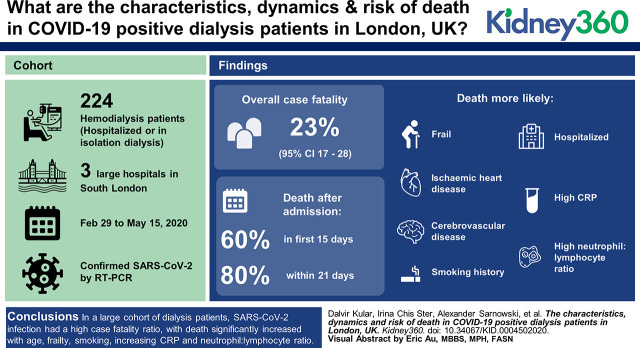
Visual Abstract
Keywords: clinical nephrology, COVID-19, frailty, hemodialysis, London, mortality, mortality risk, peritoneal dialysis, United Kingdom
Abstract
Background
Patients on dialysis with frequent comorbidities, advanced age, and frailty, who visit treatment facilities frequently, are perhaps more prone to SARS-CoV-2 infection and related death—the risk factors and dynamics of which are unknown. The aim of this study was to investigate the hospital outcomes in patients on dialysis infected with SARS-CoV-2.
Methods
Data on 224 patients on hemodialysis between February 29, 2020 and May 15, 2020 with confirmed SARS-CoV-2 were analyzed for outcomes and potential risk factors for death, using a competing risk-regression model assessed by subdistribution hazards ratio (SHR).
Results
Crude data analyses suggest an overall case-fatality ratio of 23% (95% CI, 17% to 28%) overall, but that varies across age groups from 11% (95% CI, 0.9% to 9.2%) in patients ≤50 years old and 32% (95% CI, 17% to 48%) in patients >80 years; with 60% of deaths occurring in the first 15 days and 80% within 21 days, indicating a rapid deterioration toward death after admission. Almost 90% of surviving patients were discharged within 28 days. Death was more likely than hospital discharge in patients who were more frail (WHO performance status, 3–4; SHR, 2.16 [95% CI, 1.25 to 3.74]; P=0.006), had ischemic heart disease (SHR, 2.28 [95% CI, 1.32 to 3.94]; P=0.003), cerebrovascular disease (SHR, 2.11 [95% CI, 1.20 to 3.72]; P=0.01), smoking history (SHR, 2.69 [95% CI, 1.33 to 5.45]; P=0.006), patients who were hospitalized (SHR, 10.26 [95% CI, 3.10 to 33.94]; P<0.001), and patients with high CRP (SHR, 1.35 [95% CI, 1.10 to 1.67]) and a high neutrophil:lymphocyte ratio (SHR, 1.03 [95% CI, 1.01 to 1.04], P<0.001). Our data did not support differences in the risk of death associated with sex, ethnicity, dialysis vintage, or other comorbidities. However, comparison with the entire dialysis population attending these hospitals, in which 13% were affected, revealed that patients who were non-White (62% versus 52% in all patients, P=0.001) and those with diabetes (54% versus 22%, P<0.001) were disproportionately affected.
Conclusions
This report discusses the outcomes of a large cohort of patients on dialysis. We found SARS-CoV-2 infection affected more patients with diabetes and those who were non-White, with a high case-fatality ratio, which increased significantly with age, frailty, smoking, increasing CRP, and neutrophil:lymphocyte ratio at presentation.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is similar to the viruses responsible for the SARS and Middle East respiratory syndrome epidemics in 2003 and 2013 (1). It is highly transmissible between humans and can spread easily in dialysis units, where patients are in close contact with each other and their healthcare workers at frequent and regular intervals. The populations of patients on dialysis include a high representation of individuals who are elderly, have comorbidities, and are frail (2). In addition, they may also be more susceptible to infections, due to abnormal monocyte and T lymphocyte responses (3). The Middle East respiratory syndrome epidemic demonstrated the importance of T cell immunity in fighting SARS-CoV-1 infection, and the same may be relevant for SARS-CoV-2 infection (4).
Measures to protect patients on hemodialysis (HD) have been recommended, including strict protocols for the screening, isolation, de-isolation, and management of patients within dialysis facilities (5–7). There are few reports of outcomes of coronavirus disease 2019 (COVID-19) in patients on dialysis. The case-fatality of patients on HD who are positive for COVID-19 in three HD centers in Wuhan varied between 0% and 16% (C. Li, M. Yonglong, T. Can, M. Dongdong, W. Sheng, L. Haifeng, X. Fei: An analysis on the clinical features of MHD patients with coronavirus disease 2019: A single center study. Research Square, 2020, 10.21203/rs.3.rs-18043/v1; Y. Ma, B. Diao, X. Lv, J. Zhu, W. Liang, L. Liu, W. Bu, H. Cheng, S. Zhang, L. Yang, M. Shi, G. Ding, B. Shen, H. Wang: 2019 novel coronavirus disease in hemodialysis [HD] patients: Report from one HD center in Wuhan, China. Medrxiv, 2020, 10.1101/2020.02.24.20027201v3) (8). In one HD facility in Northern Italy, the case-fatality ratio was as high as 44% (18 out of 41 patients on HD with COVID-19) from a cohort of 98 patients on HD (9). Another hospital in Brescia, Italy admitted 21 patients positive for COVID-19; five (24%) of the patients died and four were discharged from hospital (10). The same unit reported 94 patients, of whom 61% required hospital admission and 29% died (11). In a study from the United States of 59 patients, 31% died, which is very similar to a study from Spain in which 30% of 36 patients died (12,13).
The aim of this observational study was to examine variables that may be associated with risk of death in patients on HD positive for COVID-19 at three large National Health Service (NHS) hospitals in South London, during the start of the epidemic until May 15, 2020. We also present the daily incidence of COVID-19 and death in this patient cohort as well as the age-dependent, case-fatality ratio.
Materials and Methods
Participant Identification
Patients on dialysis were tested for SARS-CoV-2, by nasal and throat swab for real-time RT-PCR (RdRp gene) testing, if they were symptomatic with a persistent cough and/or fever, in accordance with guidance from Public Health England (14).
Data Collection
Data were collected for patients on dialysis infected with SARS-CoV-2 who were admitted to hospitals or isolation HD facilities across three South London NHS renal centers between February 29, 2020 and May 15, 2020. Collected data included demographics, comorbidities, World Health Organization (WHO) performance status, clinical symptoms, laboratory parameters at presentation, hospital management, and outcomes. Data were sourced from electronic clinical databases, including laboratory systems, clinical notes, and written communications. Aggregate comparative data were obtained from the UK Renal Registry. Baseline laboratory results were from the day of presentation or within 24 hours. The performance status was determined on the basis of clinical data on the patients’ usual mobility, exercise tolerance, frailty, and required assistance. WHO performance status is a simple tool for assessment of functional status and frailty, and it is used mostly in oncology for prognostication and to identify patients suitable for treatment (15,16). It estimates the patient’s daily activity and ability to perform activities of daily living using a progressive score from zero to five, where zero indicates a patient who is completely active, three for a patient capable of only limited self-care, and a value of five indicates death. Given the sample size of our data, a binary variable on the basis of WHO performance was created on the basis of disease severity, i.e., zero to two indicates less severe and three to four indicates severe frailty.
We also pulled aggregated statistics regarding the background populations, i.e., that of HD and peritoneal dialysis (PD) across the three hospitals. The data have been used to assess the characteristics distributions of our sample of patients positive for COVID-19 against those in the corresponding populations. The study was approved by NHS Research Ethics Committee 20/SW/0077 and Health Research Authority Integrated Research Application System 283130.
Statistical Methods
All the available variables have been graphically explored and summarized according to their nature, i.e., means, SDs, medians, interquartile limits, and ranges for continuous variables, and proportions for those that were categoric or binary. Log transformation has been performed for highly skewed variables, where appropriate. Daily time series of admissions and deaths (counts) are displayed graphically in Figure 1.
Figure 1.
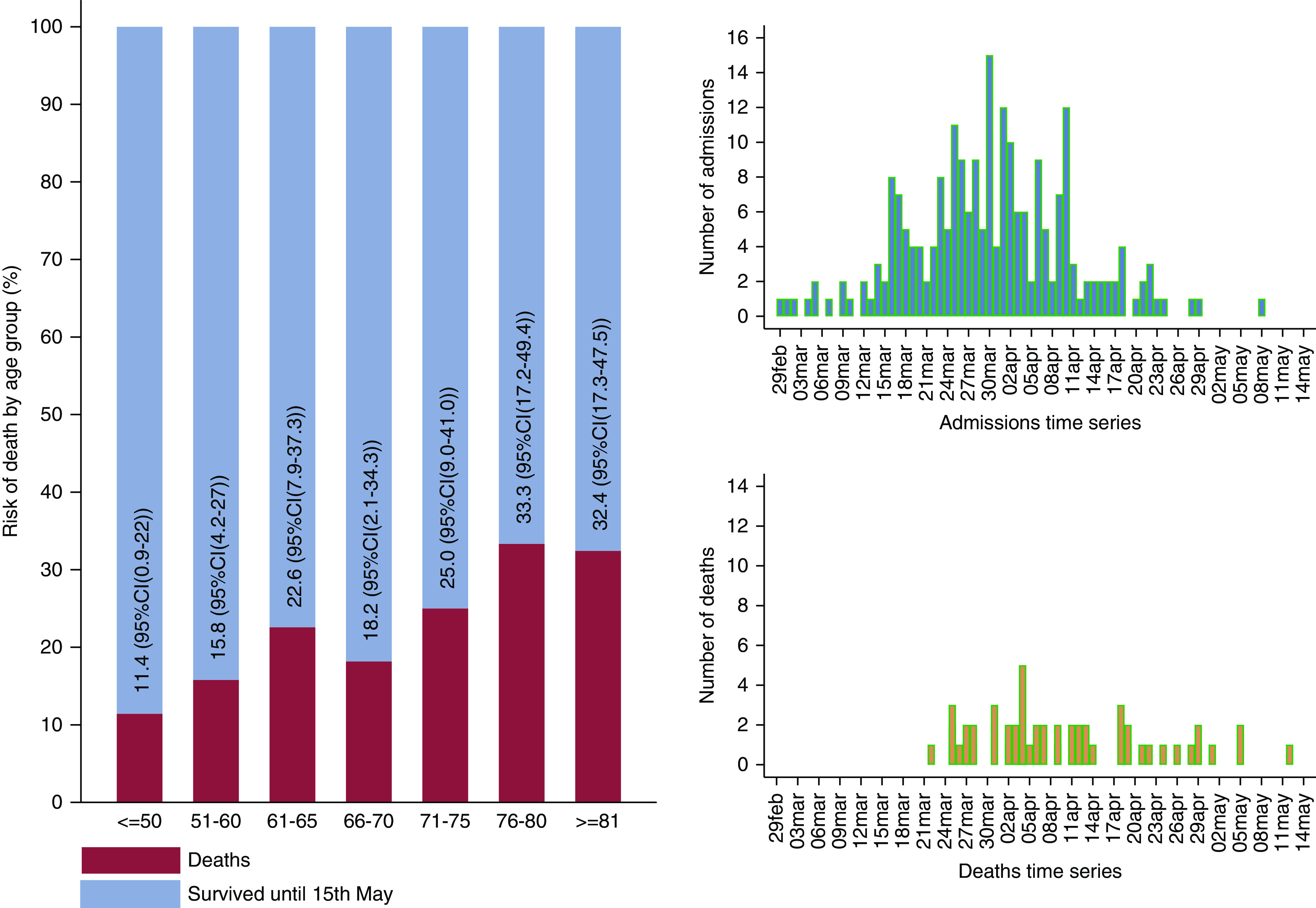
The age-dependent case-fatality ratio and the daily time series of hospital admissions and deaths in patients on hemodialysis who had coronavirus disease 2019 (COVID-19).
