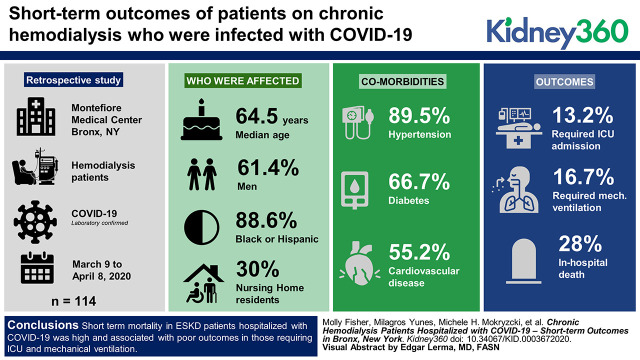
Visual Abstract
Keywords: dialysis, cardiovascular diseases, coronavirus infections, COVID-19, hospital mortality, kidney failure, chronic, New York City, renal dialysis, respiration, artificial, retrospective studies, severe acute respiratory syndrome coronavirus 2
Abstract
Background
Patients with ESKD who are on chronic hemodialysis have a high burden of comorbidities that may place them at increased risk for adverse outcomes when hospitalized with COVID-19. However, data in this unique patient population are limited. The aim of our study is to describe the clinical characteristics and short-term outcomes in patients on chronic hemodialysis who require hospitalization for COVID-19.
Methods
We performed a retrospective study of 114 patients on chronic hemodialysis who were hospitalized with COVID-19 at two major hospitals in the Bronx from March 9 to April 8, 2020 during the surge of SARS-CoV-2 infections in New York City. Patients were followed during their hospitalization through April 22, 2020. Comparisons in clinical characteristics and laboratory data were made between those who survived and those who experienced in-hospital death; short-term outcomes were reported.
Results
Median age was 64.5 years, 61% were men, and 89% were black or Hispanic. A total of 102 (90%) patients had hypertension, 76 (67%) had diabetes mellitus, 63 (55%) had cardiovascular disease, and 30% were nursing-home residents. Intensive care unit (ICU) admission was required in 13% of patients, and 17% required mechanical ventilation. In-hospital death occurred in 28% of the cohort, 87% of those requiring ICU, and nearly 100% of those requiring mechanical ventilation. A large number of in-hospital cardiac arrests were observed. Initial procalcitonin, ferritin, lactate dehydrogenase, C-reactive protein, and lymphocyte percentage were associated with in-hospital death.
Conclusions
Short-term mortality in patients on chronic hemodialysis who were hospitalized with COVID-19 was high. Outcomes in those requiring ICU and mechanical ventilation were poor, underscoring the importance of end-of-life discussions in patients with ESKD who are hospitalized with severe COVID-19 and the need for heightened awareness of acute cardiac events in the setting of COVID-19. Elevated inflammatory markers were associated with in-hospital death in patients with ESKD who were hospitalized with COVID-19.
Introduction
Coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in China in December 2019 (1,2). The global pandemic that followed has grown to millions of cases worldwide (3). The first reported case of confirmed COVID-19 in New York was on March 1, 2020 and, within weeks, New York City quickly became the United States’ epicenter for COVID-19, with >200,000 cases and 17,000 deaths (4).
Montefiore Health System (MHS) has been the largest hospital system in the Bronx providing care to patients hospitalized with COVID-19. MHS admitted its first patient with COVID-19 in early March 2020. As of June 7, 2020, >6000 patients have been hospitalized at MHS for COVID-19. Early reports identified older age and underlying chronic comorbidities as risk factors for more severe disease and death from COVID-19 (4,5). The Bronx consists of a predominantly black and Hispanic population with a disproportionate burden of comorbidities including cardiovascular disease, hypertension, diabetic mellitus, and ESKD (6–9). Compared with the other New York City boroughs, the Bronx had the highest rate of hospitalizations and death from COVID-19 at the end of April (10).
The Montefiore Nephrology Division provides care to >900 patients on chronic hemodialysis in the Bronx, including the majority of hospitalized patients on chronic hemodialysis at Montefiore Medical Center’s two main campuses, Moses and Weiler Hospitals. Data regarding outcomes in patients with ESKD on chronic hemodialysis who are hospitalized for COVID-19 are limited. The aim of our study is to describe the clinical characteristics and short-term outcomes in patients on chronic hemodialysis requiring hospitalization for COVID-19 in the Bronx during the first month of the New York City area outbreak.
Materials and Methods
Study Population
Between March 9 and April 8, 2020, we identified 114 consecutive patients with ESKD receiving chronic hemodialysis who were hospitalized with laboratory-confirmed COVID-19 at Montefiore Medical Center. Patients receiving chronic peritoneal dialysis and kidney transplant recipients were not included in the study. All patients were followed during their hospitalization through April 22, 2020, so that each patient had a minimum of 14 days follow-up for a hospital-based outcome (discharge or in-hospital death). The Albert Einstein College of Medicine institutional review board approved this study. Informed consent was waived and researchers analyzed only de-identified data.
Data from the electronic medical record were reviewed by five nephrologists. Data extracted included demographic information (age, sex, race/ethnicity), dialysis vintage, arteriovenous access, clinical signs and symptoms at presentation to the hospital, clinical comorbidities, and initial vital signs laboratory data. Laboratory tests were performed at the discretion of the treating physician. Laboratory data extracted included white blood cell count, lymphocyte percentage, D-dimer, C-reactive protein (CRP), procalcitonin, lactate dehydrogenase (LDH), ferritin, and IL-6. Information on COVID-19 treatment with hydroxychloroquine and prophylactic or therapeutic anticoagulation were noted. Data on clinical outcomes including intensive care unit (ICU) admission, need for mechanical ventilation, vasopressors, thrombotic events, in-hospital death, discharge, and hospital length of stay were obtained.
