Visual Abstract
Keywords: transplantation, antibody mediated rejection, chronic rejection, IL6 inhibition, immunosuppression, kidney transplant, rejection, tocilizumab
Abstract
Background
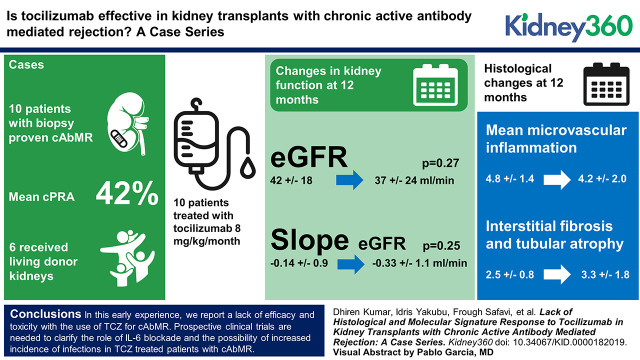
Traditional therapies for caAbMR have unclear efficacy with significant side effects in recipients of kidney transplants (KTs). A recent single-center case series suggested tocilizumab (TCZ) could stabilize renal function and improve microvascular inflammation. Here we report our findings of the use of TCZ in patients with caAbMR.
Methods
Ten adult recipients of KTs with biopsy-proven caAbMR were treated with TCZ at 8 mg/kg per month. Patients were monitored for adverse events, and therapy was interrupted in the setting of serious infections. Six patients (60%) underwent post-treatment biopsies.
Results
Patients (mean age of 43 years) were initiated on TCZ at a median of 36 months post-KT. A majority of patients were black (70%), underwent regrafts (40%), and were sensitized (mean cPRA=41%). Patients received a median of six doses of TCZ (range=3–10). At a median follow-up of 12 months (range=8–24 months), renal function did not show improvement (mean eGFR, 42±18 ml/min per 1.73 m2 to 37±24 ml/min per 1.73 m2; P=0.27). The slope of decline in eGFR remained unchanged (−0.14±0.9 to −0.33±1.1; P=0.25). There was no improvement in mean MVI (g+ptc) (4.8±1.4 to 4.2±2.0; P=0.39) scores or Molecular Microscope Diagnostic System (MMDx) AbMR scores (0.79±0.17 to 0.78±0.26; P=0.86). There was a numeric worsening of chronicity (ci+ct) scores (2.5±0.8 to 3.3±1.7; P=0.38) and MMDx atrophy fibrosis scores (0.36±0.24 to 0.58±0.15; P=0.21). Patient survival was 90%, with one patient death due to complications from a hip infection. Overall death-censored graft survival was 80%, with two graft losses in patients who had recurrent infections requiring hospitalization.
Conclusions
In this early experience, we report a lack of efficacy and toxicity with the use of TCZ for caAbMR. Prospective clinical trials are needed to clarify the role of IL-6 blockade and the possibility of increased incidence of infections in patients with caAbMR who are treated with TCZ.
Introduction
Chronic antibody-mediated rejection (cAbMR) is the leading cause of graft loss in recipients of kidney transplants. It remains the major barrier to improving long-term allograft outcomes (1,2). AbMR is primarily mediated by interactions between antibodies directed against donor endothelium. In most cases, these donor-specific antibodies (DSAs) are directed against human leukocyte antigens (HLA). The resultant chronic inflammation elicits a fibrotic response in the allograft that eventually leads to allograft dysfunction and loss (3). Therapies directed at blunting the effect of these antibodies by targeting removal, production, or preventing complement activation have shown promise in the setting of acute AbMR but have not translated into any success against cAbMR (4–6).
The proinflammatory cytokine IL-6 regulates inflammation and development of T cells, B cells, and plasma cells (7). Tocilizumab (TCZ) is an IgG1 humanized mAb specific for the IL-6 receptor (IL-6R). TCZ binds to both soluble and membrane-bound forms of the IL-6R, leading to a reduction in cytokine and acute-phase reactant production (8). TCZ has been shown to significantly reduce DSA levels in highly sensitized patients undergoing desensitization (9).
In a single-center, uncontrolled case series, Choi et al. (10) reported that TCZ stabilizes renal function, improves microvascular inflammation (MVI), and reduce DSAs in patients with chronic active AbMR (caAbMR) and transplant glomerulopathy.
Herein, we present our single-center experience on the use of TCZ in kidney transplant recipients with caAbMR who were refractory to other therapies, comparing pretreatment and post-treatment biopsy samples assessed by histology and Molecular Microscope Diagnostic System (MMDx). We were specifically interested in evidence that TCZ was suppressing disease activity measurements by histologic or MMDx criteria.
Materials and Methods
Our institutional review board approved this study. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.” Biopsy samples for molecular analyses were taken with informed consent from patients in a protocol approved by our institutional review board. We retrospectively evaluated all “kidney-alone” adult transplant patients who received TCZ with either biopsy and/or molecular confirmation of caAbMR between October 2016 and January 2018.
