Introduction
AKI has been observed in patients infected with the novel coronavirus severe acute respiratory syndrome coronavirus 2 that causes coronavirus disease 2019 (COVID-19). Recent studies in the United States have reported an incidence of AKI in the context of COVID-19 around 25%–50% of cases requiring hospitalization (1,2; L. Chan, et al, unpublished observations). Nevertheless, data remain scarce about specific characteristics of coronavirus disease 2019–associated AKI (CoV-AKI). Microscopic examination of the urinary sediment (MicrExUrSed) is a reliable clinical tool of diagnostic and prognostic value in the assessment of AKI (3–7). To date, there has been no report comprehensively describing findings of MicrExUrSed in specimens from patients with CoV-AKI. We hypothesized that MicrExUrSed may provide diagnostic information that could help in elucidating the pathogenic mechanisms of kidney injury in CoV-AKI.
Materials and Methods
We conducted a prospective observational study in patients seen in the inpatient nephrology consultation service with a diagnosis of Kidney Disease Improving Global Outcomes AKI stage ≥1 and positive nasopharyngeal swab test for severe acute respiratory syndrome coronavirus 2 RNA over a 1-month period. This study was an ancillary study of a previously reported larger cohort of 161 patients with CoV-AKI (1). Because of concerns of health care worker exposure to infected patients and rationale use of personal protective equipment (PPE), MicrExUrSed was not systematically pursued as it is routinely done outside the context of COVID-19. Nevertheless, MicrExUrSed was attempted whenever there was a suspicion for an intrinsic cause of AKI that could affect medical management and/or in clinical scenarios that were confounded by more than one possible etiology of AKI. Thus, in selected cases, urine specimens were collected and handled with PPE to perform MicrExUrSed under bright-field, dark-field, and phase contrast microscopy. At least three operators inspected each slide. Slides were assessed for significant presence of white blood cells (≥2+ dipstick, six or more per low-power field [LPF]), red blood cells (≥2+ dipstick, eight or more per LPF), acanthocytes, granular casts (GCs), renal tubular epithelial cell casts (RTECCs), and waxy casts (WxCs). Slides were assigned to a category of acute tubular injury (ATI) on the basis of either a Perazella (3,5) cast score ≥2 or a Chawla (4) cast score ≥3. Sternheimer–Malbin stain was used to enhance the visualization of casts and cells (8). The study was approved by an institutional board review with waiver of informed consent, and it was conducted in accordance with the Declaration of Helsinki.
Results
Among 161 cases of AKI, MicrExUrSed was performed in 20 (12.4%) patients (Table 1). Anuria and contact precautions related to COVID-19 were barriers to obtain more urine specimens. Eighteen (90%) patients were black, 13 (65%) were men, and the median age was 64 (49–88) years. The median serum creatinine at the time of MicrExUrSed was 4.5 (2.6–17.8) mg/dl. Seven (35%) patients had nephrotic-range proteinuria (Table 1). GCs were observed on 17 (85%) cases, of which 16 (80%) had “muddy” brown granular casts (MBGCs). A median of 5 MBGCs per LPF (1–20) were found in a median of 40% (10%–95%) of LPFs (Figure 1). WxCs were found in ten (50%) cases with a median of two (one to five) per LPF, all of whom had MBGCs also present. RTECCs were found in four (20%) cases with a median of one (one to four) per LPF (Figure 1). Two additional patients were reported by automated microscopy from the hospital laboratory to have 6 and 15 GCs per LPF (because they were not corroborated, those cases were not included in our cohort). Altogether, ATI score was assigned to 17 of 20 (85%) patients, of which 8 (40%) had AKI attributable to a form of ATI (ischemic [n=5]: hemodynamic instability or sustained hypovolemia; toxic [n=2]: rhabdomyolysis, radiocontrast; or ischemic/toxic [n=1]: hemodynamic instability and vancomycin) (Table 1). In addition, three (15%) patients had biopsy-proven ATI along with collapsing glomerulopathy. Thus, a total of 11 (55%) patients in the cohort had either clinical or histologic evidence on ATI matching the MicrExUrSed ATI findings (11 of 17; 65%). The remaining six patients with MicrExUrSed findings of ATI had been categorized as unexplained AKI given the lack of identifiable cause (Table 1). Of the three cases without MicrExUrSed evidence of ATI, acanthocytes were found in one patient with presumptive proliferative endocapillary GN (low complement C4 and positive antinuclear antibody; patient died without kidney biopsy). The remaining two cases without MicrExUrSed evidence of ATI had presented with clinical scenarios suggestive of ischemic (n=1) or ischemic/toxic ATI (n=1). In terms of cellularity, significant numbers of white blood cells and red blood cells were observed in ten (50%) and five (25%), respectively.
