Purpose of review
Chronic obstructive pulmonary disease (COPD) and COVID-19 have many potentially negative interrelationships, which may influence the course of infection and clinical outcomes. The aim of this review is to provide clinicians with an up-to-date perspective of the complex interactions between COPD and COVID-19.
Recent findings
We consider mechanisms that could increase SARS-CoV-2 infection susceptibility in COPD, including increased ACE2 expression, reduced antiviral defence and dysfunctional immunity. We review evidence that COPD is associated with worse clinical outcomes from COVID-19 in analyses that have adjusted for confounding factors, and describe the mechanisms responsible. We discuss the use of inhaled corticosteroids in the context of susceptibility to COVID-19, and consider the impact of COVID-19 on the usual care of COPD patients.
Summary
The current review highlights the evidence that COPD patients have worse outcomes from COVID-19, and the multiple mechanisms responsible.
Keywords: bacteria, coagulopathy, small airway disease, thrombosis, virus
INTRODUCTION
Coronavirus disease 2019 (COVID-19) pandemic has been caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. This highly transmissible virus [2] can cause mild viral illness, while more severe cases display systemic inflammation, pneumonia and acute severe respiratory failure [3]. COVID-19 respiratory failure is often associated with good lung compliance, implicating vascular injury and vasoconstriction as central mechanisms of respiratory insufficiency [4]. COVID-19 pneumonia appears to be caused by pulmonary exudate due to microvascular injury [5,6]. Systemic manifestations are common in severe COVID-19, with endothelial injury causing cardiac, renal and neurological complications [7]. These pulmonary and systemic disease components cause a high mortality in COVID-19 patients admitted to hospital, which is increased in older individuals and those with comorbidities including obesity and cardiovascular disease [3,8,9].
Chronic obstructive pulmonary disease (COPD) occurs in older individuals due to persistent inhalation of noxious particles, commonly from cigarette smoking [10]. The pulmonary disease includes airway inflammation and remodelling, with variable alveolar destruction (emphysema) [11]. COPD patients suffer with dyspnoea, cough and sputum production, and may experience sudden worsenings (exacerbations) that are often caused by respiratory tract infections [10]. In addition, COPD is associated with a high prevalence of comorbidities such as cardiovascular disease and diabetes, which is not surprising in an older population with a significant smoking history. COPD and COVID-19 therefore have many potentially negative interrelationships, which may lead to worse outcomes from COVID-19, including impaired pulmonary function, older age and the presence of comorbidities in COPD patients. Furthermore, COPD patients may also be more susceptible to acquiring viral infections including SARS-CoV-2 [12].
This review addresses interrelationships between COPD and COVID-19. The mechanisms that could cause increased SARS-CoV-2 infection susceptibility in COPD patients are considered. The evidence that COPD is associated with worse clinical outcomes from COVID-19 is reviewed, and whether the use of inhaled corticosteroids (ICS) alters outcomes. We also consider the impact of COVID-19 on the usual clinical care of COPD patients.
Box 1.
no caption available
SARS-COV-2 INFECTION SUSCEPTIBILITY
Cellular tropism of SARS-CoV-2 infection is determined by the expression of receptors for the virus, including angiotensin-converting enzyme 2 (ACE2) [13]. Bronchial and alveolar epithelial cells, and pulmonary endothelial cells express ACE2, and are infected with virus in COVID-19 cases [6,14]. Protein and gene expression studies have reported increased expression of ACE2 in COPD epithelial cells compared to controls [15–17], with increased expression in COPD patients with a higher BMI and more frequent exacerbations also observed [18,19]. There are inconsistent findings regarding changes in accessory protein expression, including transmembrane serine protease 2 (TMPRSS2) and furin [20]. Expression of intracellular adhesion molecule-1 (ICAM-1), the receptor for rhinovirus, is also increased in the lungs of COPD patients. [21,22] Overall, these observations suggest increased opportunities for viral entry in COPD lungs. However, this by itself is unlikely to determine the clinical outcome to infection; reduced host antiviral defence and immune system dysfunction in COPD patients may also combine to promote host permissiveness.
