Abstract
The steady rise in prescription opioids such as oxycodone has led to a virulent epidemic of widespread abuse and deaths in the US; approximately 80% of affected individuals initiate the habitual use of oxycodone by using prescription oral oxycodone. Given the importance of drug pharmacokinetics in determining abuse potential, we designed an oral operant oxycodone self-administration (SA) procedure in rats to model drug intake by most human users/abusers of oxycodone. Key aspects of the model include limited initial drug intake followed by increasing drug concentrations during extended 4-h sessions on alternating days. Sex and genetic predisposition are major determinants of human opiate abuse. Therefore, we studied females in seven inbred strains (WLI, WMI, LEW, DSS, F344, BN, SHR) and both sexes in three of these strains. All strains increased intake across serially increasing doses (0.025–0.2 mg/ml; p<0.001): the range of intakes at the final concentration of oxycodone was 0.72±0.17 – 4.84±1.42 mg/kg (mean ± SEM) - a 6.7-fold difference across strains. In LEW, WLI, and WMI strains, oxycodone intake increased significantly across all sessions in both sexes. However, in LEW and WMI male rats but not WLI, daily oxycodone intake was significantly lower across all 4-hour sessions than females (p<0.005). The estimated heritability in oxycodone intake was in the range of 0.21 – 0.41. In summary, our novel operant oral oxycodone SA model captures the strong abuse potential of oral oxycodone and demonstrates dose, sex, and strain-specific drug intake that is significantly dependent on heredity
Keywords: rat, strain, sex, oral, oxycodone, operant, heredity
Introduction
Prescription opioids such as morphine and oxycodone have long been effective analgesics. The steady rise in prescription opioids such as oxycodone has led to an epidemic of widespread abuse and deaths in the US. In 2016, ~12 million people misused opioids in the US (NSDUH 2018). In 2017, opioid overdoses caused 49,000 deaths (CDC 2018), a sharp rise from 2015 (Vadivelu et al. 2018). In 2020, during the Covid-19 pandemic (CDC 2021), drug overdoses soared to a record 93,000. Notably, approximately 80% of individuals initiate the habitual use of oxycodone by using prescription oral oxycodone (Cerdá et al. 2015; Compton & Volkow 2016).
In general, rapid intravenous infusion of abuse drugs produces greater positive subjective effects than slow infusion. This is evident for most abused drugs evaluated in primates and rats (Panlilio et al. 1998; 2001; Schindler et al. 2009; Wing & Shoaib 2013). It has been claimed that delayed absorption of oxycodone reduces its abuse liability (Kibaly et al. 2021). However, lessening behavioral effects by slowing drug release does not yield drugs that are no longer addictive. We designed an oral operant self-administration (SA) procedure in rat, a species widely used in preclinical addiction studies (Parker et al. 2013; Jaramillo & Zador 2014; Ellenbroek & Youn 2016), to model the pattern of drug intake of most human users/abusers of oxycodone, who initiate using oral tablets. While oral oxycodone SA protocols have been reported (Shaham 1993; Enga et al. 2016; Jimenez et al. 2017), either water deprivation (Enga et al. 2016) or home cage drug consumption (Shaham 1993; Jimenez et al. 2017), which affect the motivation to obtain drugs, has been required to initiate SA. In contrast, our model utilizes an operant licking procedure for oxycodone that does not require food/water restriction or prior drug consumption. Key components include limited initial drug intake followed by extended 4-h sessions on alternating days. Intermittent access produces cycles of drug-taking and withdrawal, promoting the development of addictive behavior (Kawa et al. 2016).
A dramatic rise in drug intake, which can vary greatly between individuals, often accompanies the transition from controlled drug consumption to compulsive drug intake - a cardinal feature of drug addiction (Edwards & Koob 2013). Based on classical human twin studies, half the risk for opiate use disorder (OUD) is genetic in origin (Tsuang et al. 1998; Kendler et al. 2003). Sex is also a major determinant of the pattern of opiate use, which is complex in animals. Male Sprague-Dawley rats self-administer more oxycodone than females at early stages, while females tend to self-administer more oxycodone than males at higher doses (Mavrikaki et al. 2017). Based on the impact of sex and genetics on opiate use, we further compared the pattern of drug intake in females and males from three inbred rat strains. Lewis rats (LEW) were used to establish the oral oxycodone self-administration procedure because this strain has been shown to self-administer several abused drugs, such as nicotine (Chen et al. 2012), cocaine (Valenza et al. 2016), and morphine (García-Lecumberri et al. 2011). Sex differences in oxycodone intake were further studied using the WMI and WLI rats (two inbred strains selectively bred from the Wistar Kyoto rats) that show contrasting stress responsiveness (Lim et al. 2018). In addition, females from four more inbred strains (DSS, F344, BN, SHR) were also studied. In certain strains, we see a 25-fold increase of intake throughout 19 sessions over a 33-day protocol. Additionally, significant sex and strain differences were found. The hereditary component (h2) of oxycodone intake was significant and similar to that reported for family and human twin studies (Mistry et al. 2014). The validity of our model for oral oxycodone SA is further supported by evidence of hypoalgesia immediately after SA, as well as prominent hyperalgesia during withdrawal at later time intervals. Therefore, this oral SA model captures the strong abuse potential of oral oxycodone.
