Purpose of review
The COVID-19 pandemic has led to devastating health outcomes across the world. Initially thought to primarily affect the respiratory system, there is now clear and abundant evidence that COVID-19 can impact upon the male genitourinary system and overall men's health. In this review article, we explore the potential mechanisms by which COVID-19 specifically affects men and we review the literature examining the adverse effects of the disease on men's health
Recent findings
Studies suggest that men are at higher risk for severe COVID-19 infection and death. COVID-19 infection has a negative impact on men's health including worsening semen parameters, potentially lower testosterone levels, and an increased risk of erectile dysfunction.
Summary
COVID-19 is a highly pathogenic virus that exerts adverse effects upon the male genitourinary system in myriad ways. The COVID-19 infection can impact serum testosterone, fertility, sexual function, and mental health. Fortunately, the COVID-19 vaccine is safe and effective in preventing COVID-19 infection and many of these sequelae.
Keywords: COVID-19, erectile dysfunction, male fertility, testosterone, vaccine
INTRODUCTION
After the first case of COVID-19 was identified in December 2019, this virulent pathogen spread rapidly and globally, resulting in the World Health Organization declaration of a global pandemic in March 2020 [1,2]. The COVID-19 pandemic has led to devastating health outcomes across the world, infecting almost 250 million people and resulting in over 5 million deaths [3]. Although COVID-19 was initially thought to primarily affect the respiratory system, there is now clear and abundant evidence that the virus can cause a wide range of pathology across multiple organ systems including the gastrointestinal, cardiovascular, hematological, and nervous systems [4].
Box 1.
no caption available
Since the onset of the pandemic, a growing body of evidence has examined the impact of COVID-19 upon the male genitourinary system and overall men's health. The mechanisms for these effects have not been fully elucidated but likely result from both broad (e.g. inflammatory response) and specific (e.g. androgen-dependent) pathways. As COVID-19 continues to infect men worldwide, it is imperative that we understand the adverse effects of the COVID-19 virus on men's health and how men may have less favorable health outcomes when infected. In this review article, we explore the potential mechanisms by which COVID-19 specifically effects men and we review the literature examining the adverse effects of the disease on men's health.
VIRAL PATHOGENICITY OF COVID-19
A brief review of the precise mechanisms by which COVID-19 infection occurs is essential to understand the effects of COVID-19 on men's health. Similar to other coronaviruses, COVID-19 utilizes a spike (S) protein to mediate host cell entry. The S protein binds to angiotensin-converting enzyme 2 (ACE2) in human tissue to allow for viral entry via membrane transfusion [5,6]. In addition to ACE2, COVID-19 utilizes the host cellular transmembrane serine protease 2 (TMPRSS2) to be proteolytically activated [6]. With both ACE2 and TMPRSS2 playing essential roles in the infectious mechanism of COVID-19, human tissue naturally expressing both proteins are more susceptible to the COVID-19 infection and its resulting effects.
Although ACE2 is concentrated in the lungs, heart, and kidneys, it is also found in the testis [7,8]. The ACE2 receptor is present in both testosterone-producing Leydig cells and spermatogenesis-supporting Sertoli cells, possibly rendering these cells uniquely susceptible to COVID-19 infection. Because Sertoli cells are also responsible for maintenance of the blood-testis barrier, viral infection of these cells could impair the immunoprivileged status of the testis and enable direct viral damage or inflammatory damage to germ cells. Even spermatogonia themselves have ACE-2 receptors, which may further potentiate the risk of viral infection to these germ cells [9].
TMPRSS2 is highly expressed in both the prostate and testes [9–11]. Alteration and fusion of the TMPRSS2 gene have been implicated in the pathogenesis of prostate cancer. Interestingly, the use of androgen deprivation therapy (ADT) may lead to downregulation of tissue-based TMPRSS2 expression, which could have important implications for COVID-19 infection in these men [12–14]. TMPRSS2 is also expressed within the testis, providing another potential pathway for COVID-19 mediated testicular damage.