A binary statistical outcome was defined to indicate death or discharged alive before May 15, 2020; those still under care on that date were set as censored. The analysis modeled the time since admission to discharge from care (hospital or isolation dialysis facility), or death during care (hospital or outpatient), using the Fine and Gray method for competing risk. Death is the primary statistical event of interest, and hospital discharge is assumed to be a competing event. A subdistribution hazard ratio (SHR) model has been fit to the data to account for the censored patients and to quantify the effects of each available variable on the risk of death through SHR (17–21). Predicted cumulative incidence functions are similar to the cumulative distribution functions in classic survival analysis and indicate the daily cumulative rate of death or discharge since admission in association with each potential explanatory variable. We have also built a multivariable model on the basis of the Akaike information criterion (AIC; the smaller the value the better the model), which is used on a similar number of observations in the data. Sensitivity analyses on missing data have been conducted; these results are not shown or discussed, except for smoking variables, because all others did not alter the qualitative or quantitative conclusions made on the basis of complete data. This approach is different from that of a cause-specific hazard—details on these differences have been thoroughly discussed elsewhere (18). An SHR value >1 indicates a harmful effect of the corresponding explanatory variable, <1 indicates a protective effect. Also, a steep increase in the cumulative incidence functions with time since admission corresponding to death indicates a rapid deterioration in patients who died. A P value <0.05 is interpreted as a statistically significant association. Comparisons with the UK Renal Registry COVID-19 population data for patients on dialysis have been made using elementary statistical tests according to the nature of the variables. Meta-analyses estimating pooled case-fatality ratios in the HD population from recent published studies around the world are also presented (Figure 2). All analyses have been carried out in Stata 16 (StataCorp. 2019, Stata Statistical Software: Release 16; StataCorp LLC, College Station, TX).
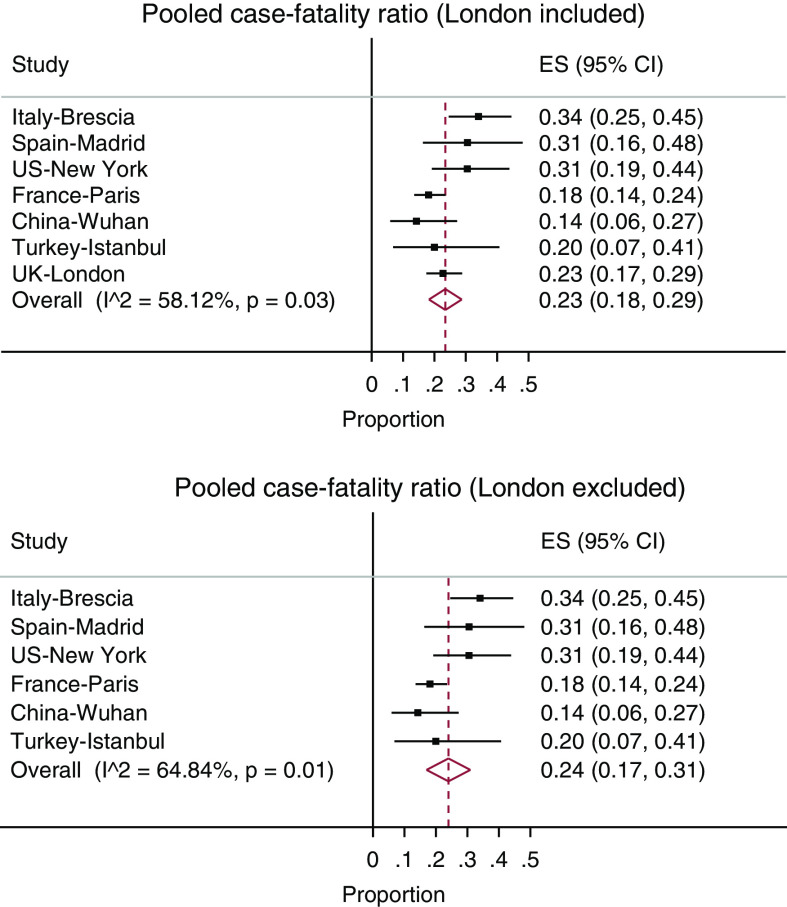
Figure 2.
Meta-analyses for the pooled case-fatality ratio (denoted by estimate [ES]) on the basis of existing research, with and without current London study (11–13,23–25).
Results
Demographics and Clinical Characteristics of Patients on HD Infected with SARS-Cov-2
Data on 224 patients on HD, with confirmed SARS-CoV-2, from three large South London NHS renal centers, admitted to hospital or isolation facility between February 29 and May 15, 2020 have been collated and analyzed to explore potential risk factors for death. Descriptive statistics for this population, survival status until May 15, and associations with SHR of death versus survival are presented in Table 1. Within this cohort of patients, 51 (23%) died, 154 (69%) were discharged alive, and 19 (9%) were still under care in hospital or an isolation facility when we stopped the data collection (censored). The first hospital admission was on February 29, and the daily time series of admissions showed a steady increase until the peak between March 30 and April 2, followed by a decline in admissions (Figure 1). The first death occurred on March 22, 2020.
Table 1.
Demographic, clinical characteristics, and hospital management features of all 224 patients positive for SARS-CoV-2 from the St George’s, King’s, and St Helier hospitals in London, collected between February 29 and May 15
| Variables | Summary Type/Category | All Patients n (% by category) | Discharged Alive n (% by category) | Died n (% by category) | Still in Care n (% by category) | Subdistribution Hazard Ratio | Adjusted Subdistribution Hazard Ratio (210 obs. [94%]) | |||
| Died versus Survived | P Value | No. | Died versus Survived | P Value | ||||||
| Total | 224 | 154 (69%) | 51 (23%) | 19 (8%) | ||||||
| Demographics | ||||||||||
| Sex | Male | 133 (59%) | 89 (58%) | 35 (69%) | 9 (47%) | 1 | ||||
| Female | 91 (41%) | 65 (42%) | 16 (31%) | 10 (53%) | 0.66 (0.36–1.20) | 0.17 | 220 | |||
| Ethnicity | ||||||||||
| Binary | White | 85 (38%) | 54 (35%) | 21 (41%) | 10 (53%) | |||||
| Other | 139 (62%) | 100 (65%) | 30 (59%) | 9 (47%) | 0.85 (0.49–1.48) | 0.57 | 220 | |||
| Detailed | White | 85 (38%) | 54 (35%) | 21 (41%) | 10 (53%) | |||||
| South Asian | 41 (18%) | 27 (18%) | 11 (22%) | 3 (16%) | ||||||
| East Asian | 8 (4%) | 5 (3%) | 3 (6%) | 0 (0%) | ||||||
| Black | 77 (34%) | 59 (38%) | 13 (25%) | 5 (26%) | ||||||
| Other | 13 (6%) | 9 (6%) | 3 (6%) | 1 (5%) | ||||||
| Smoking history | Never | 94 (42%) | 71 (46%) | 12 (24%) | 11 (58%) | 1 | ||||
| Some | 71 (32%) | 43 (28%) | 22 (43%) | 6 (32%) | 2.69 (1.33–5.45) | 0.006a | 161 | |||
| Missing | 59 (26%) | 40 (26%) | 17 (33%) | 2 (11%) | ||||||
| Missing=NO | Never | 153 (68%) | 111 (72%) | 29 (57%) | 13 (68%) | 1 | ||||
| Some | 71 (32%) | 43 (28%) | 22 (43%) | 6 (32%) | 1.78 (1.03–3.08) | 0.04a | 220 | |||
| Missing=YES | Never | 94 (42%) | 71 (46%) | 12 (24%) | 11 (58%) | 1 | ||||
| Some | 130 (58%) | 83 (54%) | 39 (76%) | 8 (42%) | 2.57 (1.34–4.93) | 0.005a | 220 | |||
| Age at admission (5-yr effect) | Mean (SD) | 65.83 (14.39) | 63.90 (14.43) | 70.47 (13.79) | 69 (12.77) | 1.16 (1.03–1.30) | 0.01a | 220 | 1.15 (1.002–1.31) | 0.05a |
| Median (Q1–Q3) | 67.5 (57–77) | 65 (57–76) | 73 (62–80) | 73 (59–81) | ||||||
| Range | 25–90 | 26–90 | 25–90 | 38–82 | ||||||
| BMI (kg/m2) | Mean (SD) | 28.2 (7.6) | 28.5 (8) | 26.9 (6.3) | 28.9 (7.9) | 0.97 (0.93–1.01) | 0.19 | 193 | ||
| Median (Q1–Q3) | 26.3 (23.1–31.2) | 26.2 (23.2–31.4) | 26.1 (21.7–30.0) | 26.6 (16.5–42) | ||||||
| Range | 16.5–57.8 | 18.7–57.8 | 17.4–49.2 | 16.5–42.1 | ||||||
| Missing | 27 (12%) | 17 (11%) | 8 (16%) | 2 (11%) | ||||||
| Comorbidities | ||||||||||
| WHO performance status | ||||||||||
| Detailed | 0 | 16 (7%) | 15 (10%) | 1 (2%) | 0 (0%) | |||||
| 1 | 51 (23%) | 42 (27%) | 6 (12%) | 3 (16%) | ||||||
| 2 | 67 (30%) | 43 (28%) | 16 (31%) | 8 (42%) | ||||||