Materials and Methods
Animals.
LEW/Hsd, F344/Hsd and SHR/Hsd breeders were purchased from Envigo. Dahl Salt Sensitive (SS/JrHsdMcwi) and Brown Norway (BN/NHsdMcwi) breeders were provided by Dr. Melinda Dwinell (Medical College of Wisconsin). Wistar Kyoto (WKY) Less Immobile (i.e WLI/Eer) and WKY More Immobile (i.e., WMI/Eer) were selected from the WKY strain (prior to full inbreeding) based on divergent responses in the forced swim test and then sib-mated for over 38 generations to create stable inbred selected lines (Will et al. 2003). WLI and WMI breeders were obtained from Dr. Eva Redei (Northwestern University). All rats were bred on our campus and group housed in a 12:12 h reversed light cycle room at the University of Tennessee Health Science Center. All experiments were conducted in the dark phase of the light cycle. Individual rats (both breeders and offspring) were identified by a radio frequency identification (RFID) tag implanted under the skin. Adult rats (PND 65–75) of both sexes were used. All procedures were approved by the Animal Care and Use Committee of The University of Tennessee Health Science Center and were conducted in accordance with the NIH Guidelines concerning the Care and Use of Laboratory Animals. The full names and sex of rats used in oxycodone self-administration and the tail immersion test are provided as Supplementory Table 1.
Drugs.
Oxycodone HCI was provided by Noramco (Wilmington, DE).
Oral operant oxycodone self-administration:
The operant chamber (Med Associates) has two lickometers: licking on the active spout that meets the requirement of a fixed ratio 5 (FR5) schedule resulted in the immediate delivery of a 60 μl oxycodone (0.025–0.2 mg/ml) to the tip of the spout. A visual cue (LED) was illuminated at the same time. A 20-s timeout period was enforced following drug delivery. Licks on the active spout during the timeout period or anytime on the inactive spout were recorded but had no programmed consequences. Rats were not food or water deprived. Rats were housed under a reverse light-cycle and tested during the dark phase. Training started with five daily 1-h sessions at 0.025 mg/ml oxycodone. Subsequent FR5 sessions were extended to 4-h but were conducted on alternate days. Starting from session 11, the dose of oxycodone was doubled every three sessions. The highest dose was 0.2 mg/ml. The number and timing of oxycodone delivery and licks on the active and inactive spouts were recorded throughout the experiment.
Tail immersion test.
Approximately 5 cm of the tip of the rat’s tail were immersed in 48±0.25 °C water. The RFID of the rat, the temperature of the water, and the latency of tail withdrawal were recorded using the tailTimer open source device we developed (Udell et al. 2021). Data were collected before oxycodone self-administration, and within 10 min of the last FR session and at 24 and 48 h after the last FR session.
Experimental design.
The LEW inbred strain (n=8 for males and n=8 for females) was used to establish the oral oxycodone self-administration procedure because this strain has been shown to self-administer several abused drugs, such as nicotine (Chen et al. 2012), cocaine (Valenza et al. 2016), and morphine (García-Lecumberri et al. 2011). Sex differences in oxycodone intake were further studied using the WMI (n=5 for males and n=6 for females) and WLI (n=5 for males and n=6 for females) inbred strains that have contrasting stress responsiveness (Lim et al. 2018). Additional female rats from BN (n=6), DSS (n=6), SHR (n=6) and F344 (n=6) were added to better estimate the heritability of oxycodone traits.
Estimate of heritability.
The between-strain variance provides a measure of additive genetic variation (VA), while within-strain variance represents environment variability (VE). An estimate of narrow-sense heritability (i.e. the proportion of total phenotypic variation that is due to the additive effects of genes, h2) for nicotine or food reward was obtained using the formula: h2 =½* VA/(½ *VA+VE) (Hegmann & Possidente 1981; Mogil et al. 1999).
Statistical analysis.
The number of licks on active and inactive spouts were transformed into log scale to fit a normal distribution. Licks, reward, and intake during acquisition were analyzed by repeated measures ANOVA, where session and spouts were treated as within subject variables. Tukey HSD was used for posthoc comparisons. Data were expressed as mean ± SEM. Statistical significance was assigned at p< 0.05. All analyses were conducted using R statistical language. Data are available on request from the authors.
Results
Adult inbred male (n = 6) and female (n = 6) LEW rats initiated oral oxycodone SA in operant chambers that have two spouts. Five licks on the active spout triggered the delivery of one drop of oxycodone (60 μl, 0.025–0.2 mg/ml, depending on the stage of SA; Fig 1A). The initial five 1-h sessions were conducted daily at 0.025 mg/ml. Subsequent sessions, lasting 4-h, were conducted on alternate days. The training dose gradually increased to 0.2 mg/ml.