COVID-19 INFECTION RISK AND SEVERITY IN MEN VERSUS WOMEN
Early studies demonstrated that while men and women were equally susceptible to COVID-19 infection, infected males had significantly greater risk for severe infection leading to intensive care unit (ICU) admission and death [15▪,16]. In a meta-analysis from Peckham et al. examining 3,111,714 global cases, males had an almost three-fold increased risk of ICU treatment (odds ratio [OR] 2.84) and death (OR 1.39) compared to females [15▪]. There are multiple potential explanations for these differences. Men may have inherent differences in systemic immune responses of both the innate and adaptive immune system, rendering them more susceptible to sequelae of infection [17]. Severity of Covid-19 infection may also relate to underlying cardiovascular comorbidities, which may be more prevalent in men [17].
Another potential explanation for sex dimorphism in COVID-19 infection involves differential and androgen-dependent expression of ACE2 and TMPRSS2, respectively. There are conflicting studies demonstrating higher ACE2 expression among women, which may be parodoxically protective, as a higher concentration of ACE2 receptors decreases the speed of viral saturation of these receptors [18,19]. In contrast, TMPRSS2 receptor expression is typically upregulated in men due to its well-established regulation by androgen-dependent promoters [16].
Okwan-Duodu et al. demonstrated that TMPRSS2 expression was higher in men, with almost three times as many cells expressing both ACE2 and TMPRSS [11]. Higher levels of androgens in men resulting in higher TMPRSS2 expression may contribute to increased severity and fatality rates of COVID-19 in men [19–22].
RELATIONSHIP BETWEEN COVID-19 AND SERUM TESTOSTERONE
Despite the well-documented role of testosterone in TMPRSS2 regulation, the broader relationship between serum testosterone and COVID-19 infection remains unclear. Some studies found that lower levels of serum testosterone among infected men were associated with worse outcomes [23,24]. Rastrelli et al. examined 31 men with COVID-19 pneumonia requiring respiratory ICU admission and found that those with lower total testosterone were more likely to need more intense care or die [23]. More recent data from Salonia et al. confirmed an association between low testosterone and higher risk of ICU admission and mortality among 286 men with symptomatic COVID-19 infection [25]. One potential explanation for low testosterone as a risk factor for COVID-19 infection is the inherent association between testosterone deficiency and comorbid conditions such as obesity and cardiovascular disease, which are also risk factors for COVID-19 disease severity [24,26–31]. Alternatively, low testosterone has been implicated in higher levels of systemic inflammatory cytokines, and this may lead to an increased inflammatory response and adverse sequelae seen in men with low testosterone who are infected with COVID-19 [32]. Although these data suggest an association between hypogonadism and severe COVID-19 infection, these studies are limited both in size and design, as preinfection testosterone levels were not assessed.
In contrast, based on the observation that TMPRSS2 is expressed in an androgen-dependent fashion, multiple studies have examined the potential protective role of ADT upon COVID-19 infection. An early study from Montopoli et al. examined 4,532 male patients and found that prostate cancer patients on ADT had lower risk of COVID-19 infection compared to those not on ADT (OR 4.05) [14]. However, newer data from Schmidt et al. queried the Cancer Consortium registry and found no difference in 30-day mortality among COVID-19 patients who did and did not receive ADT [12]. Consistent with these data, two recent meta-analysis found no protective effect of ADT against COVID-19 infection or disease severity [33,34].
