Visual Abstract
Keywords: acute kidney injury and ICU nephrology, acute kidney injury, continuous kidney replacement therapy, COVID-19, hemodialysis, hospital, intensive care unit, New York City, pandemic, peritoneal dialysis, prone ventilation
Abstract
Background
The COVID-19 pandemic strained hospital resources in New York City, including those for providing dialysis. New York University Medical Center and affiliations, including New York City Health and Hospitals/Bellevue, developed a plan to offset the increased needs for KRT. We established acute peritoneal dialysis (PD) capability, as usual dialysis modalities were overwhelmed by COVID-19 AKI.
Methods
Observational study of patients requiring KRT admitted to Bellevue Hospital during the COVID surge. Bellevue Hospital is one of the largest public hospitals in the United States, providing medical care to an underserved population. There were substantial staff, supplies, and equipment shortages. Adult patients admitted with AKI who required KRT were considered for PD. We rapidly established an acute PD program. A surgery team placed catheters at the bedside in the intensive care unit; a nephrology team delivered treatment. We provided an alternative to hemodialysis and continuous venovenous hemofiltration for treating patients in the intensive–care unit, demonstrating efficacy with outcomes comparable to standard care.
Results
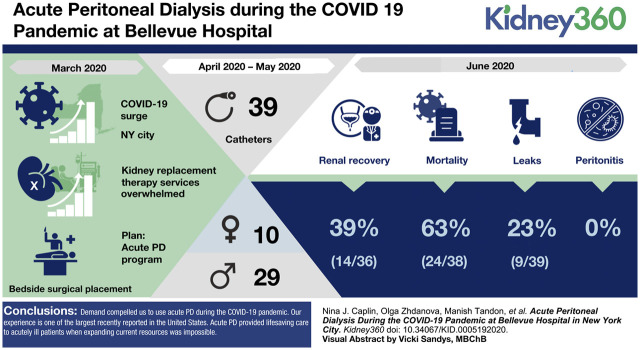
From April 8, 2020 to May 8, 2020, 39 catheters were placed into ten women and 29 men. By June 10, 39% of the patients started on PD recovered kidney function (average ages 56 years for men and 59.5 years for women); men and women who expired were an average 71.8 and 66.2 years old. No episodes of peritonitis were observed; there were nine incidents of minor leaking. Some patients were treated while ventilated in the prone position.
Conclusions
Demand compelled us to utilize acute PD during the COVID-19 pandemic. Our experience is one of the largest recently reported in the United States of which we are aware. Acute PD provided lifesaving care to acutely ill patients when expanding current resources was impossible. Our experience may help other programs to avoid rationing dialysis treatments in health crises.
Introduction
The COVID-19 pandemic created an unprecedented strain on health care systems around the world. Early data from Wuhan, China (1–3), did not report the high rates of AKI that were subsequently seen in Italy and New York. The dramatic COVID surge in March 2020 in New York City threatened to overwhelm hospital capacity (4,5) for the provision of KRT. During early March 2020, New York City Health + Hospitals/Bellevue (BH) put together an action plan (Supplemental Material) to manage the anticipated increased needs for KRT; acute peritoneal dialysis (PD) was thought to be the best option to rapidly expand the capacity to provide KRT. By April 1, 2020, it became clear that our ability to handle the surge of patients with AKI using our current modalities, intermittent hemodialysis (IHD) and continuous venovenous hemofiltration (CVVH), was insufficient. Many hemodialysis nurses were unavailable due to COVID-related illness, resulting in a shortage of trained nurses. The intensive care unit (ICU) nursing staff that performs CVVH was overtaxed because of the expansion of ICU capacity mandated by New York State. Furthermore, CVVH machines were being used at full capacity and CVVH supplies were rationed by the supplier and being rapidly depleted.
In response, we promptly implemented the plan that had been conceived weeks before the surge. Under normal circumstances in Bellevue, acute PD had not been utilized for AKI for several decades. According to the International Society of Peritoneal Dialysis guidelines, the use of PD to treat patients with AKI is an acceptable form of treatment (6). Given the urgent nature of the circumstances, we felt the necessity to establish acute PD capability as our usual KRT modalities were being overwhelmed by COVID-19 AKI. Herein, we describe the rapid and successful implementation of an acute PD program during the COVID-19 pandemic in a period from mid-March to May 2020.
