“Wisdom comes to us when it can no longer do any good”. Gabriel García Márquez, in Love in the Time of Cholera
Respiratory Disease, Respiratory Failure, and Ventilator Shortages
Those were the concerns in late February and early March, shortly before the surge of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 2019 (COVID-19) hit the United States. By late March, not only were intensive care unit beds full and respiratory failure rampant in some major cities in the United States, but there was a new enemy lurking. The tsunami started to hit. It was not volume depletion-induced serum creatinine elevations. It was severe AKI with multiple electrolyte derangements. Troops were summoned and dialysis nurses worked around the clock to try to provide clearance to patients all over hospitals. Four-hour dialysis treatments were a thing of the past; 2 hours sometimes had to suffice. While President Trump enacted the Defense Production Act to force companies to make more ventilators, kidneys and life-sustaining dialysis machines were the forgotten resources.
How did this happen? By the beginning of June, over 6.9 million patients had been diagnosed worldwide and more than 400,000 people had died. However, the early reports from China noted a relatively low incidence of AKI, some as low as 0.5%, with most studies reporting cumulative incidence in the single digits to the teens (1).
Thus, the United States was not expecting an onslaught with regard to kidney disease
Now that the initial wave of the pandemic has passed, five studies from centers in the United States have come out documenting a much higher proportion (19%–43%) of AKI in hospitalized patients with COVID-19 (Table 1).
Table 1.
Reported incidence of AKI and need for RRT in United States centers
| Centera | Paper | Study Population | n and Proportion with AKIb | Need for RRT |
| Northwell Health System | Hirsch et al. (8) | New York hospitalized (N=5449) | 1993 (37%) | 285 (5%) |
| Mount Sinai Health System | Chan et al. (9) | New York hospitalized (N=3235) | 1406 (43%) | 280 (20%) |
| Columbia | Cummings et al. (5) | New York critically ill (N=257) | NR | 79 (31%) |
| Argenziano et al. (10) | New York hospitalized (N=1000) | 288 (33%) | 117 (13.8%) | |
| Oschner Health | Mohamed et al. (2) | New Orleans hospitalized (N=575) | 161 (28%) | 89 (15%) |
NR, not recorded.
Studies of 100 patients or more.
Creatinine rise ≥0.3 mg/dl or 50% rise from baseline.
One of the United States-based studies documented experiences with AKI of hospitalized patients with COVID-19 at the Ochsner Health System in New Orleans (2). The overall incidence of AKI in this cohort was 28%, which aligns with the other United States-based reports (Table 1). Over one half of patients with AKI required acute dialysis; 98% of these patients received sustained low efficiency dialysis, and only two patients never required prolonged intermittent renal replacement therapy or continuous renal replacement therapy. These findings highlight how truly sick patients with COVID-19 were. Unique features of this study from Mohamed et al. are that the majority (71%) of the cohort were black and the authors performed manual chart reviews in an effort to ascertain the causes of AKI. As we and likely others have seen, many patients with severe AKI eventually made comfort care or died before RRT initiation. Whereas most papers consider this as “never having received dialysis,” in their paper Mohamed et al. classified these patients as “patients who died with a rising serum creatinine and oliguria” and chose to treat them as if they had required RRT. This subpopulation accounted for 21.6% of the total number of RRT patients. This unique characterization likely reduces information bias (informative censoring of the outcome of interest due to the competing risk of death or comfort care), and should be considered when comparing the incidence of severe dialysis-requiring AKI with other studies on AKI and COVID-19.
The authors should also be commended on their manual chart review of medical records and notes of >600 patients to identify the causes of AKI. Through their chart review, the authors found that 66% of patients had acute tubular injury. Urinalysis revealed a large proportion of patients with 2+ proteinuria overall (69%) of which 39% were found to be de novo proteinuria. Moreover, 69% had hematuria of which 19% was 2+ or ≥8 red blood cells/hpf. Unfortunately, this study did not report urinalysis findings in patients without AKI. A small portion of the cohort had urinary sediment microscopy performed; additional details of the urine sediment were published in a separate paper (3). The second study from the Oschner investigators is, to date, the only study in the United States to have reported urine sediment in patients with COVID-19 and AKI. Hopefully, as the potential risks of SARS-CoV-2 infectivity in urine become better understood, more urinary sediment evaluation will be performed and reported, given its diagnostic value.
Three patients underwent percutaneous kidney biopsy, of which all had collapsing glomerulopathy. This histopathologic finding has been reported in several case reports of patients with COVID-19 and AKI. Given that the majority of patients were black, it would be of interest to know the proportion of patients in this cohort that had the high-risk APOL-1 genotype, given the association between APOL1 and development of collapsing FSGS in patients with and without HIV. Postmortem studies of kidney tissue report detection of virus in the kidney tubular epithelium and podocytes (4). However, these patients are likely to have had the most severe cases of COVID-19 and, so far, there has been no detection of SAR-CoV-2 in kidney biopsies.