Beyond the impact of baseline serum testosterone upon COVID-19 pathogenicity, multiple studies have examined the impact of COVID-19 infection upon serum testosterone and gonadotropin levels. Importantly, there is significant inherent variability in these hormone levels due to a broad range of factors such as stress, circadian rhythm, and others that limit studies on this topic. An early, small study from Ma et al. of 12 patients with COVID-19 compared to age-matched controls found higher levels of luteinizing hormone (LH) in the COVID-19 patients but no difference in serum testosterone between the groups [35]. In contrast, Temiz et al. found that COVID-19 patients had lower LH, follicle stimulating hormone, and testosterone levels when compared to controls, but that these differences dissipated after treatment with an oral regimen consisting of azithromycin and hydroxychloroquine [36]. More recently, a larger study from Salonia et al. found profoundly lower testosterone levels in men with COVID-19 compared to healthy controls (10.4 vs 2.5 nmol/L, P < 0.001) [25]. The same group examined testosterone recovery 7-months after COVID-19 infection and found that 87.6% of men had increased serum testosterone compared to their baseline at the time of infection [37]. Importantly, over half of men at follow-up still had testosterone levels meeting criteria for testosterone deficiency, indicating a potential long-term association between COVID-19 and hypogonadism.
Overall, there is a growing body of literature exploring the complex relationship between testosterone and COVID-19 infection. Although these studies are limited and data regarding serum testosterone and disease severity are conflicting, there does seem to be some impact of COVID-19 infection upon circulating androgen and gonadotropin levels that warrants further investigation.
COVID-19 AND MALE FERTILITY
There are numerous potential mechanisms by which COVID-19 could impact spermatogenesis and male fertility, which have led to a large number of studies on this topic. Because both ACE2 and TMPRSS2 are found in spermatogonial stem cells, Leydig cells, and Sertoli cells, albeit at relatively low levels, there has been concern about the effect of the COVID-19 infection on male fertility [9,38]. Damage to the Sertoli-cell mediated blood-testis barrier could result in a number of downstream consequences including direct infection of spermatogonia. Indeed, studies have demonstrated rates of orchitis as high as 22% in COVID-19 infected men and about 10% of hospitalized COVID-19 infected men were found to have testicular pain, indicating a local inflammatory effect of COVID-19 upon the testis [39,40]. Additionally, high fevers and systemic inflammation are a frequent symptoms of COVID-19 infection, and prior studies have shown a high risk of impaired semen parameters in the setting of acute febrile illness [41].
Many studies have investigated the presence of COVID-19 virus in both the semen and testes of infected men. The overwhelming majority of studies have found no evidence of virus in the semen of men with COVID-19 infection with the exception of Li et al. who utilized RT-PCR testing to identify virus in 6 (15.8%) men [42–45]. A systematic review examined 8 studies and found that these 6 men were the only reported cases out of 160 total semen samples with detection of COVID-19 RNA [46]. Although further investigation is needed on a large scale, at this time it seems that COVID-19 is rarely present in semen and that it is extremely unlikely to be transmitted via intercourse. However, an autopsy study from Achua et al. found COIVD-19 virus present in testicular tissue of 1 in 6 (16.7%) patients with fatal COVID-19 infection, indicating potential for a direct impact of COVID-19 infection upon the testis [47]. Similarly, Ma et al. compared testicular biopsies of men with and without COVID-19 infection and found that infected testicles demonstrated germ cell degeneration with a histopathological pattern similar to Sertoli cell only syndrome. Furthermore, testes of infected men showed increased transcription of genes implicated in inflammatory-related processes and a down regulation of genes involved in spermatogenesis [48].
