Visual Abstract
Keywords: dialysis, arteriovenous fistula, arteriovenous graft, patency, vascular access
Abstract
Background
Arteriovenous fistulas (AVFs) for hemodialysis (HD) are often associated with better outcomes than arteriovenous grafts (AVGs). We aimed to investigate vascular access (VA) outcomes and assessed if AVF nonmaturation outweighs long-term complications of AVGs.
Methods
In this multicenter, retrospective cohort study in The Netherlands, 1- and 3-year primary, primary assisted, secondary, and functional patency rates were calculated, and the incidence of adverse events and procedures was assessed. Functional patency of RCAVFs, upper arm AVFs, and AVGs was compared using Cox analyses.
Results
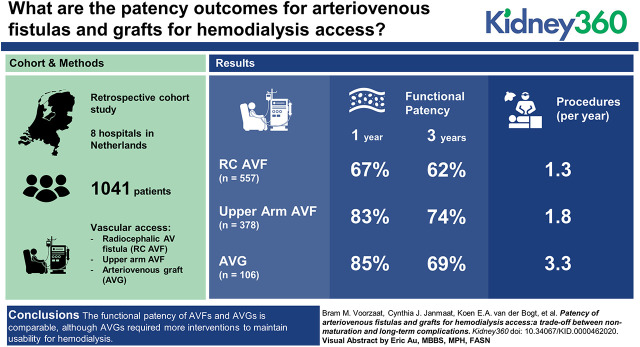
In total, 1041 patients who received their first VA were included, of whom 863 had VAs that successfully matured. These patients were analyzed with a median follow-up of 25 months. The 1-year functional patency rates were 67%±2.0% for RCAVFs, 83%±2.0% for upper arm AVFs, and 85%±3.5% for AVGs. Three-year functional patency rates were 62%±2.0% for RCAVFs, 74%±2.0% for upper arm AVFs, and 69%±5% for AVGs. AVGs required more procedures per year (3.3 per year) of functional patency when compared with upper arm AVFs (1.8 per year).
Conclusions
The functional patency of AVFs and AVGs is comparable, although AVGs required more interventions to maintain usability for HD. The choice of VA is a trade-off between short-term advantages, favoring AVGs, and long-term advantages, favoring AVFs. Which VA is most appropriate depends on the patient’s prognosis and preferences.
Introduction
Patients on maintenance hemodialysis (HD) require a reliable vascular access (VA). The European Society for Vascular Surgery and the European Best Practice Guidelines recommend to use native arteriovenous fistulas (AVFs) as the primary VA option. Indeed, AVFs are typically associated with fewer complications and longer VA survival when compared with prosthetic arteriovenous grafts (AVGs) and central venous catheters (CVCs) (1,2). However, a major disadvantage of AVFs is nonmaturation characterized by intimal hyperplasia and inadequate remodeling of the venous outflow tract, which precludes adequate use of the VA for HD. After initial successful use of an AVF, loss of patency may result from intimal hyperplasia, causing luminal narrowing and eventually resulting in thrombosis (3). As a consequence, patients on HD require multiple surgical or endovascular procedures to maintain patency or to create a new VA conduit.
Compared with AVFs, AVGs tend to have a lower primary failure rate but a lower long-term patency, requiring more procedures to maintain patency (4). The Dialysis Outcomes and Practice Patterns Study (DOPPS) on VA published in 2002 revealed large differences between the United States and Europe with regard to VA access use and outcomes (5).
We have previously reported on the maturation outcomes in a multicenter cohort of patients on HD in The Netherlands (6). The incidence of nonmaturation in our cohort was 24% for radiocephalic arteriovenous fistulas (RCAVFs) and 11% for upper arm AVFs. A primary failure rate of 6% for AVGs was observed. In this study, we report patency outcomes of arteriovenous HD access conduits in our cohort, as well as the incidence of VA-related adverse events and procedures.
Materials and Methods
Study Design and Patient Selection
Approval for the data collection was obtained from the ethics committee of the Leiden University Medical Center. Analyses were limited to RCAVFs, upper arm AVFs, and AVGs in the upper extremity. VAs were only included if no previous permanent VA was created in these patients and the clinical outcome of the VA could be retrieved. Patients were excluded if they were lost to follow-up before HD initiation. On the basis of these criteria, this study presents an analysis of the VA patency outcomes of 1041 patients from eight hospitals in The Netherlands who received their first arteriovenous VA between 1997 and 2016.
Definitions of End Points
A nonmatured VA was defined as a VA that could not be used successfully for HD or a VA that was abandoned in a patient not yet on HD (6). To standardize patency outcomes, the patency definitions as described by Sidawy et al. (7) were used. Primary patency is the time from VA creation until the first procedure, occlusion, or VA abandonment, whichever occurs first. Primary assisted patency is the time from VA creation until the first procedure to re-establish patency of an occluded VA or VA abandonment. Secondary patency is the time from VA creation until VA abandonment. Functional patency is defined as the time between first use of the VA and the abandonment of the VA. A VA was deemed successfully used for dialysis if it could be used with two-needle cannulation for three consecutive HD sessions.
