Visual Abstract
Keywords: chronic kidney disease, complications, diabetes mellitus, type 2, glucose, glycated hemoglobin A, hypoglycemia, hypoglycemic agents, prospective studies, risk factors
Abstract
Background
Glycemic management in patients with type 2 diabetes mellitus (T2DM) and CKD can become complicated. One factor that may affect treatment is hypoglycemia. Hypoglycemia risk may be increased by several biologic processes in CKD. The objective of this study was to determine the frequency, severity, and risk factors for hypoglycemia in patients with T2DM and CKD.
Methods
The design was a prospective observational study. A continuous glucose monitor (CGM) was worn by 80 patients for up to 14 days; glucose was measured every 15 minutes. Patients with T2DM and eGFR <45 ml/min were enrolled. Patients on dialysis were excluded. The primary outcome was to assess the frequency of hypoglycemic episodes during the study period. Hypoglycemic episodes were defined as a reduced glucose concentration (<70 mg/dl) lasting ≥15 minutes. Secondary outcomes included assessment of severity of hypoglycemia and risk factors for its development.
Results
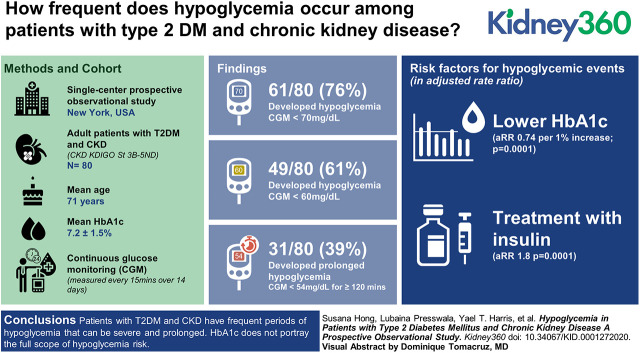
A total of 80 patients wore the CGM for a mean of 12.7±2.9 days. Hypoglycemic events occurred in 61 of 80 patients (76%) with glucose <70 mg/dl, and 49 of 80 (61%) with glucose <60 mg/dl. Prolonged hypoglycemic events (CGM glucose <54 mg/dl for ≥120 consecutive minutes) occurred in 31 patients (39%) with 118 total events. Most hypoglycemic episodes occurred overnight, from 1:00 am to 9:00 am. By multivariate analysis, lower hemoglobin A1c and treatment with insulin were two modifiable risk factors for hypoglycemic events.
Conclusions
Patients with T2DM and CKD have frequent periods of hypoglycemia that can be severe and prolonged. Hemoglobin A1c does not portray the full scope of hypoglycemia risk. This study illustrates the need for careful monitoring of glucose levels in patients with T2DM and CKD.
Introduction
The prevalence of type 2 diabetes mellitus (DM) in the United States is rising due to the obesity epidemic and aging of the population. Worldwide, up to 20% of adults >65 years of age are affected by type 2 DM (1). As many as 40% of patients with type 2 DM will develop kidney disease, and type 2 DM is the leading cause of ESKD (2). Achieving glycemic control with glucose-lowering agents improves outcomes in patients with type 2 DM, but treatment can be complicated by hypoglycemia.
Hypoglycemia is a cause of morbidity and mortality in patients with type 2 DM. When severe, it can cause confusion, seizures, coma, and death. Asymptomatic episodes have been associated with myocardial ischemia (3). In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, hypoglycemia was more common among patients with type 2 DM who were randomized to intensive treatment. The higher rate of mortality in these patients compared with those treated with a conventional glucose target has suggested that hypoglycemia may have contributed to inferior outcomes (4). Hypoglycemia also increases anxiety among patients and may hinder the ability to achieve optimal chronic glucose control (5).