Given these findings, it is not surprising that COVID-19 infection can adversely impact semen parameters. Holtmann et al. compared 18 men who had recovered from a COVID-19 infection to a control group, showing that those men with moderate symptoms had a significant decline in semen parameters (concentration and motility) compared to both controls and men who displayed mild COVID-19 symptoms [44]. Gacci et al. found that approximately 25% of men who recovered from COVID-19 had oligo-crypto-azoospermia and genital tract inflammation related to disease severity [49]. It is important to mention that there were no baseline semen parameters in the subjects for either of these studies. More recently, Hamarat et al. compared semen parameters in men before and after COVID-19 infection. They collected data on 41 men and observed a significant decrease in sperm concentration, total sperm count, and morphology after infection (repeat semen analysis was performed at least 70 days following a positive test COVID-19 infection in 63.4% of the cohort, the remainder of whom underwent repeat semen analysis prior to 70 days) [50]. Best et al. reported the longest follow-up in a small cohort of 5 men who underwent repeat analysis 3 months after their index sample. The median total sperm count was similar in the 3 month follow-up when compared to the initial analysis (18 million vs 22 million, respectively) [51]. Overall, these data suggest that semen parameters of men with COVID-19 infection may be compromised in the short term, though prospective studies with extended follow-up are needed to determine the potential long-term impact of COVID-19 on semen parameters.
SEXUAL HEALTH
COVID-19 can adversely impact male sexual health in a number of ways. Hsieh et al. performed a scoping systematic review of the literature and identified four potential pathways through which COVID-19 affected male sexual function: biologically, mentally, impaired access to care, and health disparities [52]. Indeed, a recent study from Hernandez et al. found a significant increase in sales of phosphodiesterase-5 inhibitors during the pandemic – whereas these data could be interpreted to suggest increased levels of sexual activity during the pandemic, it may also indicate increasing prevalence of sexual dysfunction. Sansone et al. compared men with (N = 25) and without (N = 75) history of COVID-19 infection and found a significantly increased prevalence of erectile dysfunction (ED) in men with history of infection (OR 5.66) [53▪].
There are a few biological mechanisms by which COVID-19 may cause ED [54]. COVID-19 can cause injury to endothelial cells resulting in endothelial dysfunction, which is a hallmark of ED [55,56]. Kresch et al. studied penile tissue from patients undergoing penile prosthesis surgery and found extracellular COVID-19 viral particles and endothelial dysfunction in men with a history of COVID-19 infection, supporting the potential role for endothelial dysfunction in COVID-19 mediated ED [57]. As noted above, the association between COVID-19 and low testosterone is not fully elucidated, but low testosterone in the setting of COVID-19 can certainly contribute to impaired erectile function, libido, and orgasmic function. Additionally, the relationship between mental health and sexual function is well described, and there are robust data suggesting an overall increased burden of mental health disorders during the pandemic, including an increase in the use of medications for these conditions such as selective serotonin reuptake inhibitors (SSRIs) [58–60]. The higher incidence of these diagnoses, along with the use of medications with adverse effects upon sexual health, has likely contributed to worsening sexual function for a large number of men, though future studies are needed to better characterize sexual function and its relation to COVID-19 infection.
COVID-19 VACCINE AND MEN'S HEALTH
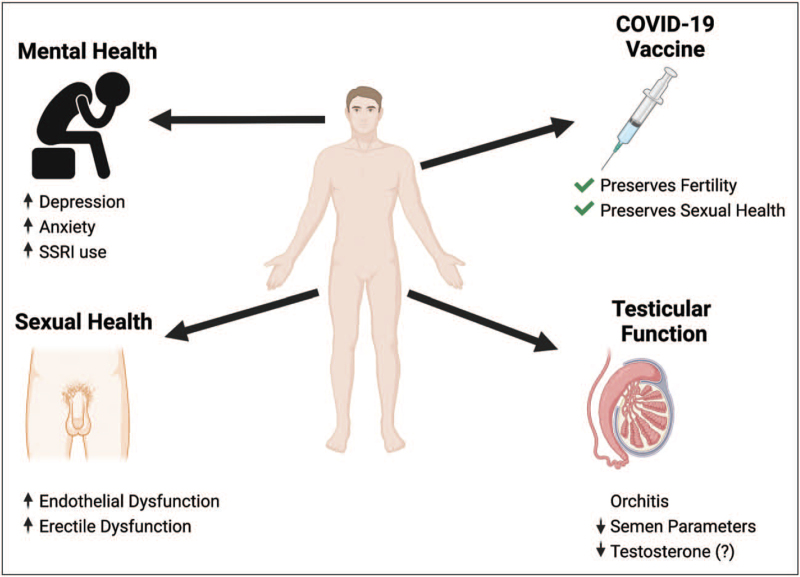