Immunosuppression and caAbMR Therapies
All patients had received induction immunosuppression with rabbit anti-thymocyte globulin (Thymoglobulin; Genzyme, Cambridge, MA) 6 mg/kg followed by standard post-transplant triple-drug immunosuppression including tacrolimus, mycophenolate mofetil (MMF; 1–2 g/d), and prednisone (tapered to 5 mg/d by 1–3 months post-transplant). Eight of ten patients had a prior episode of acute AbMR. Seven of these patients had previously been tapered off of calcineurin inhibitors (CNIs) and converted to belatacept due to significant residual interstitial fibrosis and tubular atrophy (IFTA), whereas another patient was maintained on a CNI-based regimen. The patients with prior history of acute AbMR had been treated with a combination of plasmapheresis, intravenous Ig, and rituximab and/or bortezomib.
Based on the results of the study by Choi et al. (10), we developed a protocol using TCZ as a salvage agent in patients with caAbMR. A multidisciplinary team consisting of transplant nephrologists, immunologists, pathologists, and pharmacists made a decision to treat patients with TCZ if patients diagnosed with caAbMR met the Banff 2017 revised criteria for caAbMR, which included both histologic as well as molecular assessment of tissue. We avoided TCZ use in patients who did not consent or had recent infections or malignancy.
TCZ was initiated at approximately 8 mg/kg per dose (maximum of 800 mg/dose) intravenously monthly. While on TCZ, none of the patients underwent any additional therapies for AbMR. Patients were monitored very closely for adverse events (AEs), and TCZ was stopped in the setting of serious infections requiring hospitalization.
Biopsy Processing
Biopsies were obtained under ultrasound guidance by spring-loaded needles (Bard Monopty Disposable Core Biopsy Instrument, Tempe, AZ). Paraffin sections were prepared and graded by a single internal renal pathologist. C4d staining was performed on frozen sections using a monoclonal anti-C4d antibody (Quidel, San Diego, CA).
A portion (3–4 mm) of a 16-gauge biopsy core was collected for gene expression analysis. To prevent mRNA degradation, the tissue was immediately stabilized in RNAlater (Life Technologies, Carlsbad, CA) and kept refrigerated until shipping for gene expression analysis.
Biopsy Sample Assessment
All ten patients underwent kidney biopsies before treatment with TCZ. Nine of these pretreatment biopsy samples also underwent transcriptome analysis using the MMDx (ATGAC, Edmonton, Canada) platform. One patient did not undergo pretreatment transcriptome analysis due to unavailability of the platform at the time of the biopsy. Six of the ten patients underwent post-treatment biopsies after they had received a minimum of six doses of TCZ. Five of these post-treatment biopsy samples underwent transcriptome analysis (one patient did not undergo transcriptome analysis due to degraded specimen). Four patients did not undergo post-treatment biopsies due to the following reasons: two lost their allograft to progressive renal dysfunction, one had continued decline in allograft function, and the last patient died with a functioning graft.
Biopsy specimens were graded based upon the revised Banff 2017 criteria by a single pathologist (11). Patients who were biopsied before 2017 were retrospectively regraded. An MVI score was calculated by adding the glomerulitis (g) and peritubular capillaritis (ptc) scores (g+ptc). C4d staining or presence of detectable DSAs was not considered a prerequisite for the diagnosis of AbMR. Presence of AbMR-associated gene transcript expression on biopsy tissue was regarded as valid evidence of antibody interaction with vascular endothelium. All chronic semiquantitative Banff scores were rated as zero to three as per the published criteria. A total chronicity score was calculated as the sum of four basic Banff qualifiers; chronic glomerular damage (cg), interstitial fibrosis (ci), tubular atrophy (ct), and vascular intimal thickening (cv); thus allowing for a total score ranging from zero to a maximum score of 12.
Antibody Testing
Pretransplant complement-dependent cytotoxicity assays and three-color flow cytometric crossmatching were performed for all patients at the time of transplant. DSAs were analyzed using the Luminex platform (Immucor Platform, San Diego, CA) with the use of an HLA phenotype panel (Lifematch Class I and Class II ID; Gen-Probe, San Diego, CA) and a single-antigen panel (Single Antigen Beads; Immucor Platform). Results of bead assays were measured as mean fluorescence intensity (MFI). All patients underwent repeat DSA testing at the time of initial biopsy and then at predetermined intervals after initiation of TCZ. Patients were not screened for non-HLA antibodies.
Statistical Analysis
Descriptive statistics (MedCalc Statistical Software version 19.1; MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2019) were used to estimate the frequencies, means, and medians of study variables. For comparisons of variables before and after TCZ initiation, a paired t test was used. All of the statistical tests were two sided and probability values <0.05 were considered significant.
The clinical outcomes compared were the eGFR at initiation of TCZ (T0); then at 3, 6, and 12 months; and at the most recent follow-up after therapy. Proteinuria was compared pre- and post-TCZ. Slope of eGFR was compared 12 months before and for 12 months after TCZ treatment. Histologic variables of MVI and chronicity index were compared and MMDx scores of AbMR and total rejection were compared between pre- and post-TCZ paired biopsy specimens.
Results
Demographic Characteristics
Table 1 lists the recipient and donor characteristics of the ten patients who received TCZ. The average age was 43±8.4 years. A majority of the patients were black (70%) and male (60%). Many patients (40%) were regrafts and sensitized with a mean calculated panel-reactive antibodies (cPRA) of 42% (median cPRA=32%; range=0%–100%) at the time of transplant. Most patients (60%) received living donor kidneys. The median time to treatment with TCZ was 31 months post-transplant (range=13–115 months). The median doses of TCZ received was six (range, three to ten). The mean dose of MMF was 1.775 g/d at the time of initiation of TCZ therapy.
Table 1.
