Abstract
AstraZeneca coronavirus disease 2019 (COVID-19) vaccinations have recently been implicated in thromboembolism formations. Our aim was to investigate the outcomes of patients with thromboembolic events following the AstraZeneca vaccine (ChAdOx1 nCoV-19, AZD1222). A literature search was performed from December 2019 to September 2021. Eligible studies must report participants older than 18 years vaccinated with AstraZeneca and outcomes of thromboembolic events. Pooled mean or proportion were analyzed using a random-effects model. A total of 45 unique studies (number of patients = 144, 64.6% women, mean age 21–68 years) were included. The most common presenting adverse events were headache (12.1%), intracerebral hemorrhage (7.5%), and hemiparesis (7%). The most common thromboembolic adverse events were cerebral venous sinus thrombosis (38.5%) and deep vein thrombosis/pulmonary embolism (21.1%). The most common radiologic finding were intracerebral hemorrhage and cerebral venous thrombosis. Laboratory findings included thrombocytopenia (75%) and hypofibrinogenemia (41%). On admission, 64 patients tested positive for PF4-Heparin ELISA assay (80%). Seventy-four patients were hospitalized with 22 being admitted to the ICU. A total of 78 patients recovered while 39 patients died. This meta-analysis presents evidence to suggest vaccine-induced immune thrombotic thrombocytopenia (VITT) following AstraZeneca vaccine. Clinical practice must, therefore, account for the possibility of VITT and subsequent embolic events in certain individuals’ postvaccination with adenovirus-based COVID-19 vaccines. Serum anti-PF4 suggests diagnostic value for VITT and could subsequently inform treatment choices in such instances.
Keywords: AstraZeneca, clot, coronavirus disease 2019, thromboembolic, vaccination, vaccine
Introduction
The first case of coronavirus was identified in Wuhan, China in early December 2019 [1]. The virus, part of the novel enveloped RNA betacoronavirus family, has been named as the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its associated disease coronaviruses disease 2019 (COVID-19) [2]. Since then, COVID-19 has been declared as a pandemic affecting over 224 180 411 globally with more than 4 621 205 deaths [3]. As such, efforts have been directed to combat and manage this disease.
Currently, four companies (AstraZeneca-Oxford (Cambridge, United Kingdom) Pfizer-Biontech (New York, NY, USA and Mainz, Germany), Moderna (Cambridge, Massachusetts, USA), and Johnson and Johnson (New Brunswick, New Jersey, USA)) have manufactured vaccines that have been authorized for use in the European Union. Whilst all are yet to reach approval status, emerging data from double-blinded, randomized, controlled clinical trials have persuaded the American Food and Drug Administration (FDA) to permit emergency use authorization for the Pfizer-Biontech, Moderna and Johnson and Johnson vaccines, whilst the European Medicine Agency has permitted use of the AstraZeneca vaccine, and full FDA approval for Pfizer-Biontech vaccine. Two of these vaccines are messenger RNA-based vaccines – BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) – which encode the spike protein antigen of SARS-CoV-2, encapsulated in lipid nanoparticles [4,5]. The other two vaccines, ChAdOx1 nCoV-19 (AstraZeneca) and Ad26.COV2.S (Johnson and Johnson/Janssen), are recombinant adenoviruses that encode the spike glycoprotein of SARS-CoV-2 [6].
To date, approximately 560 261 011 of vaccines have been administered in the European Union and 5.88 billion around the world, roughly 25% being ChAdOx1 nCoV-19 [7]. The expedited approval for the use of the vaccines, however, does not come without its pitfalls. In mid-February 2021, many patients reported various vaccine-related side effects [8]. A study has evaluated the safety and efficacy of the ChAdOx1 nCoV-19 based on clinical trials of 23 745 individuals randomized to either AstraZeneca vaccine or control in United Kingdom, Brazil, and South Africa [9]. The results demonstrated that most prevalent adverse events included redness, pain, headache, fatigue, and malaise. Since then, the majority of the European Union decided to temporarily halt the use of the AstraZeneca vaccine amid reports of its association with increased risk of thromboembolic events along with multiple deaths. Accordingly, a retrospective study investigated reports submitted to the EudraVigilance database [10]. Following 54,571 adverse events, 28 were associated with thromboembolic events, of which were four related mortalities.
On 13 April 2021, in a joint statement, the CDC and FDA recommended to suspend the use of Johnson and Johnson vaccines as six thromboembolic events were discovered following an administration of 6.8 million doses [11]. As more individuals continue to get vaccinated, there is an urgent need to answer questions regarding the safety of the AstraZeneca vaccine specifically pertaining to its association with increased thromboembolic adverse events. Although the results available in the literature may be sparse, it is important to recognize the urgency and time-sensitivity of this issue. To date, there is no systematic review or meta-analysis that has been conducted. Therefore, this systematic review and meta-analysis aims to provide insight into the outcomes of thromboembolic events in patients following AstraZeneca Vaccine.
Methods
Search strategy and data sources
A comprehensive search of several databases from 1 December 2019, to 1 September 2021 was conducted and limited to English language only. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Scopus, PMC Preprints, and ClinicalTrials.Gov. The search strategy was designed and conducted by a medical reference librarian. Controlled vocabulary supplemented with keywords was used to search for AstraZeneca vaccine and thromboembolic events. The actual strategy listing all search terms used and how they are combined is available in Supplementary Item 1.
Eligibility criteria and quality assessment
Eligible studies must have met all the following inclusion criteria: participants must be older than 18 years vaccinated with AstraZeneca vaccine; reports adverse events and outcomes of thromboembolic events. The methodological quality of each study was independently evaluated by two authors (R.H.M. and C.A.T.) using the methodological quality and synthesis of case series and case reports as has been previously described within the literature [12].
Statistical analysis
Means of continuous variables and rates of binary variables were pooled using the random-effects model, generic inverse variance method of DerSimonian and Laird [13]. Proportions underwent logit transformation prior to meta-analysis. The heterogeneity of effect size estimates across the studies was quantified using the Q statistic and the I2 index (P < 0.10 was considered significant). A value of I2 of 0–25% indicates minimal heterogeneity, 26–50% moderate heterogeneity, and 51–100% substantial heterogeneity. Data analysis was performed using Open Meta analyst software (CEBM, Brown University, Providence, Rhode Island, USA).
Results
Study selection and characteristics
The initial search yielded 567 potentially relevant articles from which 45 unique studies involving 144 patients met the eligibility criteria. The details of study selection process are depicted in Supplementary Item 2. The baseline characteristics of the included studies are comprehensively described in Table 1. The mean age ranged from 21 to 68 years of which 93 patients were women.
Table 1.
Baseline characteristics of included studies
| Author, year | Country | Study design | Number of subjects (n) | Sex (male) | Sex (female) | Mean age ± SD (years) |
| Aladdin et al., 2021 | Saudi Arabia | Case Report | 1 | 0 | 1 | 36.00 |
| Al-mayhani et al., 2021 | UK | Case Series | 3 | 1 | 2 | 38.33 |
| Al rawahi et al., 2021 | Oman | Case Report | 1 | 1 | 0 | 64.00 |
| Althaus et al., 2021 | Germany | Case Series | 8 | 3 | 5 | 39.50 |
| Bano et al., 2021 | UK | Case Report | 3 | 1 | 2 | 56.00 |
| Bayas et al., 2021 | Germany | Case Report | 1 | 0 | 1 | 55.00 |
| Bersinger et al., 2021 | France | Case Report | 1 | 0 | 1 | 21.00 |
| Bjornstad-tuveng et al., 2021 | Norway | Case Report | 1 | 0 | 1 | NR |
| Blauenfeldt et al., 2021 | Denmark | Case Report | 1 | 0 | 1 | 60.00 |
| Bourguignon et al., 2021 | Canada | Case Series | 3 | 2 | 1 | 68.00 |
| Castelli et al., 2021 | Italy | Case Report | 1 | 1 | 0 | 50.00 |
| Casucci et al., 2021 | Italy | Case Report | 1 | 0 | 1 | 52.00 |
| Choi et al., 2021 | South Korea | Case Report | 1 | 1 | 0 | 33.00 |
| Cliff-patel et al., 2021 | UK | Case Series | 3 | 3 | 0 | 40.66 |
| Costentin et al., 2021 | France | Case Report | 1 | 0 | 1 | 26.00 |
| D’agostino et al., 2021 | Italy | Case Report | 1 | 0 | 1 | 54.00 |
| Demichele et al., 2021 | Italy | Case Series | 2 | 0 | 2 | 56.00 |
| Dutta et al., 2021 | India | Case Report | 1 | 1 | 0 | 51.00 |
| Gabarin et al., 2021 | Canada | Case Series | 2 | 2 | 0 | 61.50 |
| Gangi et al., 2021 | UK | Case Series | 6 | 4 | 2 | 37.33 |
| Gattringer et al., 2021 | Austria | Case series | 2 | 0 | 2 | 31.50 |
| Greinacher et al., 2021 | Germany | Case Series | 11 | 2 | 9 | 36.00 |
| Guan et al., 2021 | Taiwan, ROC | Case Report | 1 | 1 | 0 | 52.00 |
| Haakonsen et al., 2021 | Norway | Case Series | 2 | 1 | 1 | NR |
| Ikenberg et al., 2021 | Germany | Case Report | 1 | 0 | 1 | NR |
| Jacob et al., 2021 | UK | Case Report | 1 | 0 | 1 | 39.00 |
| Jones et al., 2021 | Canada | Case Report | 1 | 1 | 0 | 63.00 |
| Kotal et al., 2021 | India | Case Report | 1 | 0 | 1 | 32.00 |
| Mehta et al., 2021 | UK | Case Series | 2 | 2 | 0 | 28.60 |
| Muster et al., 2021 | Austria | Case Report | 1 | 0 | 1 | 51.00 |
| Ocal et al., 2021 | Germany | Case Report | 1 | 1 | 0 | 41.00 |
| Panovska-stavridis et al., 2021 | Republic of North Macedonia | Case Report | 1 | 0 | 1 | 29.00 |
| Schultz et al., 2021 | Norway | Case Series | 5 | 1 | 4 | 40.80 |
| Scully et al., 2021 | UK | Retrospective Study | 22 | 9 | 13 | 43.00 |
| Soleimani et al., 2021 | UK | Case Series | 3 | 1 | 2 | 44.00 |
| Sorensen et al., 2021 | Denmark | Case report | 1 | 0 | 1 | 30.00 |
| Suresh, Petchey et al., 2021 | UK | Case Report | 1 | 1 | 0 | 27.00 |
| Tiede et al., 2021 | Germany | Case Series | 5 | 0 | 5 | 58.60 |
| Tobaiqy et al., 2021 | Saudi Arabia | Retrospective Descriptive Study | 28 | 9 | 19 | NA |
| Umbrello et al., 2021 | Italy | Case Report | 1 | 0 | 1 | 36.00 |
| Varona et al., 2021 | Spain | Case Report | 1 | 1 | 0 | 47.00 |
| Walter et al., 2021 | Germany | Case Report | 1 | 1 | 0 | 31.00 |
| Weidmann et al., 2021 | Norway | Case Series | 5 | 0 | 5 | 41.20 |
| Wolf et al., 2021 | Germany | Case Series | 3 | 0 | 3 | 34.70 |
| Zanferrari et al., 2021 | Italy | Case Report | 1 | 0 | 1 | 40.00 |
| Abou-Ismail et al., 2021a | USA | Case Report | 1 | 1 | 0 | 48.00 |
| Clark et al., 2021a | USA | Case Report | 1 | 0 | 1 | 40.00 |
| Dhoot et al., 2021a | USA | Case Report | 1 | 1 | 0 | 24.00 |
| Malik et al., 2021a | USA | Case Report | 1 | 0 | 1 | 43.00 |
| Muir et al., 2021a | USA | Case Report Correspondence | 1 | 0 | 1 | 48.00 |
| See et al., 2021a | USA | Case Series | 12 | 0 | 12 | NR |
NR, not reported; SD, standard deviation.
