Abstract
Sleep is essential for brain function in a surprisingly diverse set of ways. In the short term, lack of sleep leads to impaired memory and attention; in the longer term, it produces neurological dysfunction or even death. This review article discusses recent advances in understanding how sleep maintains the physiological health of the brain, via interconnected systems of neuronal activity and fluid flow. The neural dynamics that appear during sleep are intrinsically coupled to its consequences for blood flow, cerebrospinal fluid dynamics, and waste clearance. Recognizing these linked causes and consequences of sleep has shed new light on why sleep is important for such disparate aspects of brain function.
Our nightly sleep is critical for a wide array of brain functions. Missing just a single night of sleep results in memory, mood, and attentional impairments the next day (1); disrupted sleep across the lifespan is linked to neurodegeneration (2, 3). These heterogeneous effects of sleep have posed a puzzle in neuroscience – why does this brain state hold a unique role in supporting such seemingly distinct aspects of brain function?
Decades of work have shown that sleep contains unique neural dynamics linked to cognition, such as slow waves in neural activity that appear in non-rapid-eye-movement (NREM) sleep (4). More recent discoveries have shown that sleep is also a heightened state for waste removal from the brain (5, 6). Metabolic waste products are transported out of brain tissue (7) via the interstitial fluid (ISF) and cerebrospinal fluid (CSF), and sleep plays a key role in both waste regulation and flow of CSF in the brain (8, 9), which is essential for maintaining neuronal health.
These beneficial effects motivate why we spend so many hours each day asleep, as sleep’s role in basic housekeeping for the brain implicates it in broad aspects of neural function. But why is sleep linked to distinct fluid dynamics, and why does it play such a profound role in maintaining brain function?
The control circuits that govern sleep
Sleep is controlled by large-scale arousal regulatory systems that can rapidly induce states of sleep or wakefulness. Extensive circuit-based investigations have recorded from and manipulated individual brain regions and cell types, to identify their role in behavior. This approach has been powerful, identifying multiple regions in hypothalamus, brainstem, basal forebrain, and other subcortical nuclei that control sleep states (10). Key interacting circuits include the noradrenergic, dopaminergic, cholinergic, and orexinergic systems, which can induce sleep-wake transitions (10). However, these findings have raised a new question: why do so many diverse nuclei play decisive roles in whether an animal is sleeping or awake?
One possibility is that the system is simply unusually redundant; sleep is so important that the brain contains multiple switches to induce sleep states. However, a second and perhaps more likely possibility, is that these circuits represent interacting components of a larger system that induces the many distinct features of sleep, ranging from altered behavior to oscillatory neural dynamics, modulated respiratory and vascular physiology, and clearance. Notably, the circuits that regulate sleep project widely throughout the forebrain (10), and to each other, so activity within one system inevitably influences the others. Furthermore, neuromodulators such as noradrenaline and acetylcholine, which alter neuronal arousal states, also have direct actions on the vasculature.
Low-frequency neural dynamics emerge during sleep
During sleep, large-scale neural activity reorganizes into distinctive, oscillatory patterns, reflecting the wide-ranging reach of arousal regulatory circuits throughout the thalamocortical networks that generate EEG oscillations. These patterns have long been recognized to define distinct stages of sleep, separating NREM and REM. As sleep progresses into NREM, many distinct EEG patterns appear, including spindles (~11–14 Hz) and slow waves, with distinct links to memory and cognition (4). The increased low-frequency power is referred to as slow wave activity, a catch-all term that corresponds to 0.5–4 Hz EEG power, and which can reflect several low-frequency dynamics (Fig. 1a). These include individual K-complexes (occurring seconds or minutes apart), delta (1–4 Hz) waves, and the slow (0.1–1 Hz) oscillations that group and coordinate higher-frequency rhythms (11, 12). K-complexes correspond to periods of widespread suppression of neural activity lasting hundreds of milliseconds (13) (‘down-state’), typically throughout large expanses of cortex (14). In deeper NREM sleep, slow waves become continuous and rhythmic, alternating between down-states and up-states. REM sleep, by contrast, is linked to desynchronized EEG states, as well as patterns such as rapid eye movements and suppressed muscle tone (15).
Fig. 1: Low-frequency neural activity during sleep.