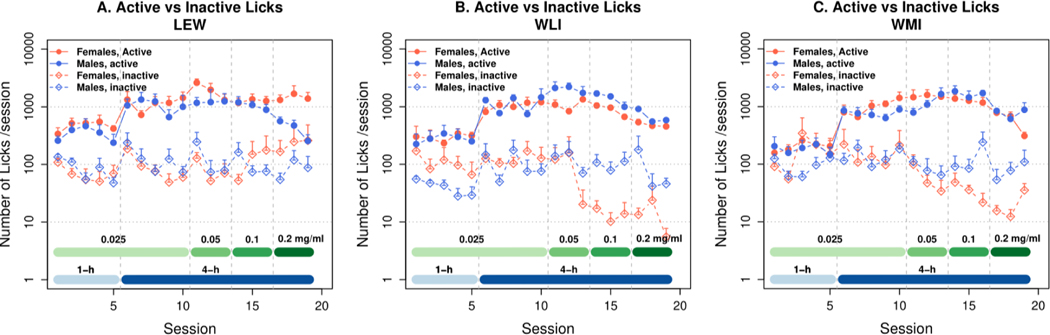
Figure 1.
Oral Oxycodone SA in inbred LEW (A) WLI (B), and WMI (C) rats (n=6/sex). Please note that the y-axis is in a logarithmic scale. Oxycodone SA (FR5) was initiated with consecutive daily 1-hour sessions. Thereafter, increasing concentrations of oxycodone were delivered in 4-hour sessions on alternating days. Detailed statistical analysis results are provided in the Results section. Active licks for oxycodone were significantly greater than inactive licks (ANOVA, male and female for active vs. inactive: ps <0.001 for both males and females in all three strains).
Both LEW male (M) and female (F) rats licked significantly more on the active than inactive spout (Fig. 1A; two-way repeated measures ANOVA found active vs. inactive: F1,193 = 232.5, p<0.001, with significant Sex x Spout interaction: F1,193 = 9.2, p<0.01). This panel also shows that active licks were greater in Lewis-F than M (F1,359 = 13.98, p<0.001), whereas inactive licks were not different (F1,355 = 0.476, p=0.49). Lastly, daily active licks significantly increased by day in both sexes (M and F by session: F24,144 = 10.0, p<0.001 and F24,152 = 3.99, p<0.001). At 0.2 mg/ml, female Lewis rats obtained 2.75 mg/kg/session - a 25-fold increase from the initial intake levels and well above the standard prescription of 80 mg/d for an adult human (~1 mg/kg).
The operant licking behavior of WLI (Fig 1B) and WMI (Fig 1C) rats are similar to that of the LEW. Two-way repeated measures ANOVA found that the main effect of Spout was significant in WLI (F1,121 = 353.4, p<0.001) and WMI (F1,125=236.7, p<0.001) rats. In addition, there were significant interactions between Spout and Sex in both WLI (F1,121 = 13.8, p<0.001) and WMI (F1,125 = 7.3, p<0.01). Lastly, daily active licks significantly increase by day in both sexes in WLI (F24,167 = 15.2, p<0.001) and WMI (F24,198 = 14.5, p<0.001).
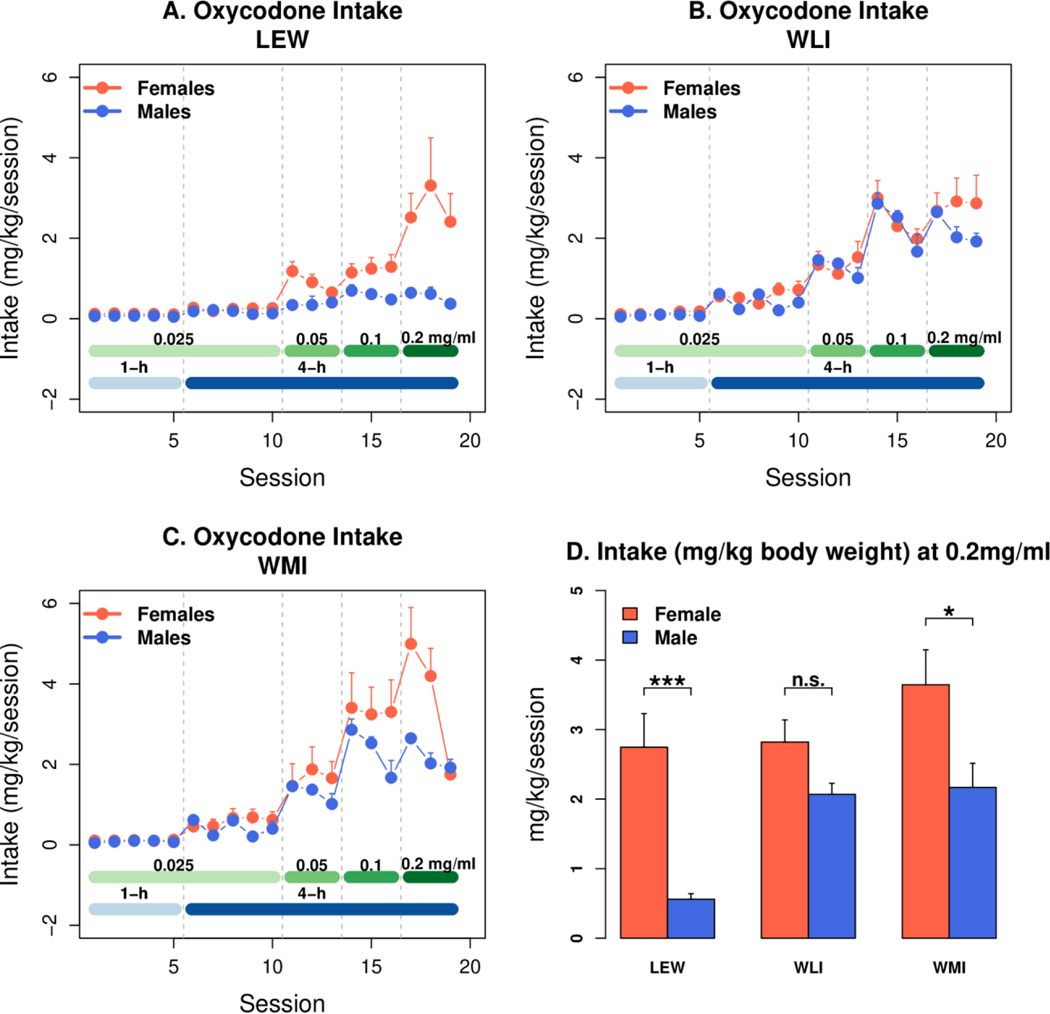
Daily oxycodone intake by session and dose in males vs females from 3 inbred strains (LEW, WLI, and WMI) were shown in Figure 2 (panels A, B, And C). Oxycodone intake increased significantly across all 20 sessions in both sexes of all three strains, including LEW-M (ANOVA: session, all rats: F18,901 = 51.7, p<2e-16; only LEW-M: F18,109 = 7.8, p<0.001). In LEW and WMI rats, daily oxycodone