collected at baseline before AVF creation included demographic data (i.e., sex, age, region of recruitment [Australia, New Zealand, and the United Kingdom collectively referred as ANZ versus Malaysia]), clinical information (i.e., body mass index; waist-hip ratio; baseline BP taken as per local practices; smoking history; cause of ESKD; presence of diabetes mellitus [DM]; hypertension; peripheral vascular disease [PVD]; ischemic heart disease; cerebrovascular disease status; presence of CVC; and medications such as statins, erythropoiesis-stimulating agents, calcium channel blockers, β-blockers, aspirin, and fish oil), and relevant blood investigations (i.e., full blood count, coagulation profile [international normalized ratio, activated partial thromboplastin time], serum calcium, phosphate, parathyroid hormone [PTH], LDL cholesterol, and glycated hemoglobin [hemoglobin A1c]) at the time of AVF creation. Recorded surgical factors included the type of anesthesia used (i.e., general, regional, or local), intraoperative heparin use, surgical expertise defined by level of training (i.e., trainee such as resident and registrar versus consultant), and type of AVF created (i.e., radiocephalic [RC], brachiocephalic, brachiobasilic, and others).
The primary outcome of AVF failure was a composite of fistula thrombosis and/or abandonment and/or cannulation failure at 12 months. Thrombosis was defined as the absence of a thrill or bruit by clinical examination and/or requirement of rescue intervention (medical thrombolysis or surgical thrombectomy). Abandonment was defined as permanent abandonment of the study AVF, including unsalvageable thrombosis of the study AVF, imaging showing that the study AVF was unusable or not amenable to any intervention for its improvement, insertion of another dialysis access (new AVF, arteriovenous graft, CVC, or peritoneal dialysis access), or ligation of the study AVF. Cannulation failure was defined as failure to successfully cannulate the study AVF during eight or more of 12 consecutive hemodialysis sessions during the cannulation assessment period (Supplemental Table 1).
Statistical Analyses
Baseline characteristics are presented as frequency (percentage), mean (± SD), or median (interquartile range), as appropriate. Differences between patients with and without AVF failure were analyzed using the independent t test or Mann–Whitney U test for continuous variables, according to data distribution. Chi-squared test and the Fisher exact test were performed for categoric variables, as appropriate.
Associations between factors and the composite outcome of AVF failure were examined by univariable and multivariable logistic regression. All patient factors that were found to be associated with AVF failure on univariable logistic regression with P values <0.2 were included in a multivariable logistic regression in model 1. DM was prespecified for inclusion in model 1 due to its clinical importance, regardless of P value on univariable logistic regression (31). Similarly, the use of study agents (open-label aspirin, randomized aspirin or matching placebo, and fish oil or matching placebo) was included in model 1 because these agents were randomized interventions from the original FAVOURED trial. Surgical factors associated with AVF failure on univariable analysis (P<0.2) or prespecified for inclusion due to its clinical importance (i.e., type of anesthesia, surgical expertise) (24) were then included to patient factors in model 1 to derive model 2. Factors with >5% missing data were excluded from multivariable logistic regression. On preliminary analysis, sex-specific differences in diastolic BP (DBP) and site of AVF created were observed and, therefore, interactions between DBP and sex, and site of AVF and sex, on AVF failures were examined. Model fit was evaluated using the Hosmer–Lemeshow test, and diagnostic accuracy was tested by the area under the operating characteristic curve (AUC). Subsequently, model 2 was applied to determine factors associated with individual, cause-specific components of AVF failure (i.e., AVF thrombosis, abandonment, and failure to cannulate at 12 months). P values <0.05 were considered statistically significant. Statistical analyses were performed with Stata version 14.1 (Stata Corporation, College Station, TX).
Results
Study Participants
All 536 participants randomized and analyzed in the original FAVOURED study were included in this study (30). Among those recruited, 334 participants were enrolled from Australia (62%), 144 from Malaysia (27%), 49 from New Zealand (9%), and nine from the United Kingdom (2%). The baseline characteristics for participants with and without AVF failure are shown in Table 1. More than 5% missing data were present for laboratory parameters, including hemoglobin A1c, LDL cholesterol, international normalized ratio, activated partial thromboplastin time, and PTH levels.
Table 1.
Baseline characteristics of the study population
| Parameter | All Participants (n=536) | Patients without AVF Failurea (n=283) | Patients with AVF Failurea (n=253) | P Value |
| Female, n (%) | 194 (36) | 89 (32) | 105 (42) | 0.02 |
| Age (yr) | 55.0±14.4 | 53.6±14.3 | 56.5±14.4 | 0.02 |
| Ethnicity, n (%) | 0.25 | |||
| White | 289 (54) | 143 (51) | 146 (58) | |
| Asian | 169 (32) | 101 (36) | 68 (27) | |