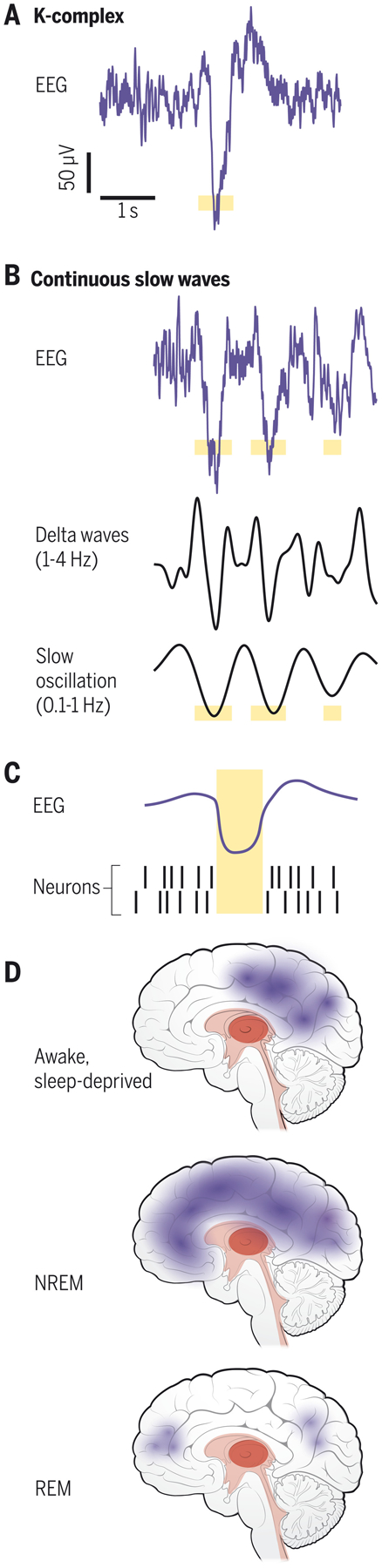
Slow wave activity (0.5–4 Hz) appears in the EEG during NREM sleep, with emergence of individual K-complexes (A) or continuous slow waves (B). Illustration of possible down-state in yellow. C) Illustration of slow waves coupled to neuronal suppression (yellow). Adapted from (13, 17). D) Low-frequency neural dynamics can occur locally in the awake brain, locally or globally in NREM, and in restricted cortical regions in REM.
While ample evidence demonstrates that brainwide activity changes during sleep, and that slow waves can be global cortical events, this should not be taken to imply that sleep is a uniform, homogenous state throughout the brain. Sleep-like slow waves can appear in local patches of cortex even within the awake brain (Fig. 1b), termed ‘local sleep’ (16). One possibility is that the brain’s need for slow waves is so strong that after lack of sleep, slow waves emerge in the awake brain despite their detrimental consequences for behavior. During NREM sleep, slow waves originally thought to represent globally coherent activity have also been found to exhibit local dynamics (17). Slow wave activity is not nearly as prominent during REM sleep, but can occur in superficial cortical layers or frontal regions (18).
Waste clearance and CSF flow in the sleeping brain
Decades of study have contributed to our understanding of the slow wave phenomena discussed above. More recent was the discovery of sleep’s role in waste clearance: molecules such as amyloid-beta are cleared from the mouse brain at far higher rates during sleep than during wakefulness (5). This solute clearance takes place via ISF and CSF flowing along blood vessels (7); however, the precise mechanisms remain a topic of controversy, with debate over the forces that drive flow and the exit routes for solutes (19–21). This observation yielded a new perspective on the importance of sleep: sleep maintains the basic physiological health of neurons by removing their potentially harmful metabolic waste.
An important consideration is that waste production rates also differ across arousal states, with higher tau and amyloid production during wakefulness in both rodents and humans (8). Sleep may thus serve as a pause in the waste generation process, allowing the clearance system time to catch up with the detritus that accumulates during wakefulness. The relative balance of these two processes – a respite from waste production, or a period of enhanced cleansing – needs further study.
Human imaging studies have provided recent support for the link between sleep and brain waste regulation. Sleep deprivation increases amyloid-beta in the brains of healthy young adults (22). Furthermore, injections of a contrast agent revealed that clearance from brain tissue is higher when participants sleep than when they are kept awake (6). This impaired waste clearance is apparent after a single night of sleep deprivation – a striking observation, given that this is a not infrequent behaviour for many individuals.