| 3 | 54 (24%) | 34 (22%) | 15 (29%) | 5 (26%) | ||||||
| 4 | 29 (13%) | 13 (8%) | 13 (25%) | 3 (16%) | ||||||
| Missing | 7 (3%) | 7 (5%) | 0 (0%) | 0 (0%) | ||||||
| Binary | 0–2 | 134 (60%) | 100 (65%) | 23 (45%) | 11 (58%) | |||||
| 3–4 | 83 (37%) | 47 (31%) | 28 (55%) | 8 (42%) | 2.16 (1.25–3.74) | 0.006a | 213 | |||
| Missing | 7 (3%) | 7 (5%) | 0 (0%) | 0 (0%) | ||||||
| History of cancer | No | 189 (84%) | 132 (86%) | 42 (82%) | 15 (79%) | |||||
| Yes | 33 (15%) | 20 (13%) | 9 (18%) | 4 (21%) | 1.25 (0.613–2.57) | 0.54 | 218 | |||
| Missing | 2 (0.89%) | 2 (1%) | 0 (0%) | 0 (0%) | ||||||
| Hypertension | No | 41 (18%) | 24 (16%) | 8 (16%) | 9 (47%) | |||||
| Yes | 182 (81%) | 129 (84%) | 43 (84%) | 10 (53%) | 1.27 (0.61–2.66) | 0.53 | 219 | |||
| Missing | 1 (0.45%) | 1 (0.65%) | 0 (0%) | 0 (0%) | ||||||
| Diabetes | No | 103 (46%) | 71 (46%) | 21 (41%) | 11 (58%) | |||||
| Yes | 120 (54%) | 82 (53%) | 30 (59%) | 8 (42%) | 1.31 (0.76–2.28) | 0.34 | 219 | |||
| Missing | 1 (0.45%) | 1 (0.65%) | 0 (0%) | 0 (0%) | ||||||
| HFrEF | No | 181 (81%) | 124 (81%) | 40 (78%) | 17 (89%) | |||||
| Yes | 40 (18%) | 27 (18%) | 11 (22%) | 2 (11%) | 1.38 (0.69–2.73) | 0.36 | 217 | |||
| Missing | 3 (1%) | 3 (2%) | 0 (0%) | 0 (0%) | ||||||
| Chronic lung disease | ||||||||||
| Detailed | No | 168 (75%) | 115 (75%) | 39 (76%) | 14 (74%) | N/A | ||||
| Asthma | 16 (7%) | 13 (8%) | 0 (0%) | 3 (16%) | ||||||
| Bronchiectasis | 1 (0.45%) | 1 (0.65%) | 0 (0%) | 0 (0%) | ||||||
| COPD | 15 (7%) | 6 (4%) | 8 (16%) | 1 (5%) | ||||||
| Fibrosis | 4 (2%) | 3 (2%) | 1 (2%) | 0 (0%) | ||||||
| Other | 17 (8%) | 13 (8%) | 3 (6%) | 1 (5%) | ||||||
| Binary | No | 168 (75%) | 115 (75%) | 39 (76%) | 14 (74%) | |||||
| Yes | 56 (25%) | 39 (25%) | 12 (24%) | 5 (26%) | 0.92 (0.48–1.77) | 0.81 | 220 | |||
| Ischemic heart disease | No | 157 (70%) | 116 (75%) | 28 (55%) | 13 (68%) | |||||
| Yes | 64 (29%) | 35 (23%) | 23 (45%) | 6 (32%) | 2.28 (1.32–3.94) | 0.003a | 217 | |||
| Missing | 3 (1%) | 3 (2%) | 0 (0%) | 0 (0%) | ||||||
| Cerebrovascular disease | No | 173 (77%) | 124 (81%) | 33 (65%) | 16 (84%) | |||||
| Yes | 49 (22%) | 28 (18%) | 18 (35%) | 3 (16%) | 2.11 (1.20–3.72) | 0.01a | 218 | |||
| Missing | 2 (0.89%) | 2 (1%) | 0 (0%) | 0 (0%) | ||||||
| Hospital management | ||||||||||
| Length of stay | Mean (SD) | 19.01 (12.4) | 17.4 (9.8) | 15.6 (10.3) | N/A | |||||
| Median (Q1-Q3) | 16 (11–23.5) | 16 (11–22) | 14 (7–19) | |||||||
| Range | 1–60 | 1–55 | 2–43 | |||||||
| Missing | 4 (2%) | 4 (3%) | 0 (0%) | |||||||
| Management | ||||||||||
| Detailed | Outpatient | 81 (36%) | 78 (51%) | 3 (6%) | 0 (0%) | 1 | 220 | |||
| Outpatient to inpatient | 28 (13%) | 18 (12%) | 6 (12%) | 4 (21%) | 6.40 (1.55–26.36) | 0.01a | 5.50 (1.33–22.79) | 0.04a | ||
| Inpatient | 115 (51%) | 58 (38%) | 42 (82%) | 15 (79%) | 11.24 (3.38–37.37) | <0.001a | 8.56 (2.54–28.83) | 0.001a | ||
| Binary | Outpatient | 81 (36%) | 78 (51%) | 3 (6%) | 0 (0%) | 1 | <0.001a | 220 | ||
| Part or total | 143 (64%) | 76 (49%) | 48 (94%) | 19 (100%) | 10.26 (3.10–33.94) | |||||
| On ACEi/ARB | No | 150 (67%) | 98 (64%) | 37 (73%) | 15 (79%) | |||||
| Yes | 70 (31%) | 53 (34%) | 14 (27%) | 3 (16%) | 0.82 (0.44–1.52) | 0.52 | 216 | |||
| Missing | 4 (2%) | 3 (2%) | 0 (0%) | 1 (5%) | ||||||
| Ceiling of care (only 143 obs) | Ward | 73 (51%) | 31 (38%) | 34 (74%) | 8 (50%) | 1 | ||||
| NIV | 24 (17%) | 17 (21%) | 5 (11%) | 2 (13%) | 0.36 (0.14–0.94) | 0.04a | 141 | |||
| Mechanical ventilation | 46 (32%) | 33 (41%) | 7 (15%) | 6 (38%) | 0.25 (0.12–0.56) | 0.001a | ||||
| Maximum breathing support | None | 67 (30%) | 64 (42%) | 0 (0%) | 3 (16%) | N/A | ||||
| Nasal cannula | 60 (27%) | 40 (26%) | 14 (27%) | 6 (32%) | ||||||
| Venturi/face mask | 32 (14%) | 6 (4%) | 23 (45%) | 3 (16%) | ||||||
| NIV | 12 (5%) | 7 (5%) | 4 (8%) | 1 (5%) | ||||||
| Mechanical ventilation | 9 (4%) | 1 (0.65%) | 6 (12%) | 2 (11%) | ||||||
| Missing | 44 (20%) | 36 (23%) | 4 (8%) | 4 (21%) | ||||||
| Dialysis access | Fistula or AVG | 124 (55%) | 89 (58%) | 24 (47%) | 11 (58%) | 1 | ||||
| Line | 98 (44%) | 64 (42%) | 26 (51%) | 8 (42%) | 1.34 (0.77–2.32) | 0.30 | 218 | |||
| Missing | 2 (0.89%) | 1 (0.65%) | 1 (2%) | 0 (0%) | ||||||
| Dialysis vintage (5-yr effect) | Mean (SD) | 4.092 (4.46) | 3.99 (4.43) | 4.44 (4.76) | 3.94 (4.46) | 1.13 (0.86–1.48) | 0.39 | 209 | ||
| Median (Q1–Q3) | 2.82 (1.11–5.46) | 2.57 (1.05–5.27) | 3.11 (1.17–5.55) | 3.94 (1.2–5.23) | ||||||
| Range | 0.003–24.7 | 0.003–24.7 | 0.022–22.9 | 0.22–16.3 | ||||||
| Missing | 11 (5%) | 8 (5%) | 2 (4%) | 1 (5%) | ||||||
| Immunosuppression | No | 197 (88%) | 135 (88%) | 47 (92%) | 15 (79%) | |||||
| Yes | 23 (10%) | 16 (10%) | 4 (8%) | 3 (16%) | 0.73 (0.27–1.94) | 0.52 | 216 | |||
| Missing | 4 (2%) | 3 (2%) | 0 (0%) | 1 (5%) | ||||||
| ICU admission | No | 207 (92%) | 148 (96%) | 44 (86%) | 15 (79%) | |||||
| Yes | 11 (5%) | 3 (2%) | 6 (12%) | 2 (11%) | ||||||
| Missing | 6 (3%) | 3 (2%) | 1 (2%) | 2 (11%) | ||||||
| Number of previous transplants | 0 | 206 (92%) | 142 (92%) | 47 (92%) | 17 (89%) | 1 | ||||
| 1–2 | 14 (6%) | 9 (6%) | 4 (8%) | 1 (5%) | 1.40 (0.54–3.67) | 0.50 | 216 | |||
| Missing | 4 (2%) | 3 (2%) | 0 (0%) | 1 (5%) | ||||||
| Transplant wait list | No | 201 (90%) | 135 (88%) | 48 (94%) | 18 (95%) | 1 | ||||
| Yes | 16 (7%) | 14 (9%) | 2 (4%) | 0 (0%) | 0.52 (0.123–2.040) | 0.36 | 215 | |||
| Missing | 7 (3%) | 5 (3%) | 1 (2%) | 1 (5%) | ||||||
| Symptoms | ||||||||||
| Fever | No | 58 (26%) | 40 (26%) | 17 (33%) | 1 (5%) | |||||
| Yes | 138 (62%) | 96 (62%) | 31 (61%) | 11 (58%) | 0.72 (0.40–1.29) | 0.27 | 193 | |||
| Missing | 28 (13%) | 18 (12%) | 3 (6%) | 7 (37%) | ||||||
| SOB | No | 105 (47%) | 85 (55%) | 17 (33%) | 3 (16%) | |||||
| Yes | 92 (41%) | 52 (34%) | 31 (61%) | 9 (47%) | 2.32 (1.29–4.17) | 0.005a | 194 | |||
| Missing | 27 (12%) | 17 (11%) | 3 (6%) | 7 (37%) | ||||||
| Dry cough | No | 116 (52%) | 84 (55%) | 25 (49%) | 7 (37%) | |||||
| Yes | 82 (37%) | 54 (35%) | 23 (45%) | 5 (26%) | 1.31 (0.74–2.29) | 0.35 | 195 | |||
| Missing | 26 (12%) | 16 (10%) | 3 (6%) | 7 (37%) | ||||||
| Productive cough | No | 160 (71%) | 111 (72%) | 39 (76%) | 10 (53%) | |||||
| Yes | 37 (17%) | 25 (16%) | 10 (20%) | 2 (11%) | 1.14 (0.57–2.26) | 0.71 | 194 | |||
| Missing | 27 (12%) | 18 (12%) | 2 (4%) | 7 (37%) | ||||||