In CKD, various factors might increase risk for hypoglycemia. With normal function, renal tubular glucose absorption and gluconeogenesis contribute approximately 20% of total body glucose (6). In kidney disease, renal glucose production is impaired. An important component of this is decreased renal gluconeogenesis. Other metabolic pathways are disrupted including diminished clearance of insulin and certain other medications (7). The circulating t1/2 of insulin increases with decreasing kidney function due to decreased excretion and metabolism. Taken together, the net effect may lead to an increased risk for hypoglycemia. In the ACCORD trial, the risk for hypoglycemia requiring medical assistance was strongly associated with elevated serum creatinine (8). Recently, Ahmad et al. (9) reported the frequent occurrence of hypoglycemia in a population with type 2 DM and CKD. For entry, patients in Ahmad et al.’s study were required to be treated with sulfonylurea or insulin, creating a cohort with enriched risk for hypoglycemia. We believed that it was important to study a broader CKD population including those treated with other diabetic medications or no diabetic medications. In this way, we could more fully understand the risk of hypoglycemia in CKD.
In this study, we sought to expand on the hypoglycemic analysis by Ahmad et al. by studying a more general population of patients with type 2 DM and CKD. In particular, we did not require patients to be on medications known to cause hypoglycemia. To accomplish this, we studied a set of 80 patients with type 2 DM that we had previously tested with continuous glucose monitoring (CGM) for assessment of glycated hemoglobin accuracy in CKD (10). The purpose of this study was to determine the frequency and severity of hypoglycemia among this same broad population of patients with type 2 DM and CKD and to identify risk factors associated with the development of hypoglycemia.
Materials and Methods
Patients
We enrolled patients from the academic nephrology practice of the Donald and Barbara Zucker School of Medicine at Hofstra/Northwell. The practice comprises 19 nephrologists and is located in western Nassau County, New York. Patients were identified as potentially eligible based on a weekly prescreen of the following week’s office patients who matched key entry criteria. All informed consent processes were carried out by the principal investigator with a witness present, in person, in the office. Our group previously reported on this group of 80 patients with type 2 DM and CKD to assess glycated hemoglobin accuracy in CKD (10).
Patients were eligible for study if they were >18 years of age, had type 2 DM, and had CKD with eGFR <45 ml/min (stages 3b–5 CKD) (calculated using the CKD–Epidemiology Collaboration equation). Exclusion criteria included a diagnosis of type 1 DM, ESKD (current dialysis treatment), hemoglobinopathies, red cell transfusions in the prior 12 weeks, hemoglobin <9 g/dl documented within the previous 3 months, daily acetaminophen use, steroid treatment within 3 months, any new medication for diabetes in the previous 2 months or any dose change in diabetes medications of >50%, and current pregnancy. Treatment with erythropoiesis-stimulating agents was permitted but the dose and frequency of administration had to be stable for 2 months.
Study Design
The initial data set was collected on day 1 of study after provision of consent. The CGM used was the Abbott Freestyle Libre Pro (Abbott Laboratories, Lake Bluff, IL). The device was configured to blind the patient to all glucose results. Glucose was measured every 15 minutes throughout the day and the results were stored in the device’s memory, providing up to 1344 measurements over 14 days. The device was placed on the patient’s upper arm according to the manufacturer’s instructions. No specific education on diet or medications (beyond what the patient had already received) was provided. While wearing the CGM, no diabetic medication could be changed unless absolutely necessary, in which case the patient would be withdrawn from study. After 14 days, patients returned to the office to have the device removed and the data downloaded. At this point, blood sampling was performed, including fasting blood glucose, hemoglobin A1c (HbA1c), serum creatinine, and additional clinical information was collected. If the device was dislodged or became inoperable, then closeout visit procedures were followed.
Statistical Analyses
Hypoglycemic events were defined as a reduced glucose concentration lasting at least 15 minutes, detected by two consecutive CGM measurements. Three different glucose concentrations were used for detecting hypoglycemia. The first definition was a glucose concentration of <70 mg/dl, which is often used in clinical practice. A second—and the primary—definition was a concentration of <60 mg/dl. This level was chosen because CGM systems tend to be less accurate at lower glucose concentrations. Fokkert et al. (11) compared the FreeStyle Libre CGM to laboratory testing for 715 glucose measurements. They found the CGM was related to the laboratory glucose by the linear equation CGM glucose=1.07×blood glucose−3.48. From this equation it can be seen that a CGM measurement <60 mg/dl is a reliable determinant of true hypoglycemia with expected blood glucose of <70 mg/dl. The third definition of hypoglycemia was the International Consensus on Use of Continuous Glucose Monitoring definition of a prolonged hypoglycemic event, which is CGM glucose levels <54 mg/dl for ≥120 consecutive minutes (12).