Why does sleep increase brain clearance? One contributing factor is that extracellular volume expands during sleep (5), which would increase the rate of molecular transport. Second, higher clearance rates occur in rodents when using anesthetics that induce high delta power (23), hinting that the neural dynamics of sleep are related to clearance. Another factor is that fluid flow patterns change during sleep. It has long been known that CSF flow in the awake human brain constantly pulses with the cardiac and respiratory cycles (24), but CSF flow during sleep has only recently been investigated.
A recent imaging study in humans repurposed classic flow-related enhancement signals in fMRI to simultaneously measure EEG, blood oxygenation, and CSF flow during sleep (9). This imaging revealed large waves of CSF flow during NREM sleep. The CSF waves were preceded by neural slow wave activity several seconds earlier and were anticorrelated with hemodynamic signals. This temporal coupling was consistent with a model in which neural activity drives CSF flow through its effects on blood volume, which in turn displaces CSF (Fig. 2a). This mechanism would explain how the intrinsic neural dynamics of sleep are linked to fluid flow.
Fig. 2: Disparate timescales of coupled neural, vascular, and CSF dynamics in sleep.
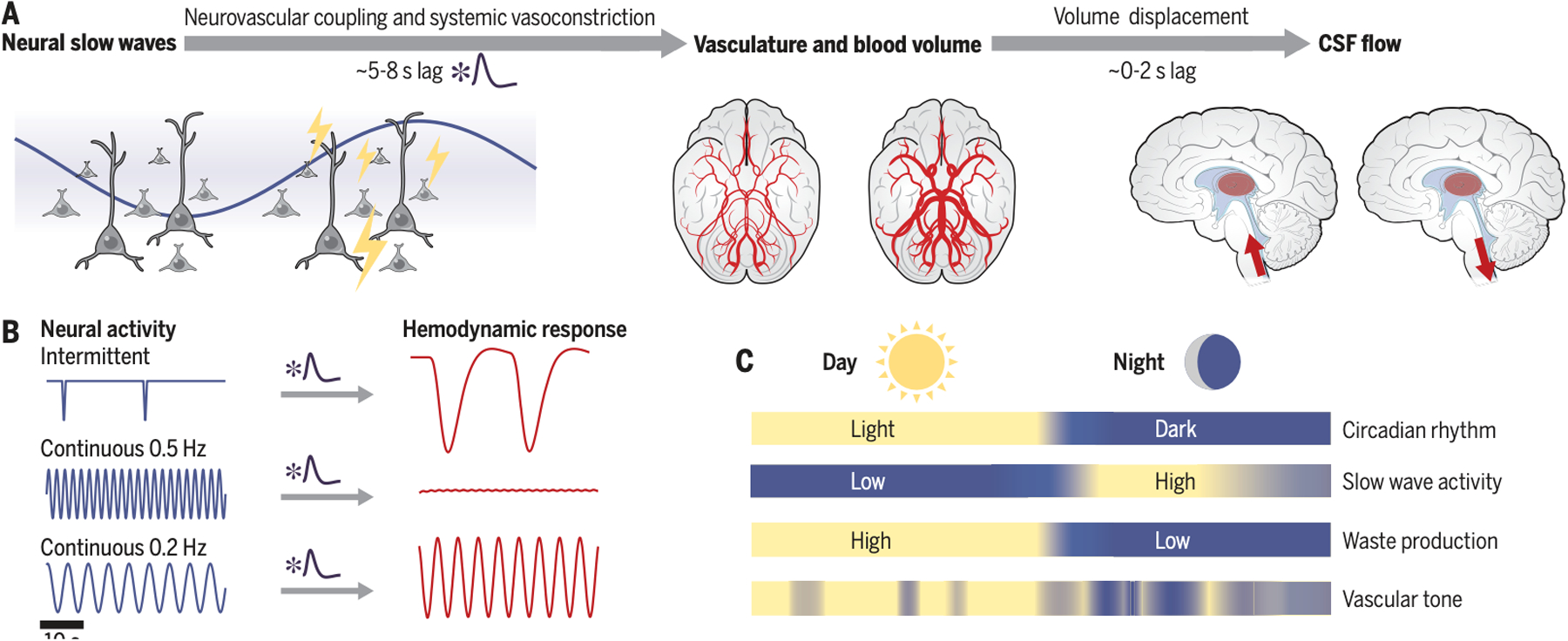
A) EEG slow wave activity (0.5–4 Hz) reflects coherent changes in neural activity. Slow coherent neural activity is linked to slow dilation and constriction of blood vessels. These blood volume changes drive CSF flow. B) Schematic illustrates that classic hemodynamic models produce slow temporal dynamics, and predict that infrequent or slow neural activity elicits larger responses. C) Multiple factors contribute on distinct timescales, including circadian rhythms and fluctuating autonomic state.