| Headache | No | 176 (79%) | 122 (79%) | 44 (86%) | 10 (53%) | |||||
| Yes | 20 (9%) | 14 (9%) | 4 (8%) | 2 (11%) | 0.76 (0.27–2.10) | 0.59 | 193 | |||
| Missing | 28 (13%) | 18 (12%) | 3 (6%) | 7 (37%) | ||||||
| Vomiting | No | 166 (74%) | 118 (77%) | 39 (76%) | 9 (47%) | |||||
| Yes | 30 (13%) | 18 (12%) | 9 (18%) | 3 (16%) | 1.29 (0.63–2.65) | 0.48 | 193 | |||
| Missing | 28 (13%) | 18 (12%) | 3 (6%) | 7 (37%) | ||||||
| Aches and pains | No | 157 (70%) | 110 (71%) | 38 (75%) | 9 (47%) | |||||
| Yes | 39 (17%) | 26 (17%) | 10 (20%) | 3 (16%) | 1.06 (0.53–2.15) | 0.86 | 193 | |||
| Missing | 28 (13%) | 18 (12%) | 3 (6%) | 7 (37%) | ||||||
| Diarrhea | No | 165 (74%) | 113 (73%) | 43 (84%) | 9 (47%) | |||||
| Yes | 30 (13%) | 23 (15%) | 5 (10%) | 2 (11%) | 0.60 (0.24–1.51) | 0.28 | 192 | |||
| Missing | 29 (13%) | 18 (12%) | 3 (6%) | 8 (42%) | ||||||
| Asymptomatic | No | 186 (83%) | 127 (82%) | 47 (92%) | 12 (63%) | |||||
| Yes | 10 (4%) | 9 (6%) | 1 (2%) | 0 (0%) | 0.37 (0.05–2.95) | 0.35 | 193 | |||
| Missing | 28 (13%) | 18 (12%) | 3 (6%) | 7 (37%) | ||||||
| Blood analyses | ||||||||||
| Hemoglobin (g/L) | Mean (SD) | 105.04 (15.28) | 104.13 (13.74) | 108.49 (18.383) | 103.06 (17.38) | 1.10 (0.99–1.22) | 0.09 | 210 | ||
| Median (Q1–Q3) | 105 (97–114) | 105 (96–113) | 107 (99–120) | 102.5 (91–116) | ||||||
| Range | 70–145 | 73–145 | 70–141 | 72–136 | ||||||
| Missing | 11 (5%) | 5 (3%) | 2 (4%) | 18 (95%) | ||||||
| CRP (mg/L) | ||||||||||
| 10-U SHR effect | Mean (SD) | 103.62 (101.8) | 89.26 (92.13) | 140.76 (116.6) | 115.14 (110.2) | 1.03 (1.01–1.05) | 0.005a | 198 | ||
| Median (Q1–Q3) | 74 (31.8–129.3) | 65 (28–103.8) | 113 (47–212) | 76.4 (38.7–183) | ||||||
| Range | 1.1–596.5 | 1.1–596.5 | 4.4–471 | 6–368 | ||||||
| Missing | 23 (10.27%) | 18 (11.69%) | 4 (7.84%) | 1 (5.26%) | ||||||
| Log scale | Mean (SD) | 4.074 (1.20) | 3.91 (1.29) | 4.51 (1.10) | 4.203 (1.22) | 1.44 (1.07–1.93) | 0.02a | 198 | ||
| Median (Q1–Q3) | 4.30 (3.46–4.86) | 4.17 (3.33–4.64) | 4.73 (3.85–5.36) | 4.32 (3.7–5.21) | ||||||
| Range | 0.095–6.39 | 0.095–6.39 | 1.48–6.15 | 1.97–5.91 | ||||||
| Missing | 23 (10%) | 18 (12%) | 4 (8%) | 1 (5%) | ||||||
| White cell count | ||||||||||
| Original scale (5-U effect) | Mean (SD) | 6.16 (3.175) | 5.65 (3.029) | 7.39 (3.158) | 6.92 (3.476) | 1.70 (1.22–2.37) | 0.002a | 210 | ||
| Median (Q1–Q3) | 5.38 (3.9–7.43) | 5.1 (3.5–6.6) | 6.9 (5.22–9.7) | 6.16 (4.27–8.1) | ||||||
| Range | 1.65–18.9 | 1.65–18.9 | 1.8–15.3 | 2.3–15.2 | ||||||
| Log scale | Mean (SD) | 1.70 (0.49) | 1.62 (0.50) | 1.90 (0.47) | 1.82 (0.50) | 2.53 (1.45–4.42) | 0.001a | 210 | ||
| Median (Q1–Q3) | 1.68 (1.36–2.01) | 1.63 (1.25–1.89) | 1.93 (1.65–2.27) | 1.81 (1.45–2.1) | ||||||
| Range | 0.5–2.94 | 0.50–2.94 | 0.59–2.73 | 0.83–2.72 | ||||||
| Missing | 11 (5%) | 8 (5%) | 2 (4%) | 1 (5%) | ||||||
| Neutrophil count (×109/L) | ||||||||||
| Original scale | Mean (SD) | 4.67 (2.99) | 4.22 (2.88) | 5.91 (3.02) | 4.98 (2.97) | 1.80 (1.26–2.56) | 0.001a | 210 | ||
| Median (Q1–Q3) | 3.8 (2.6–5.93) | 3.5 (2.3–5.2) | 5.4 (3.57–7.9) | 4.52 (3–6.1) | ||||||
| Range | 0.68–17.5 | 0.68–17.5 | 1.1–13.9 | 1.2–12.6 | ||||||
| Log scale | Mean (SD) | 1.36 (0.61) | 1.26 (0.59) | 1.64 (0.56) | 1.43 (0.64) | 2.30 (1.45–3.66) | <0.001a | 210 | ||
| Median (Q1–Q3) | 1.34 (0.96–1.78) | 1.25 (0.83–1.65) | 1.67 (1.27–2.07) | 1.51 (1.1–1.81) | ||||||
| Range | −0.39–2.86 | −0.39–2.86 | 0.10–2.63 | 0.18–2.53 | ||||||
| Missing | 11 (5%) | 8 (5%) | 2 (4%) | 1 (5%) | ||||||
| Lymphocyte count (×109/L) | ||||||||||
| Original scale | Mean (SD) | 0.903 (0.49) | 0.889 (0.403) | 0.815 (0.56) | 1.27 (0.73) | 0.59 (0.23–1.53) | 0.28 | 210 | ||
| Median (Q1–Q3) | 0.8 (0.58–1.1) | 0.8 (0.6–1.1) | 0.7 (0.4–1) | 1.2 (0.8–1.6) | ||||||
| Range | 0.1–3.6 | 0.2–2.3 | 0.1–3.4 | 0.4–3.6 | ||||||
| Log scale | Mean (SD) | −0.23 (0.52) | −0.22 (0.45) | −0.39 (0.63) | 0.10 (0.54) | 0.52 (0.31–0.86) | 0.01a | 210 | ||
| Median (Q1–Q3) | −0.22 (−0.54–0.10) | −0.22 (−1.08–0.70) | −0.36 (−0.92–0.00) | 0.18 (−0.22–0.47) | ||||||
| Range | −1.3–1.28 | −1.61–0.83 | −2.30–1.22 | −0.92–1.28 | ||||||
| Missing | 11 (5%) | 8 (5%) | 2 (4%) | 1 (5%) | ||||||
| Neut:lymp ratio | ||||||||||
| Original scale | Mean (SD) | 6.9 (8.4) | 5.7 (4.97) | 11.6 (14.3) | 4.8 (3.2) | 1.03 (1.017–1.05) | <0.001a | 210 | 1.03 (1.01–1.04) | <0.001a |
| Median (Q1–Q3) | 4.7 (3.1–7.8) | 4.30 (2.9–6.7) | 7.2 (4.2–13.4) | 4.1 (2.1–7.4) | ||||||
| Range | 0.7–93 | 0.9–32 | 0.7–93 | 0.9–11.98 | ||||||
| Log scale | Mean (SD) | 1.59 (0.80) | 1.47 (0.71) | 2.03 (0.90) | 1.33 (0.73) | 2.10 (1.54–2.87) | <0.001a | 210 | ||
| Median (Q1–Q3) | 1.54 (1.12–2.05) | 1.46 (1.05–1.91) | 1.98 (1.43–2.60) | 1.40 (0.76–1.99) | ||||||
| Range | −0.31–4.53 | −0.13–3.47 | −0.31–4.53 | −0.07–2.48 | ||||||
| Missing | 11 (5%) | 8 (5%) | 2 (4%) | 1 (5%) | ||||||
| Albumin pre-presentation (g/L) (5-U effect) | Mean (SD) | 33.66 (6.32) | 34.25 (6.01) | 31.88 (7.14) | 33 (6.26) | 0.80 (0.64–1.01) | 0.06a | 200 | ||
| Median (Q1–Q3) | 34 (30–38) | 35 (31–39) | 33 (29–37) | 33.5 (29–35) | ||||||
| Range | 13–47 | 16–47 | 13–45 | 22–43 | ||||||
| Missing | 20 (9%) | 7 (5%) | 8 (16%) | 5 (26%) | ||||||
The two columns on the right represent the univariate and adjusted effects of the corresponding raw variable on the SHR of death versus discharged alive, and the P values test the null hypothesis that that SHR is 1. An SHR value >1 indicates a harmful effect, whereas a value <1 indicates a protective effect of the corresponding variable on the left. The last column represents the most parsimonious model derived from the data. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; obs, observed; Q1-3, quartile 1-3; BMI, body mass index; WHO, World Health Organization; HFrEF, heart failure with reduced ejection fraction; COPD, chronic obstructive pulmonary disease; N/A, not applicable; ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; NIV, noninvasive ventilation; AVG, arteriovenous graft; ICU, intensive care unit; SOB, shortness of breath; CRP, C-reactive protein; SHR, subdistribution hazards ratio; Neut:lymp, neutrophil:lymphocyte. The percentages (%) sum up to 100 on columns, i.e., showing distribution of each variable within outcome category (discharged alive, dead and still in care).