The percentage of patients with hypoglycemic events and mean number of hypoglycemic events per patient were calculated and reported. The mean percentage of time with hypoglycemia was calculated as the time with reduced glucose divided by the time of all CGM measurements multiplied by 100 per patient.
Predictors of risk were studied with the outcome being number of hypoglycemic episodes, defined as glucose <60 mg/dl for at least 15 minutes adjusted for time in the study. A Poisson regression model was used to assess the relationship of risk variables to this outcome. Because the number of hypoglycemic episodes was total time of follow-up in minutes, the logarithm of time was taken as an offset term in the model. Ten patients’ medication history were not included in the risk prediction analysis due to incomplete data (medication data was available, but there were reasons to believe that it might not be complete). Variables with a P value <0.05 by univariate analysis were taken as candidates in the multivariate analysis. The final multivariate model was selected using backward selection. The rate ratio was calculated as the exponential of estimated coefficients. A two-tailed P value <0.05 was taken as evidence of statistical significance in all analyses. We conducted all analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 80 patients were enrolled and wore the CGM for a mean of 12.7±2.9 days, with 80% completing the full 14 days. All patients were included in the analysis. Patient characteristics are displayed in Table 1. Although all patients had type 2 DM, the cause of kidney disease was believed to be DM in only 63 of 80 patients. The patients were representative of CKD with diabetes except for skewing toward more of a male and non-Hispanic population.
Table 1.
Patient baseline characteristics
| Characteristics | Value |
| Total enrolled | 80 |
| Age (yr), mean (SD) | 71.3±10.9 |
| Sex | |
| Male | 61 (76%) |
| Female | 19 (24%) |
| Race | |
| White | 53 (66%) |
| Asian | 11 (14%) |
| Black | 10 (13%) |
| Unknown/not reported | 4 (5%) |
| American Indian/Alaska Native | 1 (1%) |
| More than one race/multiracial | 1 (1%) |
| Ethnicity | |
| Non-Hispanic | 77 (96%) |
| Hispanic | 2 (3%) |
| Unknown/not reported | 1 (1%) |
| Cause of CKD | |
| DM type 2 | 63 (79%) |
| Other | 17 (21%) |
| Medical history | |
| CHF | 20 (25%) |
| CAD | 26 (33%) |
| Hypertension | 77 (96%) |
| Stroke | 17 (21%) |
| History of malignancy | 6 (8%) |
| eGFR, n (%) | |
| 0 to ≤15 ml/min | 15 (19%) |
| >15 to ≤30 ml/min | 34 (43%) |
| >30 to ≤45 ml/min | 31 (39%) |
| Years of known kidney disease | 6.8±7.4 |
| Body mass index, n (%) | |
| ≤25 kg/m2 | 14 (18%) |
| >25 to ≤30 kg/m2 | 33 (41%) |
| >30 to ≤35 kg/m2 | 21 (26%) |
| >35 kg/m2 | 12 (15%) |
| Fasting glucose (mg/dl) | 160.2±59.4 |
| HbA1c (%) | 7.2±1.5 |
| Serum fructosamine, µmol/L | 304.1±57.3 |
| Medications | |
| Insulin | |
| Insulin, short acting only | 10 (13%) |
| Insulin, long acting only | 14 (18%) |
| Insulin, short and long acting | 18 (23%) |
| Sulfonylureas | 22 (28%) |
| Insulin and sulfonylureas | 8 (10%) |
| Other oral hypoglycemic agents other than SU and GLP | 27 (34%) |
| GLP-1 receptor agonists | 10 (13%) |
Adapted from Presswala et al. (10). DM, diabetes mellitus; CHF, congestive heart failure; CAD, coronary artery disease; HbA1c, hemoglobin A1c; SU, sulfonylureas; GLP, glucagon-like peptide-1 receptor agonists; GLP-1, glucagon-like peptide 1.