Table 1.
Demographic and clinical characteristics of patients with coronavirus disease 2019 and AKI with a urinary sediment specimen inspected by microscopy
| Patient | Age, yr | Sex | Race/Ethnicity | Kidney Function | Clinical Diagnosis (Cause of AKI) | Potential Etiology Culprit | Urine Studies | |||||||||
| Baseline sCr, mg/dl; eGFR, ml/min per 1.73 m2 | Type of AKI | sCr at or before Urine Microscopy, mg/dl | UPCR, g/g | Coarse Granular Casts | Waxy Casts | Renal Tubular Epithelial Cell Casts | Blood, cells per hpf | WBC, cells per hpf | Perazella Scorea | Chawla Scoreb | ||||||
| 1 | 72 | M | Black | 2.1–2.6; 27–36 | AKI over CKD 3b–4 | 3.7 | Ischemic ATI | Hemodynamic instability (large reduction in SBP) | 9.24 | c | c | d | 15 | 5 | 2 | 3 |
| 2 | 62 | W | Black | 6.5–7.3; 6–7 | AKI over CKD 5 | 17.8 | Ischemic ATI | Sustained volume depletion (poor oral intake, diarrhea) | 13.5 | e | 0 | 0 | 12 | 4 | 2 | 3 |
| 3 | 58 | M | Black | 3.1–3.2; 23–24 | AKI over CKD 4 | 5.6 | Ischemic ATI | Sustained volume depletion (hypernatremia, poor oral intake) | 0.82 | e | d | c | 1 | 8 | 3 | 4 |
| 4 | 57 | M | Black | 1.4–1.5; 58–65 | AKI over CKD 2–3a | 2.7 | Ischemic ATI | Hemodynamic instability (large reduction in SBP) | 0.69 | c | d | 0 | 13 | 3 | 1 | 3 |
| 5 | 49 | W | Black | 0.7; >100 | de novo AKI | 2.7 | Ischemic/toxic ATI | Hemodynamic instability (large reduction in SBP), vancomycin | 0.91 | 0 | 0 | 0 | 3 | 1 | 0 | 1 |
| 6 | 59 | M | Black | 4.4–4.7; 13–14 | AKI over CKD 5 | 15.1 | — | ? Sustained volume depletion (poor oral intake) | 7.25 | c | c | 0 | 0 | 2 | 2 | 3 |
| 7 | 64 | W | Black | 1.5–1.6; 39–42 | AKI over CKD 3b | 7.5 | Collapsing glomerulopathy + ATI | De novo glomerular disease | 4.58 | e | d | 0 | 1 | 6 | 2 | 3 |
| 8 | 53 | M | White | 1.6f; 45 | De novo AKIg | 1.9 | Toxic ATI | Rhabdomyolysis | 0.48 | c | 0 | 0 | 1 | 6 | 2 | 3 |
| 9 | 82 | M | Black | 3.8f; 16 | De novo AKIg | 4.5 | — | Unexplained AKI | 3.62 | c | d | 0 | 6 | 9 | 1 | 3 |
| 10 | 88 | M | Black | 1.7; 41 | AKI over CKD 3b | 6.8 | Ischemic ATI | Hemodynamic instability (rapid atrial fibrillation) | 1.11 | e | c | 0 | 7 | 1 | 2 | 3 |
| 11 | 68 | W | Black | 0.9; 76 | De novo AKI | 4.3 | Proliferative GN | De novo glomerular disease | 1.86 | 0 | 0 | 0 | 87h | 27 | 1 | 1 |