P<0.05.
The mean patient age (SD) was 66±14 years with 133 (59%) men, 85 (38%) White patients, 182 (81%) patients on hypertensives, 120 (54%) patients with diabetes, 64 (29%) patients with ischemic heart disease, 49 (22%) patients with cerebrovascular disease, 40 (18%) patients with heart failure with reduced ejection fraction, 56 (25%) patients with chronic lung disease, and 33 (15%) patients with a history of cancer (Table 1). Comparative results with the overall HD population are presented in Table 2.
Table 2.
Comparisons between the characteristics of patients with COVID-19 and the whole sample of patients on ICHD/PD across the three hospitals
| Variable | Hemodialysis Population | Peritoneal Dialysis Population | ||||||||||
| St. Helier | King’s | St. George’s | Pooled | SARS-CoV-2 Positive | P Value | St. Helier | King’s | St. George’s | Pooled | SARS-CoV-2 Positive | P Value | |
| Total (n) | 846 | 597 | 294 | 1737 | 224 | 98 | 90 | 40 | 228 | 10 | ||
| Sex | ||||||||||||
| Male | 62% | 59% | 59% | 1048 (60%) | 133 (59%) | 0.75 | 55% | 60% | 60% | 132 (58%) | 8 (80%) | 0.20 |
| Female | 28% | 41% | 42% | 689 (40%) | 91 (41%) | 45% | 40% | 40% | 96 (42%) | 2 (20%) | ||
| Ethnicity | ||||||||||||
| White | 60% | 40% | 30% | 834 (48%) | 85 (38%) | 0.001 | 76% | 38% | 48% | 127 (56%) | 5 (50% | 0.75 |
| Other | 35% | 60% | 66% | 903 (52%) | 139 (62%) | 21% | 62% | 43% | 101 (44%) | 5 (50%) | ||
| Missing | 5% | 0.01% | 4% | 3% | 0% | 10% | ||||||
| Age (yr) | ||||||||||||
| Median | 68.7 | 63.4 | 66.6 | 66.5 | 65 | 0.38 | 67.1 | 56.8 | 62.5 | 62.2 | 69.5 | 0.14 |
| Q1–Q3 | 56.4–77.7 | 53.0–75.1 | 54.6–75.6 | 57–77 | 57.7–76.5 | 45.5–72.4 | 50.9–73.8 | 59–75 | ||||
| Diabetes | ||||||||||||
| No | 1360 (78%) | 103 (46%) | 185 (81%) | 4 (40%) | ||||||||
| Yes | 12% | 36% | 22% | 377 (22%) | 120 (54%) | <0.001 | 5% | 29% | 30% | 43 (19%) | 6 (60%) | 0.004 |
The pooled proportions and numbers are weighted averages across the three hospitals. Values are given in percentages or n (%), unless otherwise specified. ICHD/PD, in-center hemodialysis/peritoneal dialysis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Q1-3, quartile 1-3.
Smoking status was reported in 165 (74%) patients and 71 (32%) were ex- or current smokers. Median (Q1–Q3) dialysis vintage was 2.82 years (1.11–5.46 years). Overall, 124 (55%) patients dialyzed with a fistula or arteriovenous graft, and 98 (44%) patients with a line. WHO performance status at the time of presentation was zero, one, or two in 134 (60%) patients, and three or four in 83 (37%) patients. Median (Q1–Q3) serum albumin at the last routine monthly blood review before presentation was 34 g/L (30–38 g/L), and 56 (34%) patients were taking an angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker at the time of COVID-19 diagnosis.
Symptoms at presentation were fever in 186 (83%), shortness of breath in 92 (41%), dry cough in 82 (37%), productive cough in 37 (17%), diarrhea in 30 (13%), vomiting in 30 (13%), headache in 20 (9%), and aches and pains in 37 (17%) patients; only ten (5%) patients were asymptomatic.
At presentation, the median (Q1–Q3) blood C-reactive protein (CRP) was 74 mg/L (32–129 mg/L), white cell count was 5.4×109/L (3.9×109–7.4×109/L), neutrophil count was 3.8×109/L (2.6×109–5.9×109/L), lymphocyte count was 0.80×109/L (0.58×109–1.1×109/L), neutrophil:lymphocyte ratio median (Q1–Q3) was 4.7 (3.1–7.8), and hemoglobin was 105 g/L (97–114 g/L).
Management of Patients on HD Infected with SARS-CoV-2
Overall, 81 (36%) of patients on HD were managed exclusively as outpatients, dialyzing initially in isolation facilities belonging to the hospitals and then being discharged to satellite units when clinically improved; 115 (52%) were cared for exclusively as inpatients; and 28 (12%) patients were managed as outpatients before hospitalization.
Of these 143 (64%) patients who were admitted to hospital, a “ceiling of care” was determined, meaning the highest level of medical intervention deemed appropriate should the patient’s clinical condition deteriorate. This decision was made by the medical team, considering the patient’s wishes and whether the patient was likely to benefit from more invasive care. A ward-based, ceiling-of-care decision was made for 73 (51%) patients, and escalation occurred for noninvasive ventilation in 24 (17%) patients and for mechanical ventilation in 46 (32%) patients.
Of the patients who were hospitalized, 92 (64%) required maximum respiratory support from respiratory support devices that could be delivered on the ceiling-of-care ward setting, including nasal cannulae and nonrebreathing masks. There were 12 patients who were hospitalized that required noninvasive ventilation. Only 11 (8%) of the patients who were hospitalized were ultimately admitted to the intensive care unit (ICU), with nine patients requiring mechanical ventilation.
At the end of follow-up, 19 (9%) patients were still inpatients because of their COVID-19-related illnesses.
Associations with the SHR of Death versus Discharge in Patients on HD Infected with SARS-CoV-2
At the end of follow-up, 51 (23%) patients on HD had unfortunately died (time series in Figure 1), 154 (69%) were discharged from either inpatient care or outpatient isolation HD, and 19 (9%) were still under clinical care.
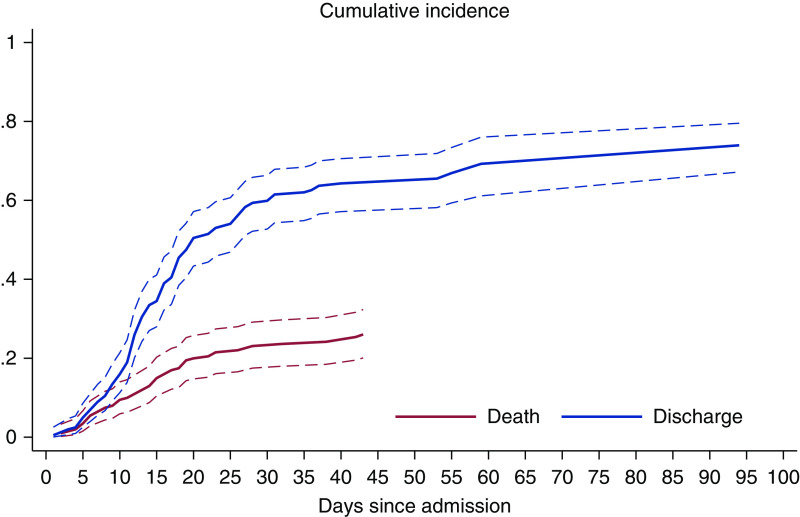
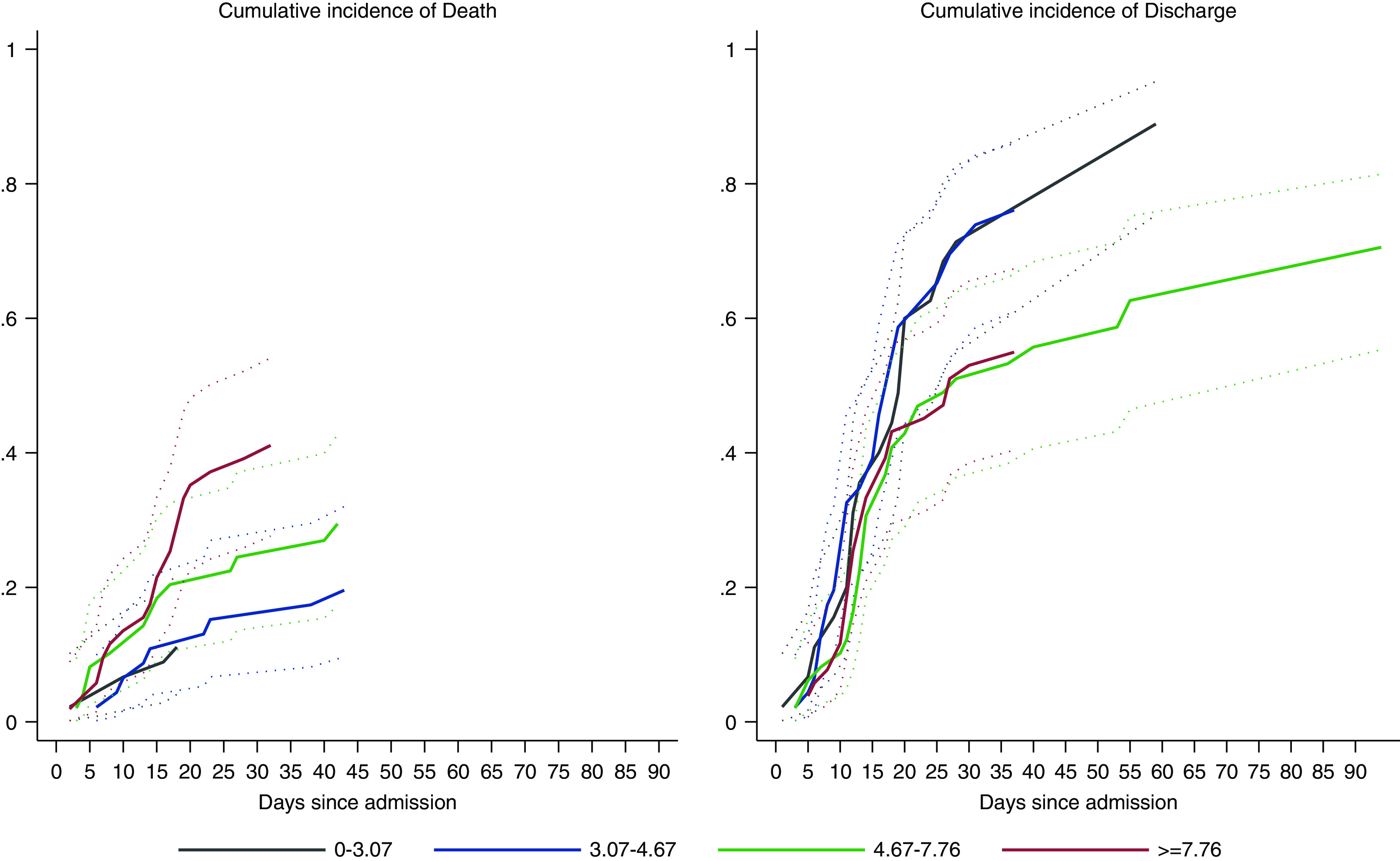
Figure 3, showing the cumulative incidence of death, suggests patients deteriorated relatively quickly, at a steadily increasing pace, during the first 23 days of admission. The daily incidence of discharge after admission increased sharply between 5 and 20 days since admission. This latter trend slowed down afterwards—driven by 38 patients who required long (21–55 days) hospitalization. The effects of age and other variables on the dynamics of death and hospital discharge can be seen in Table 1.