Early on, during the abrupt rise in COVID-19 admissions to our hospitals, we provided bedside hemodialysis to all patients infected with SARS-CoV-2 to mitigate transmission of infection within the hospital. Because this required a 1:1 dialysis nurse/patient ratio, the default frequency of hemodialysis was reduced to twice weekly in patients without hyperkalemia or signs of fluid overload. However, these patients were assessed on a daily basis to determine if additional dialysis was needed. By the end of March, we expanded our capacity by opening the inpatient hemodialysis unit 7 days per week, increasing the number of on-call hemodialysis nurses, and cohorting patients on hemodialysis who had COVID-19 on the last shift, which was followed by terminal disinfection of the unit, as per the Centers for Disease Control and Prevention recommendations. As a result, the majority of our patients on chronic hemodialysis who were hospitalized with COVID-19 were dialyzed three times per week.
Study Definitions
A confirmed case of COVID-19 was defined by a positive result on a RT-PCR assay of a specimen collected on nasopharyngeal swab from the Montefiore Virology Laboratory or a commercial laboratory used by Montefiore (Viacor, Lee’s Summit, MO).
Statistical Analyses
Descriptive statistics were used to summarize the data. Categoric variables are presented as counts and percentages. Continuous variables are presented as mean with SD and median with interquartile range (IQR) as appropriate. Comparisons in the clinical characteristics and inflammatory markers between those alive and those who died were performed using independent t tests. Regression analysis was used to determine which normally distributed independent variables influenced dependent variables. SPSS-26 software was used for statistical analysis. Significance was defined as a P value ≤0.05.
Results
Patient Demographic and Clinical Characteristics
The clinical characteristics of the cohort are shown in Table 1. From March 9 to April 8, 2020, 114 patients on chronic hemodialysis were hospitalized with COVID-19 at the two main Montefiore Medical Center campuses. The median age was 64.5 years, 61% were men, and 89% of the patients were black or Hispanic. The most common comorbidities were diabetes mellitus (67%), hypertension (90%), coronary artery disease (55%), and pulmonary disease (35%). Additionally, 31% were nursing-home residents. The median dialysis vintage was 3.2 years and 83% had an arteriovenous fistula or arteriovenous graft. The most common symptoms at initial presentation were shortness of breath (50%) and fever (45%). Less common symptoms included cough, chest pain, and diarrhea. Many patients presented with signs of sepsis on admission, 36% had a temperature ≥100.4°F, 46% had tachycardia, 49% had tachypnea, 12% had leukocytosis, and 22% had leukopenia. Admission chest x-ray findings were notable for bilateral infiltrates in 49 (43%) patients, 23 (20%) had pulmonary edema, and 12 (11%) had bilateral infiltrates and pulmonary edema. Nine (8%) patients had a normal chest x-ray on admission.
Table 1.
Clinical characteristics of patients on chronic hemodialysis who were hospitalized with coronavirus disease 2019
| Clinical Characteristic | Value | |
| n=114 | ||
| Admission location, n (%) | ||
| Home | 79 (69) | |
| Nursing home | 35 (31) | |
| Age, yr, median (IQR) | 64.5 (55 to 73) | |
| Sex, n (%) | ||
| Female | 44 (39) | |
| Male | 70 (61) | |
| Race/ethnicity, n (%) | ||
| Black | 56 (49) | |
| Hispanic | 45 (40) | |
| White | 7 (6) | |
| Asian | 6 (5) | |
| Dialysis vintage, yr, median (IQR) | 3.2 (1.1 to 5.6) | |
| Vascular access, n (%) | ||
| AVF | 83 (73) | |
| AVG | 12 (11) | |
| Central vein catheter (tunneled) | 19 (17) | |
| Clinical comorbidities, n (%) | ||
| Hypertension | 102 (90) | |
| Diabetes mellitus | 76 (67) | |
| Cardiovascular disease | 63 (55) | |
| Stroke | 25 (22) | |
| Pulmonary disease | 40 (35) | |
| Hepatitis C | 19 (17) | |
| Malignancy | 14 (12) | |
| Smoking, former or active | 58 (5) | |
| Home medication, n (%) | ||
| ACEi or ARB | 25 (22) | |
| Symptoms, n (%) | ||
| Shortness of breath | 57 (50) | |
| Fever ≥100.4°F | 51 (45) | |
| Cough | 15 (13) | |
| Chest pain | 13 (11) | |
| Altered mental status | 10 (9) | |
| Hyperkalemia (serum potassium >5.5 meq/L) | 5 (4) | |
| Diarrhea | 6 (5) | |
| Sepsis criteria on admission, n (%) | ||
| Fever ≥100.4°F | 41 (36) | |
| Heart rate >90 beats per minute | 52 (46) | |
| Respiratory rate ≥20 breaths per minute | 56 (49) | |
| White cell count >12,000, (k/μl) | 14 (12) | |
| White cell count <4000, (k/μl) | 25 (22) | |
| Admission vital signs, mean (SD) | ||
| Temperature (°F) | 99.6 (99–100.8) | |
| Heart rate (beats per minute) | 88.5 (75–104) | |
| Mean arterial pressure (mm Hg) | 96.6 (83–112) | |
| Pulse oximetry (% saturation) | 95.3 (5.5) | |
| Body mass index (kg/m2), median (IQR) | 26.9 (23 to 31) | |
| Chest x-ray findings, n (%) | ||
| Bilateral infiltrates | 49 (43) | |
| Focal infiltrate | 21 (18) | |
| Pulmonary edema | 23 (20) | |
| Pulmonary edema and bilateral infiltrates | 12 (11) | |
| Clear | 9 (8) | |
Numbers reported in table are mean (SD), median (IQR), or n (%). IQR, interquartile range; AVF, arteriovenous fistula; AVG, arteriovenous graft; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotension receptor blocker.