| 12 | 63 | M | Black | 1.2–1.3; 62–75 | De novo AKI | 9.9 | Collapsing glomerulopathy + ATI | De novo glomerular disease | 12.7 | d | 0 | 0 | 2 | 1 | 1 | 2 |
| 13 | 53 | M | Black | 1.1–1.5; 52–89 | AKI over CKD 2–3a | 7.8 | Ischemic/toxic ATI | Hemodynamic instability (large reduction in SBP), vancomycin | 0.5 | c | c | d | 4 | 0 | 2 | 3 |
| 14 | 81 | M | Black | 1.6f 46 | De novo AKIg | 3.3 | — | Unexplained AKI | 0.25 | d | 0 | 0 | 1 | 1 | 1 | 2 |
| 15 | 55 | W | White | 1.0 73 | De novo AKI | 3.5 | Ischemic ATI | Hemodynamic instability (hypotension, shock) | 0.43 | 0 | 0 | 0 | 3 | 100 | 0 | 1 |
| 16 | 63 | W | Black | 1.7–1.9; 32–37 | AKI over CKD 3b | 5.7 | Toxic ATI | IV radiocontrast | 0.12 | e | d | d | 2 | 100 | 2 | 3 |
| 17 | 74 | M | Black | 1.2–1.5; 52–69 | AKI over CKD 2–3a | 4.8 | — | Unexplained AKI | 0.34 | e | 0 | 0 | 2 | 39 | 2 | 3 |
| 18 | 73 | M | Black | 1.2–1.4; 58–69 | AKI over CKD 2–3a | 4.5 | — | Unexplained AKI | 1.31 | c | 0 | 0 | 8 | 0 | 1 | 3 |
| 19 | 65 | W | Black | 1.3; 50 | AKI over CKD 3a | 2.6 | Collapsing glomerulopathy + ATI | De novo glomerular disease | 13.6 | e | 0 | 0 | 2 | 13 | 2 | 3 |
| 20 | 71 | M | Black | 1.2–1.4; 58–70 | AKI over CKD 2–3a | 4.2 | — | Unexplained AKI | 3.15 | c | d | 0 | 2 | 17 | 2 | 3 |
CKD was defined per Kidney Disease Improving Global Outcomes guidelines. sCr, serum creatinine; UPCR, urine protein-creatinine ratio; hpf, high-power field; WBC, white blood cell; M, man; ATI, acute tubular injury; SBP, systolic BP; W, woman; —, undetermined; IV, intravenous.
Perazella score zero points: zero casts or zero renal tubular epithelial (RTE) cells; one point each: one to five casts per low-power field (lpf) or one to five RTE cells per hpf; two points each six or more casts per lpf or more than six RTE cells per hpf.
Chawla score grade 1: none (no casts or RTE); grade 2: at least one cast or RTE cell cast seen but <10% of lpf; grade 3: many casts and RTE cell casts seen on >10% but <90% of lpf; grade 4: sheets of muddy brown casts, casts, and RTE cells casts seen on >90% of lpf.
>10%–50% of lpf with presence of casts.
>0%–10% of lpf with presence of casts.
>50% of lpf with presence of casts.
Serum creatinine on admission.
Unknown baseline kidney function.
Acanthocytes seen.
Figure 1.