Studies are Ad26.COV2.S reports.
Risk of bias
Results of the quality assessment of all included studies are shown in Supplementary Table 1. All the case series were judged to be of good quality. The patients appeared to represent the whole experience of the investigator and the exposure and outcome were adequately ascertained, and the length of follow-up was adequate.
Clinical characteristics
The clinical characteristics of the patients are shown in Table 2. Among the overall population, some patients had at least one coexisting illness; frequently reported illnesses included pollen allergy (n = 6), hypothyroidism (n = 4) hypertension (n = 8), asthma (n = 3), diabetes (n = 2), and neurologic disorders (n = 2). One patient had comorbidity of Von Willebrand Disease, factor V Leiden thrombophilia, and anticardiolipin antibodies [14]. One patient had a relevant history of deep vein thrombosis (DVT) [15]. Sixteen patients were on preexisting medication prior to presentation. Some common medications included antihypertension agents [16,17] and thyroid hormone replacement agents [16,18]. The use of contraceptive methods was indicated in 15 patients. Specifically, seven patients were on the contraceptive pill [14,15,17], three patients were on hormone replacement therapy, two on hormonal intrauterine device (IUD) [14], and three patients on contraceptive vaginal ring [17] (Supplementary Item 3: Supplementary Item 4; Supplementary Item 5:).
Table 2.
Clinical characteristics of included patients
| Author, year | Existing comorbidities (n) | History of relevant disorders (n) | Preexisting medications (n) | Use of contraceptives or hormonal therapies (n) |
| Aladdin et al., 2021 | Diabetes (1) | 0 | 0 | 0 |
| Al-mayhani et al., 2021 | 0 | NR | NR | NR |
| Al rawahi et al., 2021 | Hyperlipidaemia (1), hypertension (1) | 0 | 0 | 0 |
| Althaus et al., 2021 | NR | NR | NR | NR |
| Bano et al., 2021 | Asthma (1), fibromyalgia (1), hypertension (1), obesity (1) | 0 | Anti-HTN agent (1) | HRT (1) |
| Bayas et al., 2021 | 0 | 0 | 0 | NR |
| Bersinger et al., 2021 | Chronic migraine (1) | 0 | 0 | Oral contraceptive pill (1) |
| Bjornstad-tuveng et al., 2021 | Allergy (1), iron deficiency (1) | NR | Desloratadine (1), Duroferon (1) | 0 |
| Blauenfeldt et al., 2021 | Hypertension (1), hypothyroidism (1), | NR | Anti-HTN agent (1), cholesterol-lowering medication (1), thyroid hormone replacements (1) | 0 |
| Bourguignon et al., 2021 | Diabetes (1), hypertension (1), prostate cancer (1) | Sleep apnoea (1) | Aspirin (1), heparin (1) | 0 |
| Castelli et al., 2021 | 0 | 0 | 0 | NR |
| Casucci et al., 2021 | Chronic headaches (1), Hepatitis B (1), history of breast cancer (1), ovarian cyst (1) | 0 | 0 | 0 |
| Choi et al., 2021 | 0 | 0 | 0 | 0 |
| Cliff-patel et al., 2021 | NR | NR | NR | NR |
| Costentin et al., 2021 | 0 | 0 | 0 | Oral contraceptive pill (1) |
| D’agostino et al., 2021 | Meniere's disease (1) | 0 | 0 | 0 |
| Demichele et al., 2021 | History of breast cancer (1), hypothyroidism (2) | NR | NR | NR |
| Dutta et al., 2021 | 0 | 0 | 0 | 0 |
| Gabarin et al., 2021 | 0 | NR | NR | NR |
| Gangi et al., 2021 | Asthma (1), cardiomyopathy (1), depression (2), diverticulitis (1), Guillain–Barre syndrome (1), hip arthroscopy (1), hypertension (1), PCOS (1), polycythemia (1) | 0 | 0 | Oral contraceptive pill (1) |
| Gattringer et al., 2021 | 0 | 0 | NR | NR |
| Greinacher et al., 2021 | Anticardiolipin antibodies (1), factor V Leiden (1), Von Willebrand factor (1) | NR | 0 | Hormonal IUD (2), oral contraceptive pill (1) |
| Guan et al., 2021 | 0 | NR | 0 | 0 |
| Haakonsen et al., 2021 | Hypothyroidism (1). | 0 | Thyroid hormone replacements (1) | 0 |
| Ikenberg et al., 2021 | 0 | 0 | 0 | 0 |
| Jacob et al., 2021 | 0 | 0 | 0 | 0 |
| Jones et al., 2021 | Hypertension (1), obesity (1) | 0 | Anti HTN medication (1) | NR |
| Kotal et al., 2021 | 0 | 0 | 0 | 0 |
| Mehta et al., 2021 | 0 | 0 | Amitriptyline (1), corticosteroids (1), ursodeoxycholic acid (1) | NA |
| Muster et al., 2021 | 0 | NR | NR | NR |
| Ocal et al., 2021 | NR | NR | NR | NR |
| Panovska-stavridis et al., 2021 | 0 | 0 | 0 | 0 |
| Schultz et al., 2021 | Allergy (2), asthma (1), hypertension (1) | 0 | Anti-HTN agent (1) | HRT (1), oral contraceptive pill (1), contraceptive vaginal ring (1) |
| Scully et al., 2021 | 0 | DVT (1) | 0 | 0 |
| Soleimani et al., 2021 | Bipolar disorder (1) | 0 | Lithium (1) | 0 |
| Sorensen et al., 2021 | 0 | 0 | 0 | Oral contraceptive pill (1) |
| Suresh & petchey et al., 2021 | 0 | 0 | 0 | 0 |
| Tiede et al., 2021 | 0 | NR | NR | NR |
| Tobaiqy et al., 2021 | 0 | NR | NR | NR |
| Umbrello et al., 2021 | 0 | 0 | NR | NR |
| Varona et al., 2021 | NR | 0 | NR | NR |
| Walter et al., 2021 | 0 | 0 | 0 | 0 |
| Weidmann et al., 2021 | Allergy (3), hypertension (1) | 0 | Anti HTN agent (1) | HRT (1), oral contraceptive pill (1), contraceptive vaginal ring (2) |
| Wolf et al., 2021 | 0 | 0 | 0 | 0 |
| Zanferrari et al., 2021 | 0 | 0 | 0 | 0 |
| Abou-ismail et al., 2021a | Asthma (1) | 0 | 0 | 0 |
| Clark et al., 2021a | 0 | 0 | 0 | 0 |
| Dhoot et al., 2021a | 0 | 0 | NR | NR |
| Malik et al., 2021a | Depression (1), hyperlipidaemia (1), obesity (1) | GORD (1), hyperlipidaemia (1), sleep apnoea (1) | 0 | 0 |
| Muir et al., 2021a | 0 | NR | NR | NR |
| See et al., 2021a | Hypothyroidism (1), obesity (6) | 0 | 0 | Oral Contraceptive Pill (1) |
DVT, deep vein thrombosis; IUD, intrauterine device; NR, not reported.
Studies are Ad26.COV2.S reports.
Radiological and laboratory findings
Table 3 shows the radiologic and laboratory findings on admission. The most common imaging performed were CT (n = 114) and MRI (n = 38). The most common radiologic findings were intracranial hemorrhage and cerebral venous sinus thrombosis.
Table 3.