| Aboriginal and Torres Strait Islander | 21 (4) | 9 (3) | 12 (5) | |
| Maori and Pacific Islander | 38 (7) | 20 (7) | 18 (7) | |
| Other | 19 (4) | 10 (4) | 9 (4) | |
| Region of recruitment, n (%) | 0.01 | |||
| Australia/NZ/UK | 392 (73) | 193 (68) | 199 (79) | |
| Malaysia | 144 (27) | 90 (32) | 54 (21) | |
| Cause of ESKD, n (%) | 0.90 | |||
| Diabetes mellitus | 202 (38) | 104 (37) | 98 (39) | |
| GN | 72 (13) | 40 (14) | 32 (13) | |
| Hypertension | 71 (13) | 40 (14) | 31 (12) | |
| Polycystic | 40 (8) | 23 (8) | 17 (7) | |
| Reflux | 26 (5) | 14 (5) | 12 (5) | |
| Others | 125 (23) | 62 (22) | 63 (25) | |
| BMI (kg/m2), median (interquartile range) | 27.2 (23.4–32.0) | 27.1 (23.9–31.7) | 27.5 (23.0–32.5) | 0.83 |
| Comorbidity, n (%) | ||||
| Hypertension | 475 (89) | 259 (92) | 216 (85) | 0.03 |
| DM | 243 (45) | 132 (47) | 111 (44) | 0.52 |
| IHD | 53 (10) | 24 (9) | 29 (12) | 0.25 |
| CCF | 21 (4) | 12 (4) | 9 (4) | 0.68 |
| PVD | 23 (4) | 8 (3) | 15 (6) | 0.08 |
| CVD | 17 (3) | 11 (4) | 6 (2) | 0.32 |
| Composite of IHD, CVD, and PVD | 79 (15) | 38 (13) | 41 (16) | 0.37 |
| Baseline BP (mm Hg), mean±SD | ||||
| SBP | 146.1±23.0 | 147.2±21.9 | 144.9±24.2 | 0.25 |
| DBP | 81.4±13.4 | 83.1±12.9 | 79.5±13.6 | <0.01 |
| MAP | 103.0±14.4 | 104.5±13.6 | 101.3±15.0 | 0.01 |
| PP | 64.1±20.0 | 64.1±19.6 | 65.4±20.5 | 0.45 |
| HbA1c (%), mean±SDb | 6.2±1.4 | 6.3±1.5 | 6.2±1.3 | 0.25 |
| LDL-C (mmol/L), median (interquartile range)b | 2.4 (1.8–3.1) | 2.6 (1.9–3.3) | 2.3 (1.7–3.1) | 0.07 |
| Smoking, n (%) | 0.68 | |||
| Never | 264 (49) | 137 (48) | 127 (50) | |
| Current or previous | 272 (51) | 146 (52) | 126 (50) | |
| Current or previous history of RRT, n (%) | 268 (50) | 139 (49) | 129 (51) | 0.63 |
| Presence of CVC, n (%) | 218 (41) | 107 (38) | 111 (44) | 0.15 |
| Serum albumin (g/L), median (interquartile range) | 36 (32–40) | 37 (33–41) | 36 (32–40) | 0.22 |
| PTH (pmol/L), median (interquartile range)b | 28 (16.1–45.3) | 30.2 (16.6–50.8) | 26.6 (15.2–40.8) | 0.05 |
| INR, median (interquartile range)b | 1 (1.0–1.1) | 1 (0.9–1.1) | 1 (1–1.1) | 0.58 |
| APTT, median (interquartile range)b | 30.1 (27–34) | 31 (27.7–35) | 30 (27–34) | 0.09 |
| Hemoglobin (g/L), mean±SD | 108.2±18.6 | 106.8±17.7 | 109.7±19.5 | 0.08 |
| Platelets, median (interquartile range) | 233 (191–288) | 233 (193–289) | 233 (191–287) | 0.83 |
| Medications, n (%) | ||||
| Statin | 275 (51) | 148 (52) | 127 (50) | 0.63 |
| ESA | 253 (47) | 139 (49) | 114 (45) | 0.35 |
| β-Blockers | 247 (46) | 135 (48) | 112 (44) | 0.43 |
| ACE-I/ARB | 224 (42) | 117 (41) | 107 (42) | 0.82 |
| CCB | 299 (56) | 167 (59) | 132 (52) | 0.11 |
| Surgeons, n (%) | 0.43 | |||
| Consultants | 419 (78) | 225 (80) | 194 (77) | |
| Registrars/residents | 117 (22) | 58 (21) | 59 (23) | |
| Type of anesthesia, n (%) | 0.08 | |||
| Local anesthesia | 211 (40) | 122 (43) | 89 (36) | |
| Regional/general anesthesia | 322 (60) | 161 (57) | 161 (64) | |
| AVF created, n (%) | 0.08 | |||
| Radiocephalic | 312 (58) | 168 (59) | 144 (57) | |
| Brachiocephalic | 180 (34) | 99 (35) | 81 (32) | |
| Brachiobasilic | 37 (7) | 12 (4) | 25 (10) | |
| Others | 7 (1) | 4 (1) | 3 (1) | |
| Intraoperative heparin, n (%) | 352 (66) | 184 (65) | 168 (67) | 0.73 |
| Randomization to aspirin, n (%) | 0.04 | |||
| Randomized to placebo | 194 (36) | 107 (38) | 87 (34) | |
| Randomized to aspirin | 194 (36) | 111 (39) | 83 (33) | |
| Open-label aspirin | 148 (28) | 65 (23) | 83 (33) | |
| Randomization to fish oil, n (%) | 270 (50) | 142 (50) | 128 (51) | 0.92 |
Continuous variables are presented in mean±SD if normally distributed and median (interquartile range) if non-normally distributed. Independent t test and Mann–Whitney U test performed for continuous variable when appropriate. Chi-squared test and Fisher exact test performed for categoric variables when appropriate. AVF, arteriovenous fistula; NZ, New Zealand; UK, United Kingdom; BMI, body mass index; DM, diabetes mellitus; IHD, ischemic heart disease; CCF, congestive cardiac failure; PVD, peripheral vascular disease; CVD, cerebrovascular disease; SBP, systolic BP; DB, diastolic BP; MAP, mean arterial pressure; PP, pulse pressure; HbA1c, hemoglobin A1c; LDL-C, LDL cholesterol; CVC, central venous catheter; PTH, parathyroid hormone; INR, international normalized ratio; APTT, activated partial thromboplastin time; ESA, erythropoietin-stimulating agent; ACE-I/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CCB, calcium channel blocker.
AVF failure is defined as composite of AVF thrombosis and/or failure to cannulate and/or abandonment of AVF within 12 months of AVF creation.
Variables with >5% missing data.
Predictors of AVF Failure
AVF failure occurred in 253 participants (47%); 109 participants experienced at least one episode of AVF thrombosis, 121 participants had AVF abandonment, and 212 participants experienced cannulation failure. On univariable logistic regression, female sex, older age, non-Malaysian region of recruitment, absence of hypertension, lower DBP and mean arterial pressure, higher PTH level, and open-labeled (i.e., medically required) aspirin use were statistically significantly associated with increased odds of AVF failure (Table 2). Surgical expertise, type of anesthesia used, intraoperative anticoagulation use, or the type of AVF created were not associated with AVF failure on univariable logistic regression.
Table 2.