Neurovascular physiology contributing to CSF flow
What specific vascular mechanisms might implement this observed coupling of neural slow waves and CSF flow? This question highlights a continued challenge throughout sleep research, namely that so many features of brain physiology undergo correlated changes: neural slow waves are linked to glial activity, cognitive processes, autonomic state, and vascular dynamics. Several of these processes likely contribute to coupled neural and CSF flow waves. First, neural activity elicits local changes in blood volume through neurovascular coupling (25); this relationship is the basis of most fMRI studies. Low-frequency (~0.1 Hz) neural activity can also entrain arteriolar vasomotion, leading to fluctuations in blood volume (26). Because EEG slow waves correspond to widespread suppression of cortical firing, these neuronal changes would cause decreased blood volume and increased CSF flow. Furthermore, neurovascular coupling is strengthened during NREM sleep (27), bolstering this mechanism.
Second, slow waves during sleep not only reflect local neural activity, but also are often coupled to systemic changes in vasoconstriction caused by altered neuromodulatory and autonomic state, particularly when slow waves occur as isolated events termed K-complexes (28). This systemic vasoconstriction is also coupled to brainwide hemodynamic changes and CSF flow in humans (29, 30). In addition, individual slow waves are phase-locked to slow <1 Hz oscillations (11), which are linked to autonomic modulation of blood flow (12). Indeed, this pathway may not be fully separable from neurovascular coupling; the systemic vasoconstriction may partially reflect the need for brainwide hemodynamic modulation in concert with the large changes in neuronal activity. The neuromodulators linked to sleep can also have direct effects on vasodilation – for example, the noradrenergic system modulates both sleep slow oscillations and vessel diameter (31, 32). Its effects likely depend on whether release is tonic or phasic, which differs across sleep stages and would affect subsequent CSF flow. Importantly, low-frequency (~0.1 Hz) vascular oscillations are also present at lower levels during wakefulness. Consistent with the vascular-based model (Fig. 2), these low-frequency vascular dynamics are also coupled to CSF flow during wakefulness, but with lower amplitude than sleep (33). Similarly, modulating breathing (which affects vasodilation) affects CSF flow during wakefulness (34). Thus, in this framework, the temporal properties of the vasculature are the key component that sets the timing of fluid flow, and coherent low-frequency neural activity during sleep is therefore a particularly effective driver of flow.
A key prediction of the model proposed here is that neural activity that is most effective at entraining vascular changes will drive the largest CSF flow (Fig. 2b). Low-frequency EEG dynamics during sleep display many distinct patterns (Fig. 1); this model predicts that any of these slow dynamics could drive flow, if it is linked to widespread changes in vascular dilation. For example, an isolated slow wave would be predicted to drive CSF flow more effectively than continuous slow waves, due to the slow vascular response (Fig. 2b). In addition, lower frequency oscillations (e.g., slow oscillations) are predicted to be more effective than higher frequencies (e.g., delta waves). Furthermore, slow oscillations are phase-coupled to the amplitude of higher frequency dynamics such as spindles. Studies of total sleep deprivation cannot disambiguate between the roles of distinct neural rhythms, and further study is needed to test whether different oscillations have different links to flow. Given that vascular mechanics may be the critical element governing fluid flow and clearance, it can likely be induced by multiple types of coherent neural activity or slow modulators of vasodilation. Recent work in mice supports this idea: intriguingly, presenting low-frequency (0.05 Hz) visual stimuli entrained arteriole dilation and enhanced paravascular clearance (35).
Neural and fluid dynamics at mesoscale
A key open challenge is bridging the macroscopic and microscopic scales of fluid dynamics observed in sleep. Rodent studies established clearance rates by monitoring solute transport along vessels (7, 21). Human sleep studies have observed macroscopic CSF flow in the ventricle (9), and brainwide protein accumulation (6). A major question is how these scales are linked – how does the bulk flow of CSF in the ventricles affect clearance in the tissue, and is neurovascular coupling a viable mechanism for driving solute transport out of the brain? Although experimental access at the mesoscale is challenging, computational models have shed light on these questions. Models show that the slow timescale and large amplitude of neurovascular coupling is an effective mechanism for driving solute transport along arterioles (36, 37).