The United States Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for the first COVID-19 vaccine on December 11, 2020. With the emergency authorization, a significant proportion of Americans were hesitant to get vaccinated [61]. Diaz et al. used Google Trends to explore public concerns over vaccine side effects and found a significant increase in searches targeting COVID-19 and fertility following the EUA, suggesting that a major cause of vaccine hesitancy was concern regarding the impact on fertility [62]. Fortunately, a recent study by Gonzalez et al. evaluated the semen parameters of 45 men before and 3 months after vaccination. Overall, there was no decline in sperm concentration or total motile sperm count following vaccination, and importantly, no men developed azoospermia [63▪▪]. These data provide great reassurance regarding the safety of COVID-19 vaccination with respect to future fertility. Moreover, as noted above, COVID-19 infection itself may be associated with impaired fertility; as such, COVID-19 vaccination helps to preserve reproductive function through the prevention of COVID-19 infection. There may be other protective effects of vaccination on the genitourinary tract. For example, a large retrospective study of over 10 million men demonstrated that vaccinated men were less likely to develop orchitis and/or epididymitis compared to unvaccinated men [64]. Although larger studies are forthcoming, all evidence to date suggests that the COVID-19 vaccine is a safe and effective means to prevent COVID-19 infection and preserve fertility and sexual health (Fig. 1).
FIGURE 1.
Summary of the adverse impact of COVID-19 on men's health and the protective impact of COVID-19 vaccination (SSRI, selective serotonin reuptake inhibitor).
CONCLUSION
COVID-19 is a highly pathogenic virus that exerts adverse effects upon the male genitourinary system in myriad ways. Although numerous studies have begun to detail and describe these sequelae in both the short- and long-term, the full impact of the COVID-19 pandemic on men's health has not yet fully been realized. COVID-19 infection can impact serum testosterone, fertility, sexual function, and mental health. Fortunately, the COVID-19 vaccine is safe and effective in preventing COVID-19 infection and many of these sequelae. We look forward to additional prospective, long-term studies that will further our collective understanding of the impact of COVID-19 upon men's health and the potential to mitigate these negative effects.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Organization WH. Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020 [Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. [Google Scholar]
- 3. Organization WH. Weekly operational update on COVID-19-8 November 2021 2021 [Available from: https://www.who.int/publications/m/item/weekly-operational-update-on-covid-19---8-november-2021. [Google Scholar]
- 4.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26:1017–1032. [DOI] [PubMed] [Google Scholar]
- 5.Sternberg A, Naujokat C. Structural features of coronavirus SARS-CoV-2 spike protein: targets for vaccination. Life Sci 2020; 257:118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan C, Lu W, Li K, et al. ACE2 expression in kidney and testis may cause kidney and testis infection in COVID-19 Patients. Front Med 2020; 7:563893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003; 426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells 2020; 9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YW, Lee MS, Lucht A, et al. TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol 2010; 176:2986–2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okwan-Duodu D, Lim EC, You S, Engman DM. TMPRSS2 activity may mediate sex differences in COVID-19 severity. Signal Transduct Target Ther 2021; 6:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt AL, Tucker MD, Bakouny Z, et al. Association between androgen deprivation therapy and mortality among patients with prostate cancer and COVID-19. JAMA Netw Open 2021; 4:e2134330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi J, Zhou Y, Hua J, et al. The scRNA-seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to SARS-CoV-2 infection. Int J Environ Res Public Health 2021; 18: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol 2020; 31:1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun 2020; 11:6317. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meta-analysis of 3,111,714 reported global cases of COVID-19 that demonstrated that although there is no difference in COVID-19 infections amongst sexes, males have almost 3 times higher odds (OR = 2.84) of requiring intensive care treatment and higher odds of death (OR = 1.39) compared to females.