Logistic regression of patient and surgical factors associated with arteriovenous fistula failure
| Parameter | Univariable Odds Ratio (95% CI) | P Value | Model 1a Odds Ratio (95% CI) | P Value | Model 2b Odds Ratio (95% CI) | P Value |
| Female | 1.54 (1.09 to 2.20) | 0.02 | 1.60 (1.10 to 2.32) | 0.01 | 1.79 (1.20 to 2.68) | <0.01 |
| Age (per 10 yr) | 1.15 (1.02 to 1.29) | 0.02 | 1.06 (0.92 to 1.21) | 0.42 | 1.06 (0.92 to 1.22) | 0.41 |
| Ethnicity | ||||||
| White | Ref | 0.25 | — | — | — | — |
| Asian | 0.66 (0.45 to 0.97) | |||||
| Aboriginal and Torres Strait Islander | 1.31 (0.53 to 3.19) | |||||
| Maori and Pacific Islander | 0.88 (0.45 to 1.74) | |||||
| Other | 0.88 (0.35 to 2.23) | |||||
| Region c | ||||||
| Australia/NZ/UK | Ref | 0.01 | Ref | 0.03 | Ref | 0.20 |
| Malaysia | 0.58 (0.39 to 0.86) | 0.60 (0.38 to 0.95) | 0.66 (0.34 to 1.25) | |||
| Cause of ESKD | ||||||
| DM | Ref | 0.90 | — | — | — | — |
| GN | 0.85 (0.49 to 1.46) | |||||
| Hypertension | 0.82 (0.48 to 1.42) | |||||
| APKD | 0.78 (0.40 to 1.56) | |||||
| Reflux | 0.91 (0.40 to 2.06) | |||||
| Others | 1.08 (0.69 to 1.68) | |||||
| BMI | 1.01 (0.99 to 1.04) | 0.33 | — | — | — | — |
| Hypertension | 0.54 (0.31 to 0.93) | 0.03 | — | — | — | — |
| DM | 0.89 (0.64 to 1.26) | 0.52 | 0.81 (0.55 to 1.20) | 0.29 | 0.85 (0.57 to 1.27) | 0.42 |
| IHD | 1.40 (0.79 to 2.47) | 0.25 | — | — | — | — |
| PVD | 2.17 (0.90 to 5.20) | 0.08 | 1.78 (0.70 to 4.53) | 0.23 | 1.87 (0.74 to 4.78) | 0.19 |
| CCF | 0.83 (0.35 to 2.01) | 0.68 | — | — | — | — |
| Cerebrovascular disease | 0.60 (0.22 to 1.65) | 0.32 | — | — | — | — |
| Composite of IHD, CVD, and PVD | 1.25 (0.77 to 2.01) | 0.37 | — | — | — | — |
| SBP (per 10 mm Hg) | 0.96 (0.89 to 1.03) | 0.25 | — | — | — | — |
| DBP (per 10 mm Hg) | 0.81 (0.71 to 0.93) | <0.01 | 0.84 (0.73 to 0.97) | 0.02 | 0.85 (0.74 to 0.99) | 0.04 |
| MAP (per 10 mm Hg) | 0.85 (0.76 to 0.96) | 0.01 | — | — | — | — |
| PP (per 10 mm Hg) | 1.03 (0.95 to 1.13) | 0.45 | — | — | — | — |
| HbA1c (%)d | 0.93 (0.82 to 1.05) | 0.25 | — | — | — | — |
| LDL-C (mmol/L)d | 0.86 (0.72 to 1.01) | 0.07 | — | — | — | — |
| Smoking | ||||||
| Never smoker | Ref | 0.68 | — | — | — | — |
| Current or former smoker | 0.93 (0.66 to 1.31) | |||||
| Current or previous history of kidney replacement therapy | 0.92 (0.65 to 1.29) | 0.63 | — | — | — | — |
| Presence of CVC | 1.29 (0.91 to 1.82) | 0.15 | 1.53 (1.05 to 2.23) | 0.03 | 1.49 (1.02 to 2.20) | 0.04 |
| Serum albumin (g/L) | 0.98 (0.96 to 1.01) | 0.22 | — | — | — | — |
| PTH (pmol/L)d | 0.99 (0.99 to 0.99) | 0.03 | — | — | — | — |
| INRd | 1.13 (0.57 to 2.26) | 0.73 | — | — | — | — |
| APTTd | 0.99 (0.98 to 1.01) | 0.40 | — | — | — | — |
| Hemoglobin (g/L) | 1.01 (1.00 to 1.02) | 0.08 | 1.01 (0.99 to 1.02) | 0.27 | 1.01 (0.99 to 1.02) | 0.30 |
| Platelets | 1.00 (0.99 to 1.00) | 0.85 | — | — | — | — |
| Statin use | 0.92 (0.65 to 1.29) | 0.63 | ||||
| ESA use | 0.85 (0.60 to 1.19) | 0.35 | ||||
| β-Blocker use | 0.87 (0.62 to 1.22) | 0.47 | ||||
| ACE-I/ARB use | 1.04 (0.74 to 1.47) | 0.82 | ||||
| CCB use | 0.76 (0.54 to 1.07) | 0.11 | 0.86 (0.60 to 1.25) | 0.44 | 0.84 (0.58 to 1.22) | 0.35 |
| Surgeons | ||||||
| Trainees | Ref | 0.43 | — | — | Ref | 0.66 |
| Consultant | 0.85 (0.56 to 1.28) | 0.91 (0.59 to 1.40) | ||||
| Anesthesia | ||||||
| Regional/general anesthesia | Ref | 0.08 | — | — | Ref | 0.81 |
| Local anesthesia | 0.73 (0.51 to 1.04) | 0.93 (0.54 to 1.63) | ||||
| Intraoperative heparin | 1.07 (0.74 to 1.52) | 0.73 | — | — | — | — |
| AVF type | ||||||
| Radiocephalic | Ref | 0.10 | — | — | Ref | 0.06 |
| Brachiocephalic | 0.95 (0.66 to 1.38) | 0.76 (0.50 to 1.16) | ||||
| Bachiobasilic | 2.43 (1.18 to 5.01) | 2.24 (1.10 to 5.05) | ||||
| Others | 0.88 (0.19 to 3.97) | 0.75 (0.15 to 3.71) | ||||
| Randomization to aspirin | ||||||
| Randomized to placebo | Ref | 0.04 | Ref | 0.05 | Ref | 0.03 |
| Randomized to aspirin | 0.92 (0.62 to 1.37) | 0.89 (0.59 to 1.35) | 0.85 (0.55 to 1.29) | |||
| Open-label aspirin | 1.57 (1.02 to 2.42) | 1.59 (1.00 to 2.53) | 1.60 (1.00 to 2.56) | |||
| Randomization to fish oil use | 1.02 (0.72 to 1.43) | 0.92 | 1.05 (0.73 to 1.49) | 0.80 | 1.09 (0.76 to 1.56) | 0.64 |
Single imputation for missing values of individual outcome components was performed and described in detail in the primary outcome analysis (30). Ref, reference; NZ, New Zealand; UK, United Kingdom, DM, diabetes mellitus; APKD, adult polycystic kidney disease; BMI, body mass index; IHD, ischemic heart disease; PVD, peripheral vascular disease; CCF, congestive cardiac failure; CVD, cerebrovascular disease; SBP, systolic BP; DBP, diastolic BP; MAP, mean arterial pressure; PP, pulse pressure; HbA1c, hemoglobin A1c; LDL-C, LDL cholesterol; CVC, central venous catheter; PTH, parathyroid hormone; INR, international normalized ratio; APTT, activated partial thromboplastin time; ESA, erythropoietin-stimulating agent; ACE-I/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CCB, calcium channel blocker; AVF, arteriovenous fistula.
Model 1: Multivariable logistic regression using patient factors with P<0.2 on univariable logistic analysis and <5% missing data inclusive of DM; randomization to aspirin and fish oil forced in; patient factors included are female, age, diastolic BP, region, presence of peripheral vascular disease, presence of CVC, use of CCB, and hemoglobin levels.
Model 2: Multivariable logistic regression using Model 1 with addition of surgical-related factors such as surgeons, type of anesthesia use, and type of AVF created.
Australia, New Zealand, and United Kingdom region of recruitment was grouped when compared with Malaysian region of recruitment in this analysis.
Variables with >5% missing data.
Multivariable logistic regression of patient factors, including female sex, age, region of recruitment, DBP, presence of DM, presence of PVD, presence of CVC, hemoglobin levels, use of calcium channel blockers, and randomization to aspirin and fish oil, was performed in model 1. DBP was chosen over history of hypertension and mean arterial pressure for inclusion into model 1, because it is biologically plausible and most strongly associated statistically on univariable logistic regression. Female sex (adjusted odds ratio [OR], 1.60; 95% CI, 1.10 to 2.32), presence of CVC (adjusted OR, 1.53; 95% CI, 1.05 to 2.23), and open-labeled aspirin use (adjusted OR, 1.59; 95% CI, 1.00 to 2.53) were associated with increased odds of AVF failure (Table 2). In contrast, every 10-mm Hg increase in DBP (adjusted OR, 0.84; 95% CI, 0.73 to 0.97) and Malaysian region of recruitment (adjusted OR, 0.60; 95% CI, 0.38 to 0.95; compared with ANZ) were associated with lower odds of AVF failure (Table 2).