Figure 3.
The predicted daily cumulative incidence of death and hospital discharge of patients on hemodialysis who were positive for COVID-19. The curves indicate the short and fast dynamics of death and a long time to discharge.
Patients that required admission to hospital were 10.26 (95% CI, 3.10 to 33.94) times more likely to die than patients managed exclusively as outpatients. On the basis of these data, there is not enough evidence to suggest that sex, ethnicity, body mass index, or dialysis vintage were associated with death in these patients (all corresponding P values for SHR were >0.05).
A 5-year increase in the age at admission was associated with an increase in the SHR of death versus discharge by a factor of 1.16 (95% CI, 1.03 to 1.30), P=0.01. There was an average case-fatality ratio of 23%, which exhibited heterogeneity across the age groups in this cohort, with 11% of deaths among patients <50 years of age, 33% in those 75–80 years of age, and 32% in those >80 years of age.
Smoking history was associated with an almost three times (2.69 [95% CI, 1.33 to 5.45]) increased SHR of death compared with no smoking history. Given the great deal of missing information for this variable (26%), a sensitivity analysis, in which all of these patients were assumed to be nonsmokers, still preserves the harmful effect of smoking, i.e., SHR, 1.78 (95% CI, 1.03 to 3.08), P=0.04. There is also some evidence for a higher chance of death in those with ischemic heart disease (P=0.003) and those with cerebrovascular disease (P=0.01), compared with those without these comorbidities. In addition, those with a WHO score of three to four were 2.16 (95% CI, 1.25 to 3.74) times more likely to die compared with those with a WHO score of zero to two. The data presented in this population were consistent with no effect of ACE inhibitors/angiotensin receptor blockers on the hazard of death (P=0.518).
The only evidence for an association of death with symptoms on admission was with shortness of breath (SHR, 2.32 [1.29 to 4.17]; P=0.005) (Table 1). Among patients who died, compared with patients who were discharged alive, blood CRP concentration was higher (median [Q1–Q3] 113 [47 to 212] versus 65 [28–104]), log lymphocyte count was lower, and neutrophil:lymphocyte ratio was higher (median [Q1–Q3] 7.2 [4.2–13.4] versus 4.3 [2.9–6.7]) (Figure 4). Furthermore, each unit increase in neutrophil:lymphocyte ratio was associated with a 3% (2%–5%) increase in SHR for death versus hospital discharge, and, similarly, each 10-mg/L rise in CRP was associated with a 3% (1%–5%) increased SHR of death. Our multivariable model included the predictors that remain strong (P<0.05), and for which the AIC value was the smallest. The WHO score includes elements of age, so the two confound each other, as expected. However, the model including age, neutrophil:lymphocyte ratio, and hospital management was better than including WHO score, neutrophil:lymphocyte ratio, and hospital management (AIC of 471.933 versus 473.400, respectively). The adjusted effects of these variables are only slightly modified compared with their univariate counterparts (Table 1).
Figure 4.
The dynamics of hospital death and hospital discharge in association with the neutrophil:lymphocyte ratio. High levels of this ratio are associated with high risk of in-care deaths in patients on hemodialysis positive for COVID-19. Low values of this ratio are associated with rapid and high probability of hospital discharge.
Demographics, Clinical Characteristics, and Outcomes of Patients on PD Infected with SARS-CoV-2
Among the ten patients on PD infected with SARS-CoV-2, the median (Q1–Q3) age was 69.5 years (59–75 years), eight were males, five were White, three were smokers, six were diabetic, and one was managed as an outpatient. Of the nine inpatients, three required noninvasive ventilation, two required ICU admission, and one required mechanical ventilation. Six of the admitted patients (60% of the total) died, and four were discharged alive.
Patients with SARS-CoV-2 Compared with Reference Populations
Unless otherwise specified, the reference populations are collectively those patients who have their usual dialysis provided by the South London renal centers (Table 2).
Up until May 15, 2020, 224 (approximately 13%) of all patients on HD (1727) and ten (approximately 4%) of all patients on PD (228) from the three renal centers tested positive for COVID-19. Of those that were COVID-19 positive, 51 (23%) patients on HD and six (60%) patients on PD have died, such that approximately 3% of all patients on HD and approximately 3% of all patients on PD managed at the three centers died from COVID-19 during the period of data collection.
The age data for patients positive for COVID-19 presented here were broadly consistent with that of the HD (P=0.38) and PD (P=0.14) populations, respectively, across the three hospitals. The distribution of sex in our COVID-19-positive cohort was also similar to that observed in the local dialysis populations (P=0.75 for HD and P=0.20 for PD, respectively). There was, however, a suggestion that SARS-CoV-2 infections seemed to have affected more non-White patients on HD than White patients (P=0.001; Table 2), despite no differences between case-fatality ratios supported by these data. The numbers in the PD population are too small for meaningful analyses using individual records. The proportion of patients with diabetes among the patients positive for COVID-19 is also higher than might be expected from the reference dialysis populations (54% versus 46% in HD, P<0.001, and 60% versus 19% in PD, P=0.004). Our data suggest there is some evidence that the case-fatality ratio is higher (P=0.02) in patients on PD (six of ten) than in those on HD (51/224).
Approximately 13% (224/1737) of the total dialysis population of the renal centers was infected with SARS-CoV-2, and 3% (51/1737) died by the date we stopped data collection.
The case-fatality ratio described for our patients on dialysis that tested positive for COVID-19 appears to be commensurate with national renal data (shown in Table 3) by the time of our censoring (22).
Table 3.
Local and national cumulative numbers as reported until May 15 by the UK Renal Registry
| Locality | Total RRT (n) | Hemodialysis Population | Peritoneal Dialysis Population | ||||||||
| Total ICHD (n) | SARS-CoV-2 (n) | Death (n) | Case-Fatality Ratio (%) | P Value | Total PD (n) | SARS-CoV-2 (n) | Death (n) | Case-Fatality Ratio (%) | P Value | ||
| All three hospitals | 1737 | 224 | 51 | 23% (17%, 28%) | — | 228 | 10 | 6 | 60% (30%, 90%) | — | |
| London | 14394 | 1021 | 219 | 21% (19%, 24%) | 0.67 | 44 | 12 | 27% (14%, 40%) | 0.26 | ||
| England | 56201 | 2134 | 502 | 24% (22%, 25%) | 0.80 | 78 | 25 | 32% (22%, 42%) | 0.30 | ||
| United Kingdom | 66612 | 2326 | 553 | 24% (22%, 26%) | 0.74 | 84 | 26 | 31% (21%, 41%) | 0.29 | ||
The P values are consistent with no difference between the case-fatality ratio in our sample and those in London, England, and the United Kingdom. Our data suggest some evidence that the case-fatality ratio is higher in patients on PD (six of ten) than in HD (51/224) and patients (P=0.015 according to Fisher exact test). ICHD, in-center hemodialysis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PD, peritoneal dialysis; HD, hemodialysis. The case fatality ratio are accompanied by their 95% CI, i.e., calculated using standard errors of the sample proportion.
The numbers in the patients on PD who had COVID-19 are too small for meaningful analyses using individual records, as we did for patients on HD with COVID-19.
In a meta-analysis made on the basis of another six similar studies, the case-fatality ratio was 24% (95 CI, 17%–31%) and was 23% (95% CI, 18%–29%) when including this study. There was a high level of heterogeneity in the data, mainly caused by the Wuhan, China estimate, but we felt the study should be left in the analysis (Figure 2).
Discussion
In this study of patients on dialysis infected with SARS-CoV-2, the case-fatality ratio was high: 23%. The patients who died, compared with those recovered, were older, more likely to be smokers and hospitalized, more likely to have ischemic and cerebrovascular disease, and to have worse WHO performance status. COVID-19 was observed more frequently in patients with diabetes and in those who were non-White.
The infection rate of 13% in our HD population likely represents an underestimate, because only patients with symptoms were screened; therefore, patients who were asymptomatic and those who received falsely negative PCR COVID-19 test results were missing. This has been illustrated in a recent study of 356 patients on HD, where 22% were PCR positive for COVID-19 with symptom-based screening, but the seroprevalence rate was 36%; therefore, 40% of patients with positive antibodies had been either asymptomatic or received negative results on PCR testing (26).
The effect of age is clearly visible in Figure 1, which shows that >30% of patients >75 years old died, as opposed to <15% of the patients who were <60 years of age. The case-fatality ratio presented in this report is broadly consistent with that observed in other reports of patients on dialysis with COVID-19—as seen in the meta-analysis of six studies from Europe, Asia, and North America (Figure 2)—and similar to other patients hospitalized with COVID-19 in the United Kingdom and elsewhere, but lower than in patients admitted to ICU (27).