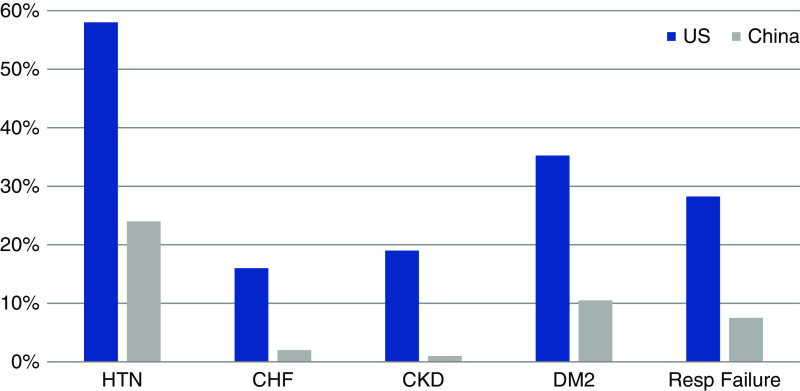
Why do the studies from China have such differing incidence and outcomes of AKI compared with those in the United States? One potential reason may be related to the much higher burden of reported comorbidities in the United States cohorts compared with the Chinese cohorts (Figure 1) (1,2,5). Many of these comorbidities have been associated with worse outcomes in hospitalized patients with COVID-19. Additionally, there may be differences in angiotensin-converting enzyme 2 expression between Asians and occidental persons in various nephron segments, including the proximal tubules, which may explain the increased risk for AKI with SARS-CoV-2 in non-Asians (6). As more reports from other countries come out, it is becoming clearer that there are indeed regional differences in the incidence of AKI, with a study from France reporting that 80% of hospitalized patients developed AKI (7). Although there are nearly 2 million confirmed cases of COVID-19 in the United States alone, data regarding kidney outcomes have only emerged from limited sites thus far (Table 1). To truly understand the epidemiology of kidney disease in patients affected by COVID-19, we will need analyses across different health systems representing heterogeneous people of varied racial, ethnic, and cultural makeup, and health systems with different levels of resources and surge capacity. One such observational study, involving 60 sites across the United States, is currently underway [Study of the Treatment and Outcomes in Critically Ill Patients with COVID-19 (STOP-COVID) NCT04343898] and may shed some light on regional differences across the United States.
Figure 1.
Proportion of reported comorbidities in studies from China and the United States. Adapted from Coca et al. (1) and updated with the United States studies listed in Table 1. HTN, hypertension; CHF, congestive heart faiolure; DM2, type 2 diabetes; Resp, respiratory.
Although much remains to be elucidated about the novel SARS-CoV-2, we need to take a step back to review how much has been achieved in a very short period of time. During the early 1980s, the United States was hit by the AIDS epidemic. It took 2 years to identify the virus, and the first reports of HIV-associated nephropathy were 3 years into the epidemic. The World Health Organization was first notified of COVID-19 on December 31, 2019. The full genetic sequence of SARS-CoV-2 was publicly shared by China on January 12, 2020. Within a 6-month time span we have had numerous epidemiologic studies, several ongoing therapeutic clinical trials, and clinical trials have already begun for potential vaccines. The speed at which these things are being achieved for SARS-CoV-2 is a testament to the progress that science has made in the past few decades.
Now that the surge has quieted in many segments of the United States, the next few months will a critical time in which to expand the investigations into COVID-19 and the kidneys. As the country sets to reopen the economy despite a lack of widespread immunity, and with an effective vaccine months away, it is likely only a matter of months before wave 2. Thus, we disagree with Gabriel García Márquez that “wisdom comes to us when it can no longer do any good.” We have the opportunity to apply insights gained from the first wave to help us manage and triage patients with COVID-19 during the next wave, or a continued plateau that may last for several months. Some critical questions to answer include the following:
(1) What is the incidence of AKI and dialysis during nonsurge conditions in patients admitted with COVID-19?
(2) What is the risk of AKI to CKD transition after COVID-19 (i.e., what proportion of patients will develop incident or progressive CKD after initial hospitalization with AKI)?
(3) Due to the high incidence of acute tubular injury in patients without clinical AKI (4), what will be the risk for CKD in COVID-19 survivors that experienced “subclinical AKI?”
(4) What are the early predictors of both acute and severe AKI, and what are the predictors of nonrecovery and long-term CKD in patients with COVID-19?
(5) What management strategies can be implemented to decrease the risk of both acute and chronic kidney outcomes?
(6) What proportion of patients will have long-lasting proteinuric kidney disease, by involvement of SARS-CoV-2 in podocytes or through “second-hits” in patients with an underlying APOL1 genotype?
Clinical investigators can take this summer reprieve and build up the infrastructure and data pipelines to try to inform the nephrology community, and the population at large, about the full landscape of COVID-19 kidney disease. We owe it to humanity to be better prepared against this devastating viral pandemic.