ICU Capabilities and Initial Challenges for KRT
Before the COVID-19 pandemic, BH had 66 ICU beds, ten ICU-capable beds in the emergency department (ED), and 780 total beds. The inpatient hemodialysis (HD) unit could accommodate 12 inpatients/d, 6 d per wk. Three portable HD machines and four CVVH machines were sufficient to provide bedside dialysis in both the ICU and non-ICU inpatients.
A total of 51 patients were seen by the Nephrology Service between January 20 and February 1, 2020; 26 were patients with ESKD who received dialysis in the inpatient unit, nine patients had ESKD who received bedside dialysis. A total of 11 patients in the ICU had AKI, five received bedside IHD, three received CVVH, and three did not require KRT. Typically, fewer than five patients per day received bedside KRT between the ICU and regular floors.
In response to the surge, 90 overflow ICU beds were established in ICUs, the ED, the endoscopy suite, and retrofitted old wards. At its peak on April 7, 2020, Bellevue had 134 ICU patients and 25 additional critical care patients in an overflow ICU area in the ED. Between March 10 and May 17, 2020, the nephrology service evaluated a total of 159 ICU patients with stage 2 or 3 AKI, most requiring KRT. This was not expected and was above the previously reported levels of COVID-19–associated AKI (4,5).
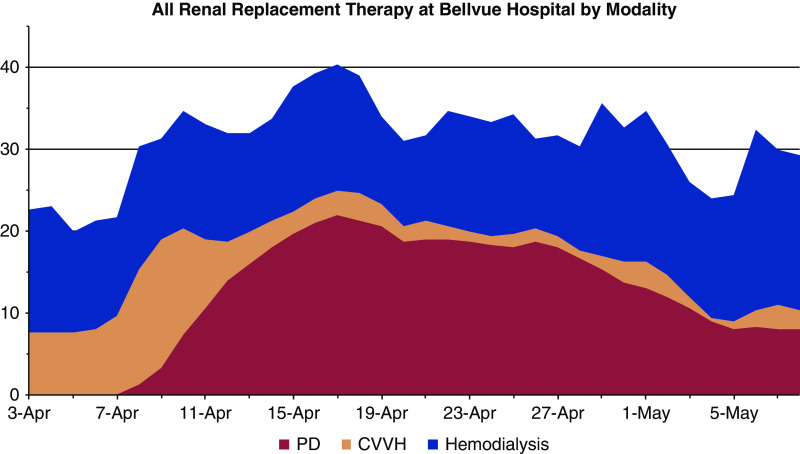
Additionally, the inpatient HD unit was closed for patients with COVID, necessitating bedside HD for all admitted patients as most patients without COVID were transferred to outside hospitals. During this time, we accommodated 35–40 inpatients requiring KRT per day, 15–20 of whom received bedside hemodialysis treatments (Figure 1).
Figure 1.
Dialysis modalities between April 3, 2020 and May 8, 2020 at Bellevue Hospital Center. Data are shown in stacked plot format and are smoothed by a 3-day rolling average to reduce the impact of day-to-day variabilities in staffing. Data shown are PD (peritoneal dialysis, red), CVVH (continuous venovenous hemofiltration, tan), and hemodialysis (blue).
The number of patients requiring KRT exceeded our baseline capacity for both IHD and CVVH. The challenges for providing IHD were (1) inadequate number of portable HD machines, (2) insufficient staffing due to illness and increased census, and (3) lack of adequate plumbing for water sources and usable drains in newly created ICU areas. Provision of CVVH was also severely limited for the same reasons.
We attempted to expand our KRT capabilities in several ways. Hemodialysis treatments were shortened, and the frequency decreased to less than three times per week for selected patients on the basis of their metabolic and volume requirements. Patients without COVID but with stable chronic HD were transferred to affiliated facilities. CVVH was expanded by performing two 10-hour accelerated venovenous hemofiltration treatments per machine in 24 hours, providing two patients with treatments each day (7,8). However, this strategy rapidly depleted CVVH disposable supplies (filters, tubing, dialysate bags, disposable bags) with no prospect of replenishment due to the nationwide rationing imposed by suppliers. Patients infected with COVID-19 also had an increased propensity to clot HD blood lines, as well as IHD and CVVH membranes and circuits, rendering these modalities useless in some patients and contributing to supply shortages. These patients experienced worsened anemia, further driving demand for blood products that were in short supply. We were facing the prospect of rationing dialysis resources.
To address these issues, we created an acute PD program. Initial planning took place in the weeks before the surge and took about 2 weeks to implement, with the first PD catheter placed on April 8, 2020. The acute PD program turned out to be instrumental in the BH response to COVID-associated AKI.