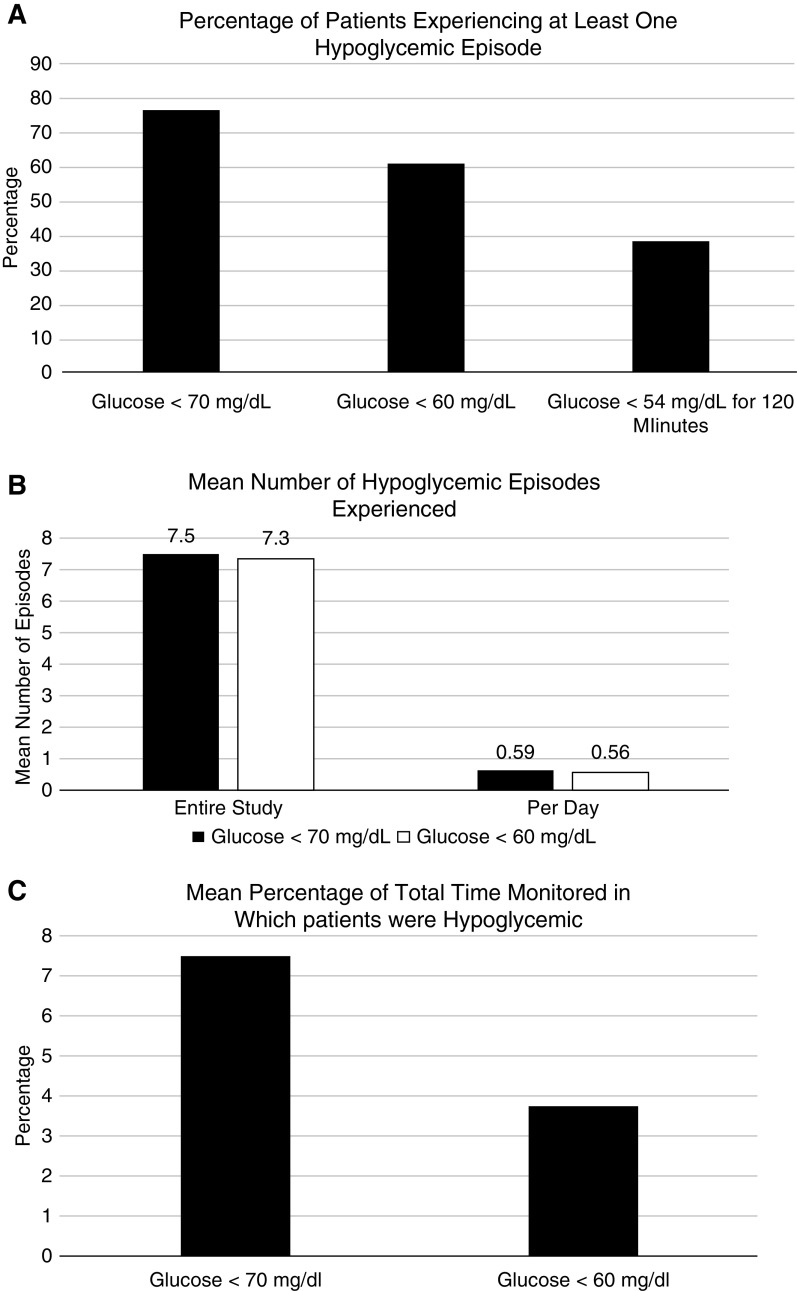
Hypoglycemic events (at least 15 minutes, two consecutive measurements) were found to be a frequent occurrence. Of the 80 patients, 61 (76%) had at least one episode of glucose <70 mg/dl, and 49 of 80 (61%) had at least one episode with glucose <60 mg/dl. The International Consensus on Use of Continuous Glucose Monitoring definition of a prolonged hypoglycemic event is CGM glucose levels <54 mg/dl for ≥120 consecutive minutes. We found this to occur in 31 patients (39%; Figure 1A), with 118 such events. The mean number of hypoglycemic events per patient was 7.5±9.0 when defined as glucose <70 mg/dl, and 7.3±6.9 when defined as glucose <60 mg/dl (Figure 1B). The mean percentage of time with glucose <70 mg/dl was 8%±10% and with glucose <60 mg/dl it was 7%±8% (Figure 1C). Related to hypoglycemia are questions relating to hyperglycemia and glucose variability. The percentage time that patients had glucose >180 mg/dl was 26.1%. Glucose variability was evaluated for the study population. The mean coefficient of variation was 33.0±9.3. The range of coefficient of variation was 17.0–58.9.