Radiologic and laboratory findings on admission
| Author, year | Imaging conducted (n) | Major MRI findings (n) | Major CT findings (n) | Thrombocytopenia (n) | Hypofibrinogenemia (n) | Mean platelet count (per μl) ± SD | Mean Prothrombin time (INR) ± SD | Mean aPTT (sec) ± SD | Mean Fibrinogen (mg/dl) | Mean D-dimer (ng/ml) ± SD |
| Aladdin et al., 2021 | Brain CT (1) | NA | NR | 1 | NR | NR | 45.00 | 98.00 | NR | NR |
| Al-mayhani et al., 2021 | CT (2), CTA (2), MRI (2), CT venogram (1) | PE (1), CVST (1), hepatic vein thrombosis (1), jugular vein thrombosis (1), iliac vein thrombosis (1), MCA occlusion (1) | MCA occlusion (2), portal vein thrombosis (1), internal carotid artery occlusion (1), CVST (1) | NR | NR | 40 333.00 ± 28 290.00 | NR | NR | NR | 23 073.33 ± 11418.24 |
| Al rawahi et al., 2021 | TTE (1), CTA abdomen (1), CTA pelvis (1), ECG (1), CXR (1), CT head with CT arteriogram and CT venogram (1) | NA | Descending aorta thrombi (1), PE (1), renal vein thrombosis with renal infarction (1), bilateral adrenal gland hemorrhage (1) | 1 | NR | 20 000.00 | 13.00 | 49.20 | 400.00 | 36 900.00 |
| Althaus et al., 2021 | NR | NR | NR | 8 | NR | 44 000.00 ± 29 800.00 | NR | 27.56 ± 7.80 | 180.00 | 18 000.00 ± 12 300.00 |
| Bano et al., 2021 | CTPA (1), CT venogram (1), CT head (1) | NA | PE (1), CVST (1), internal jugular vein thrombosis (1),subarachnoid hemorrhage (1) | 3 | NR | 23 333.00 ± 2082.00 | NR | NR | 149.70 | 20 959.00 ± 23 391.00 |
| Bayas et al., 2021 | MRI (1) | SOVT (1), ischemic stroke (1) | NA | 1 | NR | NR | NR | NR | NR | NR |
| Bersinger et al., 2021 | CT head (1), CT (1) | NA | CVST (1), jugular vein thrombosis (1), PE (1), splanchnic vein thrombosis (1), external iliac vein thrombosis (1) | 1 | NR | 61 000.00 | NR | NR | NR | NR |
| Bjornstad-tuveng et al., 2021 | CT head (1), CTA (1), transcranial Doppler (1) | NA | Intracranial Haemorrhage (1), Oedema (1) | 1 | NR | 37 000.00 | NR | 27.00 | 220.00 | NR |
| Blauenfeldt et al., 2021 | CT abdomen (1), MRI (1), CT head (1), MR angiography (1) | MCA infarction (1), internal carotid artery occlusion (1) | Adrenal hemorrhage (1), subscapular renal hematoma (1), oedema (1) | 1 | NR | 118 000.00 | NR | NA | NA | NA |
| Bourguignon et al., 2021 | CTA (1), US (1) | NA | NA | NR | NR | NR | NR | NR | NR | NR |
| Castelli et al., 2021 | CT head (1), CTA (1) | NA | Intracranial hemorrhage (1), CVST (1) | 1 | 1 | 20 000.00 | NR | 26.80 | 98.00 | NR |
| Casucci et al., 2021 | Echocardiography (1), Doppler US (1), CT head (1), CT (1), MRA (1) | Transverse sinus hypoplasia (1) | NR | NR | NR | 77 000.00 | 15.00 | 28.00 | 100.00 | 8298.00 |
| Choi et al., 2021 | Non-enhanced CT head (1), MRI venogram (1), | CVST (1) | Parietal lobe subcortical hematoma (1) subarachnoid hemorrhage (1) | 1 | 1 | 14 000.00 | 14.00 | 28.00 | 77.00 | NR |
| Cliff-patel et al., 2021 | CT (2), US (1), CT venogram (1), CTPA (2) | NA | Pyelonephritis (1), PE (3), renal vein thrombus (1), DVT (1) | 3 | NR | NR | NR | NR | NR | NR |
| Costentin et al., 2021 | MRI (1), CT (1), cervical CTA (1), transesophageal echocardiography (1) | Acute ischemic stroke | Haemorrhagic infarction (1), PE (1), portal vein thrombosis (1) | 1 | NR | 57 000.00 | NR | NR | NR | NR |
| D’agostino et al., 2021 | CT head (2), MRA (1), brain MRI (1), CTA (2) | CVST (1), ischemic cerebral artery and pontine branch lesions (1) | Intracranial Haemorrhage (1), aortic arch thrombus (1), CVST (1), splanchnic vein defects (1), adrenal hemorrhage (1), ischemic stroke (1), oedema (1) | 1 | 0 | NR | NR | 41.00 | NR | NR |
| Demichele et al., 2021 | MRI (1), whole body CT (2), TTE (2), transcranial color Doppler US (1), thorax CT (1), abdominal US (1), CT head (2), CTA (1), perfusion CT (1) | MCA occlusion (2) | Internal Carotid artery occlusion (1), MCA occlusion (1), PE (2), portal vein thrombosis (2), intrahepatic branch thrombosis (1), bilateral MCA Infarct with uncal herniation (1), RDS (1) | 2 | NR | 44 500.00 ± 30 400.00 | NR | NR | 286.00 | 5441.00 |
| Dutta et al., 2021 | MRI (1), MRI venography (1) | CVST (1) | NA | 0 | NR | NR | NR | NR | NR | NR |
| Gabarin et al., 2021 | Doppler US (2), CT chest (1), echocardiograph (1), CT Venography (1) | NA | PE (1) | NR | NR | 109 500.00 ± 9192.39 | NR | NR | NR | NR |
| Gangi et al., 2021 | angiogram (1), CTPA (2), non-contrast head CT (4), CT venography (5), brain MRI (1), abdominal US (2), CT abdomen (2), CT pelvis (2), CT chest (1), echocardiogram (1) | Venous infarct (1) | PE (2), atrial appendage thrombus (1), CVST (4), pulmonary infarct (1), splanchnic vein thrombosis (2), internal iliac artery thrombosis (1), temporal cortical venous hemorrhage (1) | 6 | NR | 59 167.00 ± 46 602.00 | NR | NR | 188 | 20 396.00 ± 26 205.23 |
| Gattringer et al., 2021 | CT head (1), CTPA (1), brain MRI (2) | CVST (1), cortical veins thrombosis (1) | Unremarkable (2) | 2 | NR | 32 500.00 ± 4950.00 | NR | NR | 62.00 | 14 200.00 |
| Greinacher et al., 2021 | CT (1) | NA | Portal vein thrombosis (1), PE (1) | 11 | 4 | 35 300.00 ± 33 865.91 | NR | 42.30 ± 13.52 | 191.50 | 36 080.00 ± 59 739.40 |
| Guan et al., 2021 | Nonenhanced CT (1), CT venogram (1) | NA | CVST (1), Internal jugular vein thrombosis (1) | 1 | NR | 99 000.00 | NR | NR | NR | NR |
| Haakonsen et al., 2021 | MRI (1) | Unremarkable (1) | NA | NR | NR | 318 500.00 ± 21 920.00 | NR | NR | NR | NA |
| Ikenberg et al., 2021 | MRI (3) | Unremarkable (1), CVST (1), intracranial hemorrhage (1) | NA | 1 | NR | 97 000.00 | NR | NR | NR | NR |
| Jacob et al., 2021 | CT head (3), CTA (1), CTA neck (1) | NA | MCA occlusion, internal carotid artery thrombosis (1) | 1 | NR | 66 000.00 | NR | NR | NR | NR |
| Jones et al., 2021 | CTA (1) | NA | PE (1), infrarenal aortic thrombus (1) | 1 | NR | 36 000.00 | NR | NR | 140 | NR |
| Kotal et al., 2021 | MRI (1), CT venogram (1), CT head (1) | Parietal hemorrhage (1) oedema (1) | Parietal lobe hematoma (1) | NR | NR | 120 000.00 | NR | NR | NR | 1105.00 |
| Mehta et al., 2021 | CT (2) | NA | CVST (2) | 2 | 2 | 24 500.00 ± 7778.20 | NR | NR | 135.00 | NR |
| Muster et al., 2021 | CTA (1), MRI venography (1), CT venography (1) | Internal iliac vein thrombosis (1) | PE (1), IVC Thrombosis (1) | 1 | NR | 37 000.00 | NR | NR | 220.00 | 34 000.00 |
| Ocal et al., 2021 | CT head (1), CTA (1), CT thorax (1), CT abdomen (1) | NA | PE (1), splanchnic vein thrombosis (1) | 1 | NR | 64 000.00 | NR | NR | NR | 42 028.00 |
| Panovska-stavridis et al., 2021 | CT head (1), contrast-enhanced MRI (1) | SOVT (1) | Unremarkable (1) | 1 | NR | 18 000.00 | NR | NR | 250.00 | 35 712.00 |
| Schultz et al., 2021 | CT head (2), CT venography (3), CT abdomen (1), MRI (1) | Compromised thoracic vertebrae/basivertebral venous drainage (1) | CVST (4) intracranial hemorrhage, (2) oedema (2), splanchnic vein Thrombosis (1), SVC constituents thrombosis (1) | 5 | 3 | 27 000.00 ± 24474.48 | NR | 27.00 ± 2.83 | 152.00 | 13 000.00 |
| Scully et al., 2021 | NR | NR | Portal vein thrombosis (2), aortic thrombosis (1) | 21 | 12 | 46 571.00 ± 32 727.00 | 13.41 ± 1.17 | 29.35 ± 5.34 | 192.72 | 33 547.00 ± 22532.00 |
| Soleimani et al., 2021 | CT head (2), CTA (1), CT abdomen (1), brain MRI (1), MRI venography (1), CT Whole (1) | CVST (1), intracranial hemorrhage (1) | Intracranial hemorrhage (2), CVST (2), PE (2), hepatic vein thrombosis (1), oedema (2), cerebral herniation (1) | 2 | NR | 96 667.00 ± 134 526.00 | 14.80 ± 0.30 | 14.80 ± 0.30 | 196.70 | 32 057.00 ± 10211.60 |
| Sorensen et al., 2021 | Noncontrast CT scan (1), MRI head (1) MR venogram (1), CTPA (1), CTA abdominal (1), CT venography (1) | Unremarkable (1) | Portal vein thrombosis (1), CVST (1) | 1 | NR | 57 000.00 | NR | NR | NR | NR |
| Suresh, Petchey et al., 2021 | Head CT (2), CT venogram (1) | NA | CVST (1) | 1 | NR | 90 000.00 | 12.90 | 27.50 | 194.00 | 340 71.00 |
| Tiede et al., 2021 | CTA (3), CT HEAD (2), BRAIN MRI (2), MR angiography (1) | CVST (1) | Intracranial hemorrhage (2), CVST (1), aortic arch thrombosis (1), splanchnic vein thrombosis (1) | 5 | NR | 49 200.00 ± 36 190.00 | NR | NR | 227.50 | 22 400.00 |