When surgical factors (surgical expertise, type of AVF created, and use of local versus general or regional anesthesia) were added (model 2), Malaysian region of recruitment was no longer associated with lower AVF failure (adjusted OR, 0.66; 95% CI, 0.34 to 1.25). Female sex (adjusted OR, 1.79; 95% CI, 1.20 to 2.68), DBP (adjusted OR, 0.85; 95% CI, 0.74 to 0.99), presence of CVC (adjusted OR, 1.49; 95% CI, 1.02 to 2.20), and open-labeled use of aspirin (adjusted OR, 1.60; 95% CI, 1.00 to 2.56) remained associated with AVF failure. None of the other surgical factors were associated with AVF failure in model 2. The Hosmer–Lemeshow goodness-of-fit values were six (P=0.65) and 8.26 (P=0.41) for model 1 and 2, respectively. Furthermore, the addition of surgical factors did not improve the prediction of AVF failure (AUC of 0.67 compared with 0.65 for model 1; P=0.08).
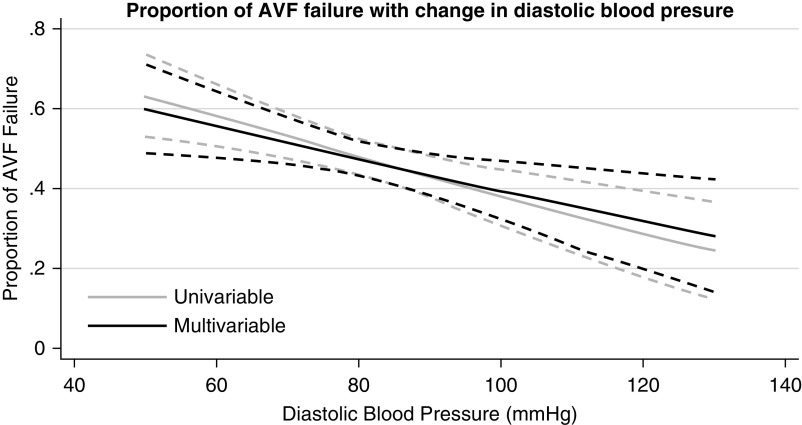
Among the factors identified to be associated with AVF failure, DBP was potentially modifiable. An inverse relationship was observed between DBP and AVF failure that remained unchanged on multivariable analysis when factors used in model 2 were added (Figure 1). The probability of AVF failure was >50% with DBP of ≤80 mm Hg (Figure 1). There was a similar trend toward greater probability of AVF failure with decreasing mean arterial pressure (Supplemental Figure 1) and, to a lesser extent, with systolic BP (Supplemental Figure 2). On the other hand, an association between higher pulse pressure and higher probability of AVF failure was observed (Supplemental Figure 3).
Figure 1.
Increase proportion of arteriovenous fistula (AVF) failure (composite outcome of AVF abandonment, thrombosis, and failure to cannulate) with decrease in diastolic BP. The light gray line reflects an almost inverse linear relationship between diastolic BP and proportion of AVF failure on univariable logistic regression, and the dashed gray line represent the 95% confidence interval at each diastolic BP level. The black line shows a similar relationship between diastolic BP and proportion of AVF failure on multivariable analysis adjusted for female sex, age, region of recruitment, presence of diabetes, presence of peripheral vascular disease, presence of central venous catheter, use of calcium channel blockers, hemoglobin levels, randomization to aspirin, randomization to fish oil, type of AVF created, surgical expertise, and type of anesthesia use; the dotted black line represents the 95% confidence interval.
Female participants had, on average, lower DBP (79.6±13.0 mm Hg) at baseline compared with males (82.4±13.5 mm Hg, P=0.02) (Supplemental Table 2), but no significant interaction between BP and sex was observed (P=0.79). It was also noted that female patients had more upper-arm AVFs created (62%), compared with only 29% (Supplemental Table 3) in males, but no significant interaction between AVF site and sex was observed (OR, 1.20; 95% CI, 0.57 to 2.55; P=0.10).
Predictors of the Individual Components of AVF Failure
The factors associated with the individual outcomes of AVF thrombosis, abandonment, and cannulation failure were different (Table 3). Female sex was associated with increased odds of AVF abandonment (adjusted OR, 2.02; 95% CI, 1.27 to 3.20) and cannulation failure (adjusted OR, 1.74; 95% CI, 1.16 to 2.61), but not AVF thrombosis. The presence of PVD was associated with increased odds of AVF abandonment (adjusted OR, 3.24; 95% CI, 1.31 to 7.98). The presence of CVC, lower DBP, and non-Malaysian region of recruitment were associated with higher odds of cannulation failure (CVC, adjusted OR, 1.66; 95% CI, 1.11 to 2.46; DBP, adjusted OR, 0.80; 95% CI, 0.68 to 0.93; region of recruitment, adjusted OR, 0.50; 95% CI, 0.26 to 0.98). Brachiocephalic AVFs were less likely to be abandoned compared with RC AVFs (adjusted OR, 0.48; 95% CI, 0.29 to 0.81), whereas brachiobasilic AVFs were more likely to fail cannulation compared with RC AVFs (adjusted OR, 2.24; 95% CI, 1.07 to 4.53).
Table 3.