intake was significantly lower across all 4-hour sessions in males compared to females, but not in WLI (strain: F2,30 = 6.5, p = 0.004. sex: F1,30 = 10.75, p = 0.002. Tukey HSD, sex/WLI, WMI, LEW: p>0.05, p = 0.0001, p = 0.0004, respectively). Additionally, oxycodone intake across all sessions was significantly less in LEW-M compared to WMI-M and WLI-M (mg/kg: F2,40 = 36.21, p<0.001). Furthermore, oxycodone intake was significantly less in males than females of LEW and WMI, but not WLI. Panel D, which shows intake at the highest dose of oxycodone (0.2 mg/kg), highlights this effect of sex on oxycodone intake. Thus, the variation in oxycodone intake by sex was strain-specific.
Figure 2.
Active spout licks for oral oxycodone SA in inbred male and female LEW (LEW), WLI and WMI rats (n=6/sex). Oxycodone SA (FR5) was initiated with consecutive daily 1-hour sessions. Thereafter, increasing concentrations of oxycodone were delivered in 4-hour sessions on alternating days. Panels A,B,C: oxycodone intake increased significantly across all 20 sessions in both sexes of all three strains, including LEW-M (ANOVA: session, all rats, p<2e-16; session, only LEW-M, p<0.001). In LEW and WMI rats, daily oxycodone intake was significantly lower across all 4-hour sessions in males compared to females, but not in WLI (strain: p = 0.004. sex: p = 0.002. Tukey HSD, sex/WLI, WMI, LEW: p>0.05, p = 0.0001, p = 0.0004, respectively). Additionally, oxycodone intake across all sessions was significantly less in LEW-M compared to WMI-M and WLI-M (mg/kg: p<0.001). Oxycodone intake was significantly less in males than females of LEW and WMI, but not WLI. Panel D: intake at the highest dose of oxycodone (0.2 mg/kg), highlights the effect of sex on oxycodone intake (*, p<0.05; ***, p<0.001).
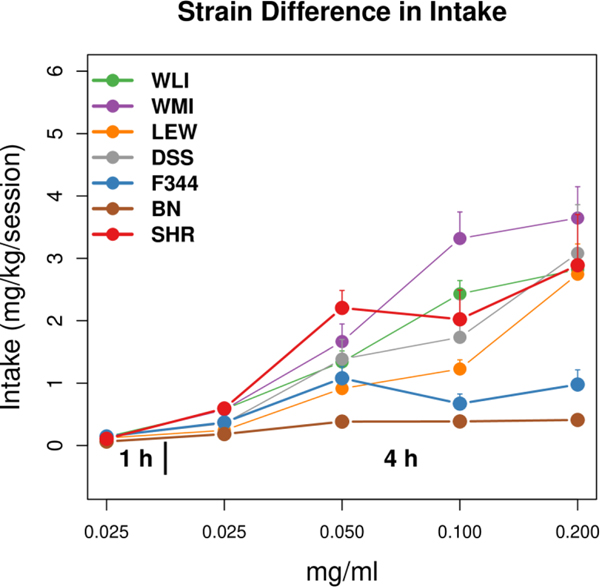
Figure 3 compared oxycodone intake per session (mg/kg/session) across all doses in females from seven inbred strains (LEW, WLI, WMI, DSS, F344, BN, SHR) using the foregoing intermittent access protocol. All strains increased intake across doses (p<0.001). The range of intakes at the final concentration of oxycodone (0.2 mg/ml) was 0.72±0.17 – 4.84±1.42 mg/kg (mean ± SEM), a 6.7-fold difference across strains. Thus, across seven female inbred rat strains, oxycodone intake was both dose and strain-dependent.