Importantly, studies measuring macroscopic CSF flow in humans have not yet specifically linked CSF velocity in the ventricles to rates of clearance. Intuitively, the idea of high-velocity CSF flow waves during sleep might be expected to increase clearance - the difference between sitting in a stagnant bath, versus waves where the water is constantly mixed and refreshed. However, this intuition has not yet been empirically confirmed, and future studies will be needed to determine the precise relationship between large-scale CSF flow and solute transport out of brain tissue.
Closing the loop: the consequences of fluid physiology for neuronal function
An emerging and tantalizing question is whether the effect of neural activity on CSF flow forms part of a bidirectional feedback loop, where each can influence the other. Several studies suggest specific routes by which fluid contents might modulate arousal. Ionic composition of the ISF can modulate neuronal firing and induce states of wakefulness or sleep (38). Amyloid and inflammatory cytokines also affect neural arousal state (39, 40). By modulating the local milieu of molecular composition of the ISF and CSF, clearance may thus affect sleep. Furthermore, individuals with genotypes linked to low aquaporin-4 expression, which forms part of the glymphatic pathway(7), show higher EEG slow wave activity (41). This observation has been proposed to reflect that EEG slow waves increase to compensate for lower clearance rates, although how such compensatory feedback might be implemented is unknown.
Second, the longer-term consequences of clearance – or lack thereof – may have more fundamental consequences for neuronal health, leading to inflammation or neurodegeneration in regions lacking adequate clearance. If clearance impairments affect the arousal regulatory circuits that induce sleep, this would further reduce sleep (42). Furthermore, as noted above, wakefulness is linked not just to lower clearance, but also higher rates of waste production in the first place (8). This ‘vicious cycle’ hypothesis could explain why disrupted sleep is linked to the development of neurodegenerative disorders.
The linked neural and vascular systems also point to a dual vulnerability in aging. The length and depth of sleep decline in aging. While some decrease in sleep is typical of healthy aging, greater sleep loss is predictive of subsequent Alzheimer’s disease pathology (2, 3). EEG slow waves in particular are implicated: patients with reduced slow wave activity show lower memory scores and increased gray matter atrophy (43). Decreased <1 Hz slow waves during sleep predict the subsequent accumulation of amyloid years later (44). While causal evidence is yet to be established, the link between EEG slow waves and fluid dynamics suggests that loss of slow waves, particularly in the lowest frequency bands, could impair clearance.
In addition to the declining neural signatures of sleep in aging, neurovascular physiology is also disrupted. Vascular dysfunction may be an early trigger for Alzheimer’s disease, as cerebral blood flow declines years prior to symptom onset (45), and may also result in a failure of sleep to drive effective clearance, because vascular dilations drive CSF flow. In support of this idea, the CSF flow imaging (9) was recently applied to a database from patients with mild cognitive impairment (MCI). Intriguingly, patients with MCI showed weaker coupling between hemodynamics and CSF flow (46), suggesting that indeed this vascular mechanism for driving CSF flow may be impaired at early stages of neurodegeneration.
Fluid physiology across sleep stages
Finally, it is unclear how distinct sleep stages differ in their contribution to fluid flow. Certainly, the mechanism discussed here addresses one part of the question, identifying how neural and fluid dynamics are linked in NREM sleep, particularly in stages such as N2 sleep where slow waves are apparent but irregularly timed. Humans spend most of their NREM sleep in stage N2 (47), and the infrequent slow wave timing in this state may be particularly effective at driving CSF flow due to the slow vascular filter (Fig. 2b). However, since local slow waves and changes in systemic vasodilation can occur even within the awake brain, the sleep-like CSF flow waves may also occur less frequently during wakefulness and lighter N1 sleep. Complicating these questions, in rodents, substages of NREM are not clearly mapped to human sleep stages, and the timing of neural oscillations and neurovascular coupling can differ. While these species-specific differences can pose challenges in translation, they also present an opportunity for dissecting how distinct neural and physiological dynamics modulate clearance systems within NREM.