Multivariable logistic regression analysis of patient and surgical factors for individual outcome components, including AVF abandonment, thrombosis, and cannulation failure at 12 mo
| Parameter | Abandonment (n=121) | Thrombosis (n=109) | Failure to Cannulate (n=212) | |||
| Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value | |
| Female sex | 2.02 (1.27 to 3.20) | <0.01 | 1.16 (0.72 to 1.89) | 0.54 | 1.74 (1.16 to 2.61) | 0.01 |
| Age (per 10 yr) | 1.01 (0.86 to 1.19) | 0.89 | 0.92 (0.77 to 1.08) | 0.30 | 1.12 (0.97 to 1.29) | 0.12 |
| Diastolic pressure (per 10 mm Hg) | 0.91 (0.76 to 1.08) | 0.27 | 0.90 (0.75 to 1.07) | 0.23 | 0.80 (0.68 to 0.93) | 0.01 |
| Region a | ||||||
| Australia/NZ/UK | Ref | 0.66 | Ref | 0.54 | Ref | 0.04 |
| Malaysia | 1.18 (0.56 to 2.47) | 1.30 (0.57 to 2.97) | 0.50 (0.26 to 0.98) | |||
| Diabetes mellitus | 0.87 (0.54 to 1.39) | 0.55 | 0.70 (0.43 to 1.14) | 0.15 | 0.92 (0.61 to 1.38) | 0.69 |
| Presence of PVD | 3.24 (1.31 to 7.98) | 0.01 | 2.46 (0.95 to 6.35) | 0.06 | 0.92 (0.37 to 2.27) | 0.85 |
| Presence of CVC | 1.03 (0.65 to 1.61) | 0.91 | 0.77 (0.48 to 1.24) | 0.27 | 1.66 (1.11 to 2.46) | 0.01 |
| Use of CCB | 0.91 (0.58 to 1.42) | 0.67 | 0.84 (0.53 to 1.32) | 0.45 | 0.88 (0.60 to 1.29) | 0.51 |
| Hemoglobin | 1.00 (0.99 to 1.01) | 0.98 | 1.00 (0.99 to 1.02) | 0.58 | 1.00 (0.99 to 1.01) | 0.48 |
| Randomization to aspirin | ||||||
| Randomized to placebo | Ref | 0.16 | Ref | 0.26 | Ref | 0.44 |
| Randomized to aspirin | 0.71 (0.42 to 1.18) | 0.99 (0.58 to 1.68) | 0.93 (0.60 to 1.43) | |||
| Open-label aspirin | 1.19 (0.70 to 2.02) | 1.50 (0.86 to 2.63) | 1.26 (0.78 to 2.03) | |||
| Randomization to fish oil use | 1.00 (0.65 to 1.52) | >0.99 | 0.86 (0.56 to 1.33) | 0.50 | 1.13 (0.78 to 1.63) | 0.53 |
| AVF type | ||||||
| Radiocephalic | Ref | 0.05 | Ref | 0.23 | Ref | 0.04 |
| Brachiocephalic | 0.48 (0.29 to 0.81) | 0.60 (0.35 to 1.02) | 0.71 (0.46 to 1.10) | |||
| Brachiobasilic | 0.95 (0.41 to 2.19) | 0.56 (0.21 to 1.53) | 2.24 (1.07 to 4.72) | |||
| Others | 1.20 (0.20 to 7.15) | 0.66 (0.07 to 5.82) | 0.89 (0.18 to 4.53) | |||
| Surgeons | ||||||
| Registrar/residents | Ref | 0.54 | Ref | 0.10 | Ref | 0.72 |
| Consultant | 0.85 (0.52 to 1.41) | 0.65 (0.40 to 1.08) | 1.08 (0.69 to 1.69) | |||
| Anesthesia | ||||||
| Regional/general anesthesia | Ref | 0.42 | Ref | 0.48 | Ref | 0.80 |
| Local anesthesia | 1.30 (0.69 to 2.45) | 0.78 (0.39 to 1.55) | 1.08 (0.62 to 1.88) | |||
Single imputation for missing values of individual outcome components was performed and described in detail in the primary outcome analysis (30). AVF, arteriovenous fistula; OR, odds ratio; NZ, New Zealand; UK, United Kingdom; Ref, reference; PVD, peripheral vascular disease; CVC, central venous catheter; CCB, calcium channel blocker.
Australia, New Zealand, and United Kingdom region of recruitment was grouped when compared with Malaysian region of recruitment in this analysis.
Discussion
This post hoc analysis of a large, multinational, multicenter, randomized controlled trial demonstrated that AVF failure was independently associated with female sex, lower DBP, presence of CVC, and medically required use of aspirin. Potentially modifiable, surgery-related factors, including surgical expertise, anesthesia, and AVF type, were not associated with AVF failure. Factors associated with individual components of AVF failure differed. Female sex, presence of PVD, and type of AVF created were associated with AVF abandonment, whereas low DBP, presence of CVC, and non-Malaysia region of recruitment were additional risk factors for cannulation failure.
The association between female sex and higher risk of AVF failure has been reported previously (12,19,32,33); however, the biologic process underpinning this observation remains uncertain. Although there is a commonly held belief that females have smaller vessel caliber and, therefore, are at increased risk of AVF failure, a retrospective sonographic evaluation of vessel size in 192 patients did not find consistent differences in vascular caliber between the two sexes (34). In this study, females were two-fold more likely to have their AVF created in their upper limb compared with their male counterparts. This may reflect physicians’ preference for upper-arm AVF in females to avoid inadequate vessel size. Unfortunately, there was no mandatory sonographic assessment of participants’ vessels in the FAVOURED trial to further explore this hypothesis. Although female participants in the FAVOURED study exhibited lower DBP, no significant interaction was observed to account for additional risk attributable to sex. Despite the increased risk of AVF failure in females, careful selection and preparation of female patients considered suitable for AVF surgery with thorough physical examination and preoperative sonographic examination of venous access may further improve outcomes (16,35).
Earlier studies suggested that tight control of systolic, diastolic, and/or mean arterial pressures preoperatively was associated with increased odds of AVF thrombosis (36,37) and early primary AVF failure (38,39). Pandey et al. (39) studied 224 patients prospectively and reported that patients with early AVF failure had lower DBP of 88.4 mm Hg, compared with 91.2 mm Hg in those with AVF success. Similarly, a retrospective review of 1051 patients found patients with early primary failure had lower DBP of 79.7 mm Hg, compared with 83.1 mm Hg in those with AVF success (38). In this study, lower DBP and mean arterial pressure presurgery were associated with an increased risk of AVF failure, with an AVF failure >50% at a DBP of ≤80 mm Hg. Higher pulse pressure, a potential measure of decreased vessel compliance, was observed to be associated with higher odds of AVF failure, but this was not statistically significant. After the creation of AVF, intradialytic hypotension has also been associated with a higher risk of AVF thrombosis (40). Lower DBP may lead to venous stasis, thereby predisposing to early AVF thrombosis, and may also reflect reduced vascular compliance associated with a wider pulse pressure, both impairing vascular remodeling and maturation (41). These findings suggest that maintaining a higher perioperative DBP may be a modifiable factor to improve AVF outcomes. However, prospective, randomized controlled studies are required to establish causality and recommend an optimal BP target, taking into consideration the overall perioperative risk profile and anticipated benefits.
The finding of an increased risk of AVF failure in patients with a CVC in this study is consistent with that reported in the Dialysis Outcomes and Practice Patterns Study (42–44). This increased risk may be related to potential hemodynamic effects and central vein stenosis associated with ipsilateral catheter placement (45). In addition, CVC predispose patients to bloodstream infection and hospitalization (46), which could indirectly predispose to AVF failure via hypotension and the inflammatory response. However, CVC use may be a surrogate of patients referred late to a nephrology service, a known predictor of AVF failure (11,44) or other unadjusted confounders.
Whereas the FAVOURED study demonstrated that neither aspirin nor fish oil use was protective against AVF failure (30), this post hoc analysis did find that patients who were required to continue on open-label aspirin for clinical indications were more likely to experience AVF failure. Although cardiovascular disease, such as ischemic heart disease and PVD, were not associated with AVF outcomes, it is possible that open-label aspirin use identified patients with more severe cardiovascular disease that was not adequately adjusted for in multivariable logistic regression.