Outcomes
Table 2 shows the clinical outcomes of patients on chronic hemodialysis who were hospitalized with COVID-19 during the study period. The majority (87%) of patients were admitted to a general medical floor, 76% received hydroxychloroquine as treatment for COVID-19, and 85% were placed on prophylactic or therapeutic anticoagulation. Ten patients clinically decompensated on the general medical floor and required intubation and transfer to the ICU. A total of 15 (13%) patients required ICU-level care, 19 (17%) patients required mechanical ventilation, and 18 patients (16%) required vasopressors. The predominant modality for RRT in this cohort was intermittent hemodialysis, with the exception of two patients who required continuous RRT and one patient who required sustained low-efficiency hemodialysis due to hemodynamic instability. Eight (7%) patients were found to have a new thrombotic event during their hospitalization. Five patients developed a clotted arteriovenous fistula requiring declotting or placement of a central vein catheter. Two patients had an acute deep venous thrombosis and one patient developed acute splenic infarcts.
Table 2.
Clinical course and outcomes in patients with ESKD on chronic hemodialysis hospitalized with COVID-19
| Clinical Course | Value |
| Hospital admission, n (%) | |
| General medical floor | 99 (87) |
| Intensive care unit | 15 (13) |
| COVID-19 Treatment, n (%) | |
| Hydroxychloroquine | 87 (76) |
| Anticoagulation, n (%) | |
| None | 17 (15) |
| Apixaban | 36 (32) |
| Heparin | 57 (50) |
| Warfarin | 4 (14) |
| Mechanical ventilation according to location, n (%) | 19 (17) |
| Intensive care unit | 14 |
| General medical floor | 5 |
| Vasopressors, n (%) | 18 (16) |
| Renal replacement modality, n (%) | |
| Intermittent hemodialysis | 111 (97) |
| Continuous RRT | 2 (2) |
| Slow low-efficiency hemodialysis | 1 (0.8) |
| New thrombotic events, n (%) | 8 (7) |
| In-hospital death, n (%) | 32 (28) |
| Intensive care unit | 13/15 (87) |
| General medical floor | 19/99 (19) |
| In-hospital death in those requiring mechanical ventilation, n (%) | 18/19 (95) |
| Terminal extubation, n (%) | 6/19 (32) |
| Causes of death, n (%) | |
| Cardiac arrest | 14 (44) |
| Respiratory failure | 14 (44) |
| Anoxic brain injury | 2 (6) |
| Hyperkalemia | 2 (6) |
| Death with a DNR order in place | 17 (53) |
| Hospitalization status in those alive, n (%) | |
| Discharges | 73 (64) |
| Still admitted at time of study | 9 (8) |
| Hospital length of stay (days), median (IQR) | |
| Discharged | 9 (6 to 12.8) |
| Died in hospital | 8 (4 to 14) |
Numbers reported in table are n (%). Hospital length of stay is reported as median (IQR). COVID-19, coronavirus disease 2019; DNR, do not resuscitate; IQR, interquartile range.
In-hospital death occurred in 32 (28%) patients in the total cohort, including 19 patients (19%) admitted to a general medical floor and 13 patients (87%) admitted or transferred to the ICU. (Figure 1, Table 2) The most common causes of death were cardiac arrest and respiratory failure. Of the patients who died, 14 (44%) had a sudden cardiac arrest requiring cardiopulmonary resuscitation, 12 were found to be in asystole, and two patients had ventricular fibrillation arrests. The causes of most cardiac arrests were hemodynamic instability and severe hypoxic respiratory failure. Two patients died from hyperkalemia. Seventeen (53%) patients who died had a “do-not-resuscitate” order in place.
Figure 1.