We additionally calculated the postcannulation primary patency defined as the time from first successful cannulation to the first subsequent procedure to maintain or re-establish patency. For procedures, the postintervention primary patency was calculated starting at the index procedure and ending at the next procedure, occlusion, or abandonment. Patients were censored if a functioning VA was abandoned due to death, transplantation, or end of follow-up. Figure 1 provides a graphical presentation of the patency measures for an example VA.
Figure 1.
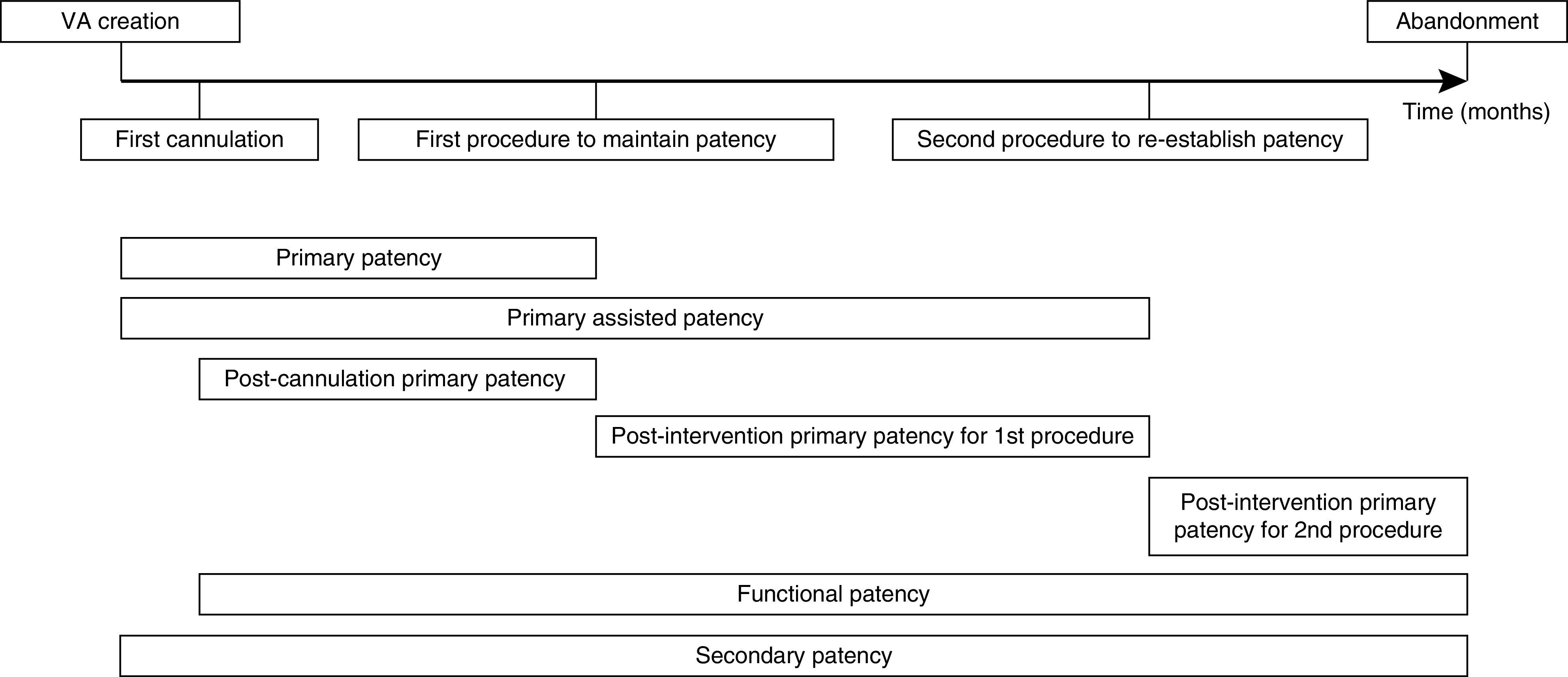
Visual example of patency measures. VA, vascular access.
If major revision surgery was performed and a new anastomosis was created between different vessels than the original VA, this was registered as abandonment of the old VA and creation of a new VA.
Statistical Analyses
Baseline characteristics are summarized as mean ± SD for continuous variables and frequency (percentage) for categorical variables. Baseline characteristics are reported only for patients receiving their first VA; those of the entire cohort were reported previously (6). Primary, primary assisted, and functional patency rates are presented as survival analyses using Kaplan–Meier curves with 1- and 3-year patency, expressed as percentage patent ± SEM. Rates of procedures and adverse events are expressed as both the number of events per year of functional patency and the proportion of VAs experiencing at least one event per event type.
Functional patency rates of the RCAVF, upper arm AVF, and AVG arteriovenous access conduits were compared using Cox regression analysis without adjustment for confounders and with adjustment for patient age, sex, body mass index, diabetes mellitus, cerebrovascular disease, coronary artery disease, peripheral vascular disease, and access configuration as covariates. IBM SPSS Statistics version 25 was used for all analyses (IBM Corp., Armonk, NY).