Materials and Methods
Planning
The use of acute PD to treat AKI requiring KRT was nonexistent before the COVID-19 pandemic at BH. Both outpatient HD and PD were outsourced at BH and rarely were patients with chronic PD treated in the hospital. However, in anticipation of a greater need for KRT during the surge of patients with COVID-19, we put together an acute PD implementation protocol (Supplemental Material) in discussion with colleagues internally and from other institutions. The protocol described the roles and responsibilities of the staff, included supply lists, guidance for PD catheter placement, and links to online training resources for performing continuous ambulatory PD, automated PD, and percutaneous catheter placement.
Training of staff was a priority because there were few able to perform PD. We had to educate ICU doctors and nurses about acute PD in AKI and the equivalence of PD to other KRT modalities, and how its use would avoid rationing (9–12).
Supply-Chain Issues
Before the surge, we accrued a list of PD supplies with the assistance of experienced PD nurses. We were able to find vendors who rapidly supplied us with solutions, transfer sets, drain bags, and PD catheters. We obtained about 100 catheters of different sizes to ensure sufficient capacity and to reduce the need to restock in the middle of the pandemic. With elective surgery suspended and operating supplies available, surgeons were able to assemble catheter insertion instrument trays. Personal protective equipment (PPE) was used according to BH infection control protocol for each patient contact, as is done for CVVH or HD or other procedures.
Surgical Support
A team of surgeons committed to providing support with insertion and management of PD catheters was an essential part of the plan. The lead surgeon (M.T.) at BH was responsible for finalizing the details of the insertion technique and acted as a point person for all procedures to increase efficiency. The team of surgeons was available around the clock, 7 days a week, allowing PD catheters to be placed typically within 12 hours of request by the nephrology team. The catheters were primarily inserted using a limited cut down to the peritoneal membrane through the rectus muscle at bedside in the ICU as all but one patient was intubated and sedated (13,14). Laparoscopic technique was not employed because of the potential aerosolization of COVID-19 particles (15).
Staffing, Staff Training, and Initial Experience
The major advantages of PD are its low-tech nature and relative ease for rapid training. This was critical given the constraints of trained nursing staff noted above. The initial PD team consisted of the lead nephrologist (N.C.), volunteer non-nephrology physicians (including pediatric ophthalmologists and a dermatologist), and ambulatory care nurses. Initially, there were no PD nurses in the hospital available to assist. Subsequently, the team grew to include two volunteer PD nurses (day 9 of our effort), nurse practitioners, and physician assistants obtained through the Federal Emergency Management Agency. The team members from this agency who were PD nurses also assisted with hands-on training and supervision. The PD prescription was managed by the PD consult service that included nephrology attendings and renal fellows in consultation with the ICU team.
An experienced PD nurse from a private outpatient dialysis unit affiliated with BH and the lead nephrologist made training videos for manual PD and for automated PD. Lessons from the nephrologist and the “homemade” training videos were used to train the new PD team. Online resources from Fresenius and Baxter (Supplemental Material) were also utilized for additional detail but were not tailored for our acute PD needs. Team members were familiar with the main aspects of sterile procedures due to their medical background and were able to effectively learn the sterile procedures needed for PD. Overall, 25 people were on the PD team and we were able to provide exchanges 24 h/d by the end of the first week.
PD Prescription and Delivery
PD catheters were flushed and used immediately after insertion with low volume exchanges (500 ml) using continuous ambulatory PD bags with heparinized dialysate. The freshly inserted catheter was flushed three times with 500 ml of 1.5% dextrose dialysate solution or until clear if bloody. Heparin, 500 U/L, was added to dialysate to prevent fibrin formation. The initial exchange volume was 500 ml of 1.5% or 2.5% dextrose solution with a dwell time of 2 hours. In the absence of leaks, we increased exchange volume by 250 ml every 2–3 exchanges for the first six exchanges then more rapidly until a volume of 2000 ml was reached, usually within the first 36 hours. In the event of leaks, dwell volume was reduced, or exchanges were held for 12 hours. The typical PD prescription was 5–8 exchanges/d, depending on dwell time, over 17 hours. As team members were added, we expanded PD exchanges to 24 hours and were able to achieve higher clearance using manual PD until cyclers were available. The typical exchange volume was 10–16 L/d when manual PD was used; exchange volume increased to 17–20 L/24-h period when cyclers were used. Adjustments to these prescriptions were made according to individual patient ultrafiltration and metabolic needs.