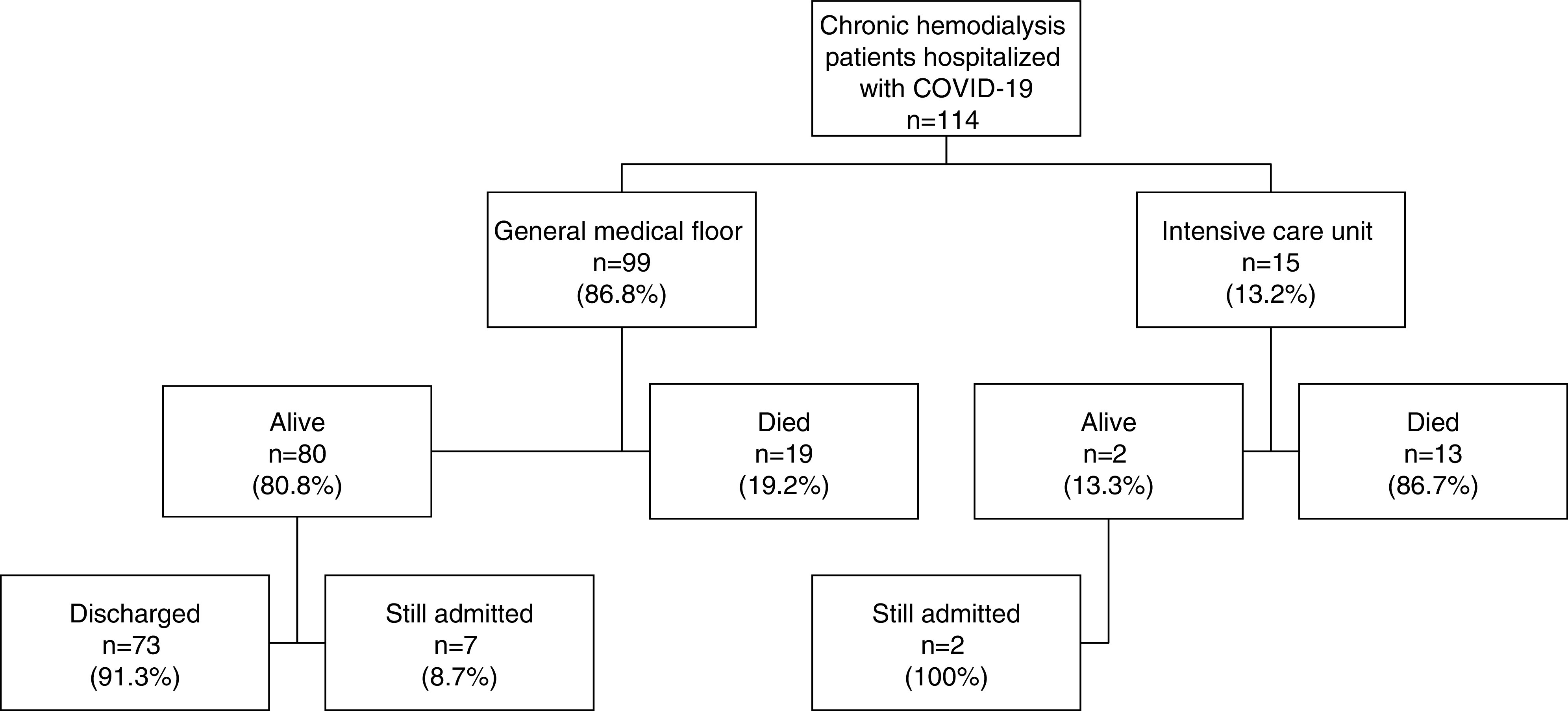
Disposition of hospitalized patients on chronic hemodialysis with coronavirus disease 2019 (COVID-19) during the study period.
A total of 19 patients required mechanical ventilation, including 14 ICU patients and five general medical floor patients, and 18 out of 19 (95%) patients requiring mechanical ventilation died. Six patients were terminally extubated after a family decision to withdraw care due to poor prognosis. The majority of the general medical floor patients that died had a do-not-resuscitate and do-not-intubate order in place at the time of death.
At the end of the study, follow-up was complete on 92% of the cohort. In-hospital death occurred in 28% of patients, 64% had been discharged, and 8% remained hospitalized. The median hospital length of stay for those who were discharged was 9 days (IQR, 6–12.8) and was 8 days (IQR, 4.3–14) for those who died.
Table 3 demonstrates comparisons in the baseline characteristics, initial vital signs, and laboratory data in those alive and those who died in the hospital. Those who died were younger compared with those alive (61 years versus 66 years, respectively). There were no differences in sex, race, dialysis access or vintage, or clinical comorbidities between those who died and those alive. Admission respiratory rate was significantly higher (P=0.03) and oxygen saturation was significantly lower (P=0.04) in those who died (Table 3). A significant difference in initial inflammatory markers was observed in those who died compared with those alive. More specifically, the initial procalcitonin, CRP, LDH, and ferritin were higher, and the lymphocyte percentage was significantly lower, in those who died. (Table 3) Although limited by the extent of missing data, IL-6 concentrations were significantly higher among those who died (n=46/114) (Table 3).
Table 3.
Comparisons in clinical characteristics, initial vital signs, and inflammatory markers in those alive compared with those who died
| Characteristic | Alive | Deceased | P Value |
| Patients, n | 82 | 32 | |
| Age, yr, median (IQR) | 66 (56 to 74) | 61 (48 to 67) | 0.05 |
| Sex, n (%) | |||
| Female | 31 (38) | 13 (41) | 0.8 |
| Male | 51 (62) | 19 (59) | |
| Race/ethnicity, n (%) | |||
| Black | 43 (52) | 19 (59) | 0.4 |
| Hispanic | 32 (82) | 9 (28) | 0.3 |
| White | 3 (57) | 3 (9) | 1.0 |
| Other | 4 (50) | 1 (3) | 0.8 |
| Dialysis vintage (yr), median (IQR) | 3.2 (1 to 6.1) | 3.2 (2.1 to 4.8) | 0.5 |
| Vascular access, n (%) | |||
| AVF | 57 (70) | 26 (81) | 0.2 |
| AVG | 9 (11) | 3 (9) | 0.8 |
| Central vein catheter (tunneled) | 16 (20) | 3 (9) | 0.2 |
| Clinical comorbidities | |||
| Hypertension, n (%) | 73 (89) | 29 (91) | 0.9 |
| Diabetes mellitus, n (%) | 53 (65) | 23 (72) | 0.5 |
| Cardiovascular disease, n (%) | 41 (57) | 16 (50) | 0.8 |
| Stroke, n (%) | 16 (20) | 9 (28) | 0.3 |
| Pulmonary disease, n (%) | 30 (37) | 10 (31) | 0.6 |
| Hepatitis C, n (%) | 14 (17) | 5 (16) | 0.9 |
| Total number of comorbidities, median (IQR) | 3 (3–5) | 3.5 (3–5) | 0.4 |
| Initial vital signs, median (IQR) | |||
| Temperature (°F) | 99.4 (98.4 to 100.8) | 100.3 (98.6 to 101.1) | 0.6 |
| Respiratory rate (breaths/min) | 19 (18 to 22) | 20 (18.3 to 23.5) | 0.03 |
| Heart rate (beats/min) | 88 (74 to 103) | 93 (76.5 to 105.5) | 0.7 |
| Pulse oximetry (%) | 96 (95 to 98) | 96 (90 to 99) | 0.04 |
| Initial laboratory data, median (IQR) | |||
| White cell count (k/µl), n=114 | 5.5 (3.9 to 7.6) | 6.7 (4.7 to 9.5) | 0.6 |
| Lymphocyte (%), n=114 | 16 (9.5 to 22) | 9.5 (6.8 to 14.3) | 0.002 |
| Procalcitonin (ng/ml), n=60 | 2.3 (1 to 6.8) | 7 (1.5 to 11.7) | 0.06 |
| C-reactive protein (mg/dl), n=102 | 9.3 (3.8 to 18.9) | 14.2 (10.2 to 27) | 0.005 |
| D-dimer (µ/ml), n=39 | 0.3 (1.9 to 4.6) | 1.4 (0.9 to 6.1) | 0.5 |
| LDH (U/L), n=106 | 329 (258 to 449) | 487.5 (346 to 613) | 0.05 |
| Ferritin (ng/ml), n=83 | 2880 (1698 to 4512) | 3812 (2839 to 8311) | 0.005 |
| IL-6 (pg/ml), n=46 | 48.2 (22.8 to 148.9) | 132 (66.3 to 234.4) | 0.04 |
Numbers reported in table are n (%). Laboratory data are reported as median (IQR) or n (%). IQR, interquartile range; AVF, arteriovenous fistula; AVG, arteriovenous graft; LDH, lactate dehydrogenase.