Figure 3.
Oxycodone intake per session across all doses in females from seven inbred strains (n=6–8 per strain), using an intermittent oral operant protocol. All strains increased intake across doses (p<0.001). The range of intakes at the final concentration of oxycodone (0.2 mg/ml) was 0.72±0.17 – 4.84±1.42 mg/kg (mean ± SEM), a 6.7-fold difference across strains. At 0.2 mg/ml, female LEW rats obtained 2.75 mg/kg/session - a 25-fold increase from the initial intake levels.
We calculated the hereditary component (h2) of several behavior characteristics (i.e. phenotypes) of oral oxycodone self-administration. The h2 for the number of active licks, oxycodone intake, and rate of increase in oxycodone intake across sessions were in the range of 0.21 – 0.41 (Table 1). Using inbred strains permits repeated sampling of the same genome (Belknap 1998) (i.e., resample in Table 1), which boosts the effective h2 (i.e., heritability of the group average). This increased the effective h2 to 0.7–0.8. In contrast, h2 for the number of inactive licks, which have no behavioral consequence, was close to zero. These data demonstrate that a significant portion of the variation in oxycodone SA amongst strains was due to genetic factors.
Table 1.
Heritability estimates
| Trait | h2 | resample, n=6 |
|---|---|---|
| Intake, 0.1 mg/ml | 0.21 | 0.62 |
| Intake, 0.2 mg/ml | 0.41 | 0.81 |
| Total Intake | 0.25 | 0.67 |
| Rate of increase in intake | 0.21 | 0.61 |
| Active licks, 0.2mg/ml | 0.29 | 0.71 |
| Inactive licks, 0.2mg/ml | 0.004 | 0.02 |
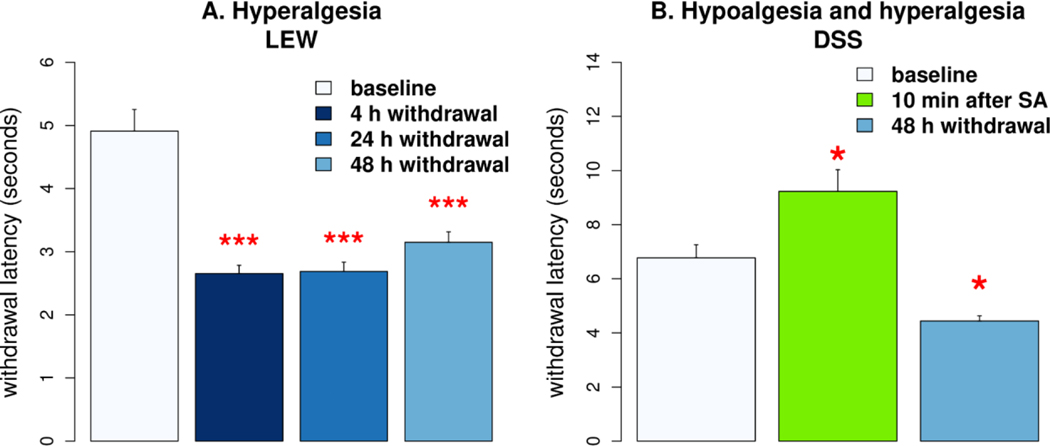
In a limited assessment of the pharmacological effects of oxycodone self-administration, we measured the latency of tail withdrawal from 48 ± 0.25 °C water in females from LEW and DSS. We measured tail withdrawal 10 min following a 4 h oxycodone SA session (0.2 mg/ml) and at later time intervals in DSS rats. Compared to baseline, measured before initiating oxycodone SA, the latency increased by 36.2% (p<0.05, Fig 4B), demonstrating the analgesia induced by orally self-administered oxycodone. Increased pain is a common sign of withdrawal from chronic opioid exposure. When tail immersion was repeated after 48 h of withdrawal from oxycodone SA, latency was reduced by 34.4% (p<0.05), indicating increased pain sensitivity. These observations align with data showing increased pain sensitivity in female LEW rats at various time points of withdrawal (Fig 4A). Overall, these data indicated that our model of oxycodone SA in inbred rats effectively modulates neural pathways regulating thermal pain, inducing time-dependent hypoalgesia and hyperalgesia.
Figure 4.
Oxycodone self-administration affects thermal pain threshold. A. Latency of withdrawal tails from 48 °C water was measured in female LEW rats (n=6). Rats were significantly more sensitive to thermal pain 4, 24, and 48 h after oxycodone SA compared to baseline. B. DSS females (n=5) significantly increased tail withdrawal latency immediately after SA. Latency was significantly reduced 48 h after withdrawal. * p<0.05, *** p<0.001, compared to baseline.