Another finding of this study was that different factors were associated with individual components of AVF failure. Whereas female sex was associated with overall poorer AVF outcomes, the presence of PVD was the predominant predictor of AVF abandonment. Participants with PVD may be at increased risk of steal syndrome or identify a population with more diffuse atherosclerosis, which might limit intervention to promote maturation and prevent AVF abandonment. Although the AVF location was not significantly associated with an increased risk of the composite outcome of AVF failure (P=0.06), it was associated with an increased risk of cannulation failure (P=0.04). Specifically, brachiobasilic AVFs were less likely to be successfully cannulated compared with RC AVFs. However, it remains unclear whether this is a statistical type-1 error, given the number of comparisons and small sample size. Alternatively, the observed association may have been due to the fact that brachiobasilic vessels tend to be deeper and technically more challenging to cannulate. It is also possible that other unadjusted factors, including prior history of AVF failure as a predictor of future AVF failure (47,48), maybe driving this association.
Despite selection of a comprehensive list of factors that might predict AVF failure, the overall diagnostic accuracy of the complete model was modest, with an AUC of 0.67. It is possible the predictive ability of the model could be further improved by including other pre- and postoperative measures, such as preoperative arterial and venous vessel size and adherence with vascular access surveillance and management protocols, which were not investigated in this study (16,20). Sonographic evaluation of artery and venous caliber has been associated with better selection of vessels suitable for AVF creation (12,13,15,16). Although postoperative care, such as dedicated protocolized surveillance to detect early AVF dysfunction (20), has yielded mixed results with respect to preventing AVF failure in studies, it is possible this may improve model prediction in this study. Whereas prediction models for AVF success are helpful, their accuracy is limited by the complexity of the process to establish a successful AVF. In particular, some practice-related factors of interest, such as surgical and cannulation expertise and techniques, are difficult to standardize and assess consistently across different studies and settings, thereby limiting the accuracy of prediction models. Beyond having a successful AVF, there should also be consideration of alternatives to AVF such as arteriovenous graft (49); alternative dialysis, such as peritoneal dialysis and even long-term tunneled dialysis catheter, depending on patient’s preferences; prior access history; expected life expectancy; anticipated dialysis start; and course of kidney replacement (3).
The strengths of this study included the use of a well-characterized study population from a randomized controlled trial, ensuring standardized and accurate collection of patient and surgical variables. A systematic and methodic approach was also taken in the analysis. However, several limitations exist, including the limited number of participants and events, insufficient granularity of data (such as severity of comorbidities, location of the CVC in relation to the AVF, history of previous failed vascular access, indication of AVF abandonment, or standardized preoperative sonographic assessment of vessel size and quality), lack of standardized BP assessment, and missing data that potentially introduced informative censoring bias. Furthermore, country-specific variation in practice patterns and expertise (e.g., surgical expertise measured as number of AVF creations as compared with phase of surgical training in this study or cannulation skills) were unavailable to better determine potentially modifiable predictors of AVF failure. This hypothesis is supported by the observation of lower risk of AVF failure among participants recruited in Malaysia compared with ANZ on univariable logistic regression, which was no longer evident when adjusting for surgical factors in model 2. Lastly, the trial population was younger with less PVD compared with the general hemodialysis population, which could limit the generalizability of the result.
Failure of newly created AVFs is a major barrier to the successful establishment of hemodialysis access and occurred in almost 50% of study participants. Female sex, low DBP, the need for aspirin therapy, and CVC dependence were associated with an increased risk of AVF failure. Avoidance of perioperative hypotension and CVCs at the time of AVF creation may help improve AVF outcomes and warrants further study.
Disclosures
A. Cass, C. Hawley, A. Irish, P. Kerr, T. Mori, E. Pascoe, P. Paul-Brent, and K. Polkinghorne report having received grants from Amgen Australia Pty Ltd., grants from Mylan EPD (at the time of funding it was known as Abbott Products Operations AG), and grant support from the National Health and Medical Research Council (NHMRC) of Australia project grant. Y. Cho has previously received research grants from Baxter Healthcare and Fresenius Medical Care, and is a current recipient of a NHMRC Early Career Fellowship. C. Hawley has previously received research grants from Baxter Healthcare and Fresenius Medical Care. D. Johnson has previously received consultancy fees, research grants, speaker’s honoraria, and travel sponsorships from Baxter Healthcare and Fresenius Medical Care. He has received consultancy fees from AstraZeneca and AWAK, and travel sponsorship from Amgen. He is also the current recipient of a NHMRC Practitioner Fellowship. T. Mori is supported by a research fellowship from the NHMRC of Australia (1042255). A. Viecelli reports having received grant support from the NHMRC of Australia (Medical Postgraduate Scholarship) and the Royal Australasian College of Physicians (Jacquot NHMRC Medical Award for Excellence and Jacquot Research Establishment Fellowship). The original conduct of the FAVOURED trial was funded by grants from the NHMRC of Australia Project Grant APP458652, Amgen Australia Pty Ltd., and Abbott Products Operations AG (now Mylan EPD). Study medication was supplied by Abbott Products Operations AG (now Mylan EPD) (fish oil and placebo) and Bayer Healthcare (aspirin and placebo) free of charge. All remaining authors have nothing to disclose.
Funding
None.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0002732020/-/DCSupplemental.
Omega 3 Fatty Acids (Fish Oils) and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED) Study Collaborative Group. Download Supplemental Appendix, PDF file, 44 KB (43.8KB, pdf)
Cannulation assessment periods. Download Supplemental Table 1, PDF file, 116 KB (116KB, pdf)
Baseline BP profile according to gender. Download Supplemental Table 2, PDF file, 116 KB (116KB, pdf)
Site of AVF created according to gender. Download Supplemental Table 3, PDF file, 116 KB (116KB, pdf)
Proportion of arteriovenous fistula failure (composite outcome of arteriovenous fistula abandonment, thrombosis and failure to cannulate) with change in mean arterial pressure. Download Supplemental Figure 1, PDF file, 116 KB (116KB, pdf)
Proportion of arteriovenous fistula failure (composite outcome of arteriovenous fistula abandonment, thrombosis and failure to cannulate) with change in systolic BP. Download Supplemental Figure 2, PDF file, 116 KB (116KB, pdf)
Proportion of arteriovenous fistula failure (composite outcome of arteriovenous fistula abandonment, thrombosis and failure to cannulate) with change in pulse pressure. Download Supplemental Figure 3, PDF file, 116 KB (116KB, pdf)
Acknowledgments
The authors gratefully acknowledge the contributions of all members of the FAVOURED Study Collaborative Group, dialysis nursing staff, trial coordinators, research staff, and most especially trial participants.
Portions of this manuscript were presented as a moderated poster at the European Renal Association–European Dialysis and Transplant Association conference 2020.
The drug manufacturer and the NHMRC of Australia had no role in study design, collection, analysis, interpretation of data, writing the report, or the decision to submit the report for publication.
The authors listed on the first page of this article constitute the FAVOURED trial writing committee.
The complete list of contributing institutions is included as a Supplemental Appendix.