In mid-April, we acquired 18 automated cyclers, which greatly eased the workload of the PD team and enabled high–volume PD for better clearance (16,17). Patients who had functioning PD catheters and were in the supine position were subsequently placed on cyclers following our initial manual prescription to ensure the catheter was functioning well.
Patients in the prone position remained on PD using manual exchanges because occasionally flow was obstructed and was more easily adjusted with manual exchanges. Obstruction of flow occurred less frequently with more experience and better coordination with the proning team. In total, seven patients received PD while being placed in the prone position for 19 h/d, one of whom recovered to her baseline kidney function. The prescription was adjusted for these patients with manual exchanges every 1 hour while supine and every 2–3 hours while prone, with a maximum 1500 ml dwell while in the prone position. We were able to successfully perform adequate manual PD on patients who were prone with minimal complications by carefully coordinating with proning teams (16,17).
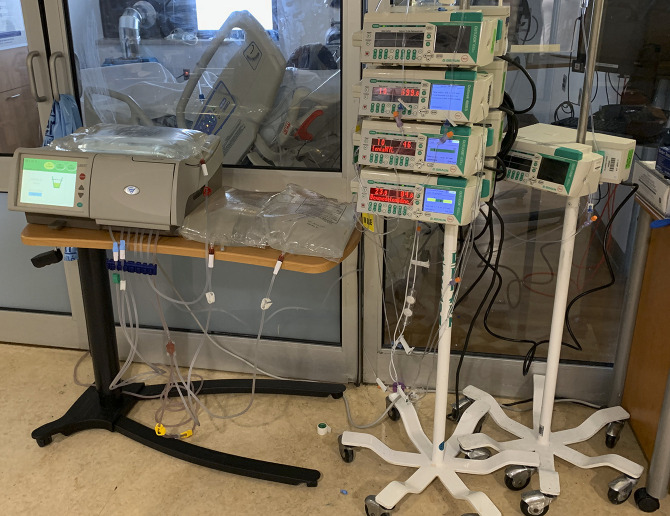
Patients were moved closer to the door, enabling the cyclers to be placed outside of the room (Figure 2), to minimize exposure of staff to COVID-19 infection and to lessen use of PPE.
Figure 2.
Placement of the cycler outside the patient room in the intensive care unit (ICU). Subsequently, drain bags were also used obviating the need for a drain line. The room is retrofitted with high-efficiency particulate absorbing (HEPA) filters to accommodate airborne isolation.
Eligibility
The renal consult team was initially consulted by the ICU team to evaluate patients with AKI. All patients with COVID, who had rapidly rising creatinine, were severely oliguric, acidotic, hypervolemic, hyperkalemic, or had uremic symptoms were considered for RRT.
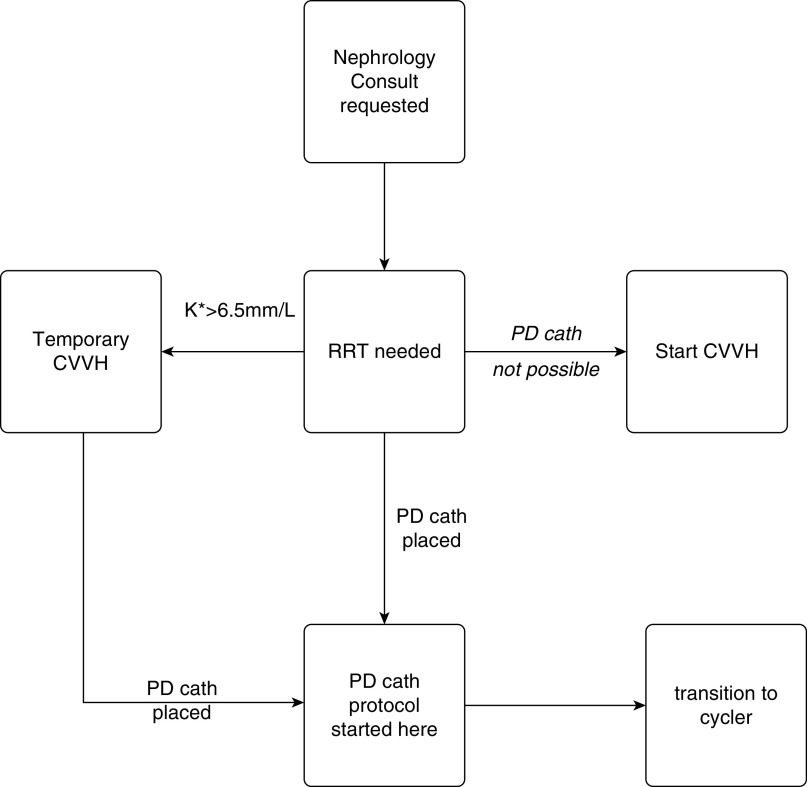
A decision-making tree for choosing dialytic modality is shown in Figure 3. All patients who needed KRT in the ICU were eligible to receive PD catheters except for those in whom we anticipated technical challenges, usually because of prior abdominal surgery, severe obesity, or known varices. All the eligible patients with PD in the ICU were intubated, sedated, and on pressor support for hypotension. If patients were hyperkalemic (serum potassium concentration >6.5 mEq/L) despite medical therapy, such that rapid dialytic removal of potassium was necessary; CVVH treatments were started while simultaneously having a PD catheter placed if they had no contraindications. There were early concerns that respiratory status might be adversely affected by PD (17,18). This did not occur in our patients. We were able to successfully place PD catheters in patients with morbid obesity, up to a body mass index of 51 kg/m2. Some patients who had been on CVVH and had no contraindications were transitioned to PD once the program was started if believed to require a prolonged hospitalization. ESKD patients in the ICU who were on CVVH because of hemodynamic instability were also considered for PD if a prolonged admission was anticipated. Additionally, because proning was not always planned, we did not consider it a contraindication (18).