In our dialysis population, smokers were more likely to die, which may be due to SARS-CoV-2 being an airborne virus that predominantly affects the lungs. Smokers and individuals with chronic obstructive pulmonary disorder have recently been reported to have increased expression of ACE-2 receptors (the site of entry of SARS-CoV-2 into cells) in small airway epithelial cells. This may explain why current and ex-smokers have poorer COVID-19-related respiratory outcomes (28). The evidence for this finding is preserved even after sensitivity analysis (Table 1).
The presence of healthy adaptive immunity, which requires the presence of healthy T and B lymphocyte populations, is important in mounting an appropriate response to viral infection, which may be defective in patients on dialysis (29). In our study, patients who died had a higher neutrophil count, lower lymphocyte (log) count, and a higher neutrophil:lymphocyte ratio at presentation. This is consistent with earlier reports in the general population, where poor prognosis was associated with low lymphocyte and higher neutrophil:lymphocyte ratio in the blood (30,31). The effects of age, neutrophil:lymphocyte ratio in the blood, and hospital management remain strong even after adjusting one for another (Table 1).
Compared with the aggregate data from the HD population in the three hospitals, a higher proportion of patients with diabetes (than patients without diabetes) and a higher proportion of patients who were non-White (compared with White) were infected with SARS-CoV-2. This is also broadly consistent with what is seen in the general population, particularly in the United Kingdom (32,33).
A major strength of our study is the investigation into the effect of the frailty score of COVID-19 in patients on HD. In this patient cohort, 51% of inpatients had an established ceiling-of-care decision for ward-based care and, within this group, approximately one in two patients died, totaling 74% of the total case fatality. As shown in Table 4, the ward-based care decision seemed appropriate, because the patients within this category were older, more frail, and comorbid than those for treatment escalation and for those that were ultimately admitted to ICU. Only one out of the nine mechanically ventilated patients were discharged alive, whereas six patients died and the other two remained ventilator dependent, indicating poor outcome.
Table 4.
Effect of clinical variable and comorbidity on ceiling of care or ICU
| Variable | Ceiling of Care | ICU Admission | |||||||
| All (n=143) | Ward (n=73) | NIV (n=24) | Mechanical Ventilation (n=46) | P Value | No. (n=207) | Yes (n=11) | Missing (n=6) | P Value | |
| Sex | |||||||||
| Male | 84 (59%) | 46 (63%) | 9 (38%) | 29 (63%) | 0.07 | 121 (58%) | 8 (73%) | 4 (67%) | 0.35 |
| Female | 59 (41%) | 27 (37%) | 15 (63%) | 17 (37%) | 86 (42%) | 3 (27%) | 2 (33%) | ||
| Ethnicity (binary) | |||||||||
| White | 58 (38%) | 28 (38%) | 13 (54%) | 17 (37%) | 0.33 | 78 (38%) | 5 (45%) | 2 (33%) | 0.61 |
| Other | 85 (62%) | 45 (62%) | 11 (46%) | 29 (63%) | 129 (62%) | 6 (55%) | 4 (67%) | ||
| Smoking history | |||||||||
| Never | 53 (37%) | 23 (32%) | 11 (46%) | 19 (41%) | 0.28 | 86 (42%) | 4 (36%) | 4 (67%) | 0.72 |
| Some | 49 (34%) | 28 (38%) | 10 (42%) | 11 (14%) | 66 (32%) | 4 (36%) | 1 (17%) | ||
| Missing | 41 (29%) | 22 (30%) | 3 (13%) | 16 (35%) | 55 (27%) | 3 (27%) | 1 (17%) | ||
| Age at admission (5-yr effect) (yr) | |||||||||
| Mean (SD) | 66.8 (14.5) | 74.8 (8.9) | 65.9 (12.0) | 54.6 (14.3) | <0.001 | 66.7 (14.0) | 49.9 (12.5) | 4 (67%) | 0.0004 |
| Median (Q1–Q3) | 70 (59–78) | 77 (70–81) | 67 (57–72.5) | 57 (44–62) | 68 (58–77) | 53 (40–61) | 2 (33%) | ||
| Range | 25–90 | 37–90 | 33–87 | 25–85 | 26–90 | 25–63 | |||
| BMI (kg/m 2 ) | |||||||||
| Mean (SD) | 27.6 (7.7) | 25.8 (4.9) | 28.1 (8.9) | 30.6 (9.8) | 0.13 | 28.5 (7.6) | 31.7 (9.9) | 0.30 | |
| Median (Q1–Q3) | 25.9 (22.3–30.1) | 25.8 (21.4–29.6) | 25.3 (22.6–29.0) | 27.8 (23.2–36.5) | 26.2 (23.1–30.7) | 29.9 (24.9–35.4) | |||
| Range | 16.5–57.8 | 16.7–38.2 | 16.5–51.7 | 18.4–52.7 | 16.5–57.8 | 21.0–49.2 | |||
| Missing | 21 (15%) | 9 (12%) | 9 (13%) | 9 (20%) | 21 (10%) | 5 (46%) | |||
| WHO performance status (binary) | |||||||||
| 0–2 | 76 (53%) | 22 (30%) | 17 (71%) | 37 (80%) | <0.001 | 120 (58%) | 8 (73%) | 6 (100%) | 0.40 |
| 3–4 | 66 (46%) | 51 (70%) | 7 (29%) | 8 (17%) | 80 (39%) | 3 (27%) | 0 (0%) | ||
| Missing | 1 (0.7%) | 0 (0%) | 0 (0%) | 1 (2%) | 7 (3%) | 0 (0%) | 0 (0%) | ||
| Neut:lymp ratio (Original scale) | |||||||||
| Mean (SD) | 8.08 (9.59) | 7.39 (6.89) | 8.81 (6.28) | 8.82 (13.91) | 0.23 | 6.31 (5.86) | 18.46 (25.54) | 0.0006 | |
| Median (Q1–Q3) | 5.30 (3.50–8.53) | 5.13 (3.08–8.44) | 7.22 (4.20–11.50) | 4.91 (3.50–8.53) | 4.60 (2.92–7.31) | 8.53 (6.23–18.19) | |||
| Range | 0.93–93 | 0.93–33.67 | 1.91–26.75 | 1.25–93 | 0.74–33.67 | 4.33–93 | |||
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 11 (5%) | 0 (0%) | |||
| History of cancer | |||||||||
| No | 121 (85%) | 62 (85%) | 19 (79%) | 40 (87%) | 0.69 | 176 (85%) | 10 (91%) | 3 (50%) | >0.99 |
| Yes | 22 (15%) | 11 (15%) | 5 (21%) | 6 (13%) | 29 (14%) | 1 (10%) | 3 (50%) | ||
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (1%) | 0 (0%) | 0 (0%) | ||
| Hypertension | |||||||||
| No | 27 (19%) | 10 (14%) | 6 (25%) | 11 (24%) | 0.27 | 37 (18%) | 3 (27%) | 1 (17%) | 0.43 |
| Yes | 116 (81%) | 63 (86%) | 18 (75%) | 35 (76%) | 169 (82%) | 8 (73%) | 5 (83%) | ||
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.50%) | 0 (0%) | 0 (0%) | ||
| Diabetes | |||||||||
| No | 72 (50%) | 32 (44%) | 13 (54%) | 27 (59%) | 0.26 | 91 (44%) | 10 (91%) | 2 (33%) | 0.003 |
| Yes | 71 (50%) | 41 (56%) | 11 (46%) | 19 (41%) | 115 (56%) | 1 (9%) | 4 (67%) | ||
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.50%) | 0 (0%) | 0 (0%) | ||
| HFrEF | |||||||||
| No | 118 (83%) | 54 (74%) | 22 (92%) | 42 (93%) | 0.01 | 164 (79%) | 11 (100%) | 6 (100%) | 0.22 |
| Yes | 25 (17%) | 20 (26%) | 2 (8%) | 3 (7%) | 40 (19%) | 0 (0%) | 0 (0%) | ||
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (1%) | 0 (0%) | 0 (0%) | ||
| Chronic lung disease=no | 102 (71%) | 51 (70%) | 15 (63%) | 36 (78%) | 0.38 | 154 (74%) | 10 (91%) | 4 (67%) | 0.30 |
| Diabetes=yes | 41 (29%) | 22 (30%) | 9 (38%) | 10 (22%) | 53 (26%) | 1 (9%) | 2 (33%) | ||
| Ischemic heart disease | |||||||||
| No | 94 (66%) | 43 (59%) | 15 (63%) | 36 (78%) | 0.08 | 144 (70%) | 9 (82%) | 4 (67%) | 0.52 |
| Yes | 49 (34%) | 30 (41%) | 9 (38%) | 10 (22%) | 60 (29%) | 2 (18%) | 2 (33%) | ||
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (1%) | 0 (0%) | 0 (0%) | ||
| Cerebrovascular disease | |||||||||
| No | 106 (74%) | 46 (63%) | 18 (75%) | 42 (91%) | 0.003 | 156 (75%) | 11 (100%) | 6 (100%) | 0.07 |
| Yes | 37 (26%) | 27 (37%) | 6 (25%) | 4 (9%) | 49 (24%) | 0 (0%) | 0 (0%) | ||
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
Tests are conducted on complete data. Values are n (%), unless otherwise stated. ICU, intensive care unit; BMI, body mass index; WHO, World Health Organization; Neut:lymp, neutrophil:lymphocyte; HFrEF, heart failure with reduced ejection fraction.
This study has several limitations. First, data were collected retrospectively through electronic health records and medical notes used for routine clinical care, and some data for those managed as outpatients were missing. We did not systematically collect detailed data on dialysis and nondialysis treatments given to patients. In the United Kingdom, the chief medical officers strongly discouraged the use of off-license treatments outside of a clinical trial. Treatment was, therefore, largely supportive unless patients participated in a clinical trial. There were 20 patients on HD and three patients on PD in this cohort who participated in the RECOVERY Trial (randomly assigned to supportive care [12 patients] or to one of four treatments: lopinavir-ritonavir [two patients], low-dose dexamethasone [three patients], hydroxychloroquine [three patients], or azithromycin [three patients]), and it is possible that these interventions may have affected their clinical course and outcomes.