Disclosures
S. Coca is a cofounder and member of the advisory board of RenalytixAI and owns equity in the same. In the past 3 years, he has received consulting fees from RenalytixAI, CHF Solutions, Quark Biopharma, Takeda Pharmaceuticals, Relypsa, Bayer, Boehringer-Ingelheim, and pulseData. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
Dr. Lili Chan’s effort and research is supported, in part, by National Institutes of Health grant R01AG066471.
Dr. Steven Coca’s effort, research, and biomarker laboratory is supported, in part, by National Institutes of Health grants U01DK106962, R01DK115562, R01HL85757, R01DK112258, U01OH011326, and R01DK126477.
Author Contributions
Both authors were responsible for conceptualization, writing the original draft, and reviewing and editing the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.kidney360.org.
See related article, “Acute Kidney Injury Associated with Coronavirus Disease 2019 in Urban New Orleans” on pages 614–622.
References
- 1.#NephJC : Acute Kidney Injury, Available at: http://www.nephjc.com/news/covidaki. Accessed June 8, 2020
- 2.Mohamed MM, Lukitsch I, Torres-Ortiz AE, Walker JB, Varghese V, Hernandez-Arroyo CF, Alqudsi M, LeDoux JR, Velez JCQ: Acute kidney injury associated with coronavirus disease 2019 in Urban New Orleans. Kidney360 1: 614–623, 2020 [DOI] [PMC free article] [PubMed]
- 3.Hernandez-Arroyo CF, Varghese V, Mohamed MMB, Velez JCQ: Urinary sediment microscopy in acute kidney injury associated with COVID-19. Kidney360 [prepublished online June 2020] 10.34067/KID.0003352020 [DOI] [PMC free article] [PubMed]
- 4.Su HUA, Yang MING, Wan CHENG, Yi LI-XIA, Tang FANG, Zhu HONG-YAN, Yi FAN, Yang HAI-CHUN, Fogo AGNES B, Nie XIU, Zhang CHUN: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98[1]: 219–227, 2020. 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, Aaron JG, Claassen J, Rabbani LE, Hastie J, Hochman BR, Salazar-Schicchi J, Yip NH, Brodie D, O’Donnell MR: Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 395: 1763–1770, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG: Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med 46: 1114–1116, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin S, Orieux A, Prevel R, Garric A, Bats M-L, Dabernat S, Camou F, Guisset O, Issa N, Mourissoux G, Dewitte A, Joannes-Boyau O, Fleureau C, Rozé H, Carrié C, Petit L, Clouzeau B, Sazio C, Bui H-N, Pillet O, Rigothier C, Vargas F, Combe C, Gruson D, Boyer A: Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019 [published online ahead of print June 6, 2020]. Clin Kidney J doi: 10.1093/ckj/sfaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch JAMIE S, Ng JIA H, Ross DANIEL W, Sharma PURVA, Shah HITESH H, Barnett RICHARD L, Hazzan AZZOUR D, Fishbane STEVEN, Jhaveri KENAR D; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium : Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98[1]: 209–218, 2020. 10.1016/j.kint.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Baweja M, Campbell K, Chun N, Chung M, Deshpande P, Farouk SS, Kaufman L, Kim T, Koncicki H, Lapsia V, Leisman S, Lu E, Meliambro K, Menon MC, Rein JL, Sharma S, Tokita J, Uribarri J, Vassalotti JA, Winston J, Mathews KS, Zhao S, Paranjpe I, Somani S, Richter F, Do R, Miotto R, Lala A, Kia A, Timsina P, Li L, Danieletto M, Golden E, Glowe P, Zweig M, Singh M, Freeman R, Chen R, Nestler E, Narula J, Just AC, Horowitz C, Aberg J, Loos RJF, Cho J, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Bottinger EP, Glicksberg BS, Coca SG, Nadkarni GN: Acute Kidney Injury in Hospitalized Patients with COVID-19. Available at: https://doi.org/10.1101/2020.05.04.20090944. Accessed June 23, 2020. medRxiv 2020
- 10.Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, Chang BP, Chau KH, Choi JJ, Gavin N, Goyal P, Mills AM, Patel AA, Romney M-LS, Safford MM, Schluger NW, Sengupta S, Sobieszczyk ME, Zucker JE, Asadourian PA, Bell FM, Boyd R, Cohen MF, Colquhoun MI, Colville LA, de Jonge JH, Dershowitz LB, Dey SA, Eiseman KA, Girvin ZP, Goni DT, Harb AA, Herzik N, Householder S, Karaaslan LE, Lee H, Lieberman E, Ling A, Lu R, Shou AY, Sisti AC, Snow ZE, Sperring CP, Xiong Y, Zhou HW, Natarajan K, Hripcsak G, Chen R: Characterization and clinical course of 1000 Patients with COVID-19 in New York: retrospective case series. Available at: https://doi.org/10.1101/2020.04.20.20072116. Accessed June 23, 2020. medRxiv 2020 [DOI] [PMC free article] [PubMed]