Figure 2 illustrates the initial and peak inflammatory markers for patients alive compared with those who died. In-hospital death was associated with higher initial procalcitonin, CRP, LDH, and ferritin, as well as peak procalcitonin LDH levels.
Figure 2.
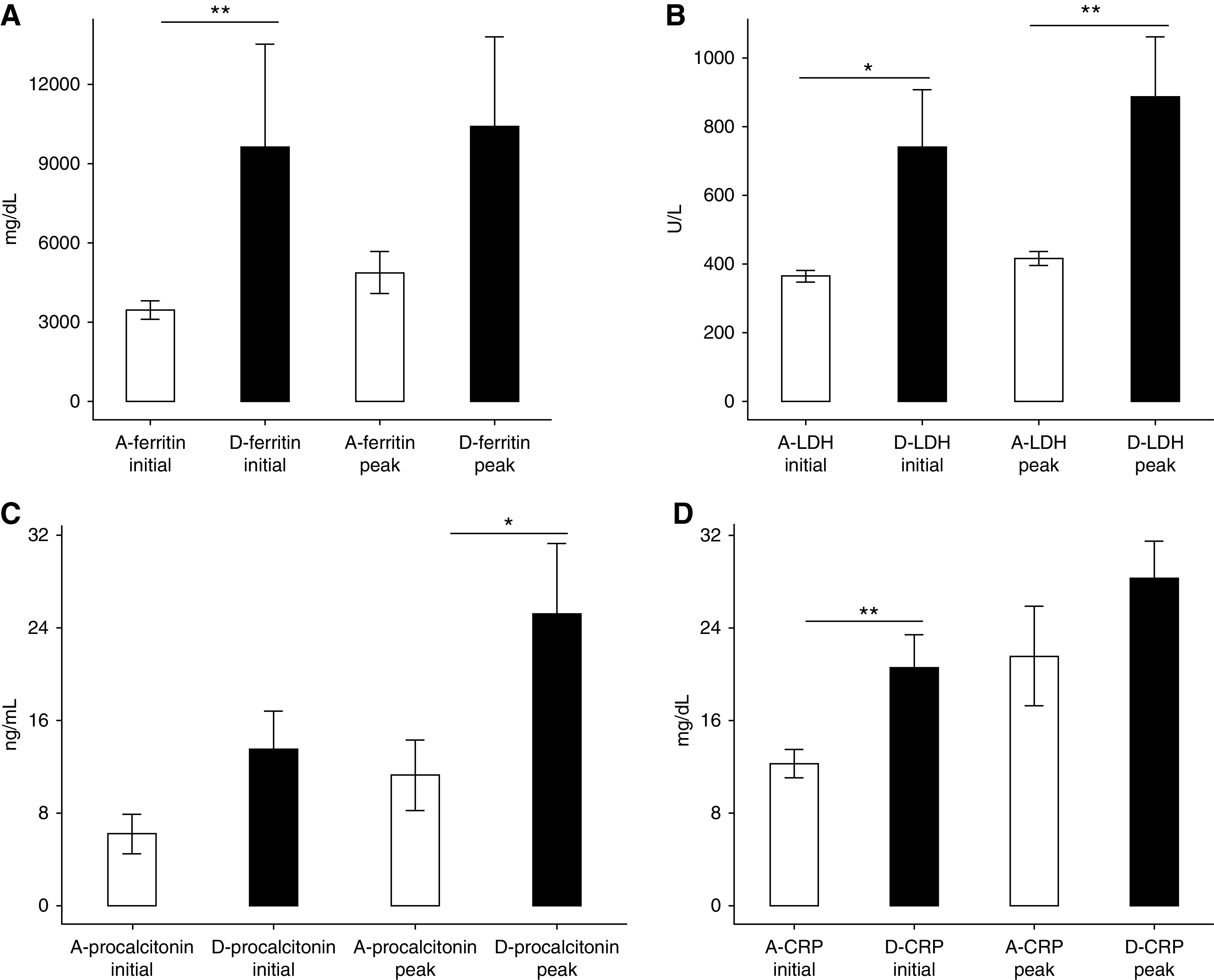
Differences in inflammatory markers in those alive compared with those who died during hospitalization, mean with SEM. (A) Initial ferritin was higher in those who died (**P=0.005). (B) Initial and peak lactate dehydrogenase (LDH) were higher in those who died (*P=0.045, **P=0.007). (C) Peak procalcitonin was higher in those who died (*P=0.05). (D) Initial C-reactive protein (CRP) was higher in those who died (**P=0.005). A, alive; D, died.