Demographics
| Characteristic | Value |
| N | 10 |
| Age in years, mean±SD | 43.3±8.4 |
| Male sex, n (%) | 6 (60) |
| Black race, n (%) | 7 (70) |
| Cause of ESKD, n (%) | |
| Hypertension | 3 (30) |
| Polycystic kidney disease | 3 (30) |
| Autoimmune kidney disease | 4 (40) |
| Average time of dialysis, yr±SD | 4.16±4.32 |
| Retransplant, n (%) | 4 (40) |
| Percentage cPRA, mean±SD | 42±42 |
| Delayed graft function, n (%) | 4 (40) |
| Positive donor-specific antibody at transplant, n (%) | 2 (20) |
| Positive donor-specific antibody at TCZ, n (%) | 8 (80) |
| Acute rejection before TCZ, n (%) | 8 (80) |
| Time to TCZ in months, mean±SD | 40±31 |
| Pre-TCZ eGFR in ml/min per 1.73 m2, mean±SD | 42±19 |
| Pre-TCZ proteinuria in g/g, mean±SD | 1.5±1.14 |
| Pre-TCZ microvascular inflammation (g+ptc), mean±SD | 4.1±1.5 |
| Pre-TCZ AbMR score (MMDx), mean±SD | 0.76±0.23 |
| Pre-TCZ TCMR score (MMDx), mean±SD | 0.04±0.09 |
| Donor characteristics | |
| Donor age in years, mean±SD | 43±13 |
| Living donor kidney transplant, n (%) | 6 (60) |
| Median kidney donor profile index, % (range)a | 44 (25–97) |
| Immunosuppression | |
| Mycophenolate+prednisone+belatacept, n (%) | 7 (70) |
| Mycophenolate+prednisone+tacrolimus, n (%) | 3 (30) |
| Mean mycophenolate dose at TCZ, g/d±SD | 1.78±0.34 |
| Median follow-up after TCZ, mo (range) | 12 (8–24) |
| Median TCZ doses, n (range) | 6 (3–10) |
cPRA, calculated panel-reactive antibody; TCZ, tocilizumab; g+ptc, glomerulitis and peritubular capillaritis score; AbMR, antibody-mediated rejection; MMDx, Molecular Microscope Diagnostic System; TCMR, T cell–mediated rejection.
Kidney Donor Profile Index calculated for deceased donor kidney transplants only.
Eight (80%) patients had a prior episode of acute AbMR. At the time of therapy with TCZ, all patients had significant evidence of persistent histologic inflammation with a mean MVI (g+ptc) score of 4.8±1.4 and chronicity with a mean IFTA (ci+ct) score of 2.5±0.84. The severe histologic scores were corroborated by the high MMDx scores for AbMR (0.76±0.23) and atrophy fibrosis (0.45±0.26). It is also important to note that our T cell–mediated rejection scores by the MMDx platform were low at 0.04±0.09, thereby indicating that we were dealing with a pure AbMR group and not a mixed rejection group.
Graft/Patient Survival
At a median follow-up of 12 months (range=8–24 months), overall death-censored graft survival was 80%. Patient survival was 90%, with one patient death due to complications from a postsurgical hip infection with a functioning graft. The two patients who lost their grafts had recurrent infections requiring hospitalization.
Renal Function
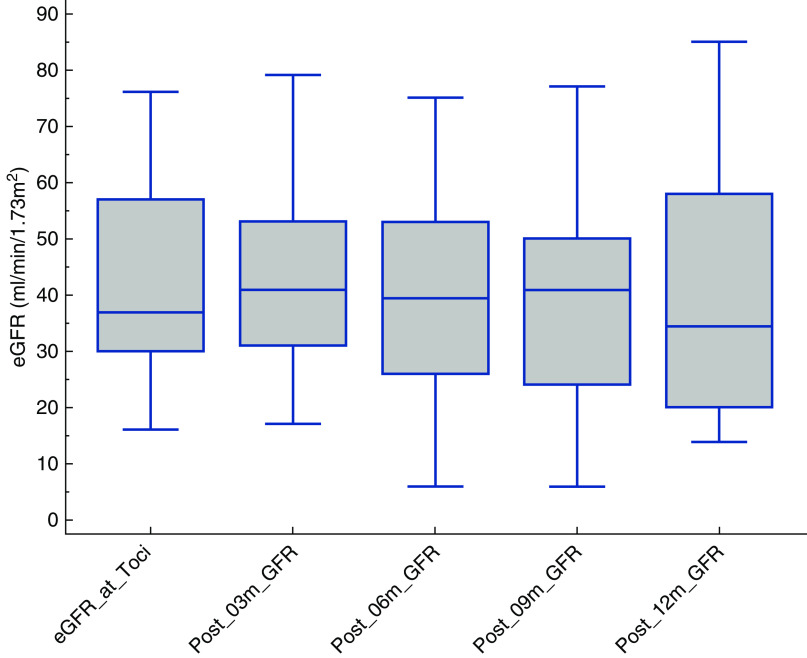
Renal function trends are depicted in Figure 1. There was no improvement in renal function throughout the follow-up period (mean eGFR of 42±19 ml/min per 1.73 m2 pre-TCZ to 39.2±19 ml/min per 1.73 m2 at 6 months [P=0.43] and 37±24 ml/min per 1.73 m2 at most recent follow-up [P=0.27]). Proteinuria remained unchanged from a pre-TCZ mean of 1.6±1.1 g/g to 1.9±2.3 g/g after therapy (P=0.70) (Table 2). The comparison of slope of decline in eGFR remained unchanged before and after treatment (−0.14±0.9 to −0.33±1.1; P=0.60) (Table 2).
