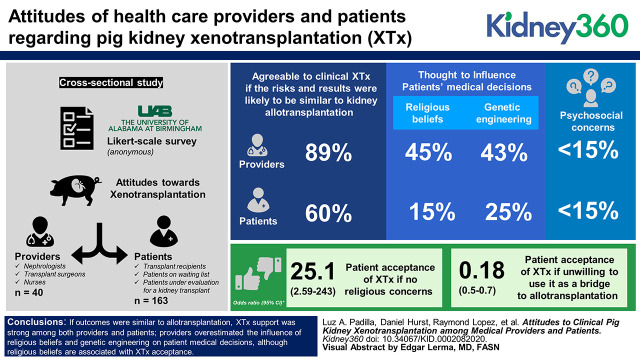
Visual Abstract
Keywords: transplantation, attitude, genetic engineering, heterografts, kidney, kidney transplantation, logistic models, patient, personality, provider, religion, surveys and questionnaires, transplantation, heterologous, xenotransplantation
Abstract
Background
In addition to governmental regulation and scientific advancements, the World Health Organization requires extensive review of local opinions before initiating clinical trials of xenotransplantation (XTx). The purpose of this study was to assess the attitudes of health care providers and patients regarding XTx.
Methods
An anonymous Likert-scale survey regarding attitudes toward XTx was distributed to pre- and post-kidney transplant patients, nephrologists, transplant surgeons, and nurses (“providers”). Patient and provider responses were described and compared. Regression analysis using patients’ responses was performed to identify factors associated with XTx acceptance.
Results
Eighty percent (32/40) of providers and 69% (113/163) of patients were agreeable to clinical XTx if the risks and results were likely to be similar to kidney allotransplantation (P<0.05). Kidney providers rated the influence of religious beliefs in medical decisions (45% versus 15%) and genetic engineering (43% versus 25%) as being more important than did patients (P<0.05). A small proportion in both groups (<15%) reported concerns about (1) potential personality changes, (2) how others would interact, (3) a perception of being “less human,” or (4) morals or ethics. Logistic regression found that the odds of patients accepting XTx were greater if they had no religious concerns (OR, 25.10; 95% CI, 2.59 to 243.00), but acceptance was less likely if they were not willing to use XTx as a bridge to allotransplantation (OR, 0.18; 95% CI, 0.51 to 0.70).
Conclusions
(1) If outcomes were similar to allotransplantation, XTx support was strong among both providers and patients; (2) providers overestimated the influence of religious beliefs and genetic engineering on patient medical decisions, although religious beliefs are associated with XTx acceptance; (3) XTx use as a bridge to allotransplant was associated with XTx acceptance; and (4) psychosocial concerns were low for either group. Future studies among other communities are warranted to assess if similar attitudes exist.
Introduction
The demand for transplant organs far outstrips the supply, forcing thousands of patients to face the possibility that they will die before a compatible organ becomes available. This shortage is particularly acute in patients needing a kidney, where the average wait time for a deceased donor is 5 years (1). The Department of Health and Human Service’s Organ Procurement and Transplantation Network data reported that an average of 20 Americans die each day awaiting transplant. In 2018, 11 of those deaths were while waiting for a kidney. Additionally, 12 people per day were removed from the kidney transplant waiting list because they were deemed too sick to undergo the rigors of surgery (2).
Xenotransplantation (XTx) may be an option in addressing the organ shortage. Recent advances in genetic modification of pigs have demonstrated recipient survival in excess of a year (3). Despite their life-saving potential, the use of animal tissues in humans creates religious, ethical, and psychosocial considerations for all involved parties (4). To begin addressing these, directives from the World Health Organization, the Food and Drug Administration, and the International XTx Association all call for public involvement and transparency in evaluating the ethics and risks of XTx (5–7).
Gauging public attitudes toward cross-species transplantation is a necessary component to preparing for, and preemptively addressing, concerns should XTx become a clinical reality. Potential ethical considerations for society include religious objections specific to porcine tissues, animal rights, and individual fears of XTx violating the very idea of “personhood” (8). However, there is a lack of studies that capture the perceptions of the other major stakeholders regarding the issue of transplant organ supply from the health care providers themselves (9). The purpose of this study was to identify attitudes, perceptions, and beliefs to XTx between different stakeholders in the renal community, who will ultimately be involved in the clinical uptake and/or care of individuals who receive a xenograft.