Discussion
Our study describes the clinical characteristics and short-term outcomes of 114 patients with ESKD on chronic hemodialysis who required hospitalization for COVID-19 at two major hospitals in the Bronx during the first month of the COVID-19 surge in New York City. The COVID-19–related in-hospital death rate observed in patients on chronic hemodialysis was higher than that observed in patients without ESKD who were hospitalized with COVID-19 at our medical center between March 11 and April 26, 2020 (28% versus 23%, respectively) (11). In-hospital death was high in hospitalized patients on chronic hemodialysis who required ICU admission and/or mechanical ventilation (87% and 95%, respectively). Several admission inflammatory markers were associated with in-hospital death including procalcitonin, CRP, LDH, and ferritin.
Inflammatory markers were higher in patients with ESKD on chronic hemodialysis who died compared with those who survived. A robust immune response leading to cytokine storm is one proposed mechanism of SARS-CoV-2 leading to severe illness and death. When compared with healthy controls, patients infected with SARS-CoV-2 had increased concentrations of proinflammatory cytokines (5). Huang et al. (5) reported elevated levels of serum granulocyte cell-stimulating factor, interferon-inducible protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein 1A, and TNFα in patients with COVID-19 requiring ICU admission, suggesting cytokine storm was associated with disease severity. A single-center study in Wuhan of 150 patients with COVID-19 demonstrated higher ferritin, LDH, and IL-6 were associated with reduced survival (12). In addition, previous coronavirus outbreaks with SARS and Middle East respiratory syndrome reported an association between elevated proinflammatory cytokine levels and severity of pulmonary inflammation and lung injury (13,14). Our findings demonstrate elevated inflammatory markers are associated with more severe disease and mortality in patients with ESKD who are on chronic hemodialysis and require hospitalization for COVID-19. Trends in these markers may help risk stratify those at risk of poor outcomes and inform treatment decisions regarding aggressive medical treatment versus end-of-life care.
Second only to cardiovascular disease, infections are a leading cause of death in the ESKD population (15). Uremia is associated with alterations in primary host defense mechanisms which may increase the risk of bacterial infections (16–22). Septicemia is the most common reason for hospitalization in patients with ESKD; it is responsible for nearly 81,000 admissions and 14,500 readmissions in 2016 (18). Furthermore, annual death rates due to pneumonia and sepsis are significantly higher in patients on dialysis compared with the general population. Patients on dialysis who are 65–74 years old have a tenfold higher risk of death from pneumonia and 100-fold higher risk of death from sepsis (23). Uremia is associated with impairment of granulocyte and lymphocyte function which may alter the immune response to SARS-CoV-2 infection (14–19). Initial studies reported increased mortality in patients with underlying comorbidities, such as hypertension and diabetes mellitus, which are common in the ESKD population (24). Therefore, there has been significant concern within the nephrology community that patients on hemodialysis with COVID-19 will be at greater risk for poor outcomes. Interestingly, a milder disease course was observed in a single-center study from China of 37 patients on hemodialysis in which no patients required ICU and no deaths were directly attributed to COVID-19 pneumonia (Y. Ma, B. Diao, X. Lv, J. Zhu, W. Liang, L. Liu, et al.: 2019 novel coronavirus disease in hemodialysis [HD] patients: report from one HD center in Wuhan, China. Medrxiv, 2019; doi:10.1101/2020.02.24.20027201). In that study, lower serum levels of inflammatory cytokines were observed in patients with ESKD compared to patients without ESKD, suggesting a weakened immune response may mitigate cytokine storm and its deleterious effects (Y. Ma, B. Diao, X. Lv, J. Zhu, W. Liang, L. Liu, et al.: 2019 novel coronavirus disease in hemodialysis [HD] patients: report from one HD center in Wuhan, China. Medrxiv, 2019; doi:10.1101/2020.02.24.20027201). However, our data suggest that a subset of hospitalized patients on chronic hemodialysis develop cytokine storm and, in these patients, this is associated with poor prognosis and high in-hospital mortality rate.
Many of the deaths in our hospitalized COVID-19 chronic hemodialysis cohort were due to cardiovascular events, including cardiac arrests due to arrhythmias and hemodynamic instability. For two patients, hyperkalemia was a contributory factor. Similarly, in a single-center study from Wuhan of patients on chronic hemodialysis with COVID-19, six of 37 (16%) deaths were due to cardiovascular or cerebrovascular causes (Y. Ma, B. Diao, X. Lv, J. Zhu, W. Liang, L. Liu, et al.: 2019 novel coronavirus disease in hemodialysis [HD] patients: report from one HD center in Wuhan, China. Medrxiv, 2019; doi:10.1101/2020.02.24.20027201). These deaths were felt to be due to complications from hyperkalemia, caused by reduced hemodialysis attendance due to fear of viral exposure. This highlights the need to closely monitor serum potassium levels, educate patients about a low potassium diet, and use prophylactic potassium binders. Compared with the general population, patients on dialysis have a higher burden of cardiovascular disease, including a ten- to 30-fold increase in cardiovascular-related mortality (25). In addition to traditional risk factors, respiratory viral illnesses are associated with cardiovascular morbidity and mortality (25). Epidemiologic studies of influenza have demonstrated an increased risk of acute myocardial infarction during viral infection (26–29). In a case series of 364 hospitalizations for acute myocardial infarction, there was a sixfold higher incidence of admissions for acute myocardial infarction during the 7-day period after laboratory-confirmed influenza infection compared with a control period 1 year before or after influenza (26). Although the pathophysiology is unclear, respiratory viruses may elicit direct cardiac damage or indirect damage through systemic effects via inflammatory cytokines and prothrombotic changes (29). Similarly, severe COVID-19 has been shown to be associated with myocarditis due to direct viral-related inflammation, microvascular thrombotic events, and cardiogenic shock (30,31). In a study of 416 patients who were hospitalized with COVID-19, almost 20% had evidence of myocardial injury which was associated with >50% in-hospital mortality (30). More than half of our ESKD cohort had cardiovascular disease, underscoring the need for vigilance in this vulnerable population hospitalized with COVID-19.