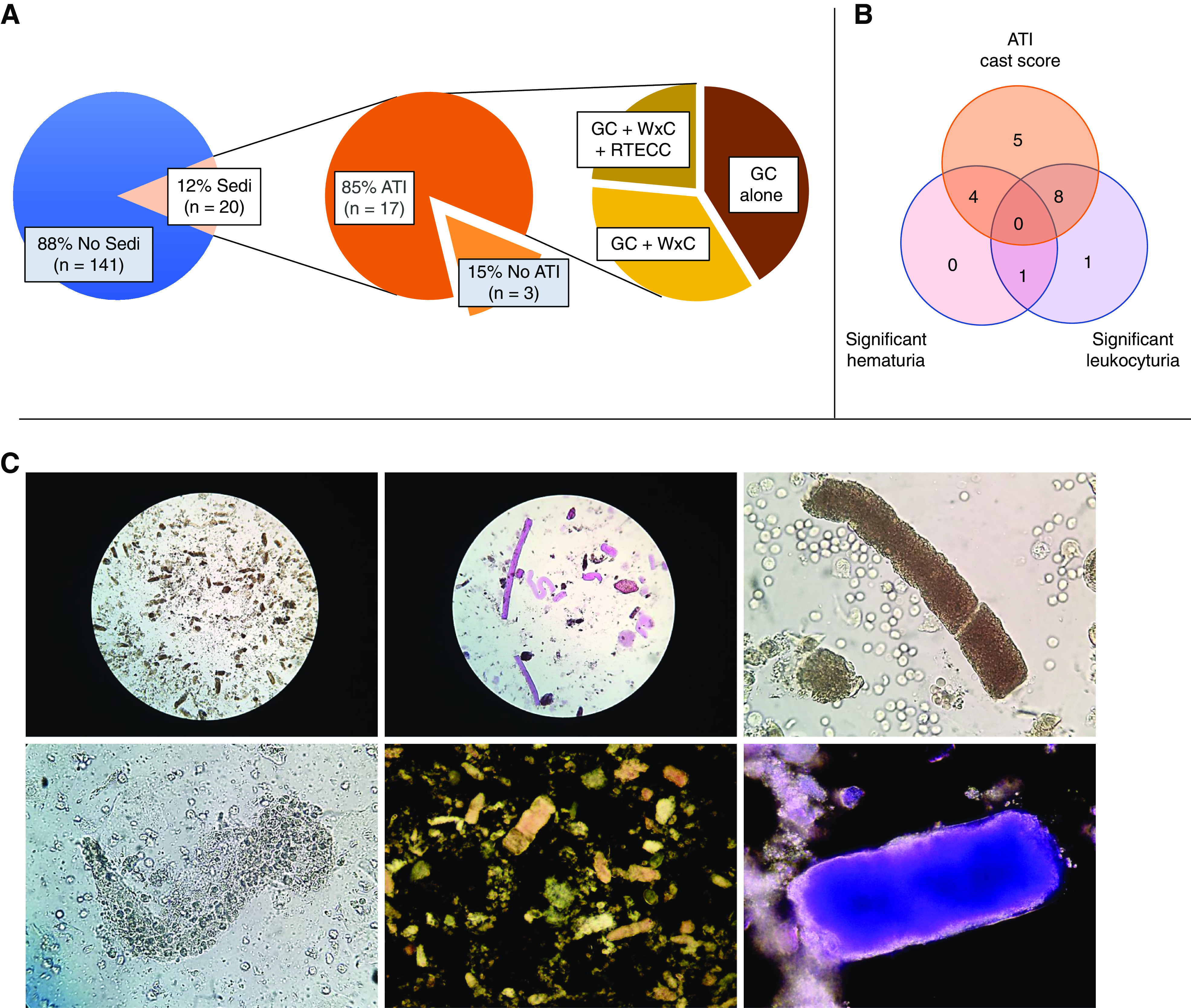
Frequency and characterisitics of urinary sediment (Sedi) findings revealing evidence of acute tubular injury (ATI). (A) Microscopic examination of the Sedi was performed in a subset of patients with coronavirus disease 2019–associated AKI (12%; 20 of 161). Findings consistent with ATI (as per Perazella and Chawla scores) were found in the majority (85%; 17 of 20). Frequency of coarse granular casts (GCs) identified either alone or along with waxy casts (WxCs) or renal tubular epithelial cell casts (RTECCs) is shown. (B) Distribution of cases with ATI cast score (as per Perazella and Chawla scores), significant hematuria, significant leukocyturia, or a combination of those elements. (C) Representative images of photomicrographs of slides of Sedi specimens examined by microscopy. (Upper left panel) Abundant muddy brown GCs under bright field at low-power magnification (×10 eyepiece and ×10 objective). (Upper center panel) Specimen treated with Sternheimer–Malbin stain showing numerous (straight and convoluted) WxCs surrounded by small fragmented coarse GCs under bright field at low-power magnification (×10 eyepiece and ×10 objective). (Upper right panel) Convoluted muddy brown GC under bright field at high-power magnification (×10 eyepiece and ×40 objective). (Lower left panel) Renal tubular epithelial cell casts under bright field at high-power magnification (×10 eyepiece and ×40 objective). (Lower center panel) Abundant coarse GCs under dark field at low-power magnification (×10 eyepiece and ×10 objective; zoomed by digital camera additional ×10). (Lower right panel) Specimen treated with Sternheimer–Malbin stain showing a muddy brown GC under dark field at high-power magnification (×10 eyepiece and ×40 objective).