Figure 1.
eGFR trend after tocilizumab. Box and whisker plot of eGFR shows no improvement in trend after initiation of tocilizumab (Toci) from time 0 to most recent follow-up.
Table 2.
Results
| Measure | N | Mean (SD) | P Value | |||
| Pre-TCZ | Post-TCZ | |||||
| Graft function | ||||||
| eGFR T0 versus T3m | 10 | 41.6 (18.8043) | 42.2 (17.6937) | 0.71 | ||
| eGFR T0 versus T6m | 10 | 41.6 (18.8043) | 39.2 (19.0193) | 0.43 | ||
| eGFR T0 versus T12m | 6 | 41.7 (20.2846) | 41 (26.6983) | 0.88 | ||
| Proteinuria T0 and Tc | 10 | 1.61 (1.1426) | 1.85 (2.3244) | 0.70 | ||
| Slope eGFR (T0−12 m versus T0+12 m) | 10 | −0.14 (0.9082) | −0.33 (1.0724) | 0.60 | ||
| Histology | ||||||
| MVI | 6 | 4.8333 (1.472) | 4.1667 (2.0412) | 0.39 | ||
| Total chronicity score | 6 | 4.3333 (1.9664) | 5.6667 (3.4448) | 0.29 | ||
| IFTA | 6 | 2.5 (0.8367) | 3.3333 (1.7512) | 0.38 | ||
| MMDx scores | ||||||
| AbMR | 5 | 0.792 (0.1681) | 0.776 (0.2615) | 0.86 | ||
| Total rejection | 5 | 0.83 (0.1454) | 0.79 (0.1488) | 0.51 | ||
| Atrophy fibrosis | 5 | 0.362 (0.2374) | 0.584 (0.1494) | 0.21 | ||
| Global disturbance | 5 | 0.884 (2.243) | 1.646 (1.2158) | 0.44 | ||
TCZ, tocilizumab; T0, at time of initiation of TCZ; T3m, 3 months after initiation of TCZ; Tc, at time of most recent followup; MVI, microvascular inflammation (glomerulitis plus peritubular capillaritis score); IFTA, interstitial fibrosis and tubular atrophy; MMDx, Molecular Microscope Diagnostic System; AbMR, antibody-mediated rejection.
Histologic Outcomes
All ten patients (100%) underwent a pretreatment surveillance biopsy. There were a total of three patients with cg=0 on light microscopy; when regraded based on Banff 2017 revised criteria, all patients had at least peritubular capillary basement membrane multilayering on electron microscopy and all had cg features on molecular diagnosis. All of the patients also had a clinical history that supported the diagnosis of AbMR. Six patients (60%) underwent post-treatment biopsies at a median time of 9 months (range=6–12 months) postinitiation of TCZ. Of the four patients who did not undergo a post-treatment biopsy, two lost their graft rapidly from recurrent infectious complications necessitating stopping TCZ and reduction of immunosuppression, another had significant decline in renal function and—in light of advanced allograft dysfunction—a biopsy was not deemed necessary, and the fourth patient died with a functioning graft from infectious complications after hip surgery. There was no improvement in histologic mean MVI (g+ptc) scores (4.8±1.4 to 4.2±2.0; P=0.39). There was a numeric worsening of histologic IFTA (ci+ct) scores (2.5±0.8 to 3.3±1.8; P=0.38) as well as overall chronicity (ci+ct+cg+cv) scores (4.3±1.9 to 5.7±3.4; P=0.29).
Transcriptome Analysis
All ten (100%) preconversion and five (50%) post-treatment surveillance biopsy specimens were subjected to intragraft mRNA-based gene expression using the MMDx platform. A comparison of molecular changes between the five pairs of pre- and post-treatment biopsies is depicted in Tables 2 and 3. Quantified gene expression scores for total rejection and AbMR remained unchanged from a mean of 0.83±0.15 to 0.79±0.15 (P=0.51) and 0.79±0.17 to 0.78±0.26 (P=0.86), respectively. Similar to histologic findings, there was a numeric worsening of molecular atrophy fibrosis and global disturbance scores from a mean of 0.36±0.24 to 0.58±0.15 (P=0.21) and 0.88±2.24 to 1.64±1.22 (P=0.44), respectively.
Table 3.