Single-center studies from China and Europe have reported in-hospital death ranging from 16% to 41% among patients with ESKD who are hospitalized with COVID-19 (24,32–35). Outcomes of COVID-19 in the United States ESKD population are limited. Two of the first reported COVID-19 deaths in the United States were in patients on hemodialysis in the State of Washington (36). A recent case series of 59 patients on dialysis from a New York City medical center reported in-hospital death was 31%, and 75% in those requiring mechanical ventilation (37). Our population is unique in that it is a predominantly minority population, consisting of mostly black and Hispanic patients. Despite worse outcomes reported in minority populations and a higher case fatality rate in the Bronx early on in the New York City case surge, the in-hospital mortality of 28% in our primarily black and Hispanic ESKD cohort was similar to that reported in white and Asian ESKD populations.
Our study’s strengths include its relatively large sample size to date of patients with ESKD who were hospitalized with COVID-19 and its minority-predominant population that has not previously been reported on. We were also able to account for follow-up of the initial hospitalization to a hospital-based outcome of death or discharge for the majority of the cohort. Limitations of our study include (1) inability to assess out-of-hospital deaths, (2) inflammatory marker tests were not performed on all patients nor at scheduled time intervals due to the retrospective observational study design, and (3) inability to adequately evaluate racial differences due to a predominantly minority population with very few white patients. Finally, this study is limited by short follow-up period. Long-term outcomes after COVID-19 infection in patients with ESKD are unknown and require further investigation.
Patients with ESKD receiving chronic hemodialysis who require hospitalization for COVID-19 are at increased risk for adverse short-term outcomes, including cardiovascular-related death. In-hospital mortality approaches 100% for patients on chronic hemodialysis with COVID-19 who require mechanical ventilation and should be considered when addressing goals of care, advance directives, and end-of-life discussions in those patients with their health care proxy. Elevated inflammatory markers correlate with disease severity and are associated with in-hospital death in this population.
Disclosures
L. Golestaneh reports other from DaVita, during the conduct of the study, other from Horizon Pharmaceuticals, and other from Cardiovascular Research Institute, outside the submitted work. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
We would like to acknowledge the dialysis nurses, physician extenders, nephrology fellows, and faculty for their hard work in providing excellent care to patients with COVID-19 during these unprecedented times. We would also like to acknowledge Dr. Victor J. Pai for his assistance with presentation of laboratory data.
Author Contributions
E. Alahiri, M. Coco, M. Fisher, M. Mokrzycki, and M. Yunes were responsible for data curation; M. Coco was responsible for software; M. Coco and M. Fisher were responsible for methodology and project administration; M. Coco, M. Fisher, and M. Mokrzycki provided supervision; M. Coco, M. Fisher, L. Golestaneh, and M. Mokrzycki conceptualized the study; M. Coco, M. Fisher, M. Mokrzycki, and M. Yunes wrote the original draft; M. Coco and L. Golestaneh were responsible for formal analysis; M. Fisher was responsible for investigation and resources; M. Fisher, M. Mokrzycki, and M. Yunes were responsible for visualization; and all authors reviewed and edited the manuscript.