There is evidence that host antiviral responses, notably interferons, are dampened in COPD patients. During experimental rhinovirus infection, the production of interferon (IFN)-α, β and λ from bronchoalveolar lavage cells was lower in COPD patients than in controls, albeit only significant for IFN-β [23]. COPD patients experienced more respiratory symptoms and also had a higher viral load and increased markers of inflammation. An immunohistochemistry study showed significantly lower bronchial epithelial and alveolar macrophage expression of IFN-β in COPD patients compared to controls [24]. Furthermore, sputum levels of IFN-β and IFN-λ are lower from COPD patients with more exacerbations, suggesting that reduced host defence against viral infection associates with worse clinical outcomes [25]. Reduced IFN production may be caused by reduced signalling by pattern recognition receptors; bronchial epithelial expression of melanoma differentiation-associated gene-5 (MDA-5) and retinoic acid-inducible gene-1 (RIG-1) are reduced in COPD patients compared with controls [24], suggesting that COPD bronchial epithelial cells are less adept at mounting antiviral responses. Indeed, Veerati et al. [26] reported that IFN-β production and antiviral gene expression by COPD epithelial cells were delayed following rhinovirus infection. Although antiviral responses using COPD lung cells exposed to SARS-CoV-2 have not been studied, evidence using other viral exposure models indicates a downregulation of interferon defence mechanisms.
Lymphopoenia and T cell dysfunction are associated with worse outcomes in COVID-19 [27]. T cells in the lungs of COPD patients display increased expression of programmed cell death protein 1 (a marker of T-cell exhaustion), while T-cell receptor (TCR) expression is decreased compared with controls [28,29]. These observations indicate that COPD T cells are dysfunctional; this is supported by reports of reduced degranulation by COPD lung T-cells in response to influenza infection, and reduced cytokine production from COPD blood T-cells following TCR activation [28,30]. T-cell dysfunction in COPD patients may cause suboptimal host defence responses to SARS-CoV-2 infection.
CLINICAL OUTCOMES IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE PATIENTS WITH COVID-19
Despite extensive published research, our knowledge concerning risk factors for developing COVID-19 and for adverse outcomes of COVID-19 has been hampered by poor methodological design of some studies coupled with inadequate consideration of confounding factors [20,31].
It was proposed that COPD or smoking (either a current or previous history) confers a protective effect against contracting COVID-19, based on the lower-than-expected prevalence of COPD and current or previous smoking history among patients diagnosed with COVID-19 [32–35]. However, patients with COPD or other conditions associated with smoking have been shielding during the pandemic, thus limiting their likelihood of contracting SARS-CoV-2 infection [20]. Furthermore, the proposed protective effects were mostly based on studies retrospectively evaluating routinely collected hospital clinical data. The quality and completeness of such clinical records could be questioned [36] during the pandemic that pressurized health systems [37] increasing the likelihood of under-reporting of smoking history or COPD.
The potential for negative effects of COPD on COVID-19 outcomes can be overestimated if confounding factors, including age, sex and comorbidities, are not fully addressed [38,39]. We previously reviewed cohort studies performed in 2020 [20], which mostly reported that COPD was a risk factor for worse clinical outcomes in hospitalized COVID-19 patients, although most did not adequately address for confounding [17]. In order to overcome potential selection bias in these studies, which can arise due to only considering the cohort of individuals admitted to hospital with COVID-19, Aveyard et al.[40▪▪] performed an analysis using a UK community-based population of more than 8 million individuals with 14 479 hospitalised with COVID-19. COPD was a significant risk factor for hospitalization and death [hazard ratio 1.54, confidence interval (CI) 1.45–1.63, and hazard ratio 1.54, CI 1.42–1.67, respectively] after adjustment for age, sex and comorbidities. The unadjusted hazard ratios were considerably higher (>5), highlighting the influence of confounding factors. Interestingly, using the adjusted model data, the risk was higher in younger COPD patients, probably reflecting the dominant poor prognostic influence of age (relative to COPD) in older individuals.