Materials and Methods
In 2019, a cross-sectional study was conducted after institutional review board approval that used a five-point Likert-scale survey to explore patient perceptions, clinical decision-making attitudes, and psychosocial beliefs related to XTx among nephrologists, kidney transplant surgeons, nurses, and pre- and post-kidney transplant patients at the University of Alabama at Birmingham. The survey was face validated by a heterogenous group of experts and pilot tested among junior and senior level nursing students from a major midwestern university regional campus. Survey candidates were then contacted via email, and 67 of the 250 candidates contacted agreed to participate (data not published). The survey was revised once after pilot testing and before this study.
Patients who were either a kidney transplant recipient, on the waiting list for a kidney, or currently being evaluated for kidney transplantation were approached at our nephrology outpatient clinics and asked if they would like to participate in our study. After informed consent, patients were left to fill out our survey in the privacy of a clinic room and, once finished, they were instructed to deposit the survey in a collection box to keep their responses anonymous.
A similar survey was constructed using Qualtrics for all providers (nephrologists, kidney transplant surgeons, and nurses who are in direct care of renal transplant patients). An email that contained a link directing the providers to our survey was sent through departmental administrative staff. All recipients received our email questionnaire three times over the course of a month. To avoid information influencing responses and to show responses with the existing level of knowledge of XTx, the survey did not contain any introductory or education materials on XTx or other scientific terms used in the survey. All online responses were anonymous and untraceable.
Responses from transplant nephrologists, kidney transplant surgeons, and kidney nurses were merged to create the kidney provider group. Frequencies, percentages, means, and SDs were calculated and used to summarize the data between the provider and patient groups. Due to a small number of responses in the questions related to patient perception and psychosocial beliefs, Likert-scale options were merged to reflect only three categories (no/possibly, undecided, and yes/probably). Differences between the two groups were compared using chi-squared tests for categoric variables and t test for continuous variables. A stepwise regression analysis for best model selection for XTx acceptance was performed with an entry threshold of P<0.1 using all clinical and survey variables in Tables 1 and 2. Once the stepwise selection showed the significant predicting variables, univariable logistic regression was performed to report strength of association between predicting variables and XTx acceptance using odds ratios (ORs) and 95% confidence intervals. XTx acceptance was defined using the question: if a pig kidney transplant had similar risks and results as a human kidney would you consider a pig kidney as a treatment option? “Probably” and “yes” responses to this question were merged into one category, and “possibly” and “no” were merged into another to create a dichotomous outcome variable. Those who answered “undecided” were not included in the regression analysis to reflect true acceptance and nonacceptance. SAS version 9.4 (SAS Institute, Cary, NC) was used for the analysis and all P values of <0.05 were considered significant.
Table 1.
Comparison of sociodemographic characteristics of kidney transplant providers and kidney patients at University of Alabama at Birminghama
| Characteristics | Kidney Providers N=40 (20%) | Kidney Patients N=163 (80%) | P Value |
| Age (mean±SD) | 43.1±11.7 | 51.7±13.3 | 0.0003 |
| Median | 42 | 52 | |
| Gender (male) | 8 (20%) | 100 (62%) | <0.0001 |
| Ethnicity | 0.30 | ||
| White | 22 (55%) | 74 (46%) | |
| Black | 13 (33%) | 74 (46%) | |
| Asian/NA/Hispanic | 5 (13%) | 14 (9%) | |
| Religion | 0.007 | ||
| Christian/Catholic | 32 (80%) | 153 (94%) | |
| Jewish, Islam, other, no religion | 8 (20%) | 9 (6%) | |
| Years of clinical practice or as kidney patient (mean±SD) | 15.4±10.1 | 4.1±6.1 | <0.001 |
| Marital status | — | ||
| Single | — | 36 (22%) | |
| Married/living with partner | — | 92 (56%) | |
| Divorced or separated | — | 27 (17%) | |
| Widowed | — | 8 (5%) | |
| Highest level of education completed | — | ||
| None/elementary | — | 4 (3%) | |
| High school | — | 97 (61%) | |
| Bachelors | — | 43 (27%) | |
| Graduate degree | — | 16 (10%) | |
| What best describes your prior medical treatment at the UAB kidney transplant program | — | ||
| Kidney transplant recipient | — | 110 (68%) | |
| On waiting list or being evaluated for a kidney transplant | — | 53 (33%) |
NA, Native American; UAB, University of Alabama at Birmingham.