This report describes the outcomes of patients on dialysis with COVID-19, who were more likely to be diabetic and non-White, from a large cohort of patients on dialysis from three NHS hospitals in South London. The case-fatality ratio among those infected with SARS-CoV-2 was high at 23%, in line with the pooled estimate from the meta-analysis. The patients who died, compared with those who survived, were older, more likely to be smokers, have cardiovascular disease, and have worse WHO performance status. The case-fatality ratio in this patient population, known to have a high burden of comorbidities, is broadly comparable with other reports in patients on dialysis who are infected with SARS-CoV-2, the United Kingdom dialysis population, and rates of hospital deaths in the United Kingdom population.
Disclosures
A. Sarnowski reports receiving grants from KIDNEYcon, during the conduct of the study; and reports holding shares in Baxter and Fresenius, outside the submitted work. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
Dr. Martin L. Ford reports receiving personal fees and nonfinancial support from AstraZeneca and other from Amgen, outside the submitted work. Dr. Richard Hull reports receiving personal fees from Napp Pharmaceuticals and personal fees from Pharmacosmos, outside the submitted work. Dr. Pauline A. Swift reports receiving personal fees from Napp Pharmaceuticals, outside the submitted work.
Author Contributions
D. Banerjee provided supervision and was responsible for visualization; D. Banerjee, D. Braide-Azikiwe, H. Cairns, I. Chis Ster, N. Cole, M. Ford, R. Hull, D. Kular, E. Lioudaki, D. Makanjuola, M. Phanish, A. Rankin, and P. Swift were responsible for data curation; D. Banerjee, D. Braide-Azikiwe, H. Cairns, I. Chis Ster, N. Cole, M. Ford, R. Hull, D. Kular, E. Lioudaki, M. Phanish, J. Popoola, A. Rankin, and P. Swift reviewed and edited the manuscript; D. Banerjee, D. Braide-Azikiwe, I. Chis Ster, D. Kular, D. Makanjuola, J. Popoola, A. Sarnowski, and P. Swift wrote the original draft; D. Banerjee and I. Chis Ster conceptualized the study and were responsible for validation; D. Banerjee, I. Chis Ster, and D. Kular were responsible for formal analysis and project administration; D. Banerjee, I. Chis Ster, D. Kular, and P. Swift were responsible for methodology; D. Banerjee, I. Chis Ster, and A. Sarnowski were responsible for resources; D. Banerjee, I. Chis Ster, D. Kular, and P. Swift were responsible for investigation.
Footnotes
D.K. and I.C.S. contributed equally to this work as first authors.
P.A.S. and D.B. contributed equally to this work as last authors.
References
- 1.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W: Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 395: 565–574, 2020. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinclair AJ, Abdelhafiz AH: Age, frailty and diabetes – triple jeopardy for vulnerability to COVID-19 infection. EClinicalMedicine 22: 100343, 2020. 10.1016/j.eclinm.2020.100343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon JW, Gollapudi S, Pahl MV, Vaziri ND: Naïve and central memory T-cell lymphopenia in end-stage renal disease. Kidney Int 70: 371–376, 2006. 10.1038/sj.ki.5001550 [DOI] [PubMed] [Google Scholar]
- 4.Channappanavar R, Zhao J, Perlman S: T cell-mediated immune response to respiratory coronaviruses. Immunol Res 59: 118–128, 2014. 10.1007/s12026-014-8534-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basile C, Combe C, Pizzarelli F, Covic A, Davenport A, Kanbay M, Kirmizis D, Schneditz D, van der Sande F, Mitra S: Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant 35: 737–741, 2020. 10.1093/ndt/gfaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudreuilh C, Kumar N, Moxham V, Hemsley C, Goldenberg S, Moutzouris DA: De-isolation of COVID-19-positive hemodialysis patients in the outpatient setting: A single-center experience. Kidney Int 98: 236–237, 2020. 10.1016/j.kint.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikizler TA: COVID-19 in dialysis patients: Adding a few more pieces to the puzzle. Kidney Int 98: 17–19, 2020. 10.1016/j.kint.2020.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang R, Liao C, He H, Hu C, Wei Z, Hong Z, Zhang C, Liao M, Shui H: COVID-19 in hemodialysis patients: A report of 5 cases. Am J Kidney Dis 76: 141–143, 2020. 10.1053/j.ajkd.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manganaro M, Baldovino S; The Working group of the Piedmont and Aosta Valley Section of the SIN: First considerations on the SARS-CoV-2 epidemic in the dialysis units of piedmont and aosta valley, Northern Italy. J Nephrol 33: 393–395, 2020. 10.1007/s40620-020-00732-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, Maffei C, Possenti S, Piva S, Latronico N, Foca E, Castelli F, Gaggia P, Movilli E, Bove S, Malberti F, Farina M, Bracchi M, Costantino EM, Bossini N, Gaggiotti M, Scolari F; Brescia Renal COVID Task Force : Management of patients on dialysis and with kidney transplantation during the SARS-COV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep 5: 580–585, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, Maffei C, Possenti S, Lucca B, Cortinovis R, Terlizzi V, Zappa M, Saccà C, Pezzini E, Calcaterra E, Piarulli P, Guerini A, Boni F, Gallico A, Mucchetti A, Affatato S, Bove S, Bracchi M, Costantino EM, Zubani R, Camerini C, Gaggia P, Movilli E, Bossini N, Gaggiotti M, Scolari F: A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int 98: 20–26, 2020. 10.1016/j.kint.2020.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valeri AM, Robbins-Juarez SY, Stevens JS, Ahn W, Rao MK, Radhakrishnan J, Gharavi AG, Mohan S, Husain SA: Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol 31: 1409–1415, 2020. 10.1681/ASN.2020040470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goicoechea M, Sánchez Cámara LA, Macías N, Muñoz de Morales A, González Rojas Ã, Bascuñana A, Arroyo D, Vega A, Abad S, Verde E, García Prieto AM, Verdalles U, Barbieri D, Felipe Delgado A, Carbayo J, Mijaylova A, Acosta A, Melero R, Tejedor A, Rodriguez Benitez P, de José AP, Rodriguez Ferrero ML, Anaya F, Rengel M, Barraca D, Luño J, Aragoncillo I: COVID-19: Clinical course and outcomes of 36 maintenance hemodialysis patients from a single center in Spain. Kidney Int 98: 27–34, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Government of the United Kingdom: COVID-19: Investigation and initial clinical management of possible cases. 2020. Available at: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection#criteria
- 15.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP: Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol 5: 649–655, 1982. 10.1097/00000421-198212000-00014 [DOI] [PubMed] [Google Scholar]
- 16.Blagden SP, Charman SC, Sharples LD, Magee LR, Gilligan D: Performance status score: Do patients and their oncologists agree? Br J Cancer 89: 1022–1027, 2003. 10.1038/sj.bjc.6601231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putter H, Fiocco M, Geskus RB: Tutorial in biostatistics: Competing risks and multi-state models. Stat Med 26: 2389–2430, 2007. 10.1002/sim.2712 [DOI] [PubMed] [Google Scholar]
- 18.Lau B, Cole SR, Gange SJ: Competing risk regression models for epidemiologic data. Am J Epidemiol 170: 244–256, 2009. 10.1093/aje/kwp107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen PK, Geskus RB, de Witte T, Putter H: Competing risks in epidemiology: Possibilities and pitfalls. Int J Epidemiol 41: 861–870, 2012. 10.1093/ije/dyr213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brock GN, Barnes C, Ramirez JA, Myers J: How to handle mortality when investigating length of hospital stay and time to clinical stability. BMC Med Res Methodol 11: 144, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 22.The Renal Association: Initial analysis of the impact of covid19 infection on patients with advanced chronic kidney disease in the UK. 2020. Available at https://renal.org/covid-19/data/. Accessed September 15, 2020
- 23.Wu J, Li J, Zhu G, Zhang Y, Bi Z, Yu Y, Huang B, Fu S, Tan Y, Sun J, Li X: Clinical features of maintenance hemodialysis patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Clin J Am Soc Nephrol 15: 1139–1145, 2020. 10.2215/CJN.04160320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aydin Bahat K, Parmaksiz E, Sert S: The clinical characteristics and course of COVID-19 in hemodialysis patients [published online ahead of print July 30, 2020]. Hemodial Int 10.1111/hdi.12861 [DOI] [PubMed] [Google Scholar]
- 25.Chawki S, Buchard A, Sakhi H, Dardim K, El Sakhawi K, Chawki M, Boulanger H, Kofman T, Dahmane D, Rieu P, Attaf D, Ahriz-Saksi S, Besson F, Boula R, Hafi A, Massoumi A, Diddaoui AZ, Fromentin L, Michaut P, Nebbad R, Desassis JF, Nicolet L, Ghazali A, Sohier-Attias J, Lamriben L, Adem A, Dupuis E, Rifard MK, Joly D, El Karoui K, Attias P; on behalf of the HD-CovIDF Study Group: Treatment impact on COVID-19 evolution in hemodialysis patients [published online ahead of print August 1, 2020]. Kidney Int 10.1016/j.kint.2020.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke C, Prendecki M, Dhutia A, Ali MA, Sajjad H, Shivakumar O, Lightstone L, Kelleher P, Pickering MC, Thomas D, Charif R, Griffith M, McAdoo SP, Willicombe M: High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol 31: 1969–1975, 2020. 10.1681/ASN.2020060827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Dunning J, Openshaw PJ, Baillie JK, Semple MG; ISARIC4C investigators:Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study. BMJ 369: m1985, 2020. 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, Dorscheid DR, Sin DD: ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur Respir J 55: 2000688, 2020. 10.1183/13993003.00688-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girndt M, Sester M, Sester U, Kaul H, Köhler H: Molecular aspects of T- and B-cell function in uremia. Kidney Int Suppl 78: S206–S211, 2001. 10.1046/j.1523-1755.2001.59780206.x [DOI] [PubMed] [Google Scholar]
- 30.Cao X: COVID-19: Immunopathology and its implications for therapy. Nat Rev Immunol 20: 269–270, 2020. 10.1038/s41577-020-0308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, Luo M, Chen L, Zhao Y: Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 81: e6–e12, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study [published correction appears in Lancet 395: 1038, 2020 10.1016/S0140-6736(20)30638-3]. Lancet 395: 1054–1062, 2020. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khunti K, Singh AK, Pareek M, Hanif W: Is ethnicity linked to incidence or outcomes of covid-19? BMJ 369: m1548, 2020. 10.1136/bmj.m1548 [DOI] [PubMed] [Google Scholar]