Summary of individual patient outcomes
| Patient No. | Donor Type | Maintenance Immunosuppression | Cause of ESKD | cPRA (%) | Regraft | Rejection Pre-TCZ | TVZ Doses (no.) | Immunologic Outcomes | Clinical Outcomes | Histologic Outcomes | Molecular Diagnostic Scores | Graft and Patient Survival Outcomes | Adverse Events | ||||||||||||||||
| Pre-DSA Class I | Pre-DSA Class II | Post-DSA Class I | Pre-total DSA (MFI) | Post-Total DSA (MFI) | Pre-Scr (mg/dl) | Pre-eGFR (ml/min per 1.73m2) | Post-Scr (mg/dl) | Post-GFR (ml/min per 1.73m2) | UPCR At TCZ (g/g) | UPCR Recent (g/g) | Pre ci+ct+cv+cg | Post ci+ct+cv+cg- | Pre g+ptc- | Post g+ptc | Pre-rejection Score | Post-rejection Score | Pre-AbMR Score | Post-AbMR Score | Death | Graft Loss | |||||||||
| 1 | LKT | T+M+Bela | IgAN | 0 | No | Yes | 7 | No | Yes | No | 13,679 | 23,294 | 2.85 | 25 | 3.32 | 20 | 1.8 | 1.5 | 7 | NA | 4 | NA | 0.83 | NA | 0.97 | NA | No | No | None |
| 2 | LKT | T+M+Bela | HTN | 24 | No | Yes | 9 | No | Yes | No | 15,470 | 5550 | 2.37 | 30 | 4.45 | 14 | 0.5 | 1.3 | 2 | 4 | 6 | 6 | 0.98 | 0.96 | 0.98 | 0.99 | No | Yes | UTI, bacteremia, diarrhea, leukopenia |
| 3 | LKT | T+M+Bela | ADPKD | 2 | No | Yes | 6 | No | No | No | 0 | 0 | 2.5 | 30 | 1.94 | 41 | 2.9 | 2.3 | 7 | 11 | 5 | 6 | 0.95 | 0.92 | 0.95 | 0.99 | No | No | Leukopenia, vaginal HSV infection |
| 4 | DDKT | T+M+Bela | DM/HTN | 100 | Yes | Yes | 3 | Yes | No | 2870 | NA | 2.02 | 42 | NA | NA | 2.3 | 0.5 | 4 | NA | 2 | NA | 0.73 | NA | 0.87 | NA | Yes | No | None | |
| 5 | LKT | T+M+Bela | HTN | 2 | No | Yes | 5 | No | Yes | No | 1640 | 0 | 2.71 | 32 | 3.02 | 28 | 1.4 | 4.9 | 5 | NA | 3 | NA | 0.83 | NA | 0.79 | NA | No | No | Bacteremia, hip infection, diarrhea |
| 6 | DDKT | T+M+P | FSGS | 97 | Yes | Yes | 10 | No | Yes | No | 8760 | 3183 | 1.36 | 76 | 1.23 | 85 | 0.1 | 0.1 | 5 | 1 | 3 | 3 | 0.65 | 0.69 | 0.6 | 0.79 | No | No | Clostridium difficile–associated diarrhea |
| 7 | DDKT | T+M+Bela | FSGS | 88 | Yes | Yes | 10 | No | Yes | No | 14,687 | 15,109 | 1.33 | 57 | 1.31 | 58 | 0.1 | 0.1 | 3 | 5 | 3 | 2 | 0.86 | 0.61 | 0.67 | 0.35 | No | No | None |
| 8 | LKT | T+M+Bela | PCKD | 62 | Yes | Yes | 6 | No | Yes | 1975 | NA | 3.56 | 16 | NA | NA | 3.5 | 7 | 5 | NA | 3 | NA | 0.4 | NA | 0.25 | NA | No | Yes | None | |
| 9 | DDKT | T+M+P | Membranous nephropathy | 0 | No | No | 6 | No | Yes | No | 3943 | 2471 | 1.89 | 45 | NA | NA | 1.5 | 0.3 | 3 | 5 | 6 | 2 | NA | 0.92 | NA | 0.88 | No | No | None |
| 10 | LKT | T+M+P | PCKD | 40 | No | No | 6 | No | No | No | 0 | 580 | 1.04 | 63 | NA | NA | 2 | 0.5 | 6 | 8 | 6 | 6 | 0.71 | 0.77 | 0.76 | 0.76 | No | No | None |
cPRA, calculated panel-reactive antibodies; TCZ, tocilizumab; DSA, donor-specific antibody; MFI, mean fluorescence intensity; Scr, serum creatinine; ci, interstitial fibrosis score; ct, tubular atrophy score; cv, vascular intimal thickening score; cg, chronic glomerular damage score; g, glomerulitis score; ptc, peritubular capillaritis score; AbMR, antibody-mediated rejection; LKT, living donor kidney transplant; T, tacrolimus; M, mycophenolate; Bela, belatacept; IgAN, IgA nephropathy; NA, not available; HTN, hypertension; UTI, urinary tract infection; ADPKD, autosomal dominant polycystic kidney disease; HSV, herpes simplex virus; DDKT, deceased donor kidney transplant; DM, diabetes; P, prednisone; PCKD, polycystic kidney disease.