Several meta-analyses have evaluated whether COPD and smoking history are risk factors for COVID-19 outcomes. Only three of them adjusted for potential confounding factors [41,42]. Izcovich et al.[41] included preferably adjusted data, without excluding nonadjusted data, and identified both COPD and current smoking status as strong predictors of worse outcomes including mortality. A sensitivity analysis, including adjusted data only, showed consistent results, but were not presented in detail. However, this meta-analysis adjusted for age, at least one comorbidity and one estimate of COVID-19 severity, such as respiratory rate. The latter violates the temporality between the prognostic factor and outcome, creating difficulties in interpretation. Xiao et al.[42] only included adjusted data in their meta-analysis, and reported an association between COPD and COVID-19 mortality [odds ratio (OR) 1.53, CI 1.29–1.8]. This meta-analysis had two important limitations: ORs were pooled with hazard ratios, which is questionable, and any adjustment strategy was acceptable regardless of the adjusting factors used. Interestingly, eight of the 11 included studies presenting adjusted ORs did not yield significant results.
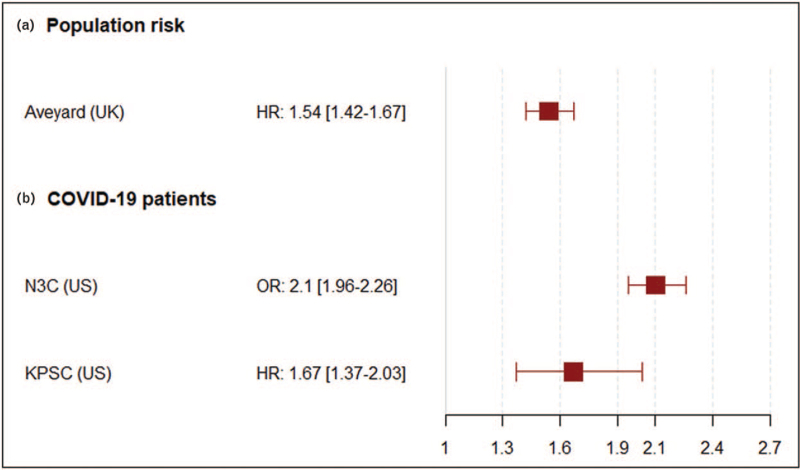
However, these meta-analyses were followed by the publication of adjusted data from larger cohorts (Fig. 1). The US National COVID Cohort Collaboration (N3C), with more than 3.4 million patients, including more than 387 000 patients with COVID-19 including more than 7500 COPD patients demonstrated an association between COPD and COVID-19 mortality (OR: 2.1, 95% CI: 1.96–2.26), after adjusting for age, sex and comorbidities [43▪▪]. Similarly, data from the Kaiser-Permanante Southern California electronic patient records based on 61 338 adults with COVID-19, including 820 patients with COPD, found a significant association of COPD with COVID-19 related 60-day mortality (hazard ratio: 1.67, 95% CI: 1.37–2.03), hospitalization (OR: 1.27, 95% CI: 1.05–1.53) and intensive respiratory support (OR: 1.49, 95% CI: 1.16–1.92), after adjusting for relevant covariates, including age, sex, ethnicity, main demographics and the Charlson's comorbidity score [44].
FIGURE 1.
Preexisting chronic obstructive pulmonary disease and adjusted COVID-19 mortality risk. Risk was evaluated (a) in a population-based cohort, (b) in extensive cohorts of patients with confirmed COVID-19.
Risk factors associated with severe COVID-19 among patients with COPD (n = 68 902) were evaluated in the Swedish National Airway Register [45]. Multivariate analyses revealed that severe COVID-19 was associated with older age, male sex, lower educational level, underweight or obesity, FEV1 less than 50% predicted and a COPD Assessment test score at least 18.
Overall, although confounding factors exist in the multiple studies on this topic, the large cohort studies of Aveyard et al.[40▪▪], the N3C and Kaiser-Permanante databases demonstrate that COPD is associated with worse outcomes from COVID-19 after adjusting for confounding factors.