Figure 1.
Hypoglycemia in CKD and type 2 diabetes. (A) With a mean of 12.7±2.9 days of wearing the continuous glucose monitor (CGM) device, with measurements every 15 minutes, this figure displays the percentage of patients experiencing at least one hypoglycemic episode of ≥15 minutes. The first column has hypoglycemia defined as glucose <70 mg/dl; the second is glucose <60 mg/dl; and the third is the percentage of patients experiencing a severe, prolonged event. (B) This figure displays the total number of hypoglycemic episodes of at least 15 minutes per patient. The first set of two vertical bars represent the number of episodes over the entire mean 12.7±2.9 days wearing the CGM device for glucose <70 mg/dl (black) and 60 mg/dl (white). The second set of vertical bars represents the mean number of hypoglycemic episodes per day per patient. (C) This figure displays the total percentage time per patient in which the patient was hypoglycemic over the course of study, for glucose <70 mg/dl and <60 mg/dl.
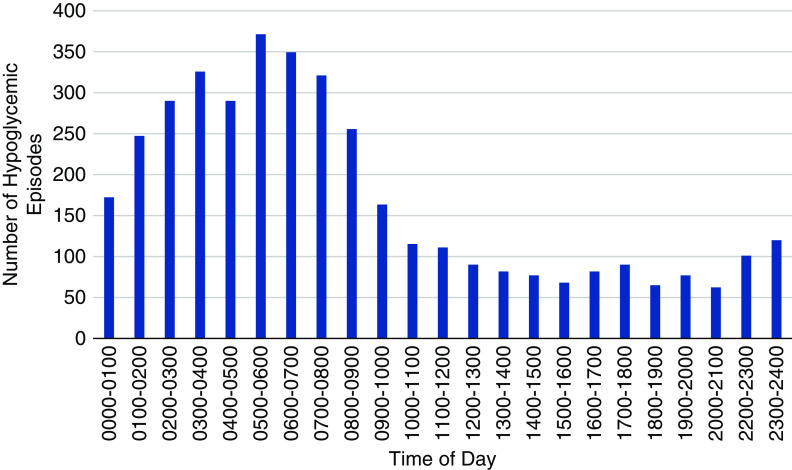
An examination of the time of day of hypoglycemic events is presented in Figure 2. From 10:00 am and throughout the day, the number of episodes was relatively low. This was particularly true in the traditional after dinner hours. Starting at approximately 23:00 pm, the number of episodes started to increase with a sharp increase in the early morning hours. From 1:00 to 9:00 am, the rate of hypoglycemic events was more than threefold higher than during the daytime hours.
Figure 2.
Most hypoglycemia occurred in the early morning hours. Number of hypoglycemic events (y axis; glucose <60 mg/dl for at least 15 minutes) by time of day (x axis). It can be seen that the greatest incidence of hypoglycemic episodes occurred in the early morning hours.
An analysis of the relationship between patient characteristics and the risk of hypoglycemic episodes (defined as glucose <60 mg/dl for at least 15 minutes adjusted for time in the study) was performed. In the univariate analysis, there were multiple predictors of hypoglycemia (Table 2). The multivariate analysis (Table 3) was more discriminating, but still resulted in ten independent predictors. Increased age and serum creatinine were associated with increased hypoglycemic risk. Conversely, increased body mass index, years of kidney disease, fasting blood glucose, hemoglobin and HbA1c were associated with lower risk. Patients without a history of stroke had a lower risk than those with stroke. Regarding medications, patients who were not currently treated with insulin had a 44% lower risk of hypoglycemia than those on insulin (P=0.0001).
Table 2.