| Tobaiqy et al., 2021 | NR | NR | NR | 4 | 1 | NR | NR | NR | NR | NR |
| Umbrello et al., 2021 | CXR (1), abdominal CT (1), trans-hepatic portal vein venography (1), mesenteric angiography (1) | NA | Splanchnic vein thrombosis (1) | 1 | NR | 133 000.00 | NR | NR | 501.00 | NR |
| Varona et al., 2021 | CT (1), MRI (1) | CVST (1), Bilateral Adrenal Haemorrhage (1) | CVST (1) | 1 | NR | 103 000.00 | NR | NR | NR | 20 506.00 |
| Walter et al., 2021 | MRI (1), catheter angiography (1), CT (1), CTA (1), US (1), transesophageal echocardiography (1) | MCA occlusion (1) | Brain infarction (1) | 0 | NR | 217 000.00 | NR | 27.50 | 270.00 | 11 000.00 |
| Weidmann et al., 2021 | Cerebral CT (9), cerebral CT with venography (1), MRI venography (3), CTPA (1), abdominal CT (1), abdominal US (1) | CVST (3), cortical vein thrombosis (2) | Subarachnoid hemorrhage (4), cerebellar hemorrhage (3), temporo-occipital hemorrhage (1), parenchymal hemorrhage (2), herniation (3), lobar hemorrhage (1), CVST (2), cortical vein thrombosis (1), PE (1), uterine vein thrombosis (1) | 5 | NR | 31 600.00 ± 22 600.00 | NR | 27.40 ± 2.50 | 134.00 | 14 600.00 ± 2300.00 |
| Wolf et al., 2021 | MRI (2), digital subtraction angiography (DSA) (3) | Intracranial hemorrhage (2), CVST (3), oedema (1) | NA | 3 | 0 | 75667.00 ± 16010.00 | NR | NR | NR | 9170.00 ± 11806.00 |
| Zanferrari et al., 2021 | CT head (1), brain MRI (1) | CVST (1) | Intracranial hemorrhage (1) | 1 | NR | NA | NR | NR | NR | 27 546.00 |
| Abou-ismail et al., 2021b | Venous duplex ultrasound (1), CT scan (1), MR venography (1), MR angiography (1) | NA | PE (1) | 1 | NR | 74 000.00 | 12.70 | 31.80 | 254.00 | 15 109.00 |
| Clark et al., 2021b | CT (1) | NA | CVST (1), PE (1) | 1 | NR | 20 000.00 | 16.00 | 26.40 | 149.00 | 27 150.00 |
| Dhoot et al., 2021b | Contrast CT (2), brain MRI (1), intravascular US guidance with intracardiac echocardiography (1) | Unremarkable (1) | Portal vein thrombosis (1), superior mesenteric vein thrombosis (1), splenic vein thrombosis (1) | 1 | NR | 66 000.00 | NR | 31.00 | 225.00 | 5250.00 |
| Malik et al., 2021b | CTA (2), MRI (1), Doppler US (1), CTPA (1), brain MRI (1), CT head (1) | None | CVST (1), PE (1), Carotid Artery Thrombosis (1) | 1 | NR | 21000.00 | 12.20 | 26.40 | 142.00 | 35 200.00 |
| Muir et al., 2021b | CT (1), CT head (1), MRI (1), MRA (1), CTA (1) | CVST (1), intracranial hemorrhage (1) | CVST (1), splanchnic vein thrombosis (1) | 1 | 1 | NA | NR | 41.00 | NA | NA |
| See et al., 2021b | CT (12) | NA | CVST (12) | 12 | NR | 45 750.00 ± 40 636.86 | NR | 25.32 ± 8.77 | 159.33 | 24 928.00 ± 32 542.75 |
| Author, year | Mean Asserachrom HPIA IgG Assay (OD) ± SD | Mean Lifecodes PF4 IgG Assay (OD) ± SD | PF4-heparin ELISA (OD) ± SD | HemosIL AcuStar HIT IgG Assay (n) | Functional HIT Assay (n) | PF4-heparin ELISA Ab (n) |
| Aladdin et al., 2021 | NR | NR | NR | NR | NR | NR |
| Al-mayhani et al., 2021 | NR | NR | NR | NR | NR | Positive (3) |
| Al rawahi et al., 2021 | NA | NR | NR | NR | NR | NR |
| Althaus et al., 2021 | NR | 2.59 ± 0.64 | 0.17 ± 0.07 | NR | NR | Positive (8) |
| Bano et al., 2021 | NR | NR | 2.64 ± 0.22 | NR | NR | Positive (3) |
| Bayas et al., 2021 | NR | NR | NR | NR | NR | Negative (1) |
| Bersinger et al., 2021 | 0 | NR | NR | NR | NR | NR |
| Bjornstad-tuveng et al., 2021 | NR | NR | NR | NR | NR | Positive (1) |
| Blauenfeldt et al., 2021 | NR | NR | NR | NR | NR | Positive (1) |
| Bourguignon et al., 2021 | NR | NR | NR | NR | NR | NR |
| Castelli et al., 2021 | NR | NR | NR | NR | NR | NR |
| Casucci et al., 2021 | NR | NR | NR | NR | NR | NR |
| Choi et al., 2021 | NR | 0.72 | NR | NR | NR | NR |
| Cliff-patel et al., 2021 | NR | NR | 1.35 | NR | NR | Positive (3) |
| Costentin et al., 2021 | NR | NR | NR | NR | NR | Positive (1) |
| D’agostino et al., 2021 | NR | NR | NR | NR | NR | NR |
| Demichele et al., 2021 | NR | 1.48 ± 0.27 | NR | NR | NR | NR |
| Dutta et al., 2021 | NR | NR | NR | NR | NR | Negative (1) |
| Gabarin et al., 2021 | NR | NR | 1.71 ± 1.02 | NR | NR | Positive (2) |
| Gangi et al., 2021 | NR | NR | 2.65 ± 0.60 | NR | NR | Positive (6) |
| Gattringer et al., 2021 | NR | NR | NR | NR | NR | NR |
| Greinacher et al., 2021 | NR | NR | NR | NR | NR | Positive (9) |
| Guan et al., 2021 | NR | NR | NR | NR | NR | Positive (1) |
| Haakonsen et al., 2021 | NR | NR | NR | NR | NR | NR |
| Ikenberg et al., 2021 | NR | NR | NR | NR | NR | Positive (1) |
| Jacob et al., 2021 | NR | 2.45 | NR | Negative (1) | NR | NR |
| Jones et al., 2021 | NR | NR | NR | Negative (1) | NR | Positive (1) |
| Kotal et al., 2021 | NR | NR | NR | NR | NR | NR |
| Mehta et al., 2021 | NR | NR | NR | NR | NR | Positive (1) |
| Muster et al., 2021 | NR | NR | NR | NR | NR | NR |
| Ocal et al., 2021 | NR | NR | NR | NR | NR | Positive (1) |
| Panovska-stavridis et al., 2021 | NR | NR | NR | NR | NR | Positive (1) |
| Schultz et al., 2021 | NR | 3.48 ± 0.34 | NR | NR | NR | Positive (5) |
| Scully et al., 2021 | 0.57 | 2.06 ± 0.71 | NR | NR (14), Negative (8) | Positive (5), Negative (2), NR (15) | NR |
| Soleimani et al., 2021 | NR | NR | NR | Negative (1) | NR | Positive (2) |
| Sorensen et al., 2021 | NR | 2.20 | NR | NR | NR | Positive (1) |
| Suresh & etchey et al., 2021 | NR | NR | 3.12 | NR | NR | Positive (1) |
| Tiede et al., 2021 | NR | NR | NR | NR | NR | Positive (5) |
| Tobaiqy et al., 2021 | NR | NR | NR | NR | NR | NR |
| Umbrello et al., 2021 | NR | NR | 2.50 | NR | NR | Positive (1) |
| Varona et al., 2021 | NR | NR | NR | NR | NR | Positive (1) |
| Walter et al., 2021 | NR | NR | NR | NR | NR | Positive (1) |
| Weidmann et al., 2021 | NR | 3.34 ± 0.46 | NR | NR | NR | NR |
| Wolf et al., 2021 | NR | NR | NR | NR | NR | Positive (3) |
| Zanferrari et al., 2021 | NR | NR | 2.59 | NR | NR | Positive (1) |
| Abou-ismail et al., 2021a | NR | NR | 3.32 | NR | NR | Positive (1) |
| Clark et al., 2021a | NR | NR | NR | Negative (1) | NR | Positive (1) |
| Dhoot et al., 2021a | NR | NR | 2.47 | NR | NR | Positive (1) |
| Malik et al., 2021a | NR | NR | 2.70 | NR | NR | Positive (1) |
| Muir et al., 2021a | NR | NR | 2.55 | NR | NR | Negative (1) |
| See et al., 2021a | NR | NR | 2.25 ± 0.612 | NR | NR | Positive (11) |
Ab, antibody; CT, computed tomography; CTA, computed tomography angiography; CVST, cerebral venous sinus thrombosis; DSA, digital subtraction angiography; HIT, heparin-induced thrombocytopenia; MCA, middle cerebral artery; MRA, magnetic resonance angiography; NA, no association; NR, not reported; OD, optical density; PE, pulmonary embolism; PF4, platelet factor 4; RDS, respiratory distress syndrome; SD, standard deviation; SOVT, superior ophthalmic vein thrombosis.
Studies are Ad26.COV2.S reports.
Values reported for one participant only within study.
On admission, the pooled mean platelet count was 63 373.552/μl (95% CI 4 3420.560–83 326.543, I2 = 97.35%). The pooled mean international normalized ratio was 1.187 (95% CI 1.127–1.247, I2 = 56.78%). Thrombocytopenia was present in one hundred and four patients with a pooled proportion of 75% (95% CI 0.648–0.831, I2 = 11.99%). Hypofibrinogenemia was present in 24 patients with a pooled proportion of 41% (95% CI 0.217–0.636, I2 = 46.87%). The pooled mean d-dimer was 21 789.399 ng/ml (95% CI 15 188.467 28 390.330, I2 = 69.82%). The pooled mean aPTT was 25.157 s (95% CI 17.014–33.299, I2 = 98.94%). The pooled mean fibrinogen was 173 613 mg/dl (95% CI 146.691–200.535, I2 = 65.57%). The pooled mean C-reactive protein was 43.653 mg/l (95% CI 4.364–82.942; I2 = 95.05%).
On admission, 53 patients of 57 had a clinical diagnosis of vaccine-induced thrombocytopenia (77%; 95% CI 0.652–0.856; I2 = 0%). On admission, 64 patients tested positive for platelet factor 4-Heparin ELISA assay (80%; 95% CI 0.692–0.877; I2 = 0%). Additionally, the pooled mean rate of platelet factor 4-heparin ELISA was 1.792 optic density (95% CI 0.070–3.513; I2 = 99.33%). Moreover, the pooled mean rate of lifecodes PF4 IgG assay was 2.592 optic density (95% CI 1.829–3.354; I2 = 95.63%).