Results
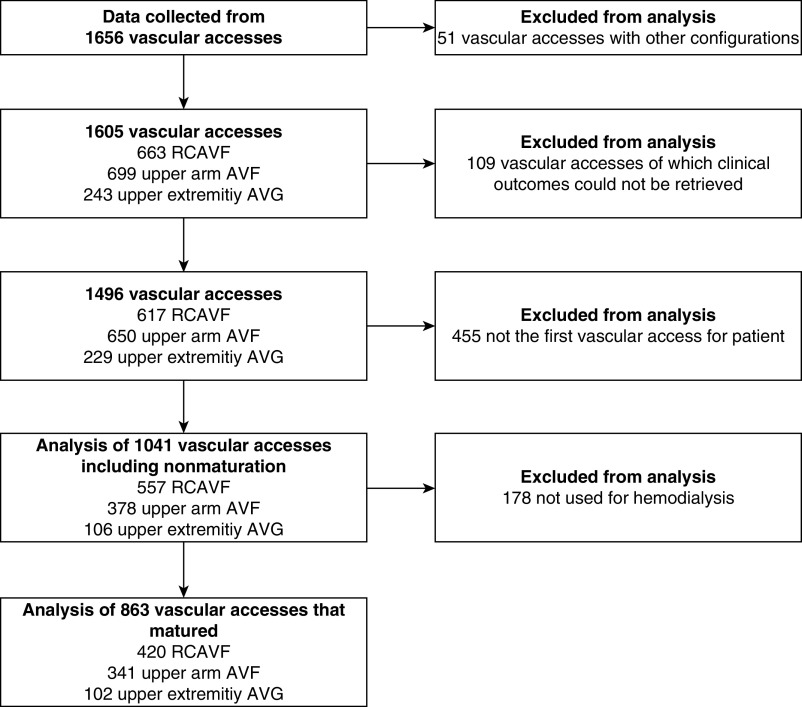
Of 1656 VAs in the original cohort, 1041 patients were included in the analysis as they received their first arteriovenous access during the study period while we could retrieve the clinical outcome parameters of their VA (Table 1). Of these 1041 VAs, 863 (83%) successfully matured (Figure 2). The median follow-up of VAs that were successfully used for HD was 25 months.
Table 1.
Baseline characteristics of patients
| Characteristic | Radiocephalic Arteriovenous Fistula, 557 | Upper Arm Arteriovenous Fistula, 378 | Arteriovenous Graft, 106 |
| Sex | |||
| Men | 392 (70.4%) | 199 (52.6%) | 44 (42.5%) |
| Women | 165 (29.6%) | 179 (47.4%) | 62 (58.5%) |
| Follow-up duration, mo | 28.6±30.1 | 24.2±21.4 | 33.5±28.7 |
| Patient age, yr | 62.7±15.0 | 63.4±14.4 | 65.4±14.0 |
| BMI, kg/m2 | 27.0±5.8 | 26.3±6.0 | 27.7±6.7 |
| Preemptive | 318 (57.1%) | 186 (49.2%) | 61 (57.5%) |
| Preoperative vein diameter (lumen), mm | 2.9±0.8 | 3.7±1.3 | 4.0±1.5 |
| Preoperative artery diameter (lumen), mm | 2.6±0.5 | 4.1±0.8 | 4.4±0.9 |
| Ipsilateral CVC | 72 (12.9%) | 69 (18.3%) | 8 (7.5%) |
| Cause of renal failure | |||
| Diabetes mellitus | 117 (21.0%) | 93 (24.6%) | 37 (34.9%) |
| Renal vascular disease | 128 (23.0%) | 76 (20.1%) | 27 (25.5%) |
| Cystic kidney disease | 44 (7.9%) | 20 (5.3%) | 4 (3.8%) |
| GN | 60 (10.8%) | 34 (9.0%) | 5 (4.7%) |
| Congenital/hereditary | 16 (2.9%) | 12 (3.2%) | 2 (1.9%) |
| Interstitial nephropathy | 35 (6.3%) | 21 (5.6%) | 5 (4.7%) |
| Multisystem disease | 25 (4.5%) | 20 (5.3%) | 3 (2.8%) |
| Other | 70 (12.6%) | 59 (15.6%) | 11 (10.4%) |
| Unknown | 62 (11.1%) | 43 (11.4%) | 12 (11.3%) |
| Comorbidities | |||
| Diabetes mellitus | 205 (36.8%) | 150 (39.7%) | 66 (62.3%) |
| Coronary artery disease | 153 (27.5%) | 105 (27.8%) | 32 (30.2%) |
| Peripheral vascular disease | 105 (18.9%) | 76 (20.1%) | 21 (19.8%) |
| Cerebrovascular disease | 83 (14.9%) | 58 (15.3%) | 15 (14.2%) |
Numbers denote mean ± SD for continuous variables or count (percentage) for categorical variables. BMI, body mass index; CVC, central venous catheter.
Figure 2.
Flowchart of inclusion and exclusion from analysis. AVF, arteriovenous fistula; AVG, arteriovenous graft; RCAVF, radiocephalic arteriovenous fistula.
Maturation and Procedures to Promote Maturation
Fifty-nine percent of RCAVFs, 79% of upper arm AVFs, and 79% of AVGs did not require any intervention before these could be used for HD.
From the 230 RCAVFs that did not mature spontaneously, the RCAVF was abandoned in 98 patients. In the remaining 132 patients, one or multiple procedures were performed to promote maturation, resulting in successful use of the RCAVF for HD in 93 patients (70% of patients in whom procedures were performed to promote maturation). In 36% of patients, a surgical revision was performed, and the remaining patients underwent an endovascular procedure.