WORSE COVID-19 CLINICAL OUTCOMES IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE PATIENTS; MECHANISTIC EXPLANATIONS
COVID-19 hypoxaemia arises due to widespread pulmonary intravascular clots and alveolar oedema, which reduces perfusion and ventilation, respectively, in the capillary bed [46]. This ventilation/perfusion (V/Q) mismatching causes hypoxic pulmonary vasoconstriction, thereby restricting blood flow to areas of impaired gas exchange [47] and causing intrapulmonary shunting of blood to other areas. Kotwica et al. [48▪] showed that increased intrapulmonary shunting was associated with worse COVID-19 outcomes, including mortality. COPD patients have compromised ventilation due to small airway disease and emphysema [11], and therefore have poor respiratory functional reserve to cope with intrapulmonary shunting, as this may divert blood to areas with impaired gas exchange due to COPD pathophysiology.
Hypoxic pulmonary vasoconstriction increases fluid shear stress in pulmonary vessels, leading to platelet aggregation and increased risk for thrombus formation [49,50]. Hypoxic pulmonary vasoconstriction occurs in COPD patients (without COVID-19) due to reduced ventilation [51], which predisposes to in-situ thrombus formation; this pathophysiology can be worsened by COVID-19. Hypoxic pulmonary vasoconstriction is associated with increased pulmonary hypertension in COPD due to vascular remodelling [52]; again, this may be further worsened by COVID-19.
Pulmonary thromboembolism is typical of severe COVID-19 [53], due to the development of thrombosis within smaller pulmonary vessels [6] due to increased coagulation and endothelial cell dysfunction [54]. COPD patients (without COVID-19) also display higher circulating pro-coagulation markers, which are further increased during exacerbations [55–58], and increased numbers of apoptotic endothelial cells and markers of endothelial cell dysfunction [59]. These data suggest increased susceptibility of COPD patients to thromboembolic events due to coagulopathy and endothelial cell dysfunction may be further worsened by COVID-19.
Secondary bacterial infection is common amongst COVID-19 patients, causing worse outcomes [60]. Many COPD patients have colonizing pathogenic bacteria in the airways during the stable state, which cause secondary bacterial infections following respiratory viral infections [61,62]. This may be due to dampened antimicrobial responses; viral infection reduces bacterial phagocytosis by COPD alveolar macrophages and reduces antimicrobial peptide release in COPD patients [61,63].
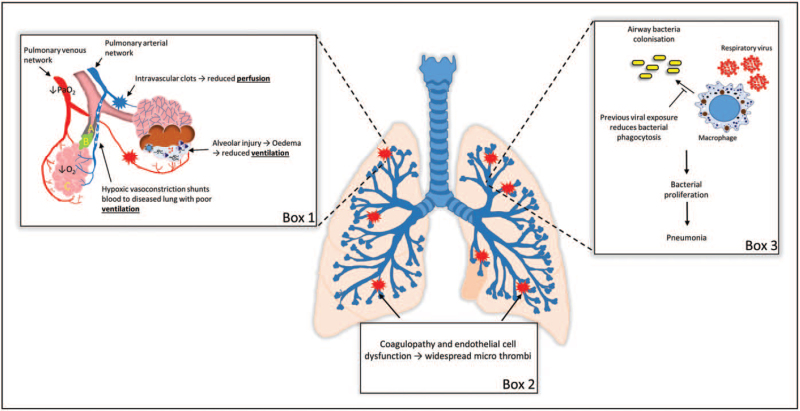
Overall, there are multiple mechanisms to explain worse COVID-19 outcomes in COPD patients. The most important mechanisms are increased risk for micro-thrombosis, the effects of intrapulmonary shunting and the potential for secondary bacterial infection (Fig. 2).
FIGURE 2.
Worse clinical outcomes from COVID-19 in chronic obstructive pulmonary disease patients. Box 1: Reduced perfusion and ventilation leads to hypoxic vasoconstriction and intrapulmonary shunting of blood to other areas of the lung. In COPD patients, this may divert blood to areas with poor ventilation as a result of COPD pathophysiology: (a) reduced lumen diameter and increased airway wall thickening; (b) mucous plugs; (c) alveolar wall destruction. Box 2: coagulopathies and endothelial cell dysfunction increase the risk of micro-thrombosis. Box 3: secondary bacterial infection as a result of reduced host defence may lead to pneumonia.
