Abstract
Kidney transplantation is the preferred treatment modality in patients with ESKD. However, there are associated complications that arise from immunosuppressive medications, infections, and associated comorbidities. Neurologic disorders frequently develop in patients who have received a kidney transplant, which in turn increases the associated morbidity and mortality. This review discusses the common neurologic disorders after kidney transplantation, including infections, cognitive decline, drug-related conditions, malignancy, seizure, and other neurologic complications.
Keywords: acute kidney injury and ICU nephrology, calcineurin inhibitors, cognitive dysfunction, comorbidity, ESKD, immunosuppression, infections, kidney failure, chronic, kidney transplantation, neurological complications, seizures
Introduction
Kidney transplantation is the desired treatment modality in patients with ESKD. It offers better survival and quality of life compared with hemodialysis and peritoneal dialysis. Despite these advantages, transplant recipients are susceptible to complications that impede the quality of life and add to the financial burden. Neurologic diseases frequently develop in recipients of kidney transplants and this increases morbidity.
Neurologic diseases after kidney transplantation are frequently underdiagnosed and have been reported in up to 30%–60% of patients (1). Notably, in a systematic review and meta-analysis by Mohammadi et al. (2), the total prevalence of neurologic disorders in 4674 patients after kidney transplantation was 8%.
This review attempts to classify the neurologic complications in kidney transplant recipients (KTRs) into the following categories: (1) preexisting neurologic conditions, (2) early neurologic complications, (3) subacute complications, and (4) late neurologic complications. We also highlight various neurologic complications related to infections, medications, malignancies, and other comorbid conditions.
Preexisting Neurologic Disease
Neurologic disease in renal failure may not be discovered until transplantation. Associated comorbidities like vascular calcification, malnutrition, chronic inflammation, cerebrovascular accidents (CVAs), diabetes mellitus, hypertension, and other underlying diseases such as SLE predispose these patients to neurologic syndromes. Ischemic stroke is responsible for both acute neurologic deficits or subclinical, gradually worsening cognitive impairments. Diabetes mellitus affects peripheral nerves resulting in painful sensory neuropathy. SLE has a wide spectrum of neurologic manifestations, for example, headache, seizures, chorea, cognitive dysfunction, myelopathy, meningitis, and mononeuropathy (3). Patients with infections like HIV can experience dementia, neuropathies, and vacuolar myelopathy (4). Long-standing uremia causes axonal, symmetrical, sensorimotor, length-dependent polyneuropathy that does not entirely resolve even after the improvement of renal function. Autonomic dysfunction is another complication of uremia, which causes orthostatic hypotension, impotence, heart rate variability, exercise intolerance, and gastrointestinal intolerance (5). Factors like age, diabetes mellitus, inflammation, stroke, oxidative stress, and decreased cerebral perfusion, leading to cerebral ischemia during hemodialysis, make patients with ESKD more vulnerable to cognitive impairment and dementia as compared with the general population (6).
Neurologic illnesses may manifest at any time after transplantation. Immediate neurologic complications occurring postrenal transplantation surgery are associated with several diagnostic possibilities. They can affect both the central nervous system (CNS) and the peripheral nervous system (PNS).
CNS Dysfunction
After transplant surgery, KTRs may exhibit mild symptoms like behavioral changes and confusion due to perioperative sedation, but symptoms may be as severe as encephalopathy or coma resulting from hypoxic-ischemic insult. The patients requiring admission to the intensive care unit may develop psychosis within 2–5 days after surgery. Neuroimaging by performing computed tomography (CT) or magnetic resonance imaging (MRI) scans aid in diagnosis (7). Psychosis usually resolves with environmental reorientation; neuroleptics are rarely required.
Electrolyte Imbalance
Abnormalities in electrolytes and acid-base balance are a regular finding after renal transplantation. The most frequent post-transplant metabolic disturbances are dysnatremia, hyperkalemia, hypomagnesemia, hypophosphatemia, and metabolic acidosis. Serum sodium of <120 mEq/L may lead to confusion, disorientation, or generalized tonic-clonic seizures. Severe hypomagnesemia may also manifest as confusion, muscle weakness, tremor, tetany, and seizures (8). The correction of any existing electrolyte imbalance improves associated neurologic symptoms. Sodium correction should be done meticulously because a rapid correction (>10 mEq/L over 24 hours) may lead to central pontine myelinolysis.
Hypertensive Encephalopathy
Hypertensive encephalopathy in the immediate post-transplant period is caused by uncontrolled high BP. It may result from drugs (steroids/calcineurin inhibitors [CNIs]), allograft rejection, renal artery stenosis, or volume overload (9). Hypertensive encephalopathy is often associated with posterior reversible encephalopathy syndrome (PRES). PRES results in characteristic findings on MRI scans which include vasogenic edema in the bilateral deep cortical and subcortical regions of the parietal and occipital lobes. Other risk factors contributing to PRES are young age, high doses of corticosteroids, CNIs, and long-standing uremia before transplant. PRES usually presents with convulsion, headache, visual defects, and altered mental state. Management involves the removal of offending drugs and antihypertensive medication for BP control (10).