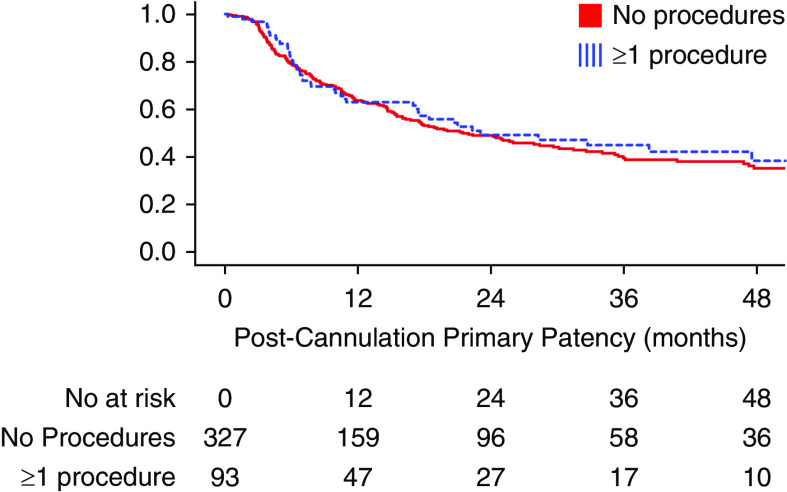
Functional patency of RCAVFs that required a procedure to assist maturation was comparable with patency of RCAVFs that matured spontaneously, with 1- and 3-year functional patency rates of 91% and 83%, respectively. The time until the first procedure after successful use of the AVF was also not different between RCAVFs that matured with or without additional procedures (Figure 3).
Figure 3.
Postcannulation primary patency of RCAVF that matured with and without interventional procedures, excluding RCAVFs that never matured.
From the group of 22 AVGs that were not suitable for cannulation, three AVGs were abandoned without additional interventions. In the remaining 19 AVGs, one or more interventions were performed, which resulted in successful use for HD in 18 patients (95%).
Patency Outcomes
The 1-year primary patency rates of VAs that matured were 51% for RCAVFs, 65% for upper arm AVFs, and 47% for AVGs (Figure 4, Table 2). Primary patency most often ended due to a procedure to maintain patency (RCAVF, 69%; upper arm AVF, 66%; and AVG, 54%) or a procedure to re-establish patency (RCAVF, 22%; upper arm AVF, 25%; and AVG, 43%). Rarely, primary patency ended with immediate VA abandonment (RCAVF, 8%; upper arm AVF, 6%; and AVG, 3%).
Figure 4.
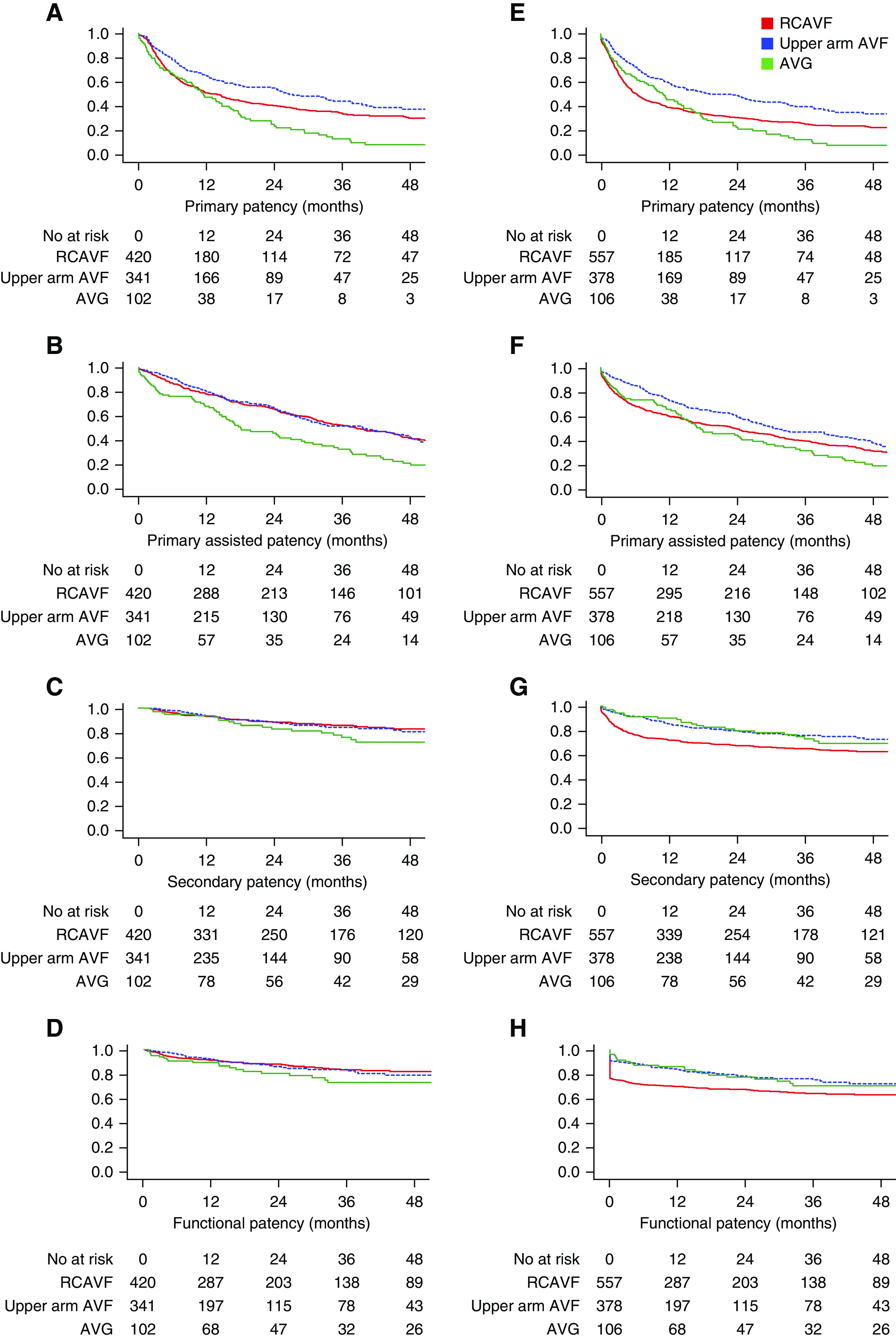
Primary, primary assisted, secondary, and functional patency for RCAVFs, upper arm AVFs, and AVGs. Patients are censored for death and transplantation. (A–D) Clinical outcomes of all VAs, excluding VAs that did not mature (n=863). (E–H) Clinical outcomes of all VAs, including nonmatured VAs (n=1041).