Counts, N, and percentages shown for all variables unless otherwise specified.
Table 2.
Attitudes, perceptions, and beliefs toward xenotransplantation among kidney transplant providers and kidney patients at University of Alabama at Birminghama
| Attitudes/Perceptions/Beliefs | Kidney Providers N=40 (20%) | Kidney Patients N=163 (80%) | P Value |
| Patient perceptions | 0.0003 | ||
| How influential are your/your patients’ religious beliefs when making medical decisions? | |||
| Never/sometimes | 18 (45%) | 121 (75%) | |
| Undecided | 4 (10%) | 17 (11%) | |
| Always/often | 18 (45%) | 24 (15%) | |
| In your opinion, would the fact that pigs used for transplantation are genetically engineered to help prevent organ rejection in a human influence you/your patients’ consideration of a pig kidney transplant? | 0.001 | ||
| Yes | 17 (43%) | 42 (25%) | |
| No | 23 (58%) | 96 (59%) | |
| Undecided | 0 (0%) | 25 (15%) | |
| Clinical decision making | |||
| If pig kidney transplantation had similar risks and results as human kidney transplantation, would you consider a pig kidney as a treatment option for you/your patients? | 0.04 | ||
| No | 2 (5%) | 10 (6%) | |
| Possibly | 4 (10%) | 14 (9%) | |
| Undecided | 2 (5%) | 26 (16%) | |
| Probably | 13 (33%) | 21 (13%) | |
| Yes | 19 (48%) | 91 (56%) | |
| If the risks and results of pig kidney transplantation were not as good as human organ transplantation, would you be prepared to recommend/accept a pig kidney as a bridge until a human donor kidney became available? | 0.57 | ||
| No | 9 (23%) | 35 (22%) | |
| Possibly | 8 (20%) | 29 (18%) | |
| Undecided | 11 (28%) | 29 (18%) | |
| Probably | 4 (10%) | 17 (11%) | |
| Yes | 8 (20%) | 50 (31%) | |
| Psychosocial beliefs | |||
| If you/your patient successfully received a pig kidney transplant, do you believe that it could change their personality? | 0.72 | ||
| No/possibly | 34 (85%) | 129 (80) | |
| Undecided | 4 (10%) | 23 (14%) | |
| Yes/probably | 2 (5%) | 10 (6%) | |
| If you/your patient successfully received a pig kidney transplant, do you believe that it could change how other people see or interact with you/your patient? | 0.38 | ||
| No/possibly | 35 (88%) | 128 (79%) | |
| Undecided | 1 (3%) | 16 (10%) | |
| Yes/probably | 4 (10%) | 18 (11%) | |
| If you/your patient received a successful pig kidney transplant, do you believe that you/they would be “less human”? | 0.86 | ||
| No/possibly | 37 (93%) | 149 (91%) | |
| Undecided | 2 (5%) | 7 (4%) | |
| Yes/probably | 1 (3%) | 7 (4%) | |
| Do you believe that there are moral and/or ethical reasons why we should not consider xenotransplantation as a form of treatment? | 0.31 | ||
| No/possibly | 32 (80%) | 135 (84%) | |
| Undecided | 4 (10%) | 20 (12%) | |
| Yes/probably | 4 (10%) | 6 (4%) | |
| Do you believe that there are religious reasons why we should not consider xenotransplantation as a form of treatment? | 0.13 | ||
| No/possibly | 28 (70%) | 132 (83%) | |
| Undecided | 6 (15%) | 18 (11%) | |
| Yes/probably | 6 (15%) | 10 (6%) | |
| Do you believe there are public health risks associated with xenotransplantation (i.e., zoonosis)? | — | ||
| No/possibly | 23 (56%) | Not asked | |
| Undecided | 12 (30%) | Not asked | |
| Yes/probably | 5 (13%) | Not asked |
Counts, N, and percentages shown for all responses.
Results
A total of 40 providers and 163 patients completed our survey. Response rate among nephrologists and kidney transplant surgeons was 61% (8/13) and 31% for kidney nurses (32/65). The average overall provider response rate was 51%. Response rate among patients was 85%. A total of 29 patients declined to participate in our study (163/192).