Figure 3.
Decision–making tree for peritoneal dialysis treatment. K, potassium.
In summary, enough PD catheters were placed to offset shortages in other modalities and allowed CVVH and hemodialysis to be done for those not suitable for PD, thereby meeting the needs of all AKI patients; all patients requiring KRT received it.
IRB Review
The study was approved by the institutional review board at New York University Langone Hospital (study i20-00809). This is a retrospective review and thus the requirement for informed consent was waived.
Results
Daily dialysis treatments, all modalities, between April 7 and May 8, 2020 ranged from 30 to 40 (Figure 1). As of May 8, 2020, 63 patients were evaluated for PD and 39 PD catheters were placed into ten women and 29 men. The average age was 59.5 years. Two patients had ESKD. Outcomes are summarized in Table 1. As of June 10, 2020, 39% of the AKI patients started on PD recovered adequate kidney function and dialysis was stopped. All patients continued on PD as long as needed or until they died. The average age of men and women who recovered renal function was 56 and 59.5 years, respectively, and for men and women who expired was 71.8 and 66.2 years, respectively. One ESKD patient who changed to PD because of vascular access complications was discharged on PD.
Table 1.
Patient outcomes by gender and age
| Outcome | Male | Female | Total |
| Patients who received PD catheters | 29 | 10 | 39 |
| Recovered before starting PD | 1 | 0 | 1 |
| ESKD | 1 | 1 | 2 |
| Recovered and had catheter removeda | 9/27 (33%) | 5/9 (56%) | 14/36 (39%) |
| Expired on PD | 20/28 (71%) | 4/10 (40%) | 24/38 (63%) |
| Average age of all patients (yr) | 59.5 | 66.2 | 59.5 |
| Average age, recovered patients (yr) | 56 | 59.5 | 57.6 |
| Average age, expired patients (yr) | 71.8 | 62.3 | 60.6 |
PD, peritoneal dialysis.
ESKD patients are not included in this calculation.
Of the 39 patients who had catheters placed, nine (23%) had transient leaks that were resolved with reduction of dwell volume. There were no cases of peritonitis (0%), tunnel infections, or exit site infections. Two catheters (5%) needed surgical revision because of poor flow, and six (15%) catheters had minimal postplacement bleeding treated with Surgicel. One patient (2.5%) with a poorly functioning catheter required conversion to HD before recovery.
PD delivery in our patient population was monitored closely by the attending nephrologists. The patients were monitored for extracellular fluid volume overload and depletion, electrolyte and urea levels, and acid-base status to assess efficiency as with the other KRT modalities. The goal ultrafiltration (UF) volume was discussed with the ICU team and dialysate solution dextrose concentrations were adjusted accordingly. We were able to routinely achieve prescribed UF rates, removing up to 5 L in a 24-hour period, on par with other modalities. Of particular note, we used lower volume exchanges to avoid respiratory compromise (16,17). PD was tolerated by ventilated patients with hemodynamic instability and did not cause blood loss or systemic infections seen with the other modalities.