PNS Dysfunction
During renal transplantation, the incidence of peripheral nerve injuries is seen in up to 5% of patients (11). The femoral nerve, lateral femoral cutaneous nerve, and the lumbosacral plexus are the commonly affected sites (12). Damage to the nerve can occur either by compression by the formation of a local hematoma or by stretching of the nerve from prolonged retraction. Rarely, acute femoral neuropathy may develop in 0.1%–3% of patients (13). It may not be noticed until the patient attempts to ambulate and is typically apparent within 24–48 hours after surgery. Ischemia, due to the “steal phenomenon” during anastomosis of the renal graft artery to the internal iliac artery, is another mechanism of nerve damage as the proximal end-to-end anastomosis diverts blood away from the vasa nervorum (14). On examination, unilateral weakness on knee extension, absent patellar reflex, and decreased sensation on the anterior medial aspect of the thigh may be found. The patient may also complain of numbness over the lateral aspect of the thigh due to injury of the lateral femoral cutaneous nerve, which is present in 2% of patients in one series (15). Lumbosacral plexopathy can also occur in cases where the internal iliac artery is used for graft revascularization. The patient complains of pain in the buttock, and examination reveals weakness of ankle dorsiflexion or proximal leg weakness. Neuropathies are usually self-limiting and resolve entirely, which can take several months; however, there may be incomplete recovery (12,13,16).
Seizure
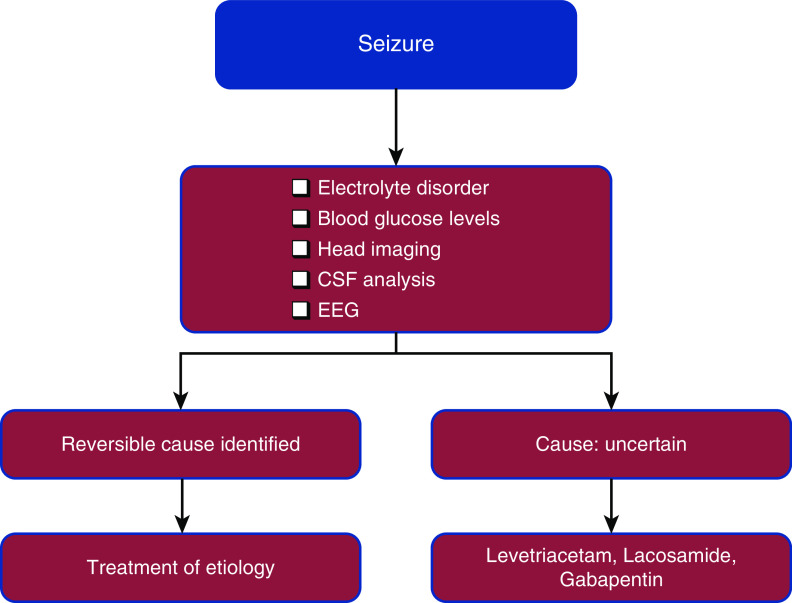
The reported incidence of seizure in KTRs is approximately 18% (17). Seizure in a KTR may be related to numerous etiologies such as electrolyte disorders, withdrawal of antiepileptic drugs (AEDs), CNI toxicity including PRES, liver dysfunction, CVAs, infections, and brain tumors. Convulsions are commonly reported during the immediate 24 hours post-transplant surgery; they are usually due to changes in plasma osmotic pressure and serum sodium (18). For identification of etiology, imaging with CT or MRI scans of the head is helpful. The electroencephalogram plays an essential role in excluding nonepileptic seizures (such as myoclonus) and determines the type of seizure. To rule out an infectious cause, cerebrospinal fluid (CSF) analysis should be performed. An approach to management of a seizure in a KTR is illustrated in Figure 1. The mainstay for seizure management in a KTR is to identify and treat the underlying etiology in addition to initiating an AED. The selection of an AED for a KTR is complicated by factors like drug interactions, tolerability, metabolism, and excretion. Drugs like barbiturates, phenytoin, and carbamazepine have significant drug interactions with immunosuppressive medications because they induce hepatic cytochrome P450 enzymes, leading to increase in the metabolism of CNIs and steroids (19). Newer AEDs like levetiracetam, lacosamide, gabapentin, and pregabalin have favorable side effect profiles and minimal drug interactions. A brief summary of the pharmacokinetics of important AEDs is shown in Table 1.
Figure 1.
An approach to management of a seizure in a renal allograft recipient. CSF, cerebrospinal fluid; EEG, electroencephalogram.
Table 1.