Outcomes of reported adverse events
The pooled mean of time to onset of first adverse event following vaccination was 8.468 days (95% CI 7.486–9.451; I2 = 79.42%) ranging from 0 to 20 days. A total of 604 adverse events were reported, 223 of which were thromboembolic events. Out of 223 thromboembolic events, 70 were central venous sinus thrombosis (CVST), 67 were pulmonary embolism (PE)/DVT, and the rest were classified as other thromboembolic events. The pooled rate of CVST was 38.5% (95% CI 0.309–0.466, I2 = 6.54%). The rate of PE/DVT was 21.1% (95% CI 0.168–0.255, I2 = 0%). Seventy-two patients presented to the Emergency Room following an adverse event with a pooled rate of 73.1% (95% CI 0.617–0.820, I2 = 0%). Seventy-four patients were hospitalized, of which 22 were admitted to the ICU. The pooled rate of patients hospitalized was 80% (95% CI 0.708–0.868, I2 = 0%) and pooled rate of ICU admission was 44.1% (95% CI 0.310–0.582, I2 = 0%). The pooled mean of time to hospitalization after vaccination was 10.065 days (95% CI 8.275–11.856, I2 = 57.93%).
Patients received various treatment modalities over the course of their stay. Twenty-one patients received platelet transfusions with a pooled rate of 34.5% (95% CI 0.251–0.452, I2 = 0%). Nineteen patients were treated with a craniectomy with a pooled rate of 33.5% (95% CI 0.244–0.441, I2 = 0%). Ten patients received low-molecular-weight heparin (LMWH) with a pooled rate of 24.6% (95% CI 0.171–0.340, I2 = 0%), whereas 12 patients received heparin with a pooled rate of 25.2% (95% CI 0.179–0.343, I2 = 0%). Lastly, nine patients were treated with a thrombectomy with a pooled rate of 22.7% (95% CI 0.157–0.317; I2 = 0%). In terms of medications, patients were treated with direct thrombin inhibitors (32.5%; 95% CI 0.236–0.428; I2 = 1.85%), factor Xa inhibitors (22.1%; 95% CI 0.153–0.309; I2 = 0%), aspirin (20.3%; 95% CI 0.136–0.292; I2 = 0%), and antibiotics (21.1%; 95% CI 0.142–0.302; I2 = 0%).
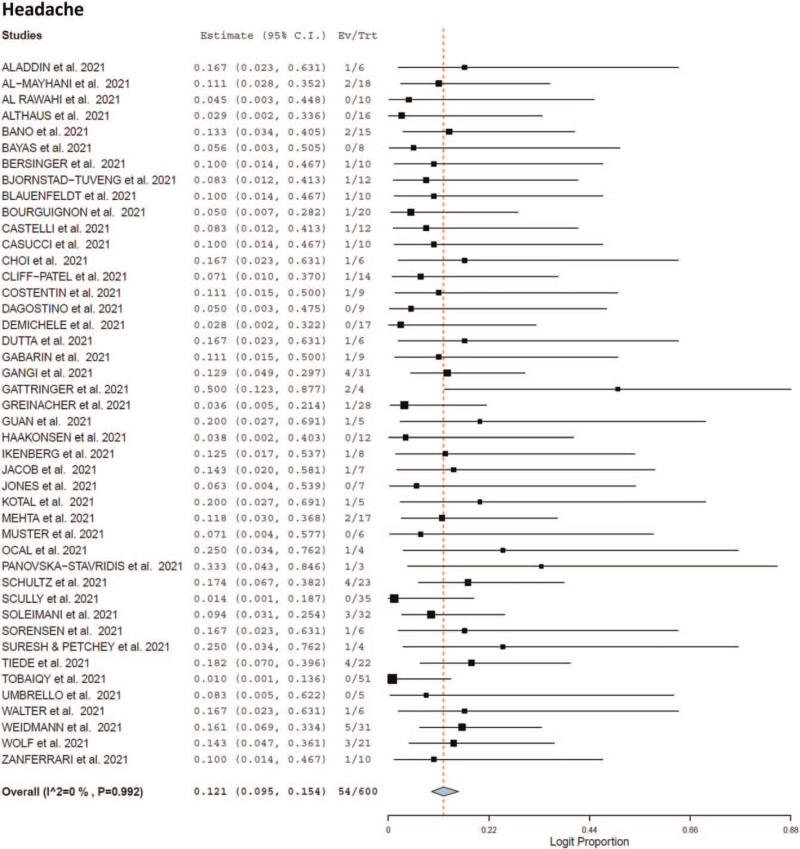
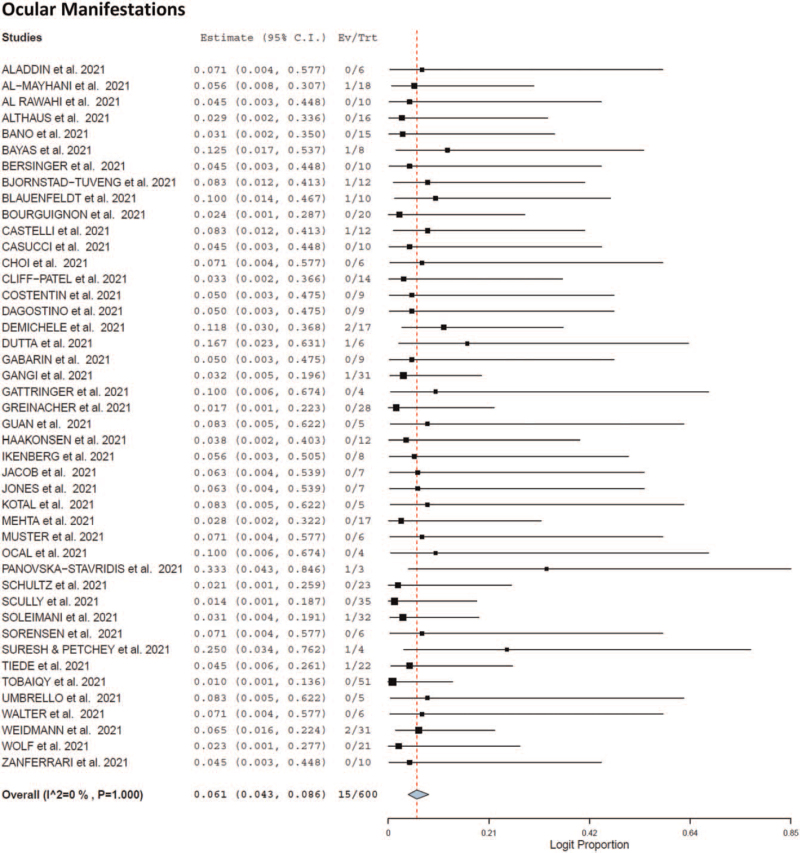
Seventy-eight patients recovered following adverse event [recovery rate 57.2%; 95% CI 0.481–0.658, I2 = 0%) whereas 39 patients died following adverse event [mortality rate 35%; 95% CI 0.270–0.440, I2 = 0%). The cause of death of the patients was unknown. As shown in Table 4 and Fig. 1 ), headache (12.1%; 95% CI 0.095–0.154, I2 = 0%), intracerebral hemorrhage (7.5%, 95% CI 0.056–0.102, I2 = 0%), hemiplegia (7%; 95% CI 0.051–0.096, I2 = 0%), fever (6.6%; 95% CI 0.047–0.091, I2 = 0%), congestive edema (5.3%; 95% CI 0.036–0.075, I2 = 0%), visual impairment (5.7%; 95% CI 0.040–0.081, I2 = 0%), and ocular manifestations (6.1%; 95% CI 0.043–0.086; I2 = 0%) were the most common reported adverse events following vaccination after first dose.
Table 4.