Table 2.
One- and 3-year primary, primary assisted, secondary, and functional patency for radiocephalic arteriovenous fistulas, upper arm arteriovenous fistulas, and arteriovenous grafts
| Patency Measure | 1-yr Patency | 3-yr Patency | ||||
| Radiocephalic Arteriovenous Fistula | Upper Arm Arteriovenous Fistula | Arteriovenous Graft | Radiocephalic Arteriovenous Fistula | Upper Arm Arteriovenous Fistula | Arteriovenous Graft | |
| Patency measures of VAs excluding VAs that did not mature (n=863), % | ||||||
| Primary | 51±3 | 65±3 | 47±5 | 35±3 | 43±3 | 13±4 |
| Primary assisted | 78±2 | 81±2 | 67±5 | 53±3 | 52±3 | 32±5 |
| Secondary | 93±1 | 94±1 | 94±3 | 86±2 | 84±3 | 78±5 |
| Functional | 91±1 | 92±2 | 89±3 | 83±2 | 83±3 | 72±6 |
| Patency measures of VAs including VAs that did not mature (n=1041), % | ||||||
| Primary | 39±2 | 59±3 | 45±5 | 26±2 | 39±3 | 12±4 |
| Primary assisted | 59±2 | 73±2 | 65±5 | 40±2 | 47±3 | 31±5 |
| Secondary | 71±2 | 85±2 | 90±3 | 64±2 | 75±3 | 75±5 |
| Functional | 67±2 | 83±2 | 85±4 | 62±2 | 74±3 | 69±5 |
Patency rates are percentage of VAs still patent ± SEM. Patients are censored for death and transplantation. VA, vascular access.
The 1-year functional patency rate of VAs that matured was 90% for all types of VAs (RCAVFs, upper arm AVFs, and AVGs). When nonmaturated VAs were included in the analysis as well, the 1-year functional patency was lower for RCAVFs at 67% compared with 83%±2% for upper arm AVFs and 85%±4% for AVGs. Functional patency rates at 3 years were 62% for RCAVFs, 74% for upper arm AVFs, and 69% for AVGs, with no statistically differences between groups (data not shown). In contrast, the functional patency of RCAVFs was significantly lower when compared with upper arm AVFs (unadjusted hazard ratio [HR], 1.6; 95% confidence interval [95% CI], 1.28 to 2.13; adjusted HR, 1.8; 95% CI, 1.4 to 2.4) or AVGs (unadjusted HR, 1.4; 95% CI, 1.0 to 2.1; adjusted HR, 1.6; 95% CI, 1.0 to 2.4).
Procedures and Adverse Events in Matured Vascular Access Conduits
Of the VAs that maturated successfully, 49% of RCAVFs, 38% of upper arm AVFs, and 68% of AVGs required at least one balloon angioplasty procedure during their lifetimes (Table 3). The event rate, expressed as the number of procedures per year of functional patency, was also different at 1.0 balloon angioplasty procedure per year for RCAVFs, 1.6 balloon angioplasty procedures per year for upper arm AVFs, and 1.8 balloon angioplasty procedures per year for AVGs. For thrombectomy procedures, these differences were more pronounced, as only 5% of AVFs required a thrombectomy to restore patency compared with 34% for AVGs. The thrombectomy rate was highest for AVGs at 1.1 per year of patency compared with 0.03 per year for RCAVFs and 0.05 per year for upper arm AVFs.
Table 3.