Kidney patients were older, predominantly male, and a higher proportion reported being Christian as their primary religion (P<0.05; Table 1). Of the kidney patients, 67% were postkidney transplant and 32% were being evaluated and on the waiting list for a kidney transplant. Most kidney patients (87%) reported to have completed either high school or bachelor’s degree.
XTx acceptance was significantly higher among providers when compared with kidney patients (80% versus 69%, respectively; P=0.04) (Table 2). Of the providers, 45% believed that a patient’s religious beliefs affect their medical decision, whereas 74% of patients reported that their religious beliefs affect their medical decisions sometimes or never (P=0.0003). Only 14% of patients reported religious beliefs as a medical decision influencer, of which all but two were Christian. Of the providers, 42% believed that the fact that a pig is genetically modified for XTx would affect patients considering a pig kidney for transplant, but only 24% of patients reported it as an issue, and 15% were undecided (P=0.001).
Logistic regression analysis showed that attitudes related to religion and using XTx as a bridge to allotransplantation were factors associated with XTx acceptance among patients (Table 3). Kidney patients who would not accept a pig kidney as a bridge until a human kidney became available were 82% less likely to accept XTx when compared with those who were willing to use a pig kidney as a bridge (OR, 0.02; 95% CI, 0.51 to 0.70). XTx acceptance was high when the person did not have any religious reasons to object to XTx, compared with those who had religious objections (OR, 25.10; 95% CI, 2.59 to 243.00). No other factors showed a significant association with XTx acceptance among patients.
Table 3.
Logistic regression analysis showing variables associated with acceptance to xenotransplantation among kidney patients at the University of Alabama at Birmingham
| Patient Questions | Odds of Acceptance among Kidney Patients |
| N (xenotransplantation acceptance rate among kidney patients) | 112 (82%) |
| If the risks and results of pig kidney transplantation were not as good as human organ transplantation, would you be prepared to recommend a pig kidney as a bridge until a human donor kidney became available? | |
| No/possibly, odds ratio (95% CI) | 0.18 (0.51 to 0.70) |
| Undecided, odds ratio (95% CI) | 0.63 (0.08 to 4.97) |
| Yes/probably | Reference |
| Do you believe that there are religious reasons why we should not consider xenotransplantation as a form of treatment for adults? | |
| No/possibly, odds ratio (95% CI) | 25.10 (2.59 to 243.00) |
| Undecided, odds ratio (95% CI) | 2.16 (0.16 to 27.92) |
| Yes/probably | Reference |
Discussion
Genetically modified pig kidney xenografts may be a way to address the high transplant wait-list time and kidney donor organ shortage (10). However, clinical uptake of XTx as a bridge to allotransplantation or as primary treatment for ESKD will depend on acceptance from their potential users. This survey collected results from 40 health care providers and 163 kidney patients regarding their attitudes toward clinical pig kidney XTx.
Our study shows strong support for XTx among both health care providers and patients if risks and outcomes are similar to those of receiving a human kidney. However, support for XTx dropped markedly when providers and patients were informed of inferior outcomes as compared to a human kidney transplant (even when the xenograft would only be used as a bridge). The strong support may be evidence of the initial excitement and intrigue for this new technology. However, the hesitation when faced with less favorable outcomes may speak to the high scientific expectations of XTx. Furthermore, this hesitation may be evidence of the current level of comfort with existing medical management and RRTs among both patients and providers. XTx will have to offer at least comparable outcomes to other clinical options for it to even be considered as treatment.
Willingness for XTx to be used as a bridge, however, was higher among patients (41%) than providers (30%). Patients may be more open to alternatives that offer change to the morbidity and mortality associated with their current treatments than providers who do not personally experience such risks. The benefits to the patient of a xenograft as a bridge therapy would include (1) no longer having to undergo dialysis, (2) possibly decreasing their wait-list mortality, and (3) perhaps provide a better quality of life. For critically ill patients, a xenograft bridge may prove lifesaving until an allograft becomes available. It is important to note that if the results of XTx only allow for its use as a bridge to allotransplantation, then XTx would not alleviate the human organ shortage. Nevertheless, not being open to XTx as a bridge was associated with reduced XTx acceptance. Future studies will be needed to explore the possible reasons that drive this attitude.