Discussion
This is the first detailed report of MicrExUrSed findings in patients with CoV-AKI. MicrExUrSed has been demonstrated to be an effective tool to differentiate prerenal AKI from ATI and to predict outcomes, such as worsening of AKI and acute need for dialysis (3–7). In our cohort, the overwhelming majority of findings from MicrExUrSed were consistent with features of ATI. Because hemodynamic instability related to shock and prolonged volume depletion from malaise and reduced oral intake are common components of the clinical course of COVID-19, it is not surprising that ATI features were dominant. Fair abundance of coarse GCs and MBGCs was seen in 75% of the cases, whereas half of the specimens revealed the presence of WxCs, and a smaller fraction contained RTECCs. Altogether, the entire spectrum of casts associated with ATI was identified in this cohort. In many cases (11 of 17), the observed urinary sediment features of ATI either aligned with a clinical event suspicious for either ischemic or toxic ATI or were corroborated by biopsy-proven ATI. The latter was found in three of three patients who underwent kidney biopsy due to nephrotic-range proteinuria and were found to have collapsing glomerulopathy along with ATI in the tissue specimens. Interestingly, in six of six cases in which no clear cause of AKI could be identified by clinical grounds, features of ATI were found, thus suggesting that a form of ATI may also be the etiology of AKI even in those cases labeled as having unexplained AKI. Our observations are in agreement with a recent report of postmortem findings in kidneys from patients deceased with COVID-19 that revealed ATI as the dominant histopathologic feature in 100% of the cases (9).
It has been suggested that cases of CoV-AKI may present with high frequency of proteinuria, hematuria, and leukocyturia (10). Furthermore, the term nephritis has been proposed to describe cases of CoV-AKI presenting with those urinary features (11). From the perspective of MicrExUrSed, findings that would validate a suspicion for either interstitial or glomerular nephritis include presence of white blood cell casts, red blood cell casts, or acanthocytes. Neither white blood cell casts nor red blood cell casts were found in any of the specimens. The specimen of only one patient revealed presence of acanthocytes, a patient with positive antinuclear antibodies and low complement C4, suggesting that the patient likely had a form of proliferative GN unrelated to COVID-19. Furthermore, coexisting urine sediment elements of ATI were found in nine of ten cases of significant leukocyturia and in four of five cases of significant hematuria. In sum, we found no definitive evidence by MicrExUrSed to support the notion that there is a form of nephritis contributing as a cause of AKI in patients with COVID-19.
As a limitation, it should be noted that our cohort is likely enriched with cases of intrinsic forms of AKI. Given the significant concern in rationalizing the use of PPE and in minimizing health care worker exposure to infected patients, MicrExUrSed was not systematically attempted in all cases of AKI. Rather, it was pursued only in cases where the clinical presentation was not entirely clear for a defined cause of AKI. Although most cases had clinical features suggestive of ischemic or toxic ATI, they also had other confounding factors that stimulated the team of providers to attempt ascertaining the diagnosis by MicrExUrSed. Some of those confounding factors not characteristic of ATI that prompted MicrExUrSed included exposure to antibiotics, hypertension, nephrotic-range proteinuria, and hematuria or pyuria reported by the hospital laboratory. Thus, the percentage of patients with CoV-AKI with MicrExUrSed findings of ATI may be over-represented in this report.