Summary of the pharmacokinetics of antiepileptic drugs
| Drugs | Metabolism | Route of Elimination | Renal Toxicity |
| Inducers of cytochrome P450 enzymes | |||
| Phenobarbital | Inducer of CYP3A | Hepatic/renal | Interstitial nephritis, anemia, hypovitaminosis D |
| Phenytoin | Inducer of CYP3A, metabolized by CYP2C9 | Renal <5% | Acute interstitial nephritis, decrease ADH release |
| Carbamazepine | Inducer of CYP3A | Hepatic | Acute interstitial nephritis, hyponatremia (SIADH) |
| Lamotrigine | Induces CYP34A | Hepatic | Acute interstitial nephritis |
| Topiramate | Weak inducer of CYP3A4 | Renal | Renal tubular acidosis, nephrolithiasis |
| Inhibitors of cytochrome P450 enzymes | |||
| Valproate | Hepatic (CYP450) metabolism, inhibits CYP2C9 | Hepatic | Tubulointerstitial nephritis, Fanconi syndrome |
| Felbamate | Inhibit CYP450 | Renal | Rare incidence of renal stone |
| No drug interactions | |||
| Gabapentin | None | Renal | — |
| Levetiracetam | None | Renal | Hypokalemia, hypomagnesemia |
CYP, cytochrome p450; ADH, antidiuretic hormone; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
Subacute Neurologic Complications
Infections
About 5%–10% of KTRs suffer from CNS-related infections at any time post-transplantation, resulting in a mortality rate of 44%–77% (20). Infections account for >30% of patients presenting with signs and symptoms of neurologic illness (21). The probability of infection from a pathogen varies with the duration post-transplantation. Infections in the early post-transplantation period (<1 month) are usually nosocomial (acquired pathogens) or donor derived. Subsequently, after 5 months, infections due to opportunistic organisms develop (22). The risk of developing an infection depends on two crucial factors: (1) epidemiologic exposure (from the community or hospital) with the organism, and (2) the net immunosuppressive state of the patient. Decreased T-cell immunity due to immunosuppressive medications is primarily responsible for infectious complications. Treatment of graft rejection, diabetes mellitus, malnutrition, and poor graft functions are other important contributing factors (23). The clinical presentation of these patients varies from fever, meningismus, and headache, to altered sensorium and seizure. In KTRs, the diagnosis of CNS infection may be challenging because they may present with minimal signs and symptoms attributable to immunosuppressive therapy. CNS infections can be categorized as meningitis, encephalitis, and focal brain abscess. Table 2 summarizes common CNS infections experienced in KTRs along with methods of diagnosis and treatment strategies (22–24).
Table 2.
Common central nervous system infections postrenal transplantation
| Disease | Organism | Diagnosis | Treatment | |
| Meningitis: headache, fever, altered mental sensorium, cranial nerve palsy, neck rigidity | Bacterial | Listeria monocytogenes, Haemophilus influenza, Neisseria meningitides, and Streptococcus pneumonia | Pleocytosis of CSF, increased protein, and reduced glucose | Antibiotics based on culture and sensitivity |
| Gram stain positive | ||||
| Rapid antigen latex agglutination test | ||||
| PCR for N. meningitides and S. pneumoniae | ||||
| Mycobacterium tuberculosis | CSF positive for acid-fast bacilli | Antitubercular therapy | ||
| Raised adenosine deaminase | ||||
| Raises total leukocyte with lymphocyte predominance, low glucose, increased protein | ||||
| Fungal | CSF shows lymphocytic or monocytic pleocytosis, elevated protein, and low glucose | |||
| Cryptococcus neoformans | Test CSF for cryptococcal antigen, stain with India ink | I.V. amphotericin B and fluconazole | ||
| Aspergillus spp. | Galactomannan assay | Voriconazole, amphotericin B | ||
| (1,3)-β-d-glucan assay | ||||
| DNA PCR assay in serum or BAL samples | ||||
| Branching, septate hyphae | ||||
| Candida spp. | Pseudohyphae and budding yeast | Fluconazole, echinocandin, or amphotericin B | ||
| Encephalitis: headache, seizures, focal neurologic deficit, altered mental status, cranial nerve palsy | Cytomegalovirus | PCR-positive CSF | Ganciclovir | |
| MRI scan showing enhancing ventriculoencephalitis | ||||
| Varicella zoster virus | MRI scan showing mixed lesions (ischemic or hemorrhagic infarcts) | Acyclovir, ganciclovir | ||
| Demyelinatinag lesions at gray-white matter junction | ||||
| PCR-positive CSF | ||||
| Human herpes virus 6 | PCR-positive CSF | Ganciclovir or foscarnet | ||
| Focal or diffuse encephalitis | ||||
| Focal brain infections: headaches, seizures, and focal neurologic deficits | Parasite | Toxoplasma gondii | MRI scan showing multiple ring-enhancing lesions, predilection to basal ganglia, thalami, and corticomedullary junction | Pyrimethamine and folinic acid |
| Bacterial | Nocardia asteroides | Gram-positive, weakly acid-fast, branching rod-shaped bacteria | Trimethoprim/sulfamethoxazole and neurosurgical intervention | |
| MRI scan showing single or multiple lesions with contrast enhancement and little mass effect | ||||
| Fungal | Mucormycosis, aspergillosis, Candida, Cryptococcosis | Begin with paranasal sinuses, producing periorbital edema and may invade the intracavernous carotid artery, cerebral artery emboli, mycotic aneurysm, and stroke | Antifungals | |
CSF, cerebrospinal fluid; I.V., intravenous; BAL, bronchoalveolar lavage; MRI, magnetic resonance imaging.