Providers overestimated the influence of religious beliefs and genetic engineering on patient decisions. In the regression model, religious beliefs were associated with XTx acceptance among patients. A significant percentage of kidney patients (94%) self-identified as Christian. The religious influence on attitudes to XTx has been studied in some detail, although much of the literature concentrates on the views of academics and trained theologians, not laypersons or patients. Protestant, Catholic, Islamic, and Jewish theologians have reported no fundamental reasons to prohibit XTx as a treatment option for those who are critically ill and in desperate need of an organ (11,12). Hence, some theologians find no issue with XTx within their faith tradition.
However, this viewpoint is not unanimous. Paris and colleagues (13) held a theologic symposium in 2017 with Jewish, Christian, and Muslim theologians, where they admitted that their opinions might not align with those of potential patients. Although the theologic perspectives on XTx are undoubtedly complex, and there have been less than favorable opinions, the recent literature indicates an acceptance of XTx among theologians.
Furthermore, providers overestimated the degree to which the genetic modification of pigs for XTx would influence patients’ decision making. It is worthwhile to note that a significant portion of patient respondents (15%) said they were undecided about whether or not genetic engineering would influence their decision to receive a xenograft, whereas zero providers were undecided on this question. Genetic engineering in itself is a complex term, and it may be that patient respondents did not comprehend the question.
This overestimation of religious and genetic-engineering beliefs from providers in our study may be evidence of provider bias. The presence of stereotypes may affect how providers may frame information about XTx to their patients, which can ultimately affect a patient’s clinical decisions and uptake of this option. Furthermore, the existence of implicit provider biases has been associated with worse outcomes for patients (14). Our study shows that acceptance is high when there are no beliefs that religious barriers exist. Developing interventions that help reduce implicit biases and address religious-belief barriers are needed to further increase acceptance to XTx. Nonetheless, from this study it is important to note that any religious barrier to XTx among kidney patients is very low.
Lastly, we show minimal psychosocial concerns for XTx in either group. Interestingly, even when kidney providers were asked if they believed there are public health risks associated with XTx (i.e., zoonosis), a majority (58%) answered no/possibly, whereas a significant portion were undecided (30%) and only a small percent responded yes (13%). Ample literature highlights the possible risk of zoonotic infection presented by XTx, yet the majority believe the risk to be low (15). This is a promising finding for future XTx acceptance in a scenario where the risk of human infection with porcine endogenous retroviruses is still unknown (16). However, it is unclear if patients share a similar attitude as providers because this question was not included in the patient survey to avoid creating a concern that could not be addressed due to privacy and anonymity of our methodology.
This study is a single-center study and the sample size is small, which may affect the relevance of our results to all kidney providers and patients in the United States. The small sample did not allow a viable regression analysis among providers. There were baseline differences between the two compared populations. For example, kidney patients were predominantly male, older, and a higher number of them were Christian. Furthermore, the majority of patients had already received a kidney. It is unclear how baseline, wait-list times, and transplant status differences may have affected responses in our study. These distinctions may simply reflect the differences between the workforce (some are out of state) and the state’s population at our institution, or it may be due to nonresponder bias. However, our response rates are high for patients and providers, and average for nursing (17,18). The survey was short, delivered electronically with reminders to providers, and administered on paper to patients to prevent such bias. Likert survey questions were not statistically validated and responses can be influenced by unmeasured variables of the subject’s personal views and inherent characteristics at the time of the survey. Although our cohort had a high level of education, it is unknown if complex scientific terms used in the survey were understood. Responses from our study may not be generalizable to those in other centers or populations with lower levels of education.
Overall, our findings would suggest that there is strong support for XTx among kidney health care providers and patients if XTx clinical outcomes are similar to current renal allotransplantation. However, if XTx outcomes prove inferior to current management options, acceptance of clinical uptake is low even as a bridge to allotransplantation. Although XTx is yet to be a clinical option, the presence of religious concerns and provider biases may be barriers that will have to be addressed to increase XTx acceptance in preparation for future clinical trials.
Disclosures
All authors have nothing to disclose.
Funding
Work on XTx at the University of Alabama at Birmingham is supported in part by a Children’s Hospital of Alabama grant, a National Institutes of Health National Institute of Allergy and Infectious Diseases grant U19 AI090959, and a United Therapeutics Corporation (Silver Spring, MD) grant to University of Alabama at Birmingham.