Cumulative incidence and event rates of adverse events and procedures
| Adverse Event or Procedure | Vascular Access Type | Event Rate | ||||
| Radiocephalic Arteriovenous Fistula (420) | Upper Arm Arteriovenous Fistula (341) | Arteriovenous Graft (102) | Radiocephalic Arteriovenous Fistula (420) | Upper Arm Arteriovenous Fistula (341) | Arteriovenous Graft (102) | |
| Adverse events | ||||||
| VA site infection | 7 (1.7%) | 15 (4.4%) | 11 (10.8%) | 0.0049±0.040 | 0.07±0.45 | 0.10±0.40 |
| Thrombosis | 69 (16.4%) | 37 (10.9%) | 36 (35.3%) | 0.46±5.1 | 0.14±0.61 | 1.83±8.34 |
| Procedures | ||||||
| Percutaneous procedure—only balloon angioplasty—still functional VA | 204 (48.6%) | 129 (37.8%) | 69 (67.6%) | 1.01±2.79 | 1.64±8.96 | 1.84±4.37 |
| Percutaneous procedure including thrombectomy—occluded VA | 21 (5.0%) | 16 (4.7%) | 35 (34.3%) | 0.03±0.24 | 0.05±0.32 | 1.13±4.04 |
| Stenting | 1 (0.2%) | 16 (4.7%) | 5 (4.9%) | 0.001±0.024 | 0.04±0.33 | 0.02±0.10 |
| Surgical revision | 80 (19.0%) | 24 (7.0%) | 21 (20.6%) | 0.29±2.62 | 0.05±0.28 | 0.31±1.55 |
| Flow reduction | 3 (0.7%) | 32 (6.2%) | 0 (0%) | 0.0043±0.060 | 0.05±0.32 | 0.00±0.00 |
Event rates are expressed as number of events per year of functional patency ± SD. VA, vascular access.
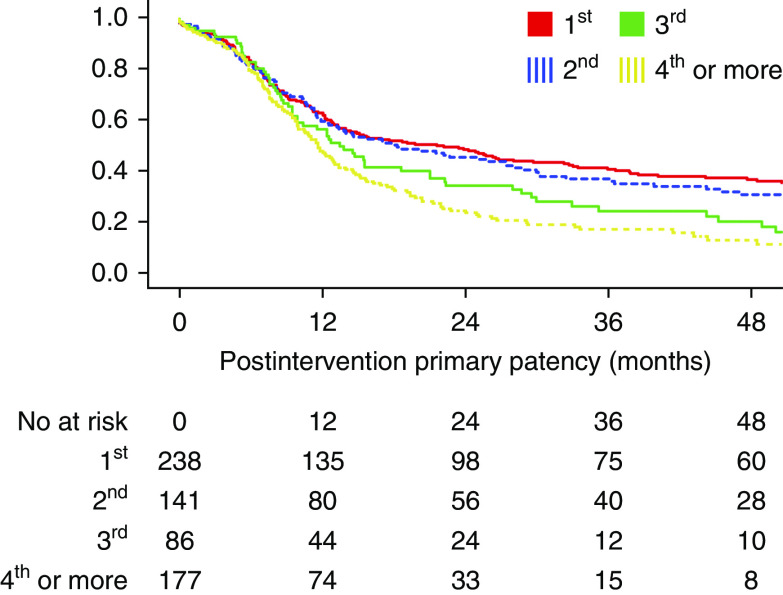
After the first procedure to maintain or re-establish patency, the 1-year postintervention primary patency rates were 63%±3.2% for RCAVFs, 60%±4.2% for upper arm AVFs, and 57%±5.5% for AVGs. After each subsequent procedure aimed at improving patency, the time until the next procedure decreased (Figure 5).
Figure 5.
Postintervention primary patency of RCAVF after subsequent procedures. Postintervention patency starts at the index procedure and ends at the next procedure, occlusion, or abandonment of the VA, and patients are censored for death and transplantation.
The number of infections per year of functional patency was highest for AVGs at 0.10 per year and lowest for RCAVFs at 0.0049 per year (Table 3). Of upper arm AVFs, 6% required flow-reducing procedure compared with 0.7% for RCAVFs. The 1-year postintervention secondary patency after these procedures was 77%±9.2%.
Subgroup Analyses
Functional patency of upper arm AVFs was significantly better for men than for women (1-year functional patency, 87%±3.0% for men versus 74%±3% for women; adjusted HR, 0.49; 95% CI, 0.30 to 0.79). This difference was mainly due to nonmaturation occurring more frequently in women. For AVGs, no differences were observed in patency between men and women (1-year functional patency, 84%±6% versus 82%±5%; adjusted HR, 0.77; 95% CI, 0.32 to 1.84). Age over 70 years was not significantly associated with functional patency of AVFs (1-year functional patency, 80%±3% in nonelderly patients and 82%±3% in elderly patients; adjusted HR, 1.57; 95% CI, 0.96 to 2.58) nor AVGs (1-year functional patency, 89%±4% in nonelderly patients and 77%±6% in elderly patients; adjusted HR, 0.32; 95% CI, 0.07 to 1.41).
Discussion
In this study, we examined patency outcomes for arteriovenous access conduits and observed that the functional patency of matured AVFs and AVGs is comparable, although AVGs required more interventions to maintain usability for HD.
Functional Patency and Nonmaturation
For VAs that maturated successfully, 1-year functional patency was approximately 90% for all types of access, whereas at 3 years, functional patency was still rather good (73% for AVGs and 83% for AVFs). This demonstrates that after an AVF has reached functional maturation, loss of functional patency is uncommon. Functional patency of RCAVFs may seem better than for AVGs, but this is only true if RCAVFs that failed to mature are not taken into account. When nonmaturated VAs are taken into account, functional patency of AVGs is superior to RCAVFs.