Reported adverse events following vaccination and common treatment modalities
| Author, year | Mean onset time of first AE post vaccination (days) ± SD | Presenting AE (n) | Total number of thromboembolic eventsa | Thromboembolic events (n) | ER admission (n) | ICU admission (n) | Hospitalization (n) | Mean time to hospitalization post vaccination (days) ± SD | Mortality |
| Aladdin et al., 2021 | 14.00 | Fever (1), headache (1), limb weakness (1), seizures (1), vomiting (1) | 1 | CVST (1) | 1 | 1 | 1 | NR | 1 |
| Al-mayhani et al., 2021 | 13.00 ± 7.55 | headache (2), speech/mouth-related issues (1), limb weakness (2), visual impairment (2), drowsiness (1), facial weakness (1), confusion (1) | 9 | Carotid artery thrombosis (1), cerebral artery thrombosis (2), CVST (1), iliac or femoral vein thrombosis (1), jugular vein thrombosis (1), PE (1), splanchnic vein thrombosis (2) | NR | NR | NR | NR | 1 |
| Al rawahi et al., 2021 | 7.00 | Fever (1), lethargy (1), malaise (1), abdominal pain (1), altered mental status (1), somnolence (1) | 3 | Aortic thrombosis (1), PE (1), renal vein thrombosis (1) | 1 | NR | 1 | 7.00 | 0 |
| Althaus et al., 2021 | 10.40 ± 5.24 | Ecchymosis/bruising/petechiae/erythema (3) | 13 | CVST (5), DVT (1), PE (4), thrombosis in other unspecified organs (3) | NR | NR | 8 | NR | 3 |
| Bano et al., 2021 | 10.67 ± 2.52 | Dyspnea (1), pain in extremities (1), headache (2), facial weakness (1), hemiparesis (1), speech/mouth-related issues (1) | 4 | CVST (2), PE (1), jugular vein thrombosis (1) | 3 | NR | 3 | 13.33 ± 3.05 | 2 |
| Bayas et al., 2021 | 1.00 | Fever (1), DIPLOPIA (1) | 1 | SOVT (1) | 0 | NR | 1 | 10.00 | 0 |
| Bersinger et al., 2021 | 9.00 | Headache (1), seizure (1) | 5 | CVST (1), iliac or femoral vein thrombosis (1), jugular vein thrombosis (1), PE (1), splanchnic vein Thrombosis (1) | 1 | 1 | 1 | 14.00 | NA |
| Bjornstad-tuveng et al., 2021 | 7.00 | Headache (1), lethargic (1), speech/mouth-related issues (1), unstable walking (1) | 2 | CVST (1), PE (1) | 0 | 1 | 1 | 10.00 | 1 |
| Blauenfeldt et al., 2021 | NR | Headache (1), abdominal pain (1), adrenal hemorrhage (1), subcapsular renal hematoma (1) | 1 | Carotid artery thrombosis (1) | 1 | 1 | 1 | 7.00 | 1 |
| Bourguignon et al., 2021 | 12.33 ± 5.51 | Pain in extremities (2), cramping (1), dyspnea (1), headache (1), confusion (1), weakness (1) | 12 | Aortic thrombus (1), carotid artery thrombosis (1), celiac artery thrombus (1), CVST (1), DVT (2), jugular vein thrombosis (1), peripheral artery thrombosis (2), PE (2), splanchnic vein thrombosis (1) | 3 | 1 | 3 | 14.67 ± 8.33 | 0 |
| Castelli et al., 2021 | 7.00 | Headache (1), speech/mouth-related issues (1), loss of lower limb strength (1), unstable walking (1), visual impairment (1) | 1 | CVST (1) | 1 | 1 | 1 | 11.00 | 1 |
| Casucci et al., 2021 | 0 | Headache (1), photophobia (1), nausea (1), chills (1), fever (1), myalgia (1), arthralgia (1), fatigue (1) | 1 | Disseminated intravascular coagulation (1) | 0 | 0 | 1 | 15.00 | 0 |
| Choi et al., 2021 | NR | Headache (1), tingling (1), vomiting (1), altered mental status (1), fever (1) | 1 | CVST (1) | 1 | 1 | 1 | 12.00 | 1 |
| Cliff-patel et al., 2021 | 13.00 ± 5.72 | Back pain (2), hematuria (1), headache (1), pain in extremities (1) loss of lower limb strength (1), dyspnea (1), chest pain (1) | 5 | DVT (1), PE (3), renal vein thrombosis (1) | NR | NR | 3 | 15.33 ± 9.00 | NA |
| Costentin et al., 2021 | 3.00 | Nausea (1), muscle ache (1), body ache (1), fatigue (1), headache (1) | 2 | PE (1), splanchnic vein thrombosis (1) | 1 | 1 | 1 | 7.00 | NR |
| D’agostino et al., 2021 | NR | Acute cerebrovascular accident (1) | 4 | Aortic arch (1), carotid artery thrombosis (1), CVST (1), Splanchnic vein thrombosis (1) | 1 | 1 | 1 | 12.00 | 1 |
| Demichele et al., 2021 | 8.00 ± 1.41 | Hemiplegia (1), ocular-related issues (2), speech/mouth-related issues (1), abdominal Pain (1) | 9 | Cerebral artery thrombosis (1), carotid artery thrombosis (3), PE (2), splanchnic vein thrombosis (3) | 2 | NR | 2 | NR | 1 |
| Dutta et al., 2021 | 6.00 | Headache (1), visual impairment (1), vomiting (1), ocular-related issues (1) | 1 | CVST (1) | NR | 0 | 1 | 14.00 | 0 |
| Gabarin et al., 2021 | 19.50 ± 16.26 | Leg swelling (1), erythema (1), headache (1), dyspnea (1), cough (1), hemoptysis (1) | 3 | DVT (2), PE (1) | 1 | 0 | 1, NR (1) | 16.00 | 0 |
| Gangi et al., 2021 | 10.33 ± 8.38 | Myocardial Infarction (1), headache (4), visual impairment/photophobia (2), dyspnea (1), hemoptysis (1), chest pain (2), abdominal pain (1), ocular-related issues (1), nausea (1) | 10 | Coronary artery thrombosis (1), CVST (4), iliac artery thrombosis (1), PE (2), pelvic artery thrombosis (1), splanchnic vein thrombosis (1) | 6 | 1 | 6 | 16.17 ± 8.06 | 0 |
| Gattringer et al., 2021 | 7.00 ± 1.41 | Headache (2) | 2 | CVST (2) | 2 | 0 | 2 | 10.00 ± 2.83 | 0 |
| Greinacher et al., 2021 | 9.30 ± 3.35 | Chills (1), fever (1), nausea (1), epigastric discomfort (1), fatigue (1), myalgia (1), headache (1) | 21 | Aortoiliac thrombosis (1), CVST (9), PE (3), splanchnic vein thrombosis (3), iliac or femoral vein thrombosis (1), inter-ventricular thrombosis (1), IVC thrombosis (1), multiple organ thrombosis (1), widespread microvascular thrombosis (1) | NR | 1 | NR | NR | 6 |
| Guan et al., 2021 | 5 | Headache (1), nausea (1), neck pain (1) | 2 | CVST (2), Jugular vein thrombosis (1) | 1 | 0 | 1 | 10.00 | 0 |
| Haakonsen et al., 2021 | NR | Fever (2), chills (2), arthralgia (1), fatigue (1) | 2 | DVT (2) | 0 | 0 | 0 | NA | 0 |
| Ikenberg et al., 2021 | 7.00 | Headache (1), myalgia (1), chills (1) | 1 | CVST (1) | 1 | NR | 1 | 7.00 | NR |
| Jacob et al., 2021 | 7.00 | Fatigue (1), altered mental status (1), headache (1), nausea (1), hemiparesis (1) | 2 | Carotid artery thrombosis (1), cerebral artery thrombosis (1) | 1 | 1 | 1 | 9.00 | 0 |
| Jones et al., 2021 | 20.00 | Dyspnea (1), insensate (1), loss of limb strength/weakness (1), pain in extremities (1) | 3 | Aortic thrombosis (1), peripheral artery thrombosis (1), PE (1) | 1 | NR | 1 | 25.00 | 0 |
| Kotal et al., 2021 | 0 | Headache (1), loss of limb strength/weakness (1), visual impairment (1) | 1 | CVST (1) | NR | 1 | 1 | 11.00 | 0 |
| Mehta et al., 2021 | NR | Headache (2) | 2 | CVST (2) | 2 | NR | NR | NR | 2 |
| Muster et al., 2021 | 8.00 | Dyspnea (1), fatigue (1), cough (1) | 3 | Iliac or femoral vein thrombosis (1), IVC thrombosis (1), PE (1) | 1 | NR | 1 | NR | NA |
| Ocal et al., 2021 | 11.00 | Headache (1) | 2 | PE (1), Splanchnic Vein Thrombosis (1) | 1 | NR | 1 | 15.00 | NA |
| Panovska-stavridis et al., 2021 | 9.00 | Headache (1), ocular-related issues (1) | 1 | SOVT (1) | 1 | NR | 1 | 10.00 | 0 |
| Schultz et al., 2021 | 7.20 ± 0.45 | Headache (3), fever (1), comatose (including reduced consciousness) (1), back pain (1), abdominal pain (1), hemiparesis (1) | 6 | CVST (4), splanchnic vein thrombosis (1), svc including constituent draining vessels (1) | 5 | 1 | 5 | 8.40 ± 1.52 | 3 |
| Scully et al., 2021 | NR | NR | 26 | Aortic thrombosis (1), CVST (13), DVT (2), jugular vein thrombosis (1), multiple organ thrombosis (1), PE (5), splanchnic vein Thrombosis (3) | 22 | NR | NR | NR | 7 |
| Soleimani et al., 2021 | 12.33 ± 1.69 | Headache (3), hemiparesis (2), pain in extremities (1) | 7 | CVST (3), Jugular Vein Thrombosis (1), PE (2), Splanchnic Vein Thrombosis (1) | 2 | 2 | 3 | 12.67 ± 5.53 | 0 |
| Sorensen et al., 2021 | 8.00 | Headache (1), malaise (1), ecchymosis (1), | 2 | CVST (1), Splanchnic Vein Thrombosis (1) | 1 | NR | 1 | 13.00 | 0 |
| Suresh & petchey et al., 2021 | 2.00 | Headache (1), ocular-related issues (1), vomiting (1) | 1 | CVST (1) | 1 | NR | 1 | NR | 1 |
| Tiede et al., 2021 | 8.40 ± 1.95 | Headache (4), somnolence (1), speech/mouth-related issues (2), hemiparesis (2), visual impairment (1), fatigue (1), ocular-related issues (1) | 6 | Aortic thrombosis (1), carotid artery thrombosis (1), CVST (1), peripheral artery thrombosis (1), splanchnic vein thrombosis (1), TMA (1) | 0 | 0 | 5 | 8.40 ± 1.96 | 0 |
| Tobaiqy et al., 2021 | NR | NR | 30 | Carotid artery thrombosis (1), CVST (1), DVT (16), pelvic vein thrombosis (2), peripheral artery thrombosis (2), PE (6), thrombophlebitis (2) | NR | NR | NR | NR | 3 |
| Umbrello et al., 2021 | NR | Abdominal pain (1), fever (1), fatigue (1), arthralgia (1) | 1 | Splanchnic vein thrombosis (1) | 1 | 1 | 1 | 17.00 | 0 |
| Varona et al., 2021 | NR | NR | 2 | CVST (1), PE (1) | NR | NR | 1 | 10.00 | 0 |
| Walter et al., 2021 | 8.00 | Headache (1), speech/mouth-related issues (1), hemiparesis (1), fatigue (1), myalgia (1) | 1 | Carotid artery thrombosis (1) | 1 | 1 | NR | NR | 0 |
| Weidmann et al., 2021 | 6.33 ± 0.58 | Headaches (5), weakness (2), hemiparesis (3), numbness (2), vomiting (1), altered mental status (1), fever (1), drowsiness (1), abdominal pain (1), nausea (2), ocular-related issues (2), speech/mouth-related issues (2), bruising (1) | 7 | CVST (5), PE (1), pelvic vein thrombosis (1) | 5 | 4 | 5 | 6.60 ± 3.36 | 4 |
| Wolf et al., 2021 | 5.33 ± 1.53 | Shivering (1), headaches (3), fever (2), epileptic seizure (1), speech/mouth-related issues (1), hemianopia (1), somnolent (1) | 3 | CVST (3) | NR | NR | 3 | NR | 0 |
| Zanferrari et al., 2021 | 2.00 | Fever (1), headache (1), speech/mouth-related Issues (1) | 1 | CVST (1) | NR | NR | 1 | 10.00 | NA |
| Abou-ismail et al., 2021b | 11.00 | Pain in extremities (1), chest pain (1) | 2 | DVT (1), PE (1) | 1 | 0 | 1 | 20.00 | 0 |
| Clark et al., 2021b | 5.00 | Headache (1), speech/mouth-related issues (1), photophobia (1), myalgia (1), dizziness (1), petechiae (1), sinus pressure (1) | 2 | CVST (1), PE (1) | 1 | 0 | 1 | 12.00 | 0 |
| Dhoot et al., 2021b | 11.00 | Abdominal pain (1), nausea (1), vomiting (1), decreased oral intake (1) | 3 | Splanchnic vein thrombosis (3) | 1 | 1 | 1 | 21.00 | 0 |
| Malik et al., 2021b | 7.00 | headache (1), fever (1), myalgia (1), chills (1), dyspnea (1), lightheadedness (1) | 3 | Carotid artery thrombosis (1), CVST (1), PE (1) | 1 | 0 | 1 | 10.00 | 0 |
| Muir et al., 2021b | 14.00 | Headache (1), malaise (1) | 2 | CVST (1), Splanchnic Vein Thrombosis (1) | 1 | NR | 1 | 17.00 | NR |
| See et al., 2021b | 8.00 ± 2.25 | Headache (11), visual impairment/photophobia (5), hemiplegia/hemiparesis (3), vomiting (6), comatose (1), fever (3), neck pain/stiffness (3), back pain (1), abdominal pain (2), lethargy (1), myalgia (3), chills/shivering (1), nausea (5), epileptic seizure (2), aphasia (1), chest pain (1), pain in extremities (2), malaise (1) | 26 | CVST (12), DVT (3), jugular vein thrombosis (6), PE (3), splanchnic vein thrombosis (2) | 12 | 10 | 12 | NR | 3 |
| Author, year | UFH | LMWH | Fondaparinux | Direct thrombin inhibitors | Direct factor Xa Inhibitor | IVIg | Corticosteroids | Platelet transfusion | Craniectomy |
| Aladdin et al., 2021 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Al-mayhani et al., 2021 | 0 | 0 | 2 | 0 | 0 | 3 | 1 | 1 | 1 |
| Al rawahi et al., 2021 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| Althaus et al., 2021 | NR | NR | NR | NR | NR | 4 | NR | NR | NR |
| Bano et al., 2021 | 0 | 2 | 1 | 1 | 0 | 1 | 2 | 3 | 1 |