- 16.Mukherjee S, Pahan K. Is COVID-19 Gender-sensitive? J Neuroimmune Pharmacol 2021; 16:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res 2020; 116:2197–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dalpiaz PL, Lamas AZ, Caliman IF, et al. Sex hormones promote opposite effects on ACE and ACE2 Activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One 2015; 10:e0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel DP, Punjani N, Guo J, et al. The impact of SARS-CoV-2 and COVID-19 on male reproduction and men's health. Fertil Steril 2021; 115:813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassau DE, Best JC, Kresch E, et al. Impact of the SARS-CoV-2 virus on male reproductive health. BJU Int 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giagulli VA, Guastamacchia E, Magrone T, et al. Worse progression of COVID-19 in men: Is testosterone a key factor? Andrology 2021; 9:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutta S, Sengupta P. SARS-CoV-2 and male infertility: possible multifaceted pathology. Reprod Sci 2021; 28:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology 2021; 9:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ory J, Lima TFN, Towe M, et al. Understanding the complex relationship between androgens and SARS-CoV2. Urology 2020; 144:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salonia A, Pontillo M, Capogrosso P, et al. Severely low testosterone in males with COVID-19: a case-control study. Andrology 2021; 9:1043–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev 2021; 37:e3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fresán U, Guevara M, Elía F, et al. Independent role of severe obesity as a risk factor for COVID-19 hospitalization: a spanish population-based cohort study. Obesity 2021; 29:29–37. [DOI] [PubMed] [Google Scholar]
- 28.Gao F, Zheng KI, Wang XB, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care 2020; 43:e72–e74. [DOI] [PubMed] [Google Scholar]
- 29.Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation 2020; 142:4–6. [DOI] [PubMed] [Google Scholar]
- 30.Parohan M, Yaghoubi S, Seraji A, et al. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male 2020; 23:1416–1424. [DOI] [PubMed] [Google Scholar]
- 31.Goha A, Mezue K, Edwards P, et al. COVID-19 and the heart: An update for clinicians. Clin Cardiol 2020; 43:1216–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pozzilli P, Lenzi A. Commentary: testosterone, a key hormone in the context of COVID-19 pandemic. Metabolism 2020; 108:154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karimi A, Nowroozi A, Alilou S, Amini E. Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer: a systematic review and meta-analysis. Urol J 2021. [DOI] [PubMed] [Google Scholar]
- 34.Sari Motlagh R, Abufaraj M, Karakiewicz PI, et al. Association between SARS-CoV-2 infection and disease severity among prostate cancer patients on androgen deprivation therapy: a systematic review and meta-analysis. World J Urol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma L, Xie W, Li D, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol 2021; 93:456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Temiz MZ, Dincer MM, Hacibey I, et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: A cross-sectional, pilot study. Andrologia 2021; 53:e13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salonia A, Pontillo M, Capogrosso P, et al. Testosterone in males with COVID-19: A 7-month cohort study. Andrology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley KE, Thomas E, Leaver M, Wells D. Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues. Fertil Steril 2020; 114:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, Huang X, Yi Z, et al. Ultrasound imaging findings of acute testicular infection in patients with coronavirus disease 2019: a single-center-based study in Wuhan, China. J Ultrasound Med 2021; 40:1787–1794. [DOI] [PubMed] [Google Scholar]
- 40.Ediz C, Tavukcu HH, Akan S, et al. Is there any association of COVID-19 with testicular pain and epididymo-orchitis? Int J Clin Pract 2021; 75:e13753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sergerie M, Mieusset R, Croute F, et al. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil Steril 2007; 88:970.e1-7. [DOI] [PubMed] [Google Scholar]