CHRONIC OBSTRUCTIVE PULMONARY DISEASE, COVID-19 AND INHALED CORTICOSTEROIDS
Experimental studies using cultured cells have shown that corticosteroids can attenuate SARS-CoV-2 replication [64,65]. The RECOVERY clinical trial (n = 6435) demonstrated that the corticosteroid dexamethasone (adminsitered orally or intravenously) decreased mortality in hospitalized COVID-19 patients requiring oxygen or mechanical ventilation [66▪▪]. In addition, a phase 2 clinical trial (n = 146) showed that inhaled budesonide administered in the community in the early stages of COVID-19 reduced the need for urgent medical care and improved the recovery time [67]. Overall, these laboratory and clinical studies support a protective role for corticosteroids against COVID-19.
A retrospective analysis of electronic health records has been performed to investigate clinical outcomes in COPD patients in the UK treated with or without ICS. The OpenSAFELY UK primary care cohort analysed mortality outcomes in COPD patients (n = 148 557); COVID-19 associated mortality was increased in ICS users versus nonusers [68▪]. Importantly, adjusting for confounding factors decreased the hazard ratio, and the fully adjusted model in patients of white ethnicity showed no detrimental effects of ICS use. Non-COVID-19 deaths were also higher in patients using ICS, highlighting the confounding effect of disease severity on mortality outcomes. Aveyard et al.[40▪▪] studied outcomes in 193 520 COPD patients using primary care records; we discussed earlier that hospitalization and mortality rates were increased in COPD patients. The authors also reported increased hospitalization and mortality rates in ICS users versus nonusers in an unadjusted analysis, but that no detrimental effect of ICS use was observed after adjustment for demographic factors and medication use.
The overall weight of scientific information does not support any detrimental effect of ICS use with regard to COVID-19 outcomes in COPD patients. Indeed, the beneficial effects of systemic and ICS administered acutely to treat COVID-19 indicates a protective effect of this drug class in this scenario. The GOLD 2020 science committee report on COVID-19 accordingly recommends that for COPD patients the use of ICS as a maintenance treatment should not be changed during this pandemic [69▪].
CLINICAL CARE OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE DURING COVID-19 PANDEMIC
The COVID-19 pandemic has caused virtual COPD outpatient care to become commonplace, minimizing exposure risks and offering time savings through less travel. However, reduced personal contact can cause problems in scenarios, such as inhaler technique training and complex discussions.
Shielding strategies reduce the risks of viral transmission, and there have been reports of decreased COPD hospitalizations for exacerbations during the COVID-19 pandemic [70]. However, avoidance of hospitals for fear of contracting COVID-19 might also contribute to these observations. Shielding has placed restrictions on the mobility and social interactions of COPD patients. In the author's personal experience, this can cause problems with physical deconditioning in some patients due to weight gain and/or loss of muscle strength, while social isolation can cause negative psychological issues.
Access to spirometry has been reduced to reduce risks associated with aerosolization of viral particles. Nevertheless, spirometry remains an essential part of COPD diagnosis and follow-up monitoring, and efforts should be made to conduct this in well tolerated environments.
CONCLUSION
This review highlights evidence from epidemiological analyses that COPD patients have worse outcomes from COVID-19. There are biological mechanisms that cause COPD patients to be more susceptible to acquiring viral infections, and to the pathophysiological consequences of COVID-19, including micro-thrombosis, the effects of intrapulmonary shunting and secondary bacterial infection. Interestingly, evidence suggests that ICS may protect against COVID-19, although this has not been directly confirmed in COPD patients. These interrelationships between COPD and COVID-19 are summarized in Table 1. A consequence of COVID-19 has been increased anxiety and isolation in COPD patients, with potentially harmful long-term consequences.
Table 1.