| Bayas et al., 2021 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Bersinger et al., 2021 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| Bjornstad-tuveng et al., 2021 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Blauenfeldt et al., 2021 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Bourguignon et al., 2021 | 1 | 0 | 2 | 1 | 1 | 3 | 0 | 0 | 0 |
| Castelli et al., 2021 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Casucci et al., 2021 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Choi et al., 2021 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Cliff-patel et al., 2021 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 0 | 0 |
| Costentin et al., 2021 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D’agostino et al., 2021 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Demichele et al., 2021 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 1 | 1 |
| Dutta et al., 2021 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gabarin et al., 2021 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 |
| Gangi et al., 2021 | 0 | 1 | 6 | 2 | 0 | 6 | 5 | 3 | 0 |
| Gattringer et al., 2021 | 0 | 0 | 0 | 4 | 0 | 2 | 2 | 0 | 0 |
| Greinacher et al., 2021 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Guan et al., 2021 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Haakonsen et al., 2021 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Ikenberg et al., 2021 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Jacob et al., 2021 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 |
| Jones et al., 2021 | 1 | 1 | 1 | 0 | 0 | 1 | NR | 1 | NR |
| Kotal et al., 2021 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| Mehta et al., 2021 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 |
| Muster et al., 2021 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Ocal et al., 2021 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Panovska-stavridis et al., 2021 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| Schultz et al., 2021 | 1 | 4 | 0 | 0 | 0 | 4 | 4 | 4 | 3 |
| Scully et al., 2021 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Soleimani et al., 2021 | 0 | 0 | 1 | 3 | 2 | 3 | 2 | 2 | 2 |
| Sorensen et al., 2021 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Suresh & petchey et al., 2021 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 |
| Tiede et al., 2021 | 1 | 0 | 0 | 4 | 0 | 3 | 0 | 0 | 2 |
| Tobaiqy et al., 2021 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Umbrello et al., 2021 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Varona et al., 2021 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Walter et al., 2021 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Weidmann et al., 2021 | 0 | 2 | 0 | 0 | 0 | 3 | 3 | 0 | 3 |
| Wolf et al., 2021 | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Zanferrari et al., 2021 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Abou-ismail et al., 2021b | 0 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 0 |
| Clark et al., 2021b | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Dhoot et al., 2021b | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| Malik et al., 2021b | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| Muir et al., 2021b | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| See et al., 2021b | 6 | 0 | 0 | 4 | 0 | 7 | 3 | 4 | 0 |
AE, adverse event; CVST, cerebral venous sinus thrombosis; DVT, deep vein thrombosis; ER, emergency room; IV, intravenous; IVC, inferior vena cava; IVIG, intravenous immunoglobulin; LMWH, low-molecular-weight heparin; NR, not reported; PE, pulmonary embolism; SD, standard deviation; SOVT, superior ophthalmic vein thrombosis; SVC, superior vena cava; UFH, unfractionated heparin.
Thromboembolic events were defined as thrombus presence upon imaging investigations or as stated by study authors. Different imaging of the same thrombus was considered a single thromboembolic event unless new findings were stated between the techniques.
Studies are Ad26.COV2.S reports.
Fig. 1.
Forest plot of the adverse effects at time of presentation.
Fig. 1 (Continued).
Forest plot of the adverse effects at time of presentation.
Fig. 1 (Continued).
Forest plot of the adverse effects at time of presentation.
Fig. 1 (Continued).
Forest plot of the adverse effects at time of presentation.
Fig. 1 (Continued).
Forest plot of the adverse effects at time of presentation.
Johnson and Johnson
Six studies demonstrated thromboembolic events following Johnson and Johnson vaccination [19,20]. Seventeen patients were included in total, of which 15 were girls. The mean age of patients was 40.6 years (range: 24–48 years). Eleven patients had preexisting comorbidities, seven of which had obesity, one had asthma, one had depression, one had hyperlipidemia, and one of which had hypothyroidism [20]. Seventeen of the patients presented and were admitted to the emergency room with thrombocytopenia. One of these patients had fibrinogenemia. Four patients were clinically diagnosed with vaccine-induced thrombocytopenia. There were 15 thromboembolic events of CVST. Moreover, out of the 17 patients who presented,15 patients out of 16 tested positive for antibody PF4-Heparin. Of the entire cohort, patients presented with 122 adverse events, of which 38 were thromboembolic events. Of the 38 thromboembolic events, 15 were CVST events and 10 were PE/DVT events, 6 were splanchnic events, and the rest were other thromboembolic events. Out of recorded reporting for 11 of these patients, 3 died and 8 recovered [20]. The baseline and clinical characteristics, radiological and laboratory findings, and outcomes of reported adverse events are reported alongside the AstraZeneca studies within Tables 1–4, respectively.
Discussion
The aim of this systematic review and meta-analysis was to investigate outcomes of thromboembolic events in patients following AstraZeneca vaccine. Forty-five AstraZeneca studies reporting on thromboembolism as an adverse event post vaccination were included, with six Johnson and Johnson studies. Within this meta-analysis, the following has been supported regarding AstraZeneca vaccine and thromboembolic events: under 60-year-olds have been the predominant age group reporting adverse events; the female sex appears to experience more adverse events than male sex; thrombocytopenia and hypofibrinogenemia appear as consistent findings in studies that report laboratory results; PF4 antibodies appear commonly present within patient serum whenever investigated. To the authors’ knowledge, this is the most recent meta-analysis to describe thromboembolism adverse events following AstraZeneca vaccine. In turn, this study may assist clinical practice in determining adenovirus COVID-19 vaccine eligibility and management.
Recently, it has become evident that adenovirus-based vector vaccines may cause autoimmune thrombosis similar to heparin-induced thrombocytopenia (HIT) [21]. This phenomenon has been termed vaccine-induced immune thrombotic thrombocytopenia (VITT) because of its shared serological profile of high antibodies to platelet factor 4 PF4–polyanion complexes, as well as clinical presentations to that of HIT in which platelet disruption leads to thrombosis development [14,17,21,22]. Clinical findings of thrombocytopenia and hypofibrinogenemia can, therefore, support suspicions of VITT, with the current meta-analysis finding thrombocytopenia prevalent in 75% of all patients who had data reported, and hypofibrinogenemia in 41%. Quantifying the estimated frequency of VITT from the included studies remains difficult, as the novel phenomenon was unknown to authors of earlier studies, with unclear guidelines for diagnosis. Now, in diagnosing VITT, guidance from the American Heart Association/American Stroke Association Stroke Council suggests complete blood counts with a peripheral smear, coagulation studies with prothrombin time, partial thromboplastin time, fibrinogen, D-dimer, and PF4 antibody ELISA [15,23]. Results of this current meta-analysis support these suggestions as included AstraZeneca studies conducting these analyses found ELISA anti-PF4 to be positive in 80% of patients tested, with corresponding abnormal patient serum samples and prolonged coagulation values (Table 3).
Upon VITT, patients have been described to present with CVST or with other arterial or venous clots [21]. The pathophysiology for thrombus formation has been previously described in detail, with the conclusion that cellular positive feedback signaling results in a hypercoagulable state post adenoviral vaccination [24]. Autoantibodies are also generated despite no heparin exposure, leading to theories of an unidentified polyanion in the adenoviral vaccine itself or infected cells causing binding to PF4 [24]. Considering this mechanism, and that prior thrombosis is not currently considered a risk factor for VITT, preemptive thrombophilia screening may not yield clinically useful information in identifying VITT susceptibility [24]. However, future studies are required to confirm this.
Due to ambiguity surrounding the exact mechanisms of VITT, there are no prominent risk factors for VITT with thromboembolism post vaccination other than female sex and age younger than 60 years [24], reflecting the demographics of patients included within the current study. It is, therefore, vital that clinicians take appropriate caution when administering adenovirus vaccination to this patient population. Outside of VITT-specific risk factors, standard thromboembolism risk factors of thrombophilia, pregnancy, the postpartum timeframe, and hormonal contraceptives are thought to apply in a general sense to patients receiving adenovirus vaccination [25,26]. However, limited reporting on these factors precluded investigations within this current analysis.
Out of embolic events, CVST was the most common with a pooled value of 38.5% in line with current reporting of embolism cases to EudraVigilance [27]. The high prevalence of CVST in turn largely explains the results of corresponding adverse event presentations. The main clinical syndromes seen with CVST are intracranial hypertension presenting as headache, focal deficits with hemiparesis and fluent aphasia, seizures, and venous hemorrhage [28,29], all of which were prevalent presenting adverse events within this meta-analysis. Consequently, presentation of these adverse events post vaccination should immediately guide clinical decision-making towards a diagnosis of VITT-related thromboembolism. The pooled onset of initial adverse event symptoms appearing approximately 10 days after vaccination is in line with current literature reporting a similar timeframe after receiving the first vaccine dose [23,30]. This highlights a clear delay in adverse event presentation post vaccination that clinicians must be aware of, with necessity for appropriate preemptive management after the first vaccine dose as compared with the second. Unfortunately, scarcity of literature precludes any further comment on outcomes of thromboembolic events following second dose vaccination.