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) is a fatal and cataclysmic condition of the CNS caused by the John Cunningham virus, a human polyoma family virus. The incidence of PML after kidney transplantation is 0.027%, with a median time of 17 months after transplant (25). Visual deficits, mental deficits (cognitive changes, emotional liability, and memory loss), motor weakness, and seizure are the usual complaints in patients with PML. Still, it can be relentlessly progressive and devastating, causing mortality within months to a year. Its spread to the brain results in cerebral white matter demyelination and oligodendrocyte lysis. MRI scans show multifocal, asymmetric lesions in cortical and subcortical areas with a minute or no mass effect or enhancement typically involving parieto-occipital regions (26). Brain biopsy is the gold standard method for diagnosis, but diagnosis can also be made by CSF analysis for the John Cunningham virus DNA using the PCR technique (27). Patients with PML have a fatal outcome and, currently, there is no effective treatment strategy. However, cytarabine and IFNs have been used as an attempt to treat PML with no clear success (28,29).
Drug Toxicity
CNIs
Tacrolimus (Tac) and cyclosporine (CsA) form the backbone of immunosuppression in a KTR. They inhibit calcineurin activation and block IL-2 production. The overall estimated frequency of neurologic adverse effects may vary from 10% to 28% (30). Various published studies have shown that Tac is more frequently and severely associated with neurotoxicity as compared with CsA (31). The timing of CNS side effects is usually within the first month after initiation, and they are more frequent at higher doses; however, side effects can occur even at therapeutic levels (32). The severity of symptoms ranges from mild (such as tremor, ataxia, agitation, confusion, and nightmares) to severe (such as encephalopathy, convulsion, and coma). Visual hallucinations and cortical blindness may also seldom occur in a KTR on CNIs (33) (Table 3). CsA and Tac may have deleterious effects on the PNS. Both the nerve and the muscle can get affected. Cases of axonal, demyelinating, and multifocal demyelinating neuropathy have been reported to be more severe with Tac (34).
Table 3.
Neurologic side effects of calcineurin inhibitors
| Severity | Central Toxicity | Peripheral Toxicity |
| Minor | Insomnia, visual disturbances, headache, and mood changes | Paraesthesias, peripheral neuropathy, and myopathy |
| Major | PRES, akinetic mutism, toxic encephalopathy, and convulsions | Axonal and demyelinating neuropathy, Guillain–Barré syndrome |
PRES, posterior reversible encephalopathy syndrome.
Mechanisms of neurotoxicity are as follows:
Calcineurin is also expressed in several areas of the brain: cerebral cortex, striatum, substantia nigra, cerebellum, and hippocampus. Both CsA and Tac are highly lipophilic and bind to LDL, so they can cross the blood-brain barrier and damage the white matter (35).
CNIs cause an increase in endothelin expression and decrease nitric oxide production. By disrupting endothelin integrity, CsA and Tac gain access to astrocytes and cerebral vascular smooth muscle, invoking vasoconstriction and vasospasm (36).
Disruption of the blood-brain barrier and cytotoxic effects on the vascular endothelium results in leakage of fluid into the interstitium, resulting in vasogenic edema.
CsA and Tac may also cause neurotoxicity by alteration of mitochondrial function and the ensuing apoptotic or necrotic cell death from activation of anaerobic glycolysis, proteases, phospholipases, and the generation of free radicals (37).
Predisposing factors for the development of CNI-induced neurotoxicities are advanced liver failure, low cholesterol levels, elevated CsA or Tac blood levels, hypomagnesemia, and steroids (33). The use of delayed-release formulations, minimum therapeutic doses, strict monitoring of blood levels, and vigilance to pharmacologic interactions may reduce drug-induced neurotoxicity. Sometimes, it may be necessary to change CNIs to mammalian target of rapamycin inhibitors or belatacept in case of severe symptoms that show no improvement.