Author Contributions
D. Cooper, V. Kumar, L. Padilla, and W. Paris conceptualized the study and were responsible for methodology; W. Paris and L. Padilla were responsible for data curation, formal analysis, project administration, and resources; D. Cooper, D. Hurst, W. Paris, and L. Padilla were responsible for investigation; L. Padilla was responsible for software and validation; W. Paris, and L. Padilla provided supervision; D. Hurst, R. Lopez, and L. Padilla wrote the original draft; and all authors reviewed and edited the manuscript.
References
- 1.American Kidney Fund: “Transplant waiting list”, Rockville, MD, American Kidney Fund, 2019. Available at: https://www.kidneyfund.org/kidney-disease/kidney-failure/treatment-of-kidney-failure/kidney-transplant/transplant-waitlist/. Accessed June 3, 2020 [Google Scholar]
- 2.Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients : 2018 Annual Data Report, Rockville, MD, Department of Health and Human Services, 2018. Available at: https://www.srtr.org/reports-tools/srtroptn-annual-data-report/
- 3.Kim S, Higginbotham L, Mathews D, Breeden C, Stephenson A, Larsen C, Ford M, Tector J, Adams A: CD4 depletion is necessary and sufficient for long-term nonhuman primate xenotransplant survival. Presented at the 2017 American Transplant Congress, Chicago, IL, April 29–May 3, 2017 [Google Scholar]
- 4.Anderson M: Xenotransplantation: A bioethical evaluation. J Med Ethics 32: 205–208, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization: Proceedings from the Fifty-Seventh World Health Assembly. Resolution WHA57.18: Human organ and tissue transplantation, Geneva, Switzerland, World Health Organization, 2004. Available at: https://www.who.int/transplantation/en/A57_R18-en.pdf?ua=1. Accessed June 3, 2020 [Google Scholar]
- 6.World Health Organization: Global consultation on regulatory requirements for xenotransplantation clinical trials. The Changsha Communiqué, Geneva, Switzerland, World Health Organization, 2008. Available at: https://www.who.int/transplantation/xeno/ChangshaCommunique.pdf?ua=1. Accessed June 3, 2020 [Google Scholar]
- 7.US Food and Drug Administration: Source animal, product, preclinical, and clinical issues concerning the use of xenotransplantation products in humans, White Oak, MD, US Food and Drug Administration, 2016. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/source-animal-product-preclinical-and-clinical-issues-concerning-use-xenotransplantation-products. Accessed June 3, 2020 [Google Scholar]
- 8.Loike JD, Kadish A: Ethical rejections of xenotransplantation? The potential and challenges of using human-pig chimeras to create organs for transplantation. EMBO Rep 19: e46337, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell C, Lipps A, Padilla L, Werkheiser Z, Cooper DKC, Paris W: Meta-analysis of public perception toward xenotransplantation. Xenotransplantation e12583, 2020 [DOI] [PubMed] [Google Scholar]
- 10.National kidney foundation: Organ Donation and Transplantation Statistics, New York, National Kidney Foundation, 2015. Available at: https://www.kidney.org/news/newsroom/factsheets/Organ-Donation-and-Transplantation-Stats. Accessed June 3, 2020 [Google Scholar]
- 11.Sautermeister J, Mathieu R, Bogner V: Xenotransplantation-theological-ethical considerations in an interdisciplinary symposium. Xenotransplantation 22: 174–182, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Mathieu R: Jewish ethics and xenotransplantation. Xenotransplantation 23: 258–268, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Paris W, Seidler Rabbi JH, Fitzgerald K, Padela AI, Cozzi E, Cooper DKC: Jewish, Christian and Muslim theological perspectives about xenotransplantation. Xenotransplant 25: e12400, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Huibregtse BM, Boardman JD: Provider bias as a function of patient genotype: Polygenic score analysis among diabetics from the health and retirement study. Obes Sci Pract 4: 448–454, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denner J, Tönjes RR: Infection barriers to successful xenotransplantation focusing on porcine endogenous retroviruses. Clin Microbiol Rev 25: 318–343, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boneva RS, Folks TM: Xenotransplantation and risks of zoonotic infections. Ann Med 36: 504–517, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Cunningham CT, Quan H, Hemmelgarn B, Noseworthy T, Beck CA, Dixon E, Samuel S, Ghali WA, Sykes LL, Jetté N: Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol 15: 32, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corner B, Lemonde M: Survey techniques for nursing studies. Can Oncol Nurs J 29: 58–60, 2019 [PMC free article] [PubMed] [Google Scholar]