Immunologic Outcomes
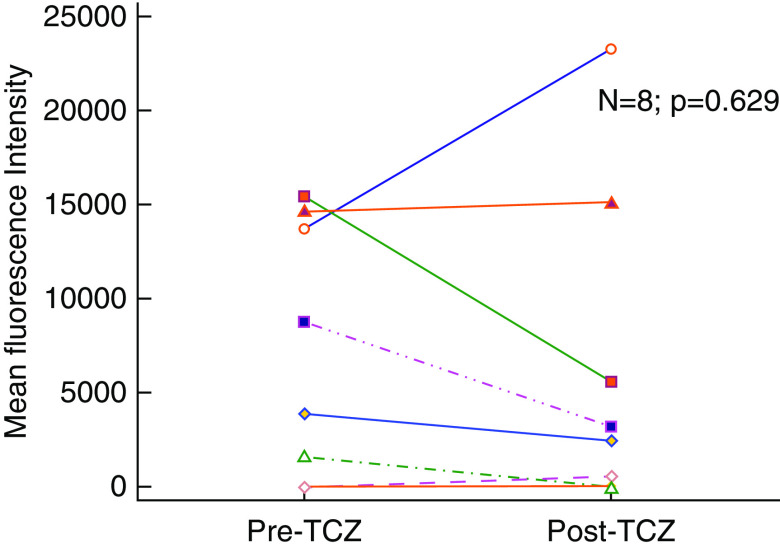
All ten patients underwent HLA DSA testing before conversion. Eight patients (80%) had evidence of anti-HLA DSA at the time of conversion. Seven (88%) of these patients had class II immunodominant DSA. Mean total DSA at the time of therapy was 7272±6698 MFI and remained unchanged at 6273±8480 MFI post-therapy (P=0.63) (Figure 2). Of the two patients who were DSA negative at the time of therapy, one developed low-grade class II DSA post-therapy and the other remained negative. Two patients did not undergo post-therapy DSA testing; one of these patients died and the other lost their allograft and returned to dialysis. None of the DSA-negative patients with caAbMR underwent testing for non-HLA antibodies.
Figure 2.
Donor-specific antibody. Comparison of donor-specific antibody shows no difference before and after therapy with tocilizumab (TCZ).
AEs
In total, nine AEs occurred over 11.6 patient-years of follow-up (0.78 per 100 person-years). Four (40%) patients had infectious AEs. All four patients required hospitalization during the treatment period for infections, mostly due to bacterial infections (three of four patients: post-operative hip infection, arteriovenous graft infection, and diarrhea-associated urinary tract infection with bacteremia). The fourth patient was hospitalized for severe vaginal and paravaginal herpes simplex virus infection. All of these patients had either cessation or interruption of TCZ therapy due to these infections.
There were no cases of fungal, cytomegalovirus, or BK polyomavirus infections post- TCZ conversion. There were also no cases of lymphomas, skin cancers, or any other malignancies observed during the follow-up period. Five (50%) patients developed leukopenia (white blood cell count <4.0×103 cells/mm3). Severe diarrhea leading to recurrent urinary tract infections occurred in one patient (10%) post-treatment. One death was observed during treatment due to infectious complications after hip replacement. However, this patient had a complicated post-transplant course, including failure to thrive before TCZ therapy, and it is difficult to attribute the cause of death solely to TCZ.
Discussion
In this study, we included ten adult recipients of renal transplants who had active cAbMR. The pretreatment biopsies of all of these patients showed significant inflammation, as manifested by high MVI scores on histology and high AbMR scores by the MMDx. The persistent inflammation resulted in a high degree of chronicity in these allografts, as represented by the high chronicity scores on histology and the MMDx.
After treatment with TCZ, we were unable to show any improvement in clinical parameters of renal function and proteinuria or histologic parameters of MVI and chronicity. We did see a high proportion of bacterial infectious complications. The incidence of leukopenia is also concerning given the unexpectedly high incidence of bacterial infections.
In contrast to our experience, the study by Choi et al. (10) (in which 36 patients with biopsy specimen-proven cAbMR underwent therapy with TCZ), reported high long-term survival rates along with stability in eGFR over 2 years. In the post-treatment biopsy specimens obtained on nine (of 36; 25%) patients, they reported a statistically significant improvement in MVI scores and C4d deposition (10). This study lacked a control group, but graft survival was higher than previously published reports of grafts with cAbMR (12,13). However, the lack of controls and the poor understanding of the natural history of AbMR makes the interpretation of this experience difficult, and no direct evidence of benefit has been established.
Before this study, the landscape of therapies against cAbMR was barren. Three randomized studies on patients with cAbMR, directed at antibody removal, production, or complement activation did not show any improvement in graft survival, graft function, or histology (4–6). In these randomized studies, there were no differences in infectious AEs; however, the BORTEJECT study had a significantly higher rate of hematologic toxicities which led to dose reduction of MMF as compared with controls (6). In addition, retrospective reviews by Bachelet et al. (14) and Piñeiro et al. (15), which rituximab for this indication, showed a higher incidence of infectious and hematologic AEs.
In our study, the lack of response could be partly explained by the degree of chronicity and MVI seen in our index pretreatment biopsy specimens. Histologic chronicity scores and MVI scores along with AbMR molecular scores are validated determinants of graft loss. In fact, AbMR molecular scores improve the ability to predict graft loss when used in conjunction with the histologic scores (16,17). When compared with the pretreatment biopsy specimens by Choi et al. (10), we find that our patient population had almost a twofold higher degree of MVI with a mean g+ptc score of 4.10±1.52 versus 1.67±1.11, and a threefold higher degree of IFTA with a mean ci+ct score of 2.80±0.79 versus 0.93±0.72. The eGFR in our patient population before treatment was numerically slightly higher when compared with the Choi et al. (10) patient population (42±19 versus 38.8 ml/min per 1.73 m2). This difference is most likely hemodynamic and can be explained by the fact that seven of our ten patients were off CNIs and maintained on belatacept, thereby removing the reduction in eGFR associated with the vasoconstrictive effects of CNIs. Although we and others have shown that belatacept is safe for patients who are highly sensitized and in fact might even result in abrogation of DSA responses (18,19), it is theoretically possible that the combination of CNIs with TCZ is more effective than belatacept with TCZ.