PRES
The overall estimated incidence of PRES in recipients of solid organ transplants is 0.5%–5%, and is more common with the use of Tac (38). MRI brain scan findings in PRES have been already mentioned above. The characteristic subcortical edema is the result of endothelial cell damage promoted by loss of autoregulation in the posterior circulation. The following criteria may help establish the diagnosis of CNI-associated PRES: (1) clinical features (headache, changes in mental status, seizures, and visual disturbances) after excluding other possible causes such as infection, metabolic disorders, and structural CNS lesions; and (2) CT or MRI brain scan characteristic findings of subcortical white matter lesions (39). This syndrome is potentially reversible and should be diagnosed as early as possible. It usually responds to cessation or lowering the dose of the drug, and additionally by controlling hypertension and convulsions.
Calcineurin-Induced Pain Syndrome
Calcineurin-induced pain syndrome is characterized by incapacitating bilateral leg pain that spares the hip areas. The reported incidence in transplant patients is 2%–14% and usually presents within the first year post-transplantation (40). The most common affected areas are the knee and feet. Symptoms may worsen with physical activity and stress, and may improve with resting. Imaging studies show bone marrow edema in affected areas. The exact mechanism is yet to be elucidated, but it is postulated that CNIs affect sensory neural function by regulating two-pore potassium channels, leading to an alteration in neuronal resting membrane potential. The syndrome is usually self-resolving but, according to some anecdotal reports, calcium channel blockers are beneficial (41).
Corticosteroids
Corticosteroids can cause neuropsychiatric symptoms such as insomnia, impaired concentration, mood changes, irritability, mania, psychosis, and depression. Symptoms usually begin within days to weeks after treatment initiation. With long-term use, the PNS can also be involved and proximal myopathy can occur, which may not completely resolve after cessation of the drug (42).
Mycophenolate Mofetil
Mycophenolate mofetil is rarely associated with neurotoxicity. Headache is one of the few side effects associated with the use of mycophenolate mofetil (43).
Mammalian Target of Rapamycin Inhibitors
Neurotoxicity is infrequent with sirolimus and everolimus. Few cases of reflex sympathetic dystrophy and PRES have been reported with sirolimus and everolimus, respectively (44,45).
Bortezomib
Bortezomib can cause painful sensory neuropathy (46).
Neurologic side effects of immunosuppressants used in kidney transplantation are shown in Table 4.
Table 4.
Neurologic side effects of immunosuppressants used in kidney transplantation
| Drug | Side Effect |
| Calcineurin inhibitors | Mild: tremor, headache, insomnia, vivid dream imagery, photophobia |
| Moderate: visual and cortical disturbances | |
| Severe: encephalopathy, convulsion, coma, and flaccid quadriparesis | |
| Steroids | Insomnia, anxiety, psychosis, myopathy, mania, depression |
| Bortezomib | Distal peripheral neuropathy |
| Mammalian target of rapamycin inhibitor | Sympathetic dystrophy, rarely PRES and possible potentiation of calcineurin-inhibitor toxicity |
| Belatacept | Central neurologic system post-transplant lymphoproliferative disorder |
| Alemtuzumab | Sensorimotor polyneuropathy and myelitis |
| Rituximab | Progressive multifocal leukoencephalopathy |
PRES, posterior reversible encephalopathy syndrome.
Chronic Neurologic Complications
Cerebrovascular Disease
CVAs, including ischemic, hemorrhagic stroke, and transient ischemic attacks, are the most common chronic neurologic complication in a KTR. United States Renal Data System data showed that the incidence of CVA in a KTR is 5% during the first year post-transplant and 9% in the second year (47). A study in 1600 KTRs revealed that 60% of patients died with a functioning graft, and stroke was the second-highest cause of mortality after infections (48). Cerebral hemorrhages can be catastrophic and fatal. Various factors like long-term use of steroids, hypertension, diabetes mellitus, smoking, old age, poor graft function, obesity, dyslipidemia, and peripheral arterial disease contribute to accelerated atherosclerosis and increased risk of stroke in a KTR. Patients with polycystic kidney disease are at a higher risk of developing a hemorrhagic stroke (49). In a KTR with ischemic stroke, other possibilities like fungal infections (aspergillosis and mucormycosis) should also be excluded because the hyphae can invade cerebral arteries with distal embolization. Correction of reversible risk factors like treatment of hypertension and dyslipidemia, cessation of smoking and alcohol, and controlling diabetes is crucial in improving outcomes. The use of anticoagulants to prevent cardioembolic stroke in patients with atrial fibrillation should be considered as per risk/benefit assessment (50).