Predictors of number of hypoglycemic events: univariate analysis
| Univariate Variable | Comparison | Estimated Coefficient (SEM) | Unadjusted Rate Ratio (95% CI) | P Value |
| Age per 1 yr | 0.01 (0.002) | 1.01 (1.007 to 1.013) | 0.0001 | |
| Sex | Female versus male | 0.46 (0.03) | 1.581 (1.481 to 1.688) | 0.0001 |
| Race | Asian versus white | −0.27 (0.05) | 0.762 (0.687 to 0.846) | 0.0001 |
| Black versus white | 0.22 (0.04) | 1.241 (1.14 to 1.35) | 0.0001 | |
| Ethnicity | Hispanic versus not Hispanic | −22.19 (79.0) | Extremely small | 0.99 |
| Body mass index per one unit (kg/m2) | −0.04 (0.003) | 0.963 (0.957 to 0.969) | 0.0001 | |
| Years of kidney diseases (per yr) | −0.09 (0.004) | 0.917 (0.909 to 0.925) | 0.0001 | |
| Fasting blood glucose (per mg/dl) | −0.01 (0.0003) | 0.989 (0.988 to 0.99) | 0.0001 | |
| Serum creatinine (per mg/dl) | 0.07 (0.008) | 1.075 (1.058 to 1.092) | 0.0001 | |
| Serum albumin (per g/L) | 0.006 (0.001) | 1.006 (1.005 to 1.008) | 0.0001 | |
| eGFR (per ml/min) | −0.008 (0.001) | 0.993 (0.99 to 0.995) | 0.0001 | |
| Hemoglobin (per g/dl) | −0.22 (0.006) | 0.805 (0.796 to 0.815) | 0.0001 | |
| HbA1c (per 1%) | −0.38 (0.01) | 0.687 (0.669 to 0.706) | 0.0001 | |
| Serum fructosamine, µmol/L | −0.004 (0.003) | 0.996 (0.995 to 0.997) | 0.0001 | |
| Average blood glucose (per 1 mg/dl) | −0.02 (0.006) | 0.977 (0.975 to 0.978) | 0.0001 | |
| CAD | Yes versus no | 0.21 (0.03) | 1.231 (1.154 to 1.312) | 0.0001 |
| CHF | Yes versus no | 0.44 (0.03) | 1.552 (1.455 to 1.655) | 0.0001 |
| Hypertension | Yes versus no | 0.88 (0.12) | 2.409 (1.912 to 3.039) | 0.0001 |
| Stroke history | Yes versus no | 0.16 (0.04) | 1.177 (1.096 to 1.264) | 0.0001 |
| History of malignancy | Yes versus no | 0.43 (0.05) | 1.531 (1.386 to 1.692) | 0.0001 |
| Medications | ||||
| Sulfonylureas | Yes versus no | 0.15 (0.03) | 1.157 (1.082 to 1.237) | 0.0001 |
| Any insulin | Yes versus no | 0.67 (0.04) | 1.949 (1.821 to 2.087) | 0.0001 |
| Insulin with SU | Yes versus no | 1.18 (0.04) | 3.267 (3.048 to 3.508) | 0.0001 |
| Oral other than SU | Yes versus no | −0.06 (0.03) | 0.945 (0.887 to 1.008) | 0.08 |
Outcome variable: number of >15 min events with glucose <60 mg/dl; n=80. HbA1c, hemoglobin A1c; CAD, coronary artery disease; CHF, congestive heart failure; SU, sulfonylureas.
Table 3.
Predictors of number of hypoglycemic events: multivariate analysis
| Variable | Comparison | Estimated Coefficient (SEM) | Adjusted Rate Ratio: (95% CI) | P Value |
| Age (per 1 yr) | 0.02 (0.002) | 1.021 (1.017 to 1.024) | 0.0001 | |
| Body mass index (per one unit kg/m2) | −0.03 (0.004) | 0.975 (0.968 to 0.983) | 0.0001 | |
| Years of kidney disease | −0.13 (0.007) | 0.881 (0.870 to 0.892) | 0.0001 | |
| Fasting blood glucose (per 1 mg/dl) | −0.005 (0.0004) | 0.995 (0.994 to 0.996) | 0.0001 | |
| Serum creatinine (per mg/dl) | 0.04 (0.02) | 1.039 (1.009 to 1.069) | 0.0001 | |
| Hemoglobin (per 1 g/dl) | −0.23 (0.01) | 0.791 (0.773 to 0.810) | 0.0001 | |
| HbA1c (per 1%) | −0.30 (0.019) | 0.742 (0.715 to 0.769) | 0.0001 | |
| Stroke history | Yes versus no | 0.20 (0.03) | 1.219 (1.142 to 1.30) | 0.0001 |
| Any insulin | Yes versus no | 0.57 (0.03) | 1.773 (1.667 to 1.883) | 0.0001 |
Outcome variable: number of >15 min events with glucose <60 mg/dl; n=80. HbA1c, hemoglobin A1c.