The results obtained in our study are in line with two large recent meta-analyses in which VA patency was investigated. Al-Jaishi et al. (8) included both upper arm and forearm AVFs. Like in our study, upper arm AVFs performed better than forearm AVFs. The incidences of primary failure was 28% for forearm AVFs and 20% for upper arm AVFs, similar to the 24% and 11%, respectively, in our cohort. The authors found a 1-year primary patency rate of 55% for forearm AVFs versus 65% for upper arm AVFs if nonmatured VAs were included. Most strikingly, forearm primary patency differed from our cohort, in which a 1-year primary patency of 39% was observed for RCAVFs and 59% was observed for upper arm AVFs. Because primary patency ends with any procedure to improve patency, this may for a significant part be the result of a “doctor’s decision,” and this outcome is sensitive to local surveillance practices and subsequent preemptive interventions. Conversely, the 1-year secondary patency was similar in the study by Al-Jaishi et al. (8) for forearm AVFs (68%) and upper arm AVFs (70%). In our cohort, 1-year secondary patency rates were slightly higher at 71% and 85%, respectively.
A more recent meta-analysis by Bylsma et al. (9) demonstrated a 1-year primary patency 64% of all AVFs, with upper arm AVFs at 69% performing better than forearm AVFs at 55%. Secondary patency rates of upper arm AVFs and forearm AVFs were similar at 67% and 71%, respectively.
Although the pooled patency rates of AVFs are relatively constant in the various large meta-analyses, there is a significant variability in patency outcomes in the included studies, which is most pronounced for primary patency. This suggests that the influence of local practices and experience in maintaining AVF patency may be significant on primary patency but less on the overall longevity of the VA.
The AVGs in our cohort had similar functional patency as autologous AVFs. This contrasts the findings from the DOPPS, where a shorter functional patency was observed for AVGs compared with AVFs, with a relative risk for AVF failure of 0.56 compared with AVGs (5). In the DOPPS, the 1-year functional patency of AVG was only 49%, which is substantially lower when compared with our study (85%). This difference might be explained by a higher proportion of AVGs created in patients predialysis in our study, as AVGs survive shorter if created in patients who initiated HD with a CVC (5). The better functional patency of AVG in our cohort might also relate to a more “aggressive” surveillance strategy. In The Netherlands, surveillance by measurements of VA flow is currently advised on a monthly basis for AVGs and a 3-monthly basis for AVFs (10). Of note, the usefulness of routine ultrasound surveillance for AVGs remains a topic of debate (11).
Elderly Patients
In concordance with previous studies (12), functional VA patency was not associated with age. This observation implies that one should not refrain from creating a permanent VA in elderly patients eligible for maintenance HD. However, the optimal VA strategy in frail elderly patients is a topic of debate as they have a higher chance of dying before reaching ESKD (13). They may be saved from the burden of preemptively creating an AVF by opting for an “early stick” AVG or CVC only when HD initiation is imminent.
Procedures Related to Patency
The incidence of procedures differed between AVFs and AVGs. Most remarkably, the fraction of AVGs for which a thrombectomy was performed was sevenfold higher than of AVFs. This observation is in agreement with data from a meta-analysis in which procedure rates from nine different studies were reported (14). All studies showed an at least twofold higher incidence of procedures per access-year for AVGs compared with AVFs, with some studies reporting differences up to sevenfold. Malik et al. (11) reviewed five clinical trials on routine ultrasound surveillance of AVGs and found that the ultrasound criteria for a hemodynamically significant stenosis differed among these studies, possibly explaining the different incidences of subsequently performed procedures. In The Netherlands, VA surveillance using flow measurements is common practice and may influence the intervention policy, resulting in more balloon angioplasty procedures to maintain patency and possibly fewer procedures to re-establish patency because unexpected VA occlusion is less likely to occur. However, as early thrombus removal is more urgent in AVFs compared with AVGs (2), the adherence to surveillance guidelines might have been lower in AVGs, resulting in a relatively high rate of procedures to restore patency.
Study Limitations
Maturation and patency of different VA configurations should be compared with caution. The choice for a specific VA configuration is on the basis of the anatomy of the patient, prior history of VA failure, and patient preference. Because current guidelines advise to start VA creation as distal as possible, most patients who received a upper arm AVF or AVG were not eligible for an RCAVF: for instance, due to more advanced vascular abnormalities. This selection bias limits the validity of direct comparisons of VA outcomes, and residual confounding after correction for patient characteristics cannot be ruled out.
In addition, it is important to notice that the loss of functional patency of a VA results not solely from pathophysiologic processes to result in VA failure but also, from the clinical decision to stop investing in a problematic VA. It is likely that upper arm AVFs and AVGs are more often a “last resort” option rather than a first choice. We assume that an RCAVF will on average be abandoned earlier as these patients will often have options to “move on” to a more proximal VA and the need to repeatedly perform procedures to maintain functional patency is less urgent, whereas the need to maintain an upper arm AVF or AVG may be more pressing.