COPD and COVID-19 interrelationships; key points
| SARS-CoV-2 infection; mechanisms of increased susceptibility in COPD | Clinical outcomes in COPD patients with COVID-19 | Worse COVID-19 clinical outcomes in COPD patients; mechanistic explanations | COPD, COVID-19 and ICS |
| Increased ACE2 expression in lung epithelium [15–17] Reduced antiviral defence [25] Dysfunctional immunity [27,29] | Confounding factors need to be controlled for in epidemiological data. Analyses adjusted for confounding variables show increased hospitalization and mortality [40▪▪,43▪▪,44] | Increased risk for micro-thrombosis due to endothelial cell dysfunction and coagulopathy [55–59] Detrimental effects of increased intra-pulmonary shunting [48▪] Secondary bacterial infection [61,62] | Corticosteroids reduce SARS-CoV-2 replication [64,65] Systemic and inhaled corticosteroids improve COVID-19 related outcomes [66▪▪,67], although not studied specifically in COPD |
Acknowledgements
DS and AG are supported by the NIHR Manchester Biomedical Research Centre.
Financial support and sponsorship
The research was supported by North West Lung Centre Charity, Manchester. This report is an independent research and the views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflicts of interest
D.S. received personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi Farmaceutici S.p.A., Cipla, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Novartis, Pfizer Inc., Pulmatrix, Teva, Theravance Biopharma, Menarini and Verona Pharma. A.H. and A.G. have no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Coronaviridae Study Group of the International Committee on Taxonomy of V. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020; 5:536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis 2020; 20:e238–e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 201:1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary postmortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020; 20:1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonaventura A, Vecchie A, Dagna L, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol 2021; 21:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring) 2020; 28:1195–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD executive summary. Eur Respir J 2017; 49:214–246. [DOI] [PubMed] [Google Scholar]
- 11.Singh D, Long G, Cancado JED, Higham A. Small airway disease in chronic obstructive pulmonary disease: insights and implications for the clinician. Curr Opin Pulm Med 2020; 26:162–168. [DOI] [PubMed] [Google Scholar]
- 12.Sajjan US. Susceptibility to viral infections in chronic obstructive pulmonary disease: role of epithelial cells. Curr Opin Pulm Med 2013; 19:125–132. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181:271–280. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Li Y, Liu Q, et al. SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov 2021; 7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai G, Bosse Y, Xiao F, Kheradmand F, Amos CI. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med 2020; 201:1557–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs M, Van Eeckhoutte HP, Wijnant SRA, et al. Increased expression of ACE2, the SARS-CoV-2 entry receptor, in alveolar and bronchial epithelium of smokers and COPD subjects. Eur Respir J 2020; 56:2378–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JC, Sausville EL, Girish V, et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev Cell 2020; 53:514–529. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higham A, Singh D. Increased ACE2 expression in bronchial epithelium of COPD patients who are overweight. Obesity (Silver Spring) 2020; 28:1586–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson A, Oberg L, Angermann B, et al. Dysregulation of COVID-19 related gene expression in the COPD lung. Respir Res 2021; 22:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higham A, Mathioudakis A, Vestbo J, Singh D. COVID-19 and COPD: a narrative review of the basic science and clinical outcomes. Eur Respir Rev 2020; 29:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zandvoort A, van der Geld YM, Jonker MR, et al. High ICAM-1 gene expression in pulmonary fibroblasts of COPD patients: a reflection of an enhanced immunological function. Eur Respir J 2006; 28:113–122. [DOI] [PubMed] [Google Scholar]