Discussion
We found that hypoglycemia occurs frequently and is often severe among patients with CKD and type 2 DM. The greatest number of events occurred overnight from midnight until the early morning hours. Prolonged, severe hypoglycemic events as defined by the International Consensus on Use of Continuous Glucose Monitoring (12) occurred in >38% of patients in 14 days of monitoring. Importantly, both lower HbA1c and use of any insulin treatment were associated with increased hypoglycemia risk.
Hypoglycemia is a cause of morbidity and mortality in patients with type 2 DM. In the ACCORD trial, hypoglycemia was more common among patients with type 2 DM who were randomized to intensive treatment. The higher rate of mortality in these patients compared with those treated with a conventional glucose target has suggested that hypoglycemia may have contributed to inferior outcomes (4). Hypoglycemia also increases anxiety among patients and may hinder the ability to achieve optimal chronic glucose control, as well as increasing the risk of mortality (13,14).
Recently, Ahmad et al. (9) reported the frequent occurrence of hypoglycemia in a population with type 2 DM and CKD. For entry, patients were required to be treated with sulfonylurea or insulin, creating a cohort with enriched risk for hypoglycemia. In this study, we extend these findings to indicate that hypoglycemia, including prolonged and severe events, is common among the more general population of patients with CKD. We note with particular concern the frequency of protracted episodes. Guidelines on the use of CGM including definition of prolonged episodes were developed by an international panel of physicians, researchers, and individuals with diabetes who are expert in CGM technologies convened by the Advanced Technologies and Treatments for Diabetes Congress. Their International Consensus on Use of Continuous Glucose Monitoring defined prolonged hypoglycemia with CGM as an event when levels are <54 mg/dl (3.0 mmol/L) for 120 consecutive minutes or more (12). We found this to occur in 38% of patients, with 118 total events in 31 patients. This occurred in only 14 days of monitoring, suggesting the substantial clinical relevance.
We found in multivariate analysis that ten different patient variables were independently associated positively or negatively with the number of hypoglycemic episodes. Some are inherent characteristics such as age, body mass index, years of kidney disease, serum creatinine, and history of stroke. We found the expected increase in hypoglycemia with insulin treatment. Surprisingly, sulfonylurea treatment was a predictor in univariate analysis, but was not independently associated with hypoglycemia risk in the multivariate analysis. An important concern is raised by the finding that an increase of 1% in HbA1c was associated with a 26% reduction in hypoglycemia risk. Although HbA1c is not always a fully accurate measure of glucose control, this still suggests that an eased vigilance as to glucose management goals might be warranted among patients with type 2 DM and CKD stages 3b–5. Our group previously reported on this same group of 80 patients with respect to assessment of glycated hemoglobin accuracy in CKD (10). We found the test to be highly accurate in these patients.
Our study does have potential limitations. The sample of 80 patients was predominately male and non-Hispanic. This is due to the characteristics of the single-center practice Northwell at Great Neck, New York and the population our center serves. Racial and sex parity would have benefited the study. It would also have been meaningful to have more robust data of a diary of self-reported symptoms during the 14 day period. A particular strength of the study was that it was not limited to patients a priori at increased risk of hypoglycemia, but rather was representative of a more general population of patients with type 2 DM and CKD.
In conclusion, we have found that hypoglycemia is common among patients with type 2 DM and CKD. Events were often severe and were prolonged in 38% of patients. Clinicians should monitor and be vigilant for the occurrence of hypoglycemia in CKD. Decisions as to HbA1c targets in these patients should weigh hypoglycemia risk, and a higher level may often be justified. Further research should be performed in larger cohorts to more completely define the problem of hypoglycemia in this patient population.
Disclosures
All authors have nothing to disclose.
Funding
None.
Acknowledgments
Dr. Yael T. Harris reports nonfinancial support from Abbot, other from Mylan, other from Novo Nordisk, and other from Sanofi, outside the submitted work.