Author Contributions
Y. Cho, C. Hawley, D. Johnson, E. Pascoe, Y. See, and A. Viecelli wrote the original draft; Y. Cho, C. Hawley, D. Johnson, E. Pascoe, and A. Viecelli provided supervision; Y. Cho, C. Hawley, E. Pascoe, Y. See, and A. Viecelli were responsible for formal analysis; C. Hawley, D. Johnson, E. Pascoe, Y. See, and A. Viecelli were responsible for methodology; C. Hawley, D. Johnson, and A. Viecelli conceptualized the study; E. Pascoe, P. Paul-Brent, and A. Viecelli were responsible for data curation; P. Paul-Brent, Y. See, and A. Viecelli were responsible for project administration; A. Viecelli was responsible for investigation; and all authors reviewed and edited the manuscript.
References
- 1.Polkinghorne KR, Chin GK, MacGinley RJ, Owen AR, Russell C, Talaulikar GS, Vale E, Lopez-Vargas PA: KHA-CARI Guideline: Vascular access - central venous catheters, arteriovenous fistulae and arteriovenous grafts. Nephrology (Carlton) 18: 701–705, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG on vascular access. Nephrol Dial Transplant 22[Suppl 2]: ii88-ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation-Kidney Dialysis Outcome Quality Initiative clinical practice guidelines for vascular access: 2019 Update. National kidney foundation-dialysis outcomes quality initiative. Am J Kidney Dis 75[Suppl 2]: S51–S164, 2019 [Google Scholar]
- 4.Lok CE: Fistula first initiative: Advantages and pitfalls. Clin J Am Soc Nephrol 2: 1043–1053, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Malas MB, Canner JK, Hicks CW, Arhuidese IJ, Zarkowsky DS, Qazi U, Schneider EB, Black JH 3rd, Segev DL, Freischlag JA: Trends in incident hemodialysis access and mortality. JAMA Surg 150: 441–448, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Feldman HI, Kobrin S, Wasserstein A: Hemodialysis vascular access morbidity. J Am Soc Nephrol 7: 523–535, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, Kosa SD, Quinn RR, Moist LM: Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 63: 464–478, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Allon M, Robbin ML: Increasing arteriovenous fistulas in hemodialysis patients: Problems and solutions. Kidney Int 62: 1109–1124, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Viecelli AK, Howell M, Tong A, Teixeira-Pinto A, O’Lone E, Ju A, Craig JC, Hooi L-S, Lee T, Lok CE, Polkinghorne KR, Quinn RR, Vacchharajani TJ, Vanholder R, Zio L, Tordoir J, Pecoits-filho R, You T, Kopperschmidt P, Smith R, Irish AB, Mori TA, Pascoe EM, Johnson DW, Hawley CM: Identifying critically important vascular access outcomes for trials in haemodialysis: An international survey with patients, caregivers and health professionals. Nephrol Dial Transplant 35: 657–668, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Lilly MP, Lynch JR, Wish JB, Huff ED, Chen SC, Armistead NC, McClellan WM: Prevalence of arteriovenous fistulas in incident hemodialysis patients: Correlation with patient factors that may be associated with maturation failure. Am J Kidney Dis 59: 541–549, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Ravani P, Marcelli D, Malberti F: Vascular access surgery managed by renal physicians: The choice of native arteriovenous fistulas for hemodialysis. Am J Kidney Dis 40: 1264–1276, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Jamil M, Usman R: Predictive parameters for successful functional maturation of native arteriovenous fistula. J Ayub Med Coll Abbottabad 27: 821–824, 2015 [PubMed] [Google Scholar]
- 13.Lockhart ME, Robbin ML, Allon M: Preoperative sonographic radial artery evaluation and correlation with subsequent radiocephalic fistula outcome. J Ultrasound Med 23: 161–168; quiz 169–171, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Lauvao LS, Ihnat DM, Goshima KR, Chavez L, Gruessner AC, Mills JL Sr.: Vein diameter is the major predictor of fistula maturation. J Vasc Surg 49: 1499–1504, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Achneck HE, Sileshi B, Li M, Partington EJ, Peterson DA, Lawson JH: Surgical aspects and biological considerations of arteriovenous fistula placement. Semin Dial 23: 25–33, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Allon M, Lockhart ME, Lilly RZ, Gallichio MH, Young CJ, Barker J, Deierhoi MH, Robbin ML: Effect of preoperative sonographic mapping on vascular access outcomes in hemodialysis patients. Kidney Int 60: 2013–2020, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Zouaghi MK, Lammouchi MA, Hassan M, Rais L, Krid M, Smaoui W, Jebali H, Kheder R, Hamida FB, Moussa FB, Fatma LB, Beji S: Determinants of patency of arteriovenous fistula in hemodialysis patients. Saudi J Kidney Dis Transpl 29: 615–622, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Polkinghorne KR, McDonald SP, Marshall MR, Atkins RC, Kerr PG: Vascular access practice patterns in the New Zealand hemodialysis population. Am J Kidney Dis 43: 696–704, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Lazarides MK, Iatrou CE, Karanikas ID, Kaperonis NM, Petras DI, Zirogiannis PN, Dayantas JN: Factors affecting the lifespan of autologous and synthetic arteriovenous access routes for haemodialysis. Eur J Surg 162: 297–301, 1996 [PubMed] [Google Scholar]
- 20.Allon M, Imrey PB, Cheung AK, Radeva M, Alpers CE, Beck GJ, Dember LM, Farber A, Greene T, Himmelfarb J, Huber TS, Kaufman JS, Kusek JW, Roy-Chaudhury P, Robbin ML, Vazquez MA, Feldman HI; Hemodialysis Fistula Maturation (HFM) Study Group : Relationships between clinical processes and arteriovenous fistula cannulation and maturation: A multicenter prospective cohort study. Am J Kidney Dis 71: 677–689, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aitken E, Jackson A, Kearns R, Steven M, Kinsella J, Clancy M, Macfarlane A: Effect of regional versus local anaesthesia on outcome after arteriovenous fistula creation: A randomised controlled trial. Lancet 388: 1067–1074, 2016 [DOI] [PubMed] [Google Scholar]
- 22.van der Linden J, Lameris TW, van den Meiracker AH, de Smet AA, Blankestijn PJ, van den Dorpel MA: Forearm venous distensibility predicts successful arteriovenous fistula. Am J Kidney Dis 47: 1013–1019, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Usta E, Elkrinawi R, Salehi-Gilani S, Adili S, Sonnentag T, Alscher M, Artunc F, Franke U: Risk factors predicting the successful function and use of autogenous arteriovenous fistulae for hemodialysis. Thorac Cardiovasc Surg 61: 438–444, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Smith GE, Souroullos P, Cayton T, Harwood A, Carradice D, Chetter IC: A systematic review and meta-analysis of systemic intraoperative anticoagulation during arteriovenous access formation for dialysis. J Vasc Access 17: 1–5, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Regus S, Almási-Sperling V, Rother U, Meyer A, Lang W: Surgeon experience affects outcome of forearm arteriovenous fistulae more than outcomes of upper-arm fistulae. J Vasc Access 18: 120–125, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Viecelli AK, O’Lone E, Sautenet B, Craig JC, Tong A, Chemla E, Hooi LS, Lee T, Lok C, Polkinghorne