- 42.Li D, Jin M, Bao P, et al. Clinical characteristics and results of semen tests among men with coronavirus disease. JAMA Netw Open 2020; 3:e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pan F, Xiao X, Guo J, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease. Fertil Steril 2020; 113:1135–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holtmann N, Edimiris P, Andree M, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril 2020; 114:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo L, Zhao S, Li W, et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology 2021; 9:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez DC, Khodamoradi K, Pai R, et al. A systematic review on the investigation of SARS-CoV-2 in Semen. Res Rep Urol 2020; 12:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Achua JK, Chu KY, Ibrahim E, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J Mens Health 2021; 39:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma X, Guan C, Chen R, et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell Mol Immunol 2021; 18:487–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gacci M, Coppi M, Baldi E, et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod 2021; 36:1520–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamarat MB, Ozkent MS, Yilmaz B, et al. Effect of SARS-CoV-2 infection on semen parameters. Can Urol Assoc J 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Best JC, Kuchakulla M, Khodamoradi K, et al. Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J Mens Health 2021; 39:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsieh TC, Edwards NC, Bhattacharyya SK, et al. The epidemic of COVID-19-related erectile dysfunction: a scoping review and healthcare perspective. Sex Med Rev 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53▪.Sansone A, Mollaioli D, Ciocca G, et al. ’Mask up to keep it up’: preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology 2021; 9:1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a retrospective study reviewing sexual health data from the Sex@COVID online survey in Italy. The study had 100 subjects (25 COVID-19 positive, 75 COVID-19 negative) and demonstrated that the prevalence of ED in COVID-19 positive men was significantly higher (28% vs 9.33%) with almost 6 times increased risk (OR 5.66) of developing ED if you get COVID-19.
- 54.Hernandez I, Gul Z, Gellad WF, Davies BJ. Marked increase in sales of erectile dysfunction medication during COVID-19. J Gen Intern Med 2021; 36:2912–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care 2020; 24:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bernard I, Limonta D, Mahal LK, Hobman TC. Endothelium infection and dysregulation by SARS-CoV-2: evidence and caveats in COVID-19. Viruses 2020; 13: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kresch E, Achua J, Saltzman R, et al. COVID-19 endothelial dysfunction can cause erectile dysfunction: histopathological, immunohistochemical, and ultrastructural study of the human penis. World J Mens Health 2021; 39:466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slomski A. Pediatric depression and anxiety doubled during the pandemic. JAMA 2021; 326:1246. [DOI] [PubMed] [Google Scholar]
- 59.Kwong ASF, Pearson RM, Adams MJ, et al. Mental health before and during the COVID-19 pandemic in two longitudinal UK population cohorts. Br J Psychiatry 2020; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milani SA, Raji MA, Chen L, Kuo YF. Trends in the use of benzodiazepines, Z-Hypnotics, and serotonergic drugs among US women and men before and during the COVID-19 pandemic. JAMA Netw Open 2021; 4:e2131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coustasse A, Kimble C, Maxik K. COVID-19 and vaccine hesitancy: a challenge the united states must overcome. J Ambul Care Manag 2021; 44:71–75. [DOI] [PubMed] [Google Scholar]
- 62.Diaz P, Reddy P, Ramasahayam R, et al. COVID-19 vaccine hesitancy linked to increased internet search queries for side effects on fertility potential in the initial rollout phase following Emergency Use Authorization. Andrologia 2021; 53:e14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63▪▪.Gonzalez DC, Nassau DE, Khodamoradi K, et al. Sperm parameters before and after COVID-19 mRNA vaccination. JAMA 2021; 326:273–274. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a single center prospective study that collected semen samples from 45 men before receiving the vaccine and then approximately 70 days after the second vaccine shot. There were no significant decreases in semen parameters.
- 64.Carto C, Nackeeran S, Ramasamy R. COVID-19 vaccination is associated with a decreased risk of orchitis and/or epididymitis in men. Andrologia 2021; e14281. [DOI] [PMC free article] [PubMed] [Google Scholar]