Author Contributions
H. Andrade Paz and V. Sakhiya were responsible for data curation; S. Fishbane, Y. Harris, S. Hong, K. Jhaveri, L. Presswala, and M. Zhang were responsible for formal analysis; S. Fishbane, Y. Harris, K. Jhaveri, L. Presswala, and I. Romao were responsible for methodology; S. Fishbane, Y. Harris, K. Jhaveri, L. Presswala, I. Romao, V. Sakhiya, and M. Zhang conceptualized the study; S. Fishbane and S. Hong wrote the original draft; S. Fishbane, S. Hong, and V. Sakhiya were responsible for investigation; and Y. Harris, K. Jhaveri, L. Presswala, D. Ross, V. Sakhiya, and M. Zhang reviewed and edited the manuscript.
References
- 1.American Diabetes Association : 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2018. Diabetes Care 41: S13–S27, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Viazzi F, Piscitelli P, Giorda C, Ceriello A, Genovese S, Russo GT, Fioretto P, Guida P, De Cosmo S, Pontremoli R; AMD-Annals Study Group : Association of kidney disease measures with risk of renal function worsening in patients with hypertension and type 2 diabetes. J Diabetes Complications 31: 419–426, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V: Association of hypoglycemia and cardiac ischemia: A study based on continuous monitoring. Diabetes Care 26: 1485–1489, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME: The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: Retrospective epidemiological analysis of the ACCORD study. BMJ 340: b4909, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cryer PE: The barrier of hypoglycemia in diabetes. Diabetes 57: 3169–3176, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer C, Gerich JE: Role of the kidney in hyperglycemia in type 2 diabetes. Curr Diab Rep 2: 237–241, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Pecoits-Filho R, Abensur H, Betônico CC, Machado AD, Parente EB, Queiroz M, Salles JE, Titan S, Vencio S: Interactions between kidney disease and diabetes: Dangerous liaisons. Diabetol Metab Syndr 8: 50, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller ME, Bonds DE, Gerstein HC, Seaquist ER, Bergenstal RM, Calles-Escandon J, Childress RD, Craven TE, Cuddihy RM, Dailey G, Feinglos MN, Ismail-Beigi F, Largay JF, O’Connor PJ, Paul T, Savage PJ, Schubart UK, Sood A, Genuth S; ACCORD Investigators : The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: Post hoc epidemiological analysis of the ACCORD study. BMJ 340: b5444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad I, Zelnick LR, Batacchi Z, Robinson N, Dighe A, Manski-Nankervis JE, Furler J, O’Neal DN, Little R, Trence D, Hirsch IB, Bansal N, de Boer IH: Hypoglycemia in People with type 2 diabetes and CKD. Clin J Am Soc Nephrol 14: 844–853, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Presswala L, Hong S, Harris Y, Romao I, Zhang M, Jhaveri KD, Sakhiya V, Fishbane S: Continuous glucose monitoring and glycemic control in patients with type 2 diabetes mellitus and CKD. Kidney Med 1: 281–287, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fokkert MJ, van Dijk PR, Edens MA, Abbes S, de Jong D, Slingerland RJ, Bilo HJ: Performance of the FreeStyle Libre Flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus. BMJ Open Diabetes Res Care 5: e000320, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, Garg S, Heinemann L, Hirsch I, Amiel SA, Beck R, Bosi E, Buckingham B, Cobelli C, Dassau E, Doyle FJ 3rd, Heller S, Hovorka R, Jia W, Jones T, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Maahs D, Murphy HR, Nørgaard K, Parkin CG, Renard E, Saboo B, Scharf M, Tamborlane WV, Weinzimer SA, Phillip M: International Consensus on use of continuous glucose monitoring. Diabetes Care 40: 1631–1640, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA: Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 35: 1897–1901, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rombopoulos G, Panitti E, Varounis C, Katsinas C, Stefanidis I, Goumenos D: A multicenter, epidemiological study of the treatment patterns, comorbidities and hypoglycemia events of patients with type 2 diabetes and moderate or severe chronic kidney disease - the ‘LEARN’ study. Curr Med Res Opin 32: 939–947, 2016 [DOI] [PubMed] [Google Scholar]