KR, Quinn RR, Vachharajani T, Vanholder R, Zuo L, Irish AB, Mori TA, Pascoe EM, Johnson DW, Hawley CM: Vascular access outcomes reported in maintenance hemodialysis trials: A systematic review. Am J Kidney Dis 71: 382–391, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Bylsma LC, Gage SM, Reichert H, Dahl SLM, Lawson JH: Arteriovenous fistulae for haemodialysis: A systematic review and meta-analysis of efficacy and safety outcomes. Eur J Vasc Endovasc Surg 54: 513–522, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Viecelli AK, Pascoe E, Polkinghorne KR, Hawley CM, Paul-Brent P-A, Badve SA, Cass A, Heritier S, Kerr PG, Mori TA, Robertson A, Hooi L-S, Irish AB; FAVOURED study team : The omega-3 fatty acids (fish oils) and aspirin in vascular access outcomes in renal disease (FAVOURED) study: The updated final trial protocol and rationale of post-initiation trial modifications. BMC Nephrol 16: 89, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irish AB, Viecelli AK, Hawley CM, Hooi LS, Pascoe EM, Paul-Brent PA, Badve SV, Mori TA, Cass A, Kerr PG, Voss D, Ong LM, Polkinghorne KR; Omega-3 Fatty Acids (Fish Oils) and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED) Study Collaborative Group : Effect of fish oil supplementation and aspirin use on arteriovenous fistula failure in patients requiring hemodialysis: A randomized clinical trial. JAMA Intern Med 177: 184–193, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Yan Y, Ye D, Yang L, Ye W, Zhan D, Zhang L, Xiao J, Zeng Y, Chen Q: A meta-analysis of the association between diabetic patients and AVF failure in dialysis. Ren Fail 40: 379–383, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masengu A, Maxwell AP, Hanko JB: Investigating clinical predictors of arteriovenous fistula functional patency in a European cohort. Clin Kidney J 9: 142–147, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee T, Qian J, Thamer M, Allon M: Gender disparities in vascular access surgical outcomes in elderly hemodialysis patients. Am J Nephrol 49: 11–19, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplin N, Sedlacek M, Teodorescu V, Falk A, Uribarri J: Venous access: Women are equal. Am J Kidney Dis 41: 429–432, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Okamuro L, Gray K, Korn A, Parrish A, Kaji A, Howell EC, Bowens N, de Virgilio C: Careful patient selection achieves high radiocephalic arteriovenous fistula patency in diabetic and female patients. Ann Vasc Surg 57: 16–21, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Irvinn J, Oldman N, Sedgwick P, Chemla E: Do blood pressure levels and other patient characteristics influence native fistula patency? Semin Dial 27: E27–E31, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Culp K, Flanigan M, Taylor L, Rothstein M: Vascular access thrombosis in new hemodialysis patients. Am J Kidney Dis 26: 341–346, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Yan Y, Su X, Zheng J, Zhang L, Yang L, Jiang Q, Chen Q: Association of preoperative mean arterial pressure with the primary failure of brescia-cimino arteriovenous fistula within the first 7 days following surgery in hemodialysis patients. Ther Apher Dial 22: 539–543, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Pandey S, Kumar M, Agrawal M, Singh M, Aggarwal A, Garg G, Agarwal S, Sankhwar S: The effects of preoperative blood pressure on early failure rate of distal arteriovenous fistulas for hemodialysis access. Hemodial Int 23: 314–318, 2019 [DOI] [PubMed] [Google Scholar]
- 40.Chang TI, Paik J, Greene T, Desai M, Bech F, Cheung AK, Chertow GM: Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol 22: 1526–1533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavish B, Izzo JL Jr.: Arterial stiffness: Going a step beyond. Am J Hypertens 29: 1223–1233, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Rayner HC, Pisoni RL, Gillespie BW, Goodkin DA, Akiba T, Akizawa T, Saito A, Young EW, Port FK; Dialysis Outcomes and Practice Patterns Study : Creation, cannulation and survival of arteriovenous fistulae: Data from the dialysis outcomes and practice patterns study. Kidney Int 63: 323–330, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Rayner HC, Besarab A, Brown WW, Disney A, Saito A, Pisoni RL: Vascular access results from the Dialysis Outcomes and Practice Patterns Study (DOPPS): Performance against Kidney Disease Outcomes Quality Initiative (K/DOQI) clinical practice guidelines. Am J Kidney Dis 44[Suppl 2]: 22–26, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, Wolfe RA, Goodkin DA, Held PJ: Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int 61: 305–316, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Ozpak B, Yilmaz Y: Arteriovenous fistulas ipsilateral to internal jugular catheters for hemodialysis have decreased patency rates. Vascular 27: 270–276, 2019 [DOI] [PubMed] [Google Scholar]
- 46.Ng LJ, Chen F, Pisoni RL, Krishnan M, Mapes D, Keen M, Bradbury BD: Hospitalization risks related to vascular access type among incident US hemodialysis patients. Nephrol Dial Transplant 26: 3659–3666, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Lee T, Ullah A, Allon M, Succop P, El-Khatib M, Munda R, Roy-Chaudhury P: Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol 6: 575–581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gibson KD, Gillen DL, Caps MT, Kohler TR, Sherrard DJ, Stehman-Breen CO: Vascular access survival and incidence of revisions: A comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg 34: 694–700, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Woo K, Ulloa J, Allon M, Carsten CG 3rd, Chemla ES, Henry ML, Huber TS, Lawson JH, Lok EC, Peden EK, Scher L, Sidawy A, Maggard-Gibbons M, Cull D: Establishing patient-specific criteria for selecting the optimal upper extremity vascular access procedure. J Vasc Surg 65: 1089–1103.e1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Omega 3 Fatty Acids (Fish Oils) and Aspirin in Vascular Access Outcomes in Renal Disease (FAVOURED) Study Collaborative Group. Download Supplemental Appendix, PDF file, 44 KB (43.8KB, pdf)
Cannulation assessment periods. Download Supplemental Table 1, PDF file, 116 KB (116KB, pdf)
Baseline BP profile according to gender. Download Supplemental Table 2, PDF file, 116 KB (116KB, pdf)
Site of AVF created according to gender. Download Supplemental Table 3, PDF file, 116 KB (116KB, pdf)
Proportion of arteriovenous fistula failure (composite outcome of arteriovenous fistula abandonment, thrombosis and failure to cannulate) with change in mean arterial pressure. Download Supplemental Figure 1, PDF file, 116 KB (116KB, pdf)
Proportion of arteriovenous fistula failure (composite outcome of arteriovenous fistula abandonment, thrombosis and failure to cannulate) with change in systolic BP. Download Supplemental Figure 2, PDF file, 116 KB (116KB, pdf)
Proportion of arteriovenous fistula failure (composite outcome of arteriovenous fistula abandonment, thrombosis and failure to cannulate) with change in pulse pressure. Download Supplemental Figure 3, PDF file, 116 KB (116KB, pdf)