As new data emerge and further define AKI as a severe complication of COVID-19, assessment, phenotyping, and risk stratification of cases of AKI are important steps that could lead to early optimization of diagnostic interventions and medical therapies. This report highlights the utility of MicrExUrSed in the context of COVID-19 to confirm a suspicion of ischemic or toxic ATI or to establish a diagnosis of an intrinsic form of AKI, and it strongly suggests that ATI is the primary form of AKI in COVID-19.
Disclosures
J.C.Q. Velez has participated in advisory board meetings for Mallinckrodt Pharmaceuticals and Retrophin and a speaker bureau for Otsuka Pharmaceuticals. None of the products related to those engagements are discussed in this manuscript. All remaining authors have nothing to disclose.
Funding
This work was supported by Ochsner Health System Clinical and Translational & Innovation Support Program grant CRISP 851111051 (to J.C.Q. Velez).
Author Contributions
J.C.Q. Velez conceptualized the study; C.F. Hernandez-Arroyo, M.M.B. Mohamed, V. Varghese, and J.C.Q. Velez were responsible for data curation; C.F. Hernandez-Arroyo, V. Varghese, and J.C.Q. Velez were responsible for investigation; C.F. Hernandez-Arroyo and J.C.Q. Velez were responsible for project administration; C.F. Hernandez-Arroyo, M.M.B. Mohamed, V. Varghese, and J.C.Q. Velez were responsible for validation; M.M.B. Mohamed was responsible for resources; M.M.B. Mohamed, V. Varghese, and J.C.Q. Velez were responsible for visualization; V. Varghese and J.C.Q. Velez were responsible for formal analysis; J.C.Q. Velez was responsible for funding acquisition; V. Varghese and J.C.Q. Velez were responsible for methodology; V. Varghese and J.C.Q. Velez were responsible for software; J.C.Q. Velez provided supervision; C.F. Hernandez-Arroyo wrote the original draft; and C.F. Hernandez-Arroyo, M.M.B. Mohamed, V. Varghese, and J.C.Q. Velez reviewed and edited the writing.
References
- 1.Mohamed MMB, Lukitsch I, Torres-Ortiz A, Walker JB, Varghese V, Hernandez-Arroyo CF, Alqudsi M, LeDoux JR, Velez JCQ: Acute kidney injury associated with coronavirus disease 2019 in urban New Orleans [published online ahead of print May 13, 2020]. Kidney360 doi: 10.34067/KID.0002652020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch JS, Ng JH, Ross DW, Sharma R, Shah HH, Barnett RL, Hazzan AD, Fishbane S, Jhaveri KD; The Northwell COVID-19 Research Consortium and The Northwell Nephrology COVID-19 Research Consortium: Acute kidney injury in patients hospitalized with COVID-19 [published online ahead of print May 13, 2020]. Kidney Int doi: 10.1016/j.kint.2020.05.006 [DOI] [Google Scholar]
- 3.Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR: Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 3: 1615–1619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla LS, Dommu A, Berger A, Shih S, Patel SS: Urinary sediment cast scoring index for acute kidney injury: A pilot study. Nephron Clin Pract 110: c145–c150, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perazella MA, Coca SG, Hall IE, Iyanam U, Koraishy M, Parikh CR: Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 5: 402–408, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perazella MA, Coca SG: Traditional urinary biomarkers in the assessment of hospital-acquired AKI. Clin J Am Soc Nephrol 7: 167–174, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Bagshaw SM, Haase M, Haase-Fielitz A, Bennett M, Devarajan P, Bellomo R: A prospective evaluation of urine microscopy in septic and non-septic acute kidney injury. Nephrol Dial Transplant 27: 582–588, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Dinda AK, Singh C, Dash SC, Tiwari SC, Aggarwal SK, Bhowmik D, Bagga A: Role of supravital staining of urine sediment and bright field microscopy in diagnosis of acute renal failure in bedside medicine. J Assoc Physicians India 48: 958–961, 2000 [PubMed] [Google Scholar]
- 9.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, Yi F, Yang H, Fogo AB, Nie X, Zhang C: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China [published online ahead of print April 9, 2020]. Kidney Int doi: 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross O, Moerer O, Weber M, Huber TB, Scheithauer S: COVID-19-associated nephritis: Early warning for disease severity and complications? Lancet 395: e87–e88, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