Cognition
Improvement in cognitive function after kidney transplantation is variable: some studies suggested improvement only to some extent, and others have indicated exacerbation of cognitive decline (51). Impairment in multiple domains like verbal learning, memory, and executive functioning was found to be higher in KTRs as compared with the general population (52). Cognitive impairment adversely affects daily living. Defective memory and executive function may lead to a breach in adherence to immunosuppressive medications. It also diminishes the quality of life and employment rates while escalating hospital admissions, financial burden, morbidity, and mortality. Metabolic and vascular changes due to prolonged exposure to comorbid medical conditions developed during the dialysis period, neurotoxicity from medications such as CNI or steroids, diabetes, higher baseline frailty, and malnutrition are some important factors responsible for cognitive impairment in a KTR (53). Improved graft function results in amelioration of processing speed, convergent thinking, and executive and attention functioning (54). Treatment of cognitive impairment involves comprehensive care and is usually supportive. It includes management of chronic comorbid conditions, treatment of other associated factors such as depression, and optimal effective immunosuppression strategies. Polypharmacy and the use of psychotropic drugs should be deterred.
CNS Malignancy
Various CNS malignancies, including post-transplant lymphoproliferative disorders, oligodendrogliomas, astrocytomas, lymphomas, and glioblastomas, have been reported in previously published literature in post-transplantation settings (55). CNS is involved in approximately 7%–15% of all post-transplant lymphoproliferative disorder cases (56). In a study by Snanoudj et al. (57) of 25 KTRs, the median duration of diagnosis post-transplant was 18 months. In contrast to this, an analysis of 34 patients of primary CNS lymphoma by Cavaliere et al. (58) reported a median time of 4.4 years post-transplantation. The most common presenting symptom was a focal neurologic deficit. Other symptoms are headache and seizures associated with raised intracranial pressure. Less frequently, visual defects and spinal cord lesions may be present. Two critical factors determining the risk of development of primary CNS lymphoma are (1) immunosuppression load of the patient, and (2) Epstein–Barr virus seropositivity. According to some studies, female sex, high lactate dehydrogenase level, poor performance status, and resistance to initial therapy are predictors of inferior survival. Treatment strategies typically involve reduction or cessation of immunosuppression medications and the use of rituximab. Systemic and intrathecal chemotherapy, radiation, and surgery may be used adjunctively according to the stage of malignancy (59).
Conclusion
Neurologic manifestations in a patient after kidney transplantation are a common and frequent cause of mortality and morbidity. It is rewarding to identify the cause and guide the treatment accordingly. Multiple etiologies need to be considered, and infection is the most common cause in post-transplant settings. A good physical examination and history help to identify most of these conditions.
Disclosures
All authors have nothing to disclose.
Funding
None.
Author Contributions
A. Bhalla conceptualized the study and was responsible for formal analysis; V. Bhargava was responsible for software and reviewed and edited the manuscript; V. Bhargava and D. Rana were responsible for resources; A. Gupta was responsible for validation; A. Gupta and D. Rana provided supervision; and P. Meena wrote the original draft.
References
- 1.Zunt JR: Central nervous system infection during immunosuppression. Neurol Clin 20: 1–v, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadi MH, Salarzaei M, Parooie F: Neurological complications after renal transplantation: A systematic review and meta-analysis. Ther Apher Dial 23: 518–528, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Tsokos GC: Systemic lupus erythematosus. N Engl J Med 365: 2110–2121, 2011 [DOI] [PubMed] [Google Scholar]
- 4.McArthur JC, Brew BJ, Nath A: Neurological complications of HIV infection. Lancet Neurol 4: 543–555, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Savica V, Musolino R, Di Leo R, Santoro D, Vita G, Bellinghieri G: Autonomic dysfunction in uremia. Am J Kidney Dis 38[Suppl 1]: S118–S121, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Kalirao P, Pederson S, Foley RN, Kolste A, Tupper D, Zaun D, Buot V, Murray AM: Cognitive impairment in peritoneal dialysis patients. Am J Kidney Dis 57: 612–620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JM, Raps EC: Neurologic complications of transplantation. Neurol Clin 16: 21–33, 1998 [DOI] [PubMed] [Google Scholar]