While using TCZ for caAbMR in our patient population, we were not able to demonstrate a clear benefit in these patients and we have concerns about the possible adverse effects of TCZ in an immunosuppressed kidney transplant population. We replicate some of the findings by Choi et al. (10), in that there was relatively little change in the GFR during the observation period, but the rate of decline was similar to the pretreatment period. In addition, our patient population had a higher proportion of infectious AEs and leukopenia. It is possible that our intervention was directed at a patient population that had a severe AbMR phenotype that would not have responded to most therapies. When we look at inflammation and chronicity on histology, our population was not a comparable patient population to the original study. At some point the graft becomes unsalvageable and, in such situations, doing less may be more. Our experience serves as a cautionary reminder that applicability of therapies associated with a high degree of toxicity should be applied to comparable patient populations and not generalized to all patients with a particular disease label. Future studies may need to incorporate quantifiable markers such as MMDx scores (20) and risk prediction scores such as iBOX scores (21). Using an unsupervised machine learning approach, Aubert et al. (22) have identified five different cAbMR archetypes, each with distinct allograft survival profiles. These quantifiable scores are complementary and provide much needed granularity and precision to a very heterogeneous disease process such as AbMR.
The limitations of our study stem from the fact that this was a retrospective review of a small cohort of patients that lacked a control group. Although all of our patients had pretreatment biopsies, we only had six pairs of pre- and post-treatment biopsy specimens to compare. However, because three of the four patients who were not biopsied had a significant decline in allograft function, it is unlikely this additional information would have made any significant difference to our current findings.
The results of our study suggest that therapy with TCZ for caAbMR is not efficacious for patients with a high degree of inflammation and chronicity on the index biopsy specimens. Future studies on caAbMR should focus on appropriate risk stratification of patients and the understanding that AbMR is not one disease process but most likely a continuum for disease progression. Machine learning has shown that there are different archetypes of AbMR that may have completely different natural disease progression with or without therapy (23). There remains a need for accuracy and precision in identifying patients that may or may not respond to therapy so that we can still meet our overarching tenet of first do no harm.
Disclosures
G. Gupta reports personal fees from Alexion, CareDx, and Mallinckrodt; grants from Gilead; and nonfinancial support from Thermo Fisher outside the submitted work. P. Halloran reports ownership interest in Transcriptome Sciences. I. Yakubu reports personal fees from Veloxis Pharmaceuticals outside the submitted work. L. Kamal, P. Kimball, A. King, D. Kumar, M. Levy, D. Massey, I. Moinuddin, and F. Safavi have nothing to disclose.
Funding
None.
ACKNOWLEDGMENTS
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. Furthermore, each author certifies that this material or similar material has not been and will not be submitted to or published in any other publication.
AUTHOR CONTRIBUTIONS
G. Gupta, L. Kamal, A. King, and D. Kumar conceptualized the study; G. Gupta, L. Kamal, P. Kimball, A. King, D. Kumar, D. Massey, I. Moinuddin, F. Safavi, and I. Yakubu were responsible for data curation; G. Gupta, P. Halloran, D. Kumar, D. Massey, F. Safavi, and I. Yakubu were responsible for formal analysis; G. Gupta and D. Kumar were responsible for investigation; G. Gupta, D. Kumar, F. Safavi, and I. Yakubu, wrote the original draft; P. Kimball, G. Gupta, P. Halloran, A. King, M. Levy, and D. Massey were responsible for resources; A. King was responsible for methodology; G. Gupta provided supervision; and all authors reviewed and edited the manuscript.
References
- 1.Halloran PF, Reeve JP, Pereira AB, Hidalgo LG, Famulski KS: Antibody-mediated rejection, T cell-mediated rejection, and the injury-repair response: New insights from the genome Canada studies of kidney transplant biopsies. Kidney Int 85: 258–264, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Sellarés J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, Famulski K, Matas A, Halloran PF: Understanding the causes of kidney transplant failure: The dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 12: 388–399, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Loupy A, Hill GS, Jordan SC: The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 8: 348–357, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni S, Kirkiles-Smith NC, Deng YH, Formica RN, Moeckel G, Broecker V, Bow L, Tomlin R, Pober JS: Eculizumab therapy for chronic antibody-mediated injury in kidney transplant recipients: A pilot randomized controlled trial. Am J Transplant 17: 682–691, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Moreso F, Crespo M, Ruiz JC, Torres A, Gutierrez-Dalmau A, Osuna A, Perelló M, Pascual J, Torres IB, Redondo-Pachón D, Rodrigo E, Lopez-Hoyos M, Seron D: Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: A multicenter, prospective, randomized, double-blind clinical trial. Am J Transplant 18: 927–935, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Eskandary F, Regele H, Baumann L, Bond G, Kozakowski N, Wahrmann M, Hidalgo LG, Haslacher H, Kaltenecker CC, Aretin