Discussion
In general, a high level of individual variation in susceptibility to addiction is characteristic of human drug abuse (Anthony et al. 1994). Experimental models that embody multiple drug abuse behavioral parameters have validated these observations, showing that full blown addiction is manifest in fewer than 20% of individual rats given access to cocaine (Deroche-Gamonet et al. 2004). This aligns with observations in humans (Anthony et al. 1994). Thus, no single factor amongst the genetic, environmental and gene x environment interactions absolutely determines susceptibility within an individual. We developed a novel model of oxycodone SA to identify and explore these factors in depth by using inbred rat strains bred in our vivarium, each of which manifests highly reproducible drug-taking behaviors. Heritable variation in the predisposition to take oral oxycodone (h2 = 0.21 – 0.41 and effective h2 = 0.7–0.8) was found at levels reported in human family and twin studies of opiate use disorder (OUD) (Tsuang et al. 1998; Kendler et al. 2003). This variation in genetic risk along with consistent breeding and postnatal environmental conditions most likely account for the consistent differences in drug-taking observed between strains.
Intravenous oxycodone SA has been reported in mice (Zhang et al. 2017) and rats (Pravetoni et al. 2014; Mavrikaki et al. 2017). Extended access (4–12h/session) SA protocols have also been reported for mice (Zhang et al. 2014) and rats (Wade et al. 2015). Since pharmacokinetics contribute to drug abuse liability in both humans and rats, our model is optimized for oral SA - the route of delivery that initiates opiate use disorder (OUD) in humans. The model utilizes an operant licking procedure for oxycodone that does not require the food/water restriction or prior drug consumption used in other paradigms (Shaham 1993; Enga et al. 2016; Jimenez et al. 2017). Initially, rats access oxycodone for only 1-hour in five consecutive daily sessions. This is followed by extended 4-h sessions on alternating days with drug concentration increasing every 3 sessions. Intermittent access produces cycles of drug taking and withdrawal that promote the development of addictive behavior (Allain et al. 2015). In females from 5 of 7 strains (Fig. 3), we observed an approximate 25-fold increase in oral oxycodone intake, which increased progressively with dose across training sessions; the rate of dose-dependent increase in oxycodone intake was heritable across strains (h2 = 0.21, Table 1). This is analogous to the dramatic rise in drug intake, which can vary greatly between individuals, and often accompanies the transition from controlled drug consumption to compulsive drug intake - a cardinal feature of drug addiction (Edwards & Koob 2013).
Sex is also a major determinant of the pattern of opiate use, which is complex in humans and rodents. The incidence of OUD is higher in men than women (Lee & Ho 2013). Yet addicted women progress more rapidly from initial use to dependence than men (Brady & Randall 1999; Becker & Koob 2016). The picture is also complex in rats. Male Sprague-Dawley rats self-administer more oxycodone than females at early stages, while females tend to consume more oxycodone at higher doses (Mavrikaki et al. 2017). We found that sex was a significant determinant of oral oxycodone intake in 2 of the 3 strains where males and females were compared. In two of these strains shown in Fig. 2, female oxycodone intake/session at 0.2 mg/ml significantly exceeded that in males. In LEW rats, the sex difference in intake was apparent across all drug concentrations, whereas the separation between sexes occurred only at the highest concentration in WMI. Thus, sex is a strain-specific determinant of oral oxycodone intake.
Although the efficacy of oral oxycodone in rats may be controversial, two lines of evidence from the current study demonstrate that oral oxycodone affects brain function. First, the strain and sex-specific effects of oral operant oxycodone SA, including the strongly heritable preference for the drug spout but not the inactive spout (Table 1), show that oxycodone is an effective positive reinforcer of operant drug-taking behavior. Second, oral oxycodone SA affects pain thresholds. Briefly following a session of oral intake, the thermal pain threshold was significantly elevated (Fig. 4B; i.e., DSS rats), as expected from the activation of μ opioid receptors by opiate agonists. Conversely, 4–48 hours post oxycodone intake (Fig 4A,B), both LEW and DSS rats manifest a reduction in thermal pain threshold, consistent with opiate withdrawal in dependent animals chronically exposed to opiates.
Several potential limitations of this study are worth considering. (i) Our experiments evaluated oxycodone doses to 0.2 mg/ml because pilot studies showed that rats consumed significantly less oxycodone at 0.4 mg/ml. Since oxycodone has a bitter taste, we cannot distinguish between reduced drug intake at higher doses due to the bitter taste or to other pharmacological effects, or a combination of these factors. (ii) In addition to establishing a method for operant oxycodone intake, we sought to estimate the heritability of behavioral traits associated with voluntary oxycodone intake in rats, in order to establish the feasibility of genetic mapping studies. In line with many studies in rodents (Dumas et al. 2000; Richards et al. 2013; Vanderlinden et al. 2014; Kantak et al. 2021), we estimated heritability in only one sex. We previously reported that estimates of heritability for nicotine intake are similar between males and females (Chen et al. 2012; Han et al. 2017), even when nicotine self-administration was conducted using two different methods; this suggested that genetic influences on drug taking behavior were shared between males and females. (iii) Lastly, in order to obtain a more accurate estimate of heritability, we studied additional strains. Two of these strains (i.e., BN and F344) were resistant to oxycodone intake. Genetic findings in human (Saccone et al. 2009) and animal studies (Fowler et al. 2011; Chen et al. 2014) frequently involve genes that confer resistance to drug intake. Hence, these two strains are relevant to future genetic studies.