- 22.Shukla SD, Mahmood MQ, Weston S, et al. The main rhinovirus respiratory tract adhesion site (ICAM-1) is upregulated in smokers and patients with chronic airflow limitation (CAL). Respir Res 2017; 18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallia P, Message SD, Gielen V, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 2011; 183:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Valero J, Olloquequi J, Montes JF, et al. Deficient pulmonary IFN-beta expression in COPD patients. PLoS One 2019; 14:e0217803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singanayagam A, Loo SL, Calderazzo M, et al. Antiviral immunity is impaired in COPD patients with frequent exacerbations. Am J Physiol Lung Cell Mol Physiol 2019; 317:L893–L903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veerati PC, Troy NM, Reid AT, et al. Airway epithelial cell immunity is delayed during rhinovirus infection in asthma and COPD. Front Immunol 2020; 11:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol 2020; 11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKendry RT, Spalluto CM, Burke H, et al. Dysregulation of antiviral function of CD8(+) T cells in the chronic obstructive pulmonary disease lung. Role of the PD-1-PD-L1 axis. Am J Respir Crit Care Med 2016; 193:642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grundy S, Plumb J, Lea S, et al. Down regulation of T cell receptor expression in COPD pulmonary CD8 cells. PLoS One 2013; 8:e71629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts MEP, Higgs BW, Brohawn P, et al. CD4+ T-cell profiles and peripheral blood ex-vivo responses to T-Cell directed stimulation delineate COPD phenotypes. Chronic Obstr Pulm Dis 2015; 2:268–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathioudakis AG, Fally M, Hashad R, et al. COVID-19 clinical trials: unravelling a methodological Gordian knot. Am J Respir Crit Care Med 2020; 202:635–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farsalinos K, Angelopoulou A, Alexandris N, Poulas K. COVID-19 and the nicotinic cholinergic system. Eur Respir J 2020; 56:1589–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with COVID-19 in New York: retrospective case series. BMJ 2020; 369:m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One 2020; 15:e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathioudakis A, Rousalova I, Gagnat AA, et al. How to keep good clinical records. Breathe (Sheff) 2016; 12:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira D, Miranda R, Leuschner P, et al. OpenEHR modeling: improving clinical records during the COVID-19 pandemic. Health Technol (Berl) 2021; 11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yates T, Zaccardi F, Islam N, et al. Obesity, chronic disease, age, and in-hospital mortality in patients with covid-19: analysis of ISARIC clinical characterisation protocol UK cohort. BMC Infect Dis 2021; 21:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis. Management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J 2019; 53:1900164. [DOI] [PubMed] [Google Scholar]
- 40▪▪.Aveyard P, Gao M, Lindson N, et al. Association between preexisting respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med 2021; 9:909–923. [DOI] [PMC free article] [PubMed] [Google Scholar]; UK community-based population of more than 8 million individuals with 14 479 hospitalized with COVID-19.
- 41.Izcovich A, Ragusa MA, Tortosa F, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PLoS One 2020; 15:e0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiao WW, Xu J, Shi L, et al. Is chronic obstructive pulmonary disease an independent predictor for adverse outcomes in coronavirus disease 2019 patients? Eur Rev Med Pharmacol Sci 2020; 24:11421–11427. [DOI] [PubMed] [Google Scholar]
- 43▪▪.Meza D, Khuder B, Bailey JI, et al. Mortality from COVID-19 in patients with COPD: a US study in the N3C Data Enclave. Int J Chron Obstruct Pulmon Dis 2021; 16:2323–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]; The US National COVID Cohort Collaboration (N3C), with more than 3.4 million patients, including more than 387 000 patients with COVID-19 including more than 7500 COPD patients.
- 44.Huang BZ, Chen Z, Sidell MA, et al. Asthma disease status, COPD, and COVID-19 severity in a large multiethnic population. J Allergy Clin Immunol Pract 2021; 3621–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stridsman C, Vanfleteren L, Konradsen JR, et al. Predictors of severe COVID-19 in a registry-based Swedish cohort of patients with chronic obstructive pulmonary disease (COPD). Eur Respir J 2021; 1920–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis 2020; 20:1365–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersson J, Glenny RW. Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J 2014; 44:1023–1041. [DOI] [PubMed] [Google Scholar]
- 48▪.Kotwica A, Knights H, Mayor N, et al. Intrapulmonary shunt measured by bedside pulse oximetry predicts worse outcomes in severe COVID-19. Eur Respir J 2021; 57: [DOI] [PMC free article] [PubMed] [Google Scholar]; Increased intrapulmonary shunting was associated with worse COVID-19 outcomes, including mortality.