With this protocol and a large team of people who were able to perform many exchanges per day, we were able to maintain adequate clearance in the acute PD patients. Other than the one patient who switched to hemodialysis due to catheter malfunction, no PD patient required supplemental dialytic support with hemodialysis or CVVH.
Discussion
New York City was the epicenter for COVID-19 infections in the United States in mid-March until end of May 2020. BH, the largest public hospital in New York City and the tertiary referral hospital for the Health and Hospitals Corporation network of New York City public hospitals, was particularly taxed. The number of COVID–associated AKI patients overwhelmed our typically used dialysis modalities, compelling us to start an acute PD program to provide adequate KRT. To our knowledge, our experience with acute PD at a single hospital is the largest reported during the pandemic and one of the largest case series of acute PD reported in the United States in recent years.
Acute PD as a modality to treat KRT has become underutilized. We summarize some of the limitations to the use of various modalities in Table 2. Several studies and meta-analyses show PD to be noninferior to IHD or CVVH (16,17). It also continues to be used widely in children (19–21). Nevertheless, there is a reluctance to use PD to treat adult patients in the ICU in the United States. The reasons for this underutilization may be a lack of familiarity with the technique by nephrologists, intensivists, and nursing staff, and the ease of ordering CVVH by the physicians. Unease about the certainty of UF and clearance potential and misconceptions regarding complications or effectiveness despite many positive trials also contribute (21). We observed a mortality rate of 63% for patients with stage 3 AKI receiving PD, comparable to or less than the mortality reported in other series of patients with COVID with stage 3 AKI, suggesting we were able to deliver adequate therapy (5,22,23). Furthermore, we were able to achieve this with a negligible complication rate, which is a tribute to the skill of the surgical team and the scrupulous technique of the PD and nursing staff.
Table 2.
Limitations of different KRT modalities during the COVID-19 surge
| Resource | HD | CVVH | Acute PD |
| Acceptance | Familiar, commonly used | Familiar, commonly used | Not used in Bellevue except rarely for chronic PD patients |
| Nursing staff | Limited trained dialysis nurses and many sick from COVID-19 | ICU nurses trained in CVVH but ICU nurses overwhelmed with increased patient numbers | ICU nurses trained in PD, but rarely used it. Required retraining effort when already overwhelmed |
| Non-nursing Staff | Difficult to train acutely | Difficult to train. Trained non-ICU nurses but needed significant oversight by the ICU nurses | Easier to train medical staff. Nephrologist-trained deployed MDs, PAs, and non-dialysis nurses |
| MD staffing | Adequate number of nephrologists to oversee | Adequate number of nephrologists and intensivists to oversee | Increased nephrology staff needed to implement the program. Surgeons available due to canceled elective cases |
| Nondisposable equipment | Fixed number of dialysis machines | Fixed number of CVVH machines | Not needed for manual PD, initially no cyclers, obtained 18 cyclers |
| Disposable equipment | Adequate supplies | Filters depleted by clotting and using machines for two people per d. Filters and fluid rationed by supplier |
|
| Disease–related limitations | Hemodynamic instability | Hypercoagulability with significant number of clotted filters | Hypermetabolic, prone positioning was used, laparoscopic placement avoided, ARDS on ventilator |
| Benefits | Easy placement of dialysis catheter | Easy placement of dialysis catheters |
|
| Potential risks | Sepsis, clotting, blood loss, hypotension, bioincompatible membrane | Sepsis, clotting, blood loss, hypocalcemia, alkalosis, potentially bioincompatible | Infection (peritonitis, tunnel infection), leak, inflow/outflow problems, hyperglycemia, bleeding at surgical site |
| Other issues | Requires plumbing in room | Not available in the ED overflow ICU | 24 h before peritoneum primed for effective dialysis |
HD, hemodialysis; CVVH, continuous venovenous hemofiltration; PD, peritoneal dialysis; ICU, intensive care units; MD, medical doctors; PA, physician assistant; OR, operating rooms; ARDS, acute respiratory distress syndrome; ED, emergency department.
Our experience provides a roadmap for responses to future crises with heavy burdens of AKI. It demonstrates that the rapid development of PD capability is a viable alternative to reliance on expanding hemodialysis and CVVH capacity, and can be implemented in centers with minimal prior experience with PD. There are several advantages of this approach. We reduced our reliance on a single source of consumable supplies, a critical factor if future crises challenge typical supply chains in the same fashion as COVID-19. The simplicity of PD allows rapid training of traditional and nontraditional medical staff to deliver PD, which is not possible with more technically complex hemodialysis and CVVH options. The use of automated cyclers further simplified delivery and limited the number of patient contacts per day, thereby reducing provider risk compared to hemodialysis and CVVH and preserving PPE. Lastly, PD can be delivered manually and is not limited by the availability of dedicated machines, or electrical power.