Footnotes
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team : A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19 : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John Hopkins Coronavirus Resource Center: COVID 19 United States cases by county, 2020. Available at: https://coronavirus.jhu.edu/us-map. Accessed June 7, 2020
- 4.Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, Prill M, Chai SJ, Kirley PD, Alden NB, Kawasaki B, Yousey-Hindes K, Niccolai L, Anderson EJ, Openo KP, Weigel A, Monroe ML, Ryan P, Henderson J, Kim S, Como-Sabetti K, Lynfield R, Sosin D, Torres S, Muse A, Bennett NM, Billing L, Sutton M, West N, Schaffner W, Talbot HK, Aquino C, George A, Budd A, Brammer L, Langley G, Hall AJ, Fry A: Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019— COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 69: 458–464, 2020. Available at: https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm. Accessed June 6, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B: Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet 395: 496, 2020]. Lancet 395: 497–506, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.United States Renal Data System (USRDS): USRDS Renal Data Extraction and Referencing (RenDER) System. Available at: https://www.usrds.org/render/xquery_result.phtml. Accessed June 8, 2020
- 7.Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, Hargreaves MK, Blot WJ: Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health 97: 2260–2267, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr., Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ; AASK Study InvestigatorsCRIC Study Investigators : APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med 369: 2183–2196, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Census Bureau: QuickFacts, Bronx County (Bronx Borough), New York. 2010. Available at: https://www.census.gov/quickfacts/fact/table/bronxcountybronxboroughnewyork/RHI225218#RHI225218. Accessed June 8, 2020
- 10.Wadhera RK, Wadhera P, Gaba P, Figueroa JF, Joynt Maddox KE, Yeh RW, Shen C: Variation in COVID-19 hospitalizations and deaths across New York city boroughs. JAMA 323: 2192–2195, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns T, et al.: Acute kidney injury in hospitalized patients with and without COVID-19 infection: A comparison study. J Am Soc Nephrol 31: 2145–2157, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruan Q, Yang K, Wang W, Jiang L, Song J: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China [published correction appears in Intensive Care Med 46: 1294–1297, 2020]. Intensive Care Med 46: 846–848, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, Lit LC, Hui DS, Chan MH, Chung SS, Sung JJ: Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 136: 95–103, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA: MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 104: 8–13, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Renal Data System (USRDS): 2018 USRDS Annual Data Report Volume 2: ESRD in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018. Available at: https://www.usrds.org/2018/download/2018_Volume_2_ESRD_in_the_US.pdf. Accessed June 23, 2020 [Google Scholar]
- 16.Girndt M, Sester M, Sester U, Kaul H, Köhler H: Molecular aspects of T- and B-cell function in uremia. Kidney Int Suppl 78: S206–S211, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Girndt M, Sester U, Sester M, Kaul H, Köhler H: Impaired cellular immune function in patients with end-stage renal failure. Nephrol Dial Transplant 14: 2807–2810, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Vaziri ND, Pahl MV, Crum A, Norris K: Effect of uremia on structure and function of immune system. J Ren Nutr 22: 149–156, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hörl WH: Neutrophil function and infections in uremia. Am J Kidney Dis 33: xlv–xlviii, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Lewis SL, Van Epps DE: Neutrophil and monocyte alterations in chronic dialysis patients. Am J Kidney Dis 9: 381–395, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Schaier M, Leick A, Uhlmann L, Kälble F, Morath C, Eckstein V, Ho A, Mueller-Tidow C, Meuer S, Mahnke K, Sommerer C, Zeier M, Steinborn A: End-stage renal disease, dialysis, kidney transplantation and their impact on CD4+ T-cell differentiation. Immunology 155: 211–224, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoen B, Paul-Dauphin A, Hestin D, Kessler M: EPIBACDIAL: A multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol 9: 869–876, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Jaber BL: Bacterial infections in hemodialysis patients: pathogenesis and prevention. Kidney Int 67: 2508–2519, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study [published correction appears in Lancet 395: 1038, 2020]. Lancet 395: 1054–1062, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American heart association councils on kidney in cardiovascular disease, high blood pressure Research, clinical cardiology, and epidemiology and prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kwong JC, Schwartz KL, Campitelli MA, Chung H, Crowcroft NS, Karnauchow T, Katz K, Ko DT, McGeer AJ, McNally D, Richardson DC, Rosella LC, Simor A, Smieja M, Zahariadis G, Gubbay JB: Acute myocardial infarction after laboratory-confirmed influenza infection. N Engl J Med 378: 345–353, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Madjid M, Miller CC, Zarubaev VV, Marinich IG, Kiselev OI, Lobzin YV, Filippov AE, Casscells SW 3rd: Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: Results from 8 years of autopsies in 34,892 subjects. Eur Heart J 28: 1205–1210, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warren-Gash C, Smeeth L, Hayward AC: Influenza as a trigger for acute myocardial infarction or death from cardiovascular disease: A systematic review. Lancet Infect Dis 9: 601–610, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Nguyen JL, Yang W, Ito K, Matte TD, Shaman J, Kinney PL: Seasonal influenza infections and cardiovascular disease mortality. JAMA Cardiol 1: 274–281, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C: Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China [published online ahead of print Mar 25, 2020]. JAMA Cardiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z: Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) [published online ahead of print Mar 27, 2020]. JAMA Cardiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong F, Tang H, Liu L, Tu C, Tian JB, Lei CT, Liu J, Dong JW, Chen WL, Wang XH, Luo D, Shi M, Miao XP, Zhang C: Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China [published online ahead of print May 8, 2020]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarpioni R, Manini A, Valsania T, De Amicis S, Albertazzi V, Melfa L, Ricardi M, Rocca C: Covid-19 and its impact on nephropathic patients: the experience at ospedale “Guglielmo da Saliceto” in piacenza. G Ital Nefrol 37: 2020–vol2, 2020 [PubMed] [Google Scholar]
- 34.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Pola A, Maffei C, Possenti S, Piva S, Latronico N, Focà E, Castelli F, Gaggia P, Movilli E, Bove S, Malberti F, Farina M, Bracchi M, Costantino EM, Bossini N, Gaggiotti M, Scolari F; Brescia Renal COVID Task Force : Management of patients on dialysis and with kidney transplant during SARS-COV-2 (COVID-19) Pandemic in Brescia, Italy. Kidney Int Rep 5: 580–585, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goicoechea M, Sanchez Camara LA, Macias N, Munoz de Morales A, Gonzalez Rojas A, Bascunana A, Arroyo D, Vega A, Abad S, Verde E, Garcia Prieto AM, Verdalles U, Barbieri D, Delgado AF, Carbayo J, Mijaylova A, Acosta A, Melero R, Tejedor A, Rodriguez Benitez P, Perez de Jose A, Rodriguez Ferraro ML, Ananya F, Rengel M, Barraca D, Luño J, Aragoncillo I: COVID-19: Clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int 98: 27–34, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M: Characteristics and outcomes of 21 critically Ill patients with COVID-19 in Washington state. JAMA 323: 1612–1614, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valeri AM, Robbins-Juarez SY, Stevens JS, Wooin A, Rao MK, Radhakrishnan J, Gharavi AG, Mohan S, Husain SA: Presentation and outcomes of patients with ESKD and COVID-19 [published online ahead of print May 28, 2020]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]