AstraZeneca has not been the only COVID-19 vaccine to present with thromboembolic events, as six studies from the USA reporting embolic events following the Johnson and Johnson vaccine were included as a subgrouping within the current meta-analysis. All patients reported similar clinical pictures to that of AstraZeneca vaccine patients, in that headaches were the most common presenting adverse event, followed by thromboembolic events that saw a high prevalence of CVST. Additionally, tested patients were 93.8% positive for ELISA anti-PF4, similar to AstraZeneca reports (Table 3). Given that both AstraZeneca ChAdOx1 nCov-19 and Johnson and Johnson/Janssen Ad26.COV2.S are nonreplicating adenovirus vector–based DNA vaccines [19], it is expected that the clinical course and laboratory results would share similarities. Slight differences have been shown in delayed clinical manifestations for Ad26.COV2.S, with lower D-dimer and activated partial thromboplastin time levels [31]. However, all other clinical characteristics remain comparable [31]. Subsequently, the findings of this meta-analysis further indicate a similarity between these two vaccines in the pathogenesis of VITT leading to thromboembolic events [20].
As of now, recommended treatment for confirmed VITT according to guidelines includes the avoidance of heparin (both unfractionated heparin and LMWH) and platelet transfusions [21]. Instead, the UK's expert Hematology panel has advised anticoagulating with nonheparin-based therapies depending on the patient's drug profile and situation [24,32]. These include direct oral anticoagulants (DOACs), such as dabigatran, apixaban, rivaroxaban, edoxaban, and fondaparinux, as well as parenteral direct thrombin inhibitors (e.g. bivalirudin and argatroban) [24]. Furthermore, urgent use of high-dose intravenous immunoglobulin at rate of 1 g/kg of body weight daily for 2 days has also been suggested [21,32,33]. In delays of initiating intravenous immunoglobin, steroid administration has been advised [32], whilst in cases of declining fibrinogen levels below 1.5 g/l, fibrinogen concentrate, or cryoprecipitate should be considered [32]. These treatment modalities were largely seen within the current meta-analysis, reflecting current developing clinical practice (Table 4).
Whilst this meta-analysis reports on arising thromboembolic events, it should be noted that these cases have thus far been a rarity in opposition to the current widespread trials and live administrations, which have not yet reported such events [9,30,34,35]. At present, approximately 21 400 000 Johnson and Johnson vaccines have been administered within the USA, whilst 500 000 000 Astrazeneca vaccines have been administered within Europe. The sample size included within this meta-analysis is, therefore, miniscule in comparison to patients safely vaccinated with adenoviral-based vaccines. In addition, the risk of CVST associated with COVID-19 infection is considerably greater than that associated with vaccination [36], which should dissuade any vaccine hesitancy.
Conclusion
Considering the rapid pace at which both COVID-19 vaccine trials and adverse event outcome reporting is occurring, the limitations of this current systematic review and meta-analysis must be acknowledged. The most pressing of these is the lack of high-quality data in the included studies. Due to the urgent timeline for data extraction and the complications surrounding VITT, many cases had incomplete documentation of the epidemiological history, laboratory values, particularly about anti-PF4 levels, and outcomes including cause of mortalities. In addition, despite pooled information providing clinical benefit, there was a reliance on case series or case reports arising within the preceding months of this meta-analysis being performed. Furthermore, vaccinated patients who were asymptomatic or had mild adverse events, and who did not require hospitalization, were not accounted for because of publication bias. This prevented calculations on true incidence rates of thromboembolism from evolving cases or discrepancies in reporting. Lastly, because of the nature of the virus and the urgent need for more studies, this meta-analysis might have missed emerging studies recently published in the literature, particularly in languages other than English.
Nevertheless, this meta-analysis presents novel evidence of VITT-related thromboembolism following AstraZeneca vaccine in certain individuals. Female individuals under 60 years of age seem as the primary group affected; however, male individuals have also been shown to be as susceptible. Further investigations are warranted into the mechanisms of potential thromboembolic formation following adenovirus vector-based DNA vaccine administration and causes for predisposition towards CVST specifically. Such elucidations may identify suitability for adenovirus vaccination and better guide clinical practice. At present, serum anti-PF4 suggests diagnostic value for VITT, and can inform treatment choices.
Acknowledgements
We would like to thank Larry J. Prokop MS, MAR for the literature search.
Author contributions: R.H.M., C.A.T., and O.D. conceived and designed the study, reviewed the literature, collected, analyzed and interpreted the data, and drafted the manuscript. R.H.M., C.A.T., and O.D. conceived and designed the study, and critically revised the manuscript. R.H.M., C.A.T., H.N., B.L.S., R.S.D., K.S., A.B., and O.D. reviewed the literature, collected, analyzed, and interpreted the data, and drafted the manuscript. All authors read and approved the final manuscript.
Data availability statement: with publication, the data set used for this meta-analysis will be shared upon request from the study authors
Ethical approval: this systematic review and meta-analysis does not require ethical approval
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 2016; 24:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease (COVID-19) situation reports. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. [Accessed 12 April 2021] [Google Scholar]
- 4.Wang F, Kream RM, Stefano GB. An evidence based perspective on mRNA-SARS-CoV-2 vaccine development. Med Sci Monit 2020; 26:e924700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang N-N, Li X-F, Deng Y-Q, Zhao H, Huang YJ, Yang G, et al. A thermostable mRNA vaccine against COVID-19. Cell 2020; 182:1271.e16–1283.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu F-C, Guan X-H, Li Y-H, Huang JY, Jiang T, Hou LH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020; 396:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Commission. Available at: https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/safe-covid-19-vaccines-europeans_en#figures-on-vaccination. [Accessed 12 April 2021] [Google Scholar]
- 8.Kim SH, Wi YM, Yun SY, Ryu JS, Shin JM, Lee EH, et al. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci 2021; 36:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021; 397:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobaiqy M, Elkout H, MacLure K. Analysis of thrombotic adverse reactions of COVID-19 AstraZeneca vaccine reported to EudraVigilance database. Vaccines (Basel) 2021; 9:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/media/releases/2021/s0413-JJ-vaccine.html. [Accessed 13 April 2021] [Google Scholar]
- 12.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018; 23:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 14.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. New Engl J Med 2021; 384:2092–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384:2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blauenfeldt RA, Kristensen SR, Ernstsen SL, Kristensen CCH, Simonsen CZ, Hvas A-M. Thrombocytopenia with acute ischemic stroke and bleeding in a patient newly vaccinated with an adenoviral vector-based COVID-19 vaccine. J Thromb Haemost 2021; 19:1771–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz NH, Sørvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021; 384:2124–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haakonsen HB, Nystedt A. Deep vein thrombosis more than two weeks after vaccination against COVID-19. Tidsskr Nor Laegeforen 2021; 141: [DOI] [PubMed] [Google Scholar]
- 19.Muir K-L, Kallam A, Koepsell SA, Gundabolu K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N Engl J Med 2021; 384:1964–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.See I, Su JR, Lale A, Woo EJ, Guh AY, Shimabukuro TT, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 2021; 325:2448–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai M, Grill A, Ivers N, Maltsev A, Miller KJ, Razak F et al on behalf of the Drugs & Biologics Clinical Practice Guidelines Working Group and the Ontario COVID-19 Science Advisory Table. Vaccine-induced prothrombotic immune thrombocytopenia (VIPIT) following AstraZeneca COVID-19 vaccination. Science Briefs of the Ontario COVID-19 Science Advisory Table. 2021. [Google Scholar]
- 22.Baroletti SA, Goldhaber SZ. Heparin-induced thrombocytopenia. Circulation 2006; 114:e355–e356. [DOI] [PubMed] [Google Scholar]
- 23.Furie KL, Cushman M, Elkind MSV, Lyden PD, Saposnik G. American Heart Association/American Stroke Association Stroke Council Leadership. Diagnosis and management of cerebral venous sinus thrombosis with vaccine-induced immune thrombotic thrombocytopenia. Stroke 2021; 52:2478–2482. [DOI] [PubMed] [Google Scholar]
- 24.Rizk JG, Gupta A, Sardar P, et al. Clinical characteristics and pharmacological management of COVID-19 vaccine-induced immune thrombotic thrombocytopenia with cerebral venous sinus thrombosis: a review. JAMA Cardiol 2021; 6:1451–1460. [DOI] [PubMed] [Google Scholar]
- 25.Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med 2021; 27:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saposnik G, Barinagarrementeria F, Brown RD, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis. Stroke 2011; 42:1158–1192. [DOI] [PubMed] [Google Scholar]
- 27. AstraZeneca's COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. Available at: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood. [Accessed 13 April 2021] [Google Scholar]
- 28.Idiculla PS, Gurala D, Palanisamy M, Vijayakumar R, Dhandapani S, Nagarajan E. Cerebral venous thrombosis: a comprehensive review. Eur Neurol 2020; 83:369–379. [DOI] [PubMed] [Google Scholar]
- 29.Piazza G. Cerebral venous thrombosis. Circulation 2012; 125:1704–1709. [DOI] [PubMed] [Google Scholar]
- 30.Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020; 396:1979–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hwang J, Lee SB, Lee SW, Lee MH, Koyanagi A, Jacob L, et al. Comparison of vaccine-induced thrombotic events between ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines. J Autoimmun 2021; 122:102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suresh P, Petchey W. ChAdOx1 nCOV-19 vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis (CVST). BMJ Case Rep 2021; 14:e243931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldenburg J, Klamroth R, Langer F, Albisetti M, von Auer C, Ay C, et al. Diagnosis and management of vaccine-related thrombosis following AstraZeneca COVID-19 vaccination: guidance statement from the GTH. Hamostaseologie 2021; 41:184–189. [DOI] [PubMed] [Google Scholar]
- 34.Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, et al. Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020; 396:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaacs D. The precautionary principle, the AstraZeneca COVID-19 vaccine and mixed messaging. J Paediatr Child Health 2021; 57:472–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taquet M, Husain M, Geddes JR, Luciano S, Harrison PJ. Cerebral venous thrombosis and portal vein thrombosis: A retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine 2021; 39:101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.