Key elements required for successful implementation include organization of a multidisciplinary team including nephrology, surgical, and nursing, development of standard protocols, education of ICU staff, and a resource for rapid training. We believe that adoption of the steps outlined may be key to avoiding the need to ration KRT in future waves of COVID-19 or other health crises and should be considered for programs considering how to ensure adequate responses. We advocate that acute PD can and should be used in acutely ill patients. During times of shortages, it can be used to offset other modalities when expanding current resources is impossible.
Our experience demonstrates that establishing an acute PD program during a crisis is possible and can be lifesaving.
Disclosures
D. Charytan has consulted for Fresenius Medical Care and Medtronic, has received research support from Bioporto and Medtronic, and has received fees related to service on a trial committee from PLC Medical. D. Goldfarb has consulted for AstraZeneca. All remaining authors have nothing to disclose.
Funding
None.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0005192020/-/DCSupplemental.
Guide for acute peritoneal dialysis for treatment of renal failure in COVID + patients. Download Supplemental Material, PDF file, 3.3 MB (3.2MB, pdf)
Introduction.
PD Candidates.
COVID-19 Concerns.
PD Protocol.
Procedure.
Common PD issues.
PD catheter placement.
PD training videos.
PD supplies.
Acknowledgments
We thank all the PD team members, especially Dr. Joyce Kadji, for all their hard work and dedication during this difficult time. We are grateful for the support of the Bellevue Hospital administration in obtaining the needed supplies, thank Fresenius for supplying PD equipment during the crisis, and thank Dr. Kevin H. Gardner for comments on the manuscript. Thanks to Dr. Jaime Uribarri for all his ongoing advice and support.
Author Contributions
B. Dyal was responsible for the methodology; B. Gelb conceptualized the study; D. Bails was responsible for the resources; D.M. Charytan conceptualized the study, and was responsible for the methodology, resources, visualization, and the review and editing of the writing; D. Patel was responsible for the investigation and methodology; D.S. Goldfarb conceptualized the study, and was responsible for the review and editing of the writing; F. Ranjeeta was responsible for the investigation; H. Chawla was responsible for the data curation and review and editing of the writing; J. Benstein conceptualized the study, and was responsible for the review and editing of the writing; J. Scherer was responsible for data curation, investigation, methodology, and the review and editing of the writing; L. Joseph was responsible for the investigation; M. Tandon was responsible for the investigation, methodology, and writing of the original draft; N.J. Caplin conceptualized the study, conducted the formal analysis, investigation, project administration, resources, supervision, and wrote the original draft; N. Thompson was responsible for data curation, methodology, and project administration; O. Zhdanova conceptualized the study, curated the data, conducted the investigation, and wrote the original draft; Q. Soomro was responsible for the investigation and methodology; R. Amerling was responsible for the investigation and the review and editing of the writing; S. Iyer was responsible for data curation; S. Joshi was responsible for the data curation and investigation.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study [published correction appears in Lancet 395: 1038, 2020 10.1016/S0140-6736(20)30606-1]. Lancet 395: 1054–1062, 2020. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Li X, Chen H, Yan S, Li D, Li Y, Gong Z: Coronavirus disease 19 infection does not result in acute kidney injury: An analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol 51: 343–348, 2020. 10.1159/000507471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldfarb DS, Benstein JA, Zhdanova O, Hammer E, Block CA, Caplin NJ, Thompson N, Charytan DM: Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol 15: 880–882, 2020. 10.2215/CJN.05180420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium: Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullis B, Abdelraheem M, Abrahams G, Balbi A, Cruz DN, Frishberg Y, Koch V, McCulloch M, Numanoglu A, Nourse P, Pecoits-Filho R, Ponce D, Warady B, Yeates K, Finkelstein FO: Peritoneal dialysis for acute kidney injury. Perit Dial Int 34: 494–517, 2014. 10.3747/pdi.2013.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allegretti AS, Endres P, Parris T, Zhao S, May M, Sylvia-Reardon M, Bezreh N, Culbert-Costley R, Ananian L, Roberts RJ, Lopez N, Charytan DM, Tolkoff-Rubin N: Accelerated venovenous hemofiltration as a transitional renal replacement therapy in the intensive care unit. Am J Nephrol 51: 318–326, 2020. 10.1159/000506412 [DOI] [PubMed] [Google Scholar]