- 49.Sakao S. Chronic obstructive pulmonary disease and the early stage of cor pulmonale: a perspective in treatment with pulmonary arterial hypertension-approved drugs. Respir Investig 2019; 57:325–329. [DOI] [PubMed] [Google Scholar]
- 50.Casa LD, Deaton DH, Ku DN. Role of high shear rate in thrombosis. J Vasc Surg 2015; 61:1068–1080. [DOI] [PubMed] [Google Scholar]
- 51.Sakao S, Voelkel NF, Tatsumi K. The vascular bed in COPD: pulmonary hypertension and pulmonary vascular alterations. Eur Respir Rev 2014; 23:350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bogaard HJ. Hypoxic pulmonary vasoconstriction in COPD-associated pulmonary hypertension: been there, done that? Eur Respir J 2017; 50:1191–1194. [DOI] [PubMed] [Google Scholar]
- 53.Jalde FC, Beckman MO, Svensson AM, et al. Widespread parenchymal abnormalities and pulmonary embolism on contrast-enhanced CT predict disease severity and mortality in hospitalized COVID-19 patients. Front Med (Lausanne) 2021; 8:666723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spiezia L, Boscolo A, Poletto F, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost 2020; 120:998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashitani J, Mukae H, Arimura Y, Matsukura S. Elevated plasma procoagulant and fibrinolytic markers in patients with chronic obstructive pulmonary disease. Intern Med 2002; 41:181–185. [DOI] [PubMed] [Google Scholar]
- 56.Husebo GR, Gabazza EC, D’Alessandro Gabazza C, et al. Coagulation markers as predictors for clinical events in COPD. Respirology 2021; 26:342–351. [DOI] [PubMed] [Google Scholar]
- 57.Liu M, Hu R, Jiang X, Mei X. Coagulation dysfunction in patients with AECOPD and its relation to infection and hypercapnia. J Clin Lab Anal 2021; 35:e23733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaidyula VR, Criner GJ, Grabianowski C, Rao AK. Circulating tissue factor procoagulant activity is elevated in stable moderate to severe chronic obstructive pulmonary disease. Thromb Res 2009; 124:259–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cella G, Sbarai A, Mazzaro G, et al. Plasma markers of endothelial dysfunction in chronic obstructive pulmonary disease. Clin Appl Thromb Hemost 2001; 7:205–208. [DOI] [PubMed] [Google Scholar]
- 60.De Santis V, Corona A, Vitale D, et al. Bacterial infections in critically ill patients with SARS-2-COVID-19 infection: results of a prospective observational multicenter study. Infection 2021; 14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallia P, Footitt J, Sotero R, et al. Rhinovirus infection induces degradation of antimicrobial peptides and secondary bacterial infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012; 186:1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Z, Maschera B, Lea S, et al. Airway host-microbiome interactions in chronic obstructive pulmonary disease. Respir Res 2019; 20:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finney LJ, Belchamber KBR, Fenwick PS, et al. Human rhinovirus impairs the innate immune response to bacteria in alveolar macrophages in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2019; 199:1496–1507. [DOI] [PubMed] [Google Scholar]
- 64.Heinen N, Meister TL, Klohn M, et al. Antiviral effect of budesonide against SARS-CoV-2. Viruses 2021; 13:1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsuyama S, Kawase M, Nao N, et al. The inhaled steroid Ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J Virol 2020; 95:1648–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66▪▪.Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dexamethasone decreased mortality in hospitalized COVID-19 patients requiring oxygen or mechanical ventilation.
- 67.Yu LM, Bafadhel M, Dorward J, et al. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet 2021; 398:843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68▪.Schultze A, Walker AJ, MacKenna B, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med 2020; 8:1106–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]; When adjusted for confounders, this study showed no detrimental effects of ICS use.
- 69▪.Halpin DMG, Criner GJ, Papi A, et al. Global Initiative for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2021; 203:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of literature on COPD and COVID19, as well as clinical recommendations.
- 70.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Reduction in hospitalised COPD exacerbations during COVID-19: a systematic review and meta-analysis. PLoS One 2021; 16:e0255659. [DOI] [PMC free article] [PubMed] [Google Scholar]