MB, Oberbauer R, Posch M, Staudenherz A, Handisurya A, Reeve J, Halloran PF, Böhmig GA: A randomized trial of bortezomib in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol 29: 591–605, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka T, Kishimoto T: The biology and medical implications of interleukin-6. Cancer Immunol Res 2: 288–294, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Jordan SC, Choi J, Kim I, Wu G, Toyoda M, Shin B, Vo A: Interleukin-6, A cytokine critical to mediation of inflammation, autoimmunity and allograft rejection: Therapeutic implications of IL-6 receptor blockade. Transplantation 101: 32–44, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Vo AA, Choi J, Kim I, Louie S, Cisneros K, Kahwaji J, Toyoda M, Ge S, Haas M, Puliyanda D, Reinsmoen N, Peng A, Villicana R, Jordan SC: A phase I/II trial of the interleukin-6 receptor-specific humanized monoclonal (tocilizumab) + intravenous immunoglobulin in difficult to desensitize patients. Transplantation 99: 2356–2363, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Choi J, Aubert O, Vo A, Loupy A, Haas M, Puliyanda D, Kim I, Louie S, Kang A, Peng A, Kahwaji J, Reinsmoen N, Toyoda M, Jordan SC: Assessment of tocilizumab (Anti-Interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant 17: 2381–2389, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, Nankivell BJ, Halloran PF, Colvin RB, Akalin E, Alachkar N, Bagnasco S, Bouatou Y, Becker JU, Cornell LD, van Huyen JPD, Gibson IW, Kraus ES, Mannon RB, Naesens M, Nickeleit V, Nickerson P, Segev DL, Singh HK, Stegall M, Randhawa P, Racusen L, Solez K, Mengel M: The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redfield RR, Ellis TM, Zhong W, Scalea JR, Zens TJ, Mandelbrot D, Muth BL, Panzer S, Samaniego M, Kaufman DB, Astor BC, Djamali A: Current outcomes of chronic active antibody mediated rejection - A large single center retrospective review using the updated BANFF 2013 criteria. Hum Immunol 77: 346–352, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Halloran PF, Merino Lopez M, Barreto Pereira A: Identifying subphenotypes of antibody-mediated rejection in kidney transplants. Am J Transplant 16: 908–920, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Bachelet T, Nodimar C, Taupin JL, Lepreux S, Moreau K, Morel D, Guidicelli G, Couzi L, Merville P: Intravenous immunoglobulins and rituximab therapy for severe transplant glomerulopathy in chronic antibody-mediated rejection: A pilot study. Clin Transplant 29: 439–446, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Piñeiro GJ, De Sousa-Amorim E, Solé M, Ríos J, Lozano M, Cofán F, Ventura-Aguiar P, Cucchiari D, Revuelta I, Cid J, Palou E, Campistol JM, Oppenheimer F, Rovira J, Diekmann F: Rituximab, plasma exchange and immunoglobulins: An ineffective treatment for chronic active antibody-mediated rejection. BMC Nephrol 19: 261, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loupy A, Lefaucheur C, Vernerey D, Chang J, Hidalgo LG, Beuscart T, Verine J, Aubert O, Dubleumortier S, Duong van Huyen JP, Jouven X, Glotz D, Legendre C, Halloran PF: Molecular microscope strategy to improve risk stratification in early antibody-mediated kidney allograft rejection. J Am Soc Nephrol 25: 2267–2277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vusser K, Lerut E, Kuypers D, Vanrenterghem Y, Jochmans I, Monbaliu D, Pirenne J, Naesens M: The predictive value of kidney allograft baseline biopsies for long-term graft survival. J Am Soc Nephrol 24: 1913–1923, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta G, Kumar D, Cotterell A, Sharma A, Posner M, Ren Q, Posner S, King A: Improvement in renal function in high immunologic risk kidney transplant recipients switched from tacrolimus to belatacept. Transplantation 98: 457, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, Moal MC, Mondragon-Ramirez GA, Kothari J, Polinsky MS, Meier-Kriesche HU, Munier S, Larsen CP: Belatacept and long-term outcomes in kidney transplantation. N Engl J Med 374: 333–343, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Halloran PF, Reeve J, Akalin E, Aubert O, Bohmig GA, Brennan D, Bromberg J, Einecke G, Eskandary F, Gosset C, Duong Van Huyen JP, Gupta G, Lefaucheur C, Malone A, Mannon RB, Seron D, Sellares J, Weir M, Loupy A: Real time central assessment of kidney transplant indication biopsies by microarrays: The INTERCOMEX study. Am J Transplant 17: 2851–2862, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Loupy A, Aubert O, Orandi BJ, Naesens M, Bouatou Y, Raynaud M, Divard G, Jackson AM, Viglietti D, Giral M, Kamar N, Thaunat O, Morelon E, Delahousse M, Kuypers D, Hertig A, Rondeau E, Bailly E, Eskandary F, Böhmig G, Gupta G, Glotz D, Legendre C, Montgomery RA, Stegall MD, Empana JP, Jouven X, Segev DL, Lefaucheur C: Prediction system for risk of allograft loss in patients receiving kidney transplants: International derivation and validation study. BMJ 366: l4923, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aubert O, Higgins S, Bouatou Y, Yoo D, Raynaud M, Viglietti D, Rabant M, Hidalgo L, Glotz D, Legendre C, Delahousse M, Shah N, Sis B, Campbell P, Mengel M, Jouven X, Van Huyen JD, Lefaucheur C, Loupy A: Archetype Analysis identifies distinct profiles in renal transplant recipients with transplant glomerulopathy associated with allograft survival. J Am Soc Nephrol 30: 625–639, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeve J, Böhmig GA, Eskandary F, Einecke G, Lefaucheur C, Loupy A, Halloran PF; MMDx-Kidney study group : Assessing rejection-related disease in kidney transplant biopsies based on archetypal analysis of molecular phenotypes. JCI Insight 2: 94197, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]