- 8.Gashti CN, Salcedo S, Robinson V, Rodby RA: Accelerated venovenous hemofiltration: Early technical and clinical experience. Am J Kidney Dis 51: 804–810, 2008. 10.1053/j.ajkd.2008.01.012 [DOI] [PubMed] [Google Scholar]
- 9.Al-Hwiesh A, Abdul-Rahman I, Finkelstein F, Divino-Filho J, Qutub H, Al-Audah N, Abdelrahman A, El-Fakhrany N, Nasr El-Din M, El-Salamony T, Noor A, Al-Shahrani M, Al-Otaibi K: Acute kidney injury in critically ill patients: A prospective randomized study of tidal peritoneal dialysis versus continuous renal replacement therapy. Ther Apher Dial 22: 371–379, 2018. 10.1111/1744-9987.12660 [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Zhang L, Liu GJ, Fu P: Peritoneal dialysis for acute kidney injury. Cochrane Database Syst Rev 12: CD011457, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponce D, Balbi A, Cullis B: Acute PD: Evidence, guidelines, and controversies☆. Semin Nephrol 37: 103–112, 2017. 10.1016/j.semnephrol.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 12.Gabriel DP, Nascimento GV, Caramori JT, Martim LC, Barretti P, Balbi AL: Peritoneal dialysis in acute renal failure. Ren Fail 28: 451–456, 2006. 10.1080/08860220600781245 [DOI] [PubMed] [Google Scholar]
- 13.Vigiola Cruz M, Bellorin O, Srivatana V, Afaneh C: Safety and efficacy of bedside peritoneal dialysis catheter placement in the COVID-19 era: Initial experience at a New York city hospital. World J Surg 44: 2464–2470, 2020. 10.1007/s00268-020-05600-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Shamy O, Sharma S, Winston J, Uribarri J: Peritoneal dialysis during the coronavirus disease-2019 (COVID-19) pandemic: Acute inpatient and maintenance outpatient experiences. Kidney Med 2: 377–380, 2020. 10.1016/j.xkme.2020.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadi SA, Guidolin K, Caycedo-Marulanda A, Sharkawy A, Spinelli A, Quereshy FA, Okrainec A: Current evidence for minimally invasive surgery during the COVID-19 pandemic and risk mitigation strategies: A narrative review. Ann Surg 272: e118–e124, 2020. 10.1097/SLA.0000000000004010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida CP, Balbi AL, Ponce D: Effect of peritoneal dialysis vs. haemodialysis on respiratory mechanics in acute kidney injury patients. Clin Exp Nephrol 22: 1420–1426, 2018. 10.1007/s10157-018-1598-7 [DOI] [PubMed] [Google Scholar]
- 17.Almeida CP, Ponce D, de Marchi AC, Balbi AL: Effect of peritoneal dialysis on respiratory mechanics in acute kidney injury patients. Perit Dial Int 34: 544–549, 2014. 10.3747/pdi.2013.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klisnick A, Souweine B, Filaire M, Wauquier JP, Gazuy N, Deteix P, Baguet JC: Peritoneal dialysis in a patient receiving mechanical ventilation in prone position. Perit Dial Int 18: 536–538, 1998. 10.1177/089686089801800516 [DOI] [PubMed] [Google Scholar]
- 19.Ponce D, Balbi AL, Amerling R: Advances in peritoneal dialysis in acute kidney injury. Blood Purif 34: 107–116, 2012. 10.1159/000341648 [DOI] [PubMed] [Google Scholar]
- 20.Vasudevan A, Phadke K, Yap HK: Peritoneal dialysis for the management of pediatric patients with acute kidney injury. Pediatr Nephrol 32: 1145–1156, 2017. 10.1007/s00467-016-3482-6 [DOI] [PubMed] [Google Scholar]
- 21.Srivatana V, Aggarwal V, Finkelstein FO, Naljayan M, Crabtree JH, Perl J: Peritoneal dialysis for acute kidney injury treatment in the United States: Brought to you by the COVID-19 pandemic. Kidney360 1: 410–415, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, Ma Z, Huang Y, Liu W, Yao Y, Zeng R, Xu G: Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020. 10.1681/ASN.2020030276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Guide for acute peritoneal dialysis for treatment of renal failure in COVID + patients. Download Supplemental Material, PDF file, 3.3 MB (3.2MB, pdf)