The identification of targets and individualized treatments for those afflicted with OUD requires a much deeper appreciation of individual differences in CNS responses to abused opiates such as oxycodone. Defining the source of these individual differences will require mechanistic knowledge of the impact of environmental and genetic factors that predispose individuals to compulsively take increasing doses of such drugs - eventuating in opiate addiction in a minority of individuals. In view of the virulent epidemic of OUD, frequently involving oxycodone, we developed a novel, robust model of oral oxycodone self-administration, ultimately to identify the genetic determinants of vulnerability to OUD. In this report, we have shown that this model readily detects both strain and sex-dependent differences in oxycodone SA over a range of increasing oral concentrations in 4-hour extended access sessions on alternating days. These features of the model are germane to human oxycodone OUD, which increases the potential relevance of genes identified in this model for human OUD.
Supplementary Material
Acknowledgements:
The authors are grateful to Melinda Dwinell Ph.D., Medical College of Wisconsin, for providing breeder pairs for many of the inbred strains studied in this report. The authors thank Tengfei Wang for his help in maintaining the breeding colony. This study was supported by funds available through the University of Tennessee Health Science Center and by NIH NIDA U01 DA-053672 (Burt M. Sharp and Hao Chen are multiple principal investigators) and NIDA R01 DA-048017 (Hao Chen, Eva E. Redei and Megan K. Mulligan are multiple principal investigators). No commercial funds were utilized.
Footnotes
Data Availability: The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Conflict of interest: The authors have no conflict of interests to declare.
References
- Allain F, Minogianis E-A, Roberts DCS & Samaha A-N (2015) How fast and how often: The pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev 56, 166–179. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA & Kessler RC (1994) Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol 2, 244–268. [Google Scholar]
- Becker JB & Koob GF (2016) Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68, 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK (1998) Effect of within-strain sample size on QTL detection and mapping using recombinant inbred mouse strains. Behav Genet 28, 29–38. [DOI] [PubMed] [Google Scholar]
- Brady KT & Randall CL (1999) Gender differences in substance use disorders. Psychiatr Clin North Am 22, 241–252. [DOI] [PubMed] [Google Scholar]
- CDC. (2018) Products - Vital Statistics Rapid Release - Provisional Drug Overdose Data. URL https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- CDC. (2021) Products - Vital Statistics Rapid Release - Provisional Drug Overdose Data [WWW Document]. URL https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- Cerdá M, Santaella J, Marshall BDL, Kim JH & Martins SS (2015) Nonmedical Prescription Opioid Use in Childhood and Early Adolescence Predicts Transitions to Heroin Use in Young Adulthood: A National Study. J Pediatr 167, 605–12.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hiler KA, Tolley EA, Matta SG & Sharp BM (2012) Genetic factors control nicotine self-administration in isogenic adolescent rat strains. PLoS One 7, e44234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Luo R, Gong S, Matta SG & Sharp BM (2014) Protection genes in nucleus accumbens shell affect vulnerability to nicotine self-administration across isogenic strains of adolescent rat. PLoS One 9, e86214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM & Volkow ND (2016) Improving Outcomes for Persons With Opioid Use Disorders: Buprenorphine Implants to Improve Adherence and Access to Care. JAMA. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D. & Piazza PV (2004) Evidence for addiction-like behavior in the rat. Science 305, 1014–1017. [DOI] [PubMed] [Google Scholar]
- Dumas P, Sun Y, Corbeil G, Tremblay S, Pausova Z, Kren V, Krenova D, Pravenec M, Hamet P. & Tremblay J. (2000) Mapping of quantitative trait loci (QTL) of differential stress gene expression in rat recombinant inbred strains. J Hypertens 18, 545–551. [DOI] [PubMed] [Google Scholar]
- Edwards S. & Koob GF (2013) Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol 24, 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek B. & Youn J. (2016) Rodent models in neuroscience research: is it a rat race? Dis Model Mech 9, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enga RM, Jackson A, Damaj MI & Beardsley PM (2016) Oxycodone physical dependence and its oral self-administration in C57BL/6J mice. Eur J Pharmacol 789, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ & Kenny PJ (2011) Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Lecumberri C, Torres I, Martín S, Crespo JA, Miguéns M, Nicanor C, Higuera-Matas A. & Ambrosio E. (2011) Strain differences in the dose-response relationship for morphine self-administration and impulsive choice between Lewis and Fischer 344 rats. J Psychopharmacol 25, 783–791. [DOI] [PubMed] [Google Scholar]
- Han W, Wang T. & Chen H. (2017) Social learning promotes nicotine self-administration by facilitating the extinction of conditioned aversion in isogenic strains of rats. Sci Rep 7, 8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegmann JP & Possidente B. (1981) Estimating genetic correlations from inbred strains. Behav Genet 11, 103–114. [DOI] [PubMed] [Google Scholar]