- 8.De Waele L, Van Gaal P-J, Abramowicz D: Electrolytes disturbances after kidney transplantation. Acta Clin Belg 74: 48–52, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Stott VL, Hurrell MA, Anderson TJ: Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Intern Med J 35: 83–90, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Haughey D, Narsipur SS: Posterior reversible encephalopathy syndrome after renal transplant: a simple solution for a complicated patient. Case Rep Nephrol Dial 5: 20–25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cengiz N, Adibelli Z, Yakupoğlu YK, Türker H: Neurological complications after renal transplantation: a retrospective clinical study. Noro Psikiyatri Arsivi 52: 331–335, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Veer H, Coosemans W, Pirenne J, Monbaliu D: Acute femoral neuropathy: a rare complication after renal transplantation. Transplant Proc 42: 4384–4388, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Li QS, Huo WQ, Nie ZL, Wang HF, Liang PH, Jin FS: Acute femoral neuropathy following renal transplantation: a retrospective, multicenter study in China. Transplant Proc 42: 1699–1703, 2010 [DOI] [PubMed] [Google Scholar]
- 14.van der Voort van Zyp NCMG, Davin JC, Idu M, Aronson DC: [Kidney transplant survival rates and surgical complications in kidney transplants in children; experiences in the Emma Children’s Hospital AMC]. Ned Tijdschr Geneeskd 149: 584–588, 2005 [PubMed] [Google Scholar]
- 15.Dhillon SS, Sarac E: Lumbosacral plexopathy after dual kidney transplantation. Am J Kidney Dis 36: 1045–1048, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Sharma KR, Cross J, Santiago F, Ayyar DR, Burke G 3rd: Incidence of acute femoral neuropathy following renal transplantation. Arch Neurol 59: 541–545, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Lin P, Tian X, Wang X: Seizures after transplantation. Seizure 61: 177–185, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Senzolo M, Ferronato C, Burra P: Neurologic complications after solid organ transplantation [published correction appears in Transpl Int 22: 507, 2009]. Transpl Int 22: 269–278, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Títoff V, Moury HN, Títoff IB, Kelly KM: Seizures, antiepileptic drugs, and CKD. Am J Kidney Dis 73: 90–101, 2019 [DOI] [PubMed] [Google Scholar]
- 20.Fishman JA: Infection in solid-organ transplant recipients. N Engl J Med 357: 2601–2614, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Tunkel AR, Glaser CA, Bloch KC, Sejvar JJ, Marra CM, Roos KL, Hartman BJ, Kaplan SL, Scheld WM, Whitley RJ; Infectious Diseases Society of America : The management of encephalitis: Clinical practice guidelines by the infectious diseases society of America. Clin Infect Dis 47: 303–327, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Fishman JA: Infection in organ transplantation. Am J Transplant 17: 856–879, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Khoury JA, Brennan DC: Infectious complications in kidney transplant recipients: review of the literature. Saudi J Kidney Dis Transpl 16: 453–497, 2005 [PubMed] [Google Scholar]
- 24.Hu JH, Zhao H, Huang YP, Zhang X, Gao HN, Yang MF, Fan J, Ma WH: Opportunistic posttransplantation virus infections in renal transplant recipients. Transplant Proc 43: 3715–3719, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Sarbu N, Shih RY, Jones RV, Horkayne-Szakaly I, Oleaga L, Smirniotopoulos JG: White matter diseases with radiologic-pathologic correlation. Radiographics 36: 1426–1447, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Koralnik IJ: New insights into progressive multifocal leukoencephalopathy. Curr Opin Neurol 17: 365–370, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Kirby JE, Qian Q: Effective use of JC virus PCR for diagnosis of progressive multifocal leukoencephalopathy. J Med Microbiol 58: 253–255, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Aksamit AJ: Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J Neurovirol 7: 386–390, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Sospedra M, Schippling S, Yousef S, Jelcic I, Bofill-Mas S, Planas R, Stellmann JP, Demina V, Cinque P, Garcea R, Croughs T, Girones R, Martin R: Treating progressive multifocal leukoencephalopathy with interleukin 7 and vaccination with JC virus capsid protein VP1. Clin Infect Dis 59: 1588–1592, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anghel D, Tanasescu R, Campeanu A, Lupescu I, Podda G, Bajenaru O: Neurotoxicity of immunosuppressive therapies in organ transplantation. Maedica (Buchar) 8: 170–175, 2013 [PMC free article] [PubMed] [Google Scholar]
- 31.Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS: A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation 63: 977–983, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Bechstein WO: Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int 13: 313–326, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Wijdicks EF: Neurotoxicity of immunosuppressive drugs. Liver Transpl 7: 937–942, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Renard D, Gauthier T, Venetz JP, Buclin T, Kuntzer T: Late onset tacrolimus-induced life-threatening polyneuropathy in a kidney transplant recipient patient. Clin Kidney J 5: 323–326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serkova NJ, Christians U, Benet LZ: Biochemical mechanisms of cyclosporine neurotoxicity. Mol Interv 4: 97–107, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Illsinger S, Janzen N, Lücke T, Bednarczyk J, Schmidt KH, Hoy L, Sander J, Das AM: Cyclosporine A: Impact on mitochondrial function in endothelial cells. Clin Transplant 25: 584–593, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Asai A, Qiu J, Narita Y, Chi S, Saito N, Shinoura N, Hamada H, Kuchino Y, Kirino T: High level calcineurin activity predisposes neuronal cells to apoptosis. J Biol Chem 274: 34450–34458, 1999 [DOI] [PubMed] [Google Scholar]