Further Directions
Although patency outcomes of different VA configurations that have reached maturation are comparable, nonmaturation remains the Achilles’ heel of RCAVFs. Functional patency of RCAVFs in our study remained lower than of AVGs. The price to pay was a two- to threefold higher incidence of procedures required to maintain patency in patients with AVGs. In other words, VA planning is a trade-off between short-term outcomes, including nonmaturation and the odds of creating an unnecessary VA, and long-term outcomes, including functional patency and the number of procedures involved. The decisions of which VA to create and when to create it should be individualized, taking the short- and long-term properties of each VA into account while considering the patient’s prognosis and preferences. Performing a randomized controlled trial that randomizes patients to RCAVFs, upper arm AVFs, or AVGs as their first permanent VA may finally elucidate the performance of these configurations.
Disclosures
All authors have nothing to disclose.
Funding
This research received unrestricted funding from Proteon Therapeutics Inc.
Acknowledgments
Patency outcomes from this study were presented at the 11th Congress of the Vascular Access Society in Rotterdam, The Netherlands on April 11–13, 2019.
Author Contributions
C. Janmaat, J. Rotmans, K. van der Bogt, and B. Voorzaat conceptualized the study; K. van der Bogt and B. Voorzaat were responsible for data curation; F. Dekker, C. Janmaat, and B. Voorzaat were responsible for formal analysis; J. Rotmans, K. van der Bogt, and B. Voorzaat were responsible for investigation; F. Dekker, C. Janmaat, J. Rotmans, and B. Voorzaat were responsible for methodology; J. Rotmans and B. Voorzaat were responsible for project administration; B. Voorzaat was responsible for software and visualization; J. Rotmans was responsible for funding acquisition and resources; J. Rotmans provided supervision; C. Janmaat, J. Rotmans, K. van der Bogt, and B. Voorzaat wrote the original draft; and F. Dekker, C. Janmaat, J. Rotmans, K. van der Bogt, and B. Voorzaat reviewed and edited the manuscript.
References
- 1.Tordoir J, Canaud B, Haage P, Konner K, Basci A, Fouque D, Kooman J, Martin-Malo A, Pedrini L, Pizzarelli F, Tattersall J, Vennegoor M, Wanner C, Wee Pt, et al. R: EBPG on vascular access. Nephrol Dial Transplant 22[Suppl 2]: ii88–ii117, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Schmidli J, Widmer MK, Basile C, de Donato G, Gallieni M, Gibbons CP, Haage P, Hamilton G, Hedin U, Kamper L, Lazarides MK, Lindsey B, Mestres G, Pegoraro M, Roy J, Setacci C, Shemesh D, Tordoir JHM, van Loon M, Kolh P, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Lindholt J, Naylor R, Vega de Ceniga M, Vermassen F, Verzini F, Mohaupt M, Ricco JB, Roca-Tey R; ESVS Guidelines Committee; ESVS Guidelines Reviewers : Editor’s choice—vascular access: 2018 Clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 55: 757–818, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Rothuizen TC, Wong C, Quax PHA, van Zonneveld AJ, Rabelink TJ, Rotmans JI: Arteriovenous access failure: More than just intimal hyperplasia? Nephrol Dial Transplant 28: 1085–1092, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, Harris J, Moist L: Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 8: 810–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, Wolfe RA, Goodkin DA, Held PJ: Vascular access use in Europe and the United States: Results from the DOPPS. Kidney Int 61: 305–316, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Voorzaat BM, van der Bogt KEA, Janmaat CJ, van Schaik J, Dekker FW, Rotmans JI; Dutch Vascular Access Study Group : Arteriovenous fistula maturation failure in a large cohort of hemodialysis patients in The Netherlands. World J Surg 42: 1895–1903, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sidawy AN, Gray R, Besarab A, Henry M, Ascher E, Silva M Jr., Miller A, Scher L, Trerotola S, Gregory RT, Rutherford RB, Kent KC: Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg 35: 603–610, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, Kosa SD, Quinn RR, Moist LM: Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 63: 464–478, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Bylsma LC, Gage SM, Reichert H, Dahl SLM, Lawson JH: Arteriovenous fistulae for haemodialysis: A systematic review and meta-analysis of efficacy and safety outcomes. Eur J Vasc Endovasc Surg 54: 513–522, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Betjes M, Hoogeveen E, Tordoir J: Dutch Federation for Nephrology—Guideline on Vascular Access 2009 [in Dutch], 2009. Available at: https://www.nefro.nl/sites/www.nefro.nl/files/richlijnen/Richtlijn-Vaattoegang-2009%5B1%5D.pdf. Accessed April 19, 2018
- 11.Malik J, Kudlicka J, Novakova L, Adamec J, Malikova H, Kavan J: Surveillance of arteriovenous accesses with the use of duplex Doppler ultrasonography. J Vasc Access 15[Suppl 7]: S28–S32, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Jennings WC, Landis L, Taubman KE, Parker DE: Creating functional autogenous vascular access in older patients. J Vasc Surg 53: 713–719, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Moist LM, Lok CE, Vachharajani TJ, Xi W, AlJaishi A, Polkinghorne KR, Vazquez M, Lee TC: Optimal hemodialysis vascular access in the elderly patient. Semin Dial 25: 640–648, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allon M: Current management of vascular access. Clin J Am Soc Nephrol 2: 786–800, 2007 [DOI] [PubMed] [Google Scholar]