- Jaramillo S. & Zador AM (2014) Mice and rats achieve similar levels of performance in an adaptive decision-making task. Front Syst Neurosci 8, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez SM, Healy AF, Coelho MA, Brown CN, Kippin TE & Szumlinski KK (2017) Variability in prescription opioid intake and reinforcement amongst 129 substrains. Genes Brain Behav 16, 709–724. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Stots C, Mathieson E. & Bryant CD (2021) Spontaneously Hypertensive Rat substrains show differences in model traits for addiction risk and cocaine self-administration: Implications for a novel rat reduced complexity cross. Behav Brain Res 411, 113406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS & Robinson TE (2016) Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 233, 3587–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA & Neale MC (2003) Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry 160, 687–695. [DOI] [PubMed] [Google Scholar]
- Kibaly C, Alderete JA, Liu SH, Nasef HS, Law P-Y, Evans CJ & Cahill CM (2021) Oxycodone in the Opioid Epidemic: High “Liking”, “Wanting”, and Abuse Liability. Cell Mol Neurobiol 41, 899–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW-S & Ho I-K (2013) Sex differences in opioid analgesia and addiction: interactions among opioid receptors and estrogen receptors. Mol Pain 9, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PH, Shi G, Wang T, Jenz ST, Mulligan MK, Redei EE & Chen H. (2018) Genetic Model to Study the Co-Morbid Phenotypes of Increased Alcohol Intake and Prior Stress-Induced Enhanced Fear Memory. Front Genet 9, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Rathmell JP, Swedberg MD, Jonzon B. & Norsten-Höög C. (2001) Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther 299, 1056–1065. [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D. & Chartoff E. (2017) Oxycodone self-administration in male and female rats. Psychopharmacology 234, 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry CJ, Bawor M, Desai D, Marsh DC & Samaan Z. (2014) Genetics of Opioid Dependence: A Review of the Genetic Contribution to Opioid Dependence. Curr Psychiatry Rev 10, 156–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM & Devor M. (1999) Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80, 67–82. [DOI] [PubMed] [Google Scholar]
- NSDUH. (2018) National Survey on Drug Use and Health [WWW Document]. URL https://nsduhweb.rti.org/respweb/homepage.cfm
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ & Schindler CW (1998) Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology 137, 253–258. [DOI] [PubMed] [Google Scholar]
- Parker CC, Chen H, Flagel SB, Geurts AM, Richards JB, Robinson TE, Solberg Woods LC & Palmer AA (2013) Rats are the smart choice: Rationale for a renewed focus on rats in behavioral genetics. Neuropharmacology 76 Pt B, 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L. & LeSage MG (2014) Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One 9, e101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Lloyd DR, Kuehlewind B, Militello L, Paredez M, Solberg Woods L. & Palmer AA (2013) Strong genetic influences on measures of behavioral-regulation among inbred rat strains. Genes Brain Behav 12, 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Wang JC, Breslau N, Johnson EO, Hatsukami D, Saccone SF, Grucza RA, Sun L, Duan W, Budde J, Culverhouse RC, Fox L, Hinrichs AL, Steinbach JH, Wu M, Rice JP, Goate AM & Bierut LJ (2009) The CHRNA5-CHRNA3-CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African-Americans and in European-Americans. Cancer Res 69, 6848–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV & Thorndike EB (2009) Effect of rate of delivery of intravenous cocaine on self-administration in rats. Pharmacol Biochem Behav 93, 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y. (1993) Immobilization stress-induced oral opioid self-administration and withdrawal in rats: role of conditioning factors and the effect of stress on “relapse” to opioid drugs. Psychopharmacology 111, 477–485. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R. & Eaves L. (1998) Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry 55, 967–972. [DOI] [PubMed] [Google Scholar]
- Udell ME, Ni J, Garcia Martinez A, Mulligan MK, Redei EE & Chen H. (2021) TailTimer: A device for automating data collection in the rodent tail immersion assay. PLoS One 16, e0256264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadivelu N, Kai AM, Kodumudi V, Sramcik J. & Kaye AD (2018) The Opioid Crisis: a Comprehensive Overview. Curr Pain Headache Rep 22, 16. [DOI] [PubMed] [Google Scholar]
- Valenza M, Picetti R, Yuferov V, Butelman ER & Kreek MJ (2016) Strain and cocaine-induced differential opioid gene expression may predispose Lewis but not Fischer rats to escalate cocaine self-administration. Neuropharmacology 105, 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderlinden LA, Saba LM, Printz MP, Flodman P, Koob G, Richardson HN, Hoffman PL & Tabakoff B. (2014) Is the alcohol deprivation effect genetically mediated? Studies with HXB/BXH recombinant inbred rat strains. Alcohol Clin Exp Res 38, 2148–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO & Koob GF (2015) Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology 40, 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will CC, Aird F. & Redei EE (2003) Selectively bred Wistar–Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry 8, 925–932. [DOI] [PubMed] [Google Scholar]
- Wing VC & Shoaib M. (2013) Effect of infusion rate on intravenous nicotine self-administration in rats. Behav Pharmacol 24, 517–522. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liang Y, Levran O, Randesi M, Yuferov V, Zhao C. & Kreek MJ (2017) Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: a RNA sequencing study. Psychopharmacology 234, 2259–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mayer-Blackwell B, Schlussman SD, Randesi M, Butelman ER, Ho A, Ott J. & Kreek MJ (2014) Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology 231, 1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.