- 38.McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, Teksam M: Posterior reversible encephalopathy syndrome: Incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 189: 904–912, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Song T, Rao Z, Tan Q, Qiu Y, Liu J, Huang Z, Wang X, Lin T: Calcineurin inhibitors associated posterior reversible encephalopathy syndrome in solid organ transplantation: report of 2 cases and literature review. Medicine (Baltimore) 95: e3173, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grotz WH, Breitenfeldt MK, Braune SW, Allmann KH, Krause TM, Rump JA, Schollmeyer PJ: Calcineurin-inhibitor induced pain syndrome (CIPS): a severe disabling complication after organ transplantation. Transpl Int 14: 16–23, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Coates PTH, Tie M, Russ GR, Mathew TH: Transient bone marrow edema in renal transplantation: a distinct post-transplantation syndrome with a characteristic MRI appearance. Am J Transplant 2: 467–470, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Ciriaco M, Ventrice P, Russo G, Scicchitano M, Mazzitello G, Scicchitano F, Russo E: Corticosteroid-related central nervous system side effects. J Pharmacol Pharmacother 4[Suppl 1]: S94–S98, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Draper HM: Depressive disorder associated with mycophenolate mofetil. Pharmacotherapy 28: 136–139, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Molina MG, Diekmann F, Burgos D, Cabello M, Lopez V, Oppenheimer F, Navarro A, Campistol J: Sympathetic dystrophy associated with sirolimus therapy. Transplantation 85: 290–292, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Tsagalou EP, Anastasiou-Nana MI, Margari ZJ, Vassilopoulos D: Possible everolimus-induced, severe, reversible encephalopathy after cardiac transplantation. J Heart Lung Transplant 26: 661–664, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Bilińska M, Usnarska-Zubkiewicz L, Pokryszko-Dragan A: Bortezomib-induced painful neuropathy in patients with multiple myeloma. Contemp Oncol (Pozn) 17: 421–426, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US Renal Data System (USRDS) : USRDS 2014 Annual Data Report: Volume One: Chronic Kidney Disease in the United States and Volume Two: End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2014 [Google Scholar]
- 48.Shimmura H, Tanabe K, Tokumoto T, Ishida H, Ishikawa N, Miyamoto N, Shimizu T, Shirakawa H, Setoguchi K, Teraoka S, Toma H: Analysis of cause of death with a functioning graft: A single-center experience. Transplant Proc 36: 2026–2029, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Huang ST, Yu TM, Chuang YW, Chung MC, Wang CY, Fu PK, Ke TY, Li CY, Lin CL, Wu MJ, Kao CH: The risk of stroke in kidney transplant recipients with end-stage kidney disease. Int J Environ Res Public Health 16: 326, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seoane-Pillado MT, Pita-Fernández S, Valdés-Cañedo F, Seijo-Bestilleiro R, Pértega-Díaz S, Fernández-Rivera C, Alonso-Hernández Á, González-Martín C, Balboa-Barreiro V: Incidence of cardiovascular events and associated risk factors in kidney transplant patients: A competing risks survival analysis. BMC Cardiovasc Disord 17: 72, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chu NM, Gross AL, Shaffer AA, Haugen CE, Norman SP, Xue QL, Sharrett AR, Carlson MC, Bandeen-Roche K, Segev DL, McAdams-DeMarco MA: Frailty and changes in cognitive function after kidney transplantation. J Am Soc Nephrol 30: 336–345, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta A, Mahnken JD, Johnson DK, Thomas TS, Subramaniam D, Polshak T, Gani I, John Chen G, Burns JM, Sarnak MJ: Prevalence and correlates of cognitive impairment in kidney transplant recipients. BMC Nephrol 18: 158, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radić J, Ljutić D, Radić M, Kovačić V, Dodig-Ćurković K, Šain M: Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am J Nephrol 34: 399–406, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Joshee P, Wood AG, Wood ER, Grunfeld EA: Meta-analysis of cognitive functioning in patients following kidney transplantation. Nephrol Dial Transplant 33: 1268–1277, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott K: Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation 80: 1233–1243, 2005 [DOI] [PubMed] [Google Scholar]
- 56.Au WY, Hung KN, Loong F, Ma SK: Patients presenting with CNS lesions. Case 3. Sequential myeloproliferative disease and glioblastoma multiforme in a renal transplant recipient. J Clin Oncol 21: 4062–4063, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Snanoudj R, Durrbach A, Leblond V, Caillard S, Hurault De Ligny B, Noel C, Rondeau E, Moulin B, Mamzer-Bruneel MF, Lacroix C, Charpentier B: Primary brain lymphomas after kidney transplantation: Presentation and outcome. Transplantation 76: 930–937, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Cavaliere R, Petroni G, Lopes MB, Schiff D; International Primary Central Nervous System Lymphoma Collaborative Group : Primary central nervous system post-transplantation lymphoproliferative disorder: an International Primary Central Nervous System Lymphoma Collaborative Group Report. Cancer 116: 863–870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Al-Mansour Z, Nelson BP, Evens AM: Post-transplant lymphoproliferative disease (PTLD): Risk factors, diagnosis, and current treatment strategies. Curr Hematol Malig Rep 8: 173–183, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]