In contrast, how clearance and CSF dynamics change during REM sleep is still unknown. Brainwide hyperemic patterns have been recently observed in rodents during REM sleep (48), and arterioles demonstrate unusually large dilations (27). These large fluctuations in blood volume may also drive CSF flow, but this possibility has not yet been tested. Because slow waves are not prominent in REM sleep, this suggests a distinct mechanism drives fluid dynamics during REM. One possibility is direct action of the neuromodulators linked to REM on the vasculature; for example, the cholinergic system, which is highly active in REM, can also have direct vasodilatory effects. Furthermore, circadian cycles also affect clearance (Fig. 2c) (49, 50). Neural activity alone thus cannot explain the full picture of clearance during sleep, and more work is necessary to understand how these mechanisms act together.
Outlook and open questions
Sleep has diverse effects on the brain; neural activity and cognition are transformed, systemic and autonomic physiology shifts, and critical housekeeping processes support neuronal health. While these processes have often been studied separately, they are intrinsically linked through their mechanistic origins and physiological consequences. The neural dynamics that appear during sleep in turn shape vascular and CSF flow, which feed back into these neural dynamics.
These converging results point to key open questions. First, what are the neural circuits that control clearance and fluid flow during sleep? While the work outlined above highlights a role for neural activity, how the diversity of specific neural signatures and sleep stages modulate fluid dynamics is not well understood. Given the astonishing number of neural circuit pathways that have been shown to control sleep, future work should identify how their interactions shape not only neural activity, but also vascular dynamics, CSF flow and clearance.
Recently developed technologies are increasingly making such brainwide, multimodal imaging studies possible. The ability to record at large scales in animal models enables studying the joint, spontaneous dynamics across the circuits that shape sleep. In human neuroscience, the impressive recent advances in the spatiotemporal resolution of fMRI also place many of these questions within reach. With these new technologies, the field is poised to make major advances on how these distributed dynamics interact to produce sleep states.
A second key challenge is to understand the mechanistic links between these interacting neuronal and non-neuronal systems, which pose a challenge for experimental investigation. Given that so many features of sleep are strongly correlated, dissecting causal relationships is difficult, and many conventional approaches include assumptions that preclude discovering these links. fMRI studies often will simply regress out one feature, such as respiration, but this presumes that these systemic physiological dynamics are a purely confounding factor – when in fact, during sleep, neural state is often collinear with and drives systemic physiology. Systems neuroscience approaches often manipulate one circuit to make statements about causality, but can miss the cascade of subsequent activity that results from focal manipulations. Directly modulating neural activity can sometimes produce effects unlike those that occur spontaneously. In addition to the neural and fluid dynamics outlined here, sleep also serves diverse other functions for the brain, ranging from synaptic homeostasis to glial function to memory and dreaming. Considering brain physiology during sleep as an interconnected dynamic system, via multimodal studies that simultaneously capture distinct aspects of sleep, is a promising perspective for understanding how these processes interact.
The results of such studies will be critical for interpreting how sleep is linked to neurological and psychiatric disorders. The decline of sleep in neurodegenerative disorders is now clearly established, highlighting the need to identify precise consequences of sleep for brain health. The relationship between clearance and psychiatric disorders is a much less explored area, but merits further study, because disordered sleep is a signature of several psychiatric disorders (51). Achieving mechanistic understanding is needed not just to probe and predict neural function linked to sleep, but to identify targets that may enable sleep-based interventions for improving brain health and clinical outcomes.
Ultimately, many factors act together to produce the effects of sleep, including coherent neural activity, vascular dynamics, and CSF and ISF flow. While these interacting components can make it challenging to probe individual mechanisms experimentally, considering these dynamics as a whole can reveal their biophysical links. Sleep’s powerful modulation of these many interconnected brain systems underlies its diverse and wide-ranging effects in maintaining cognition and healthy brain function.
Acknowledgments:
The author gratefully acknowledges discussions with Maiken Nedergaard, Robert Stickgold, and Jakob Voigts.
Funding:
The NIH R01-AG070135, R01-AT011429, 1907 Trailblazer Award, Brain and Behavior Research Foundation, Searle Scholar Award, One Mind Rising Star Award, and Simons Collaboration on Plasticity in the Aging Brain.
Footnotes
Competing interests: The author is a co-inventor on a patent application for a CSF imaging technique, and has received speaking fees from Roche.
References and Notes:
- 1.Krause AJ et al. , Nat Rev Neurosci. 18, 404 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucey BP et al. , Science Translational Medicine. 11, eaau6550 (2019).30626715 [Google Scholar]
- 3.Sabia S et al. , Nature Communications. 12, 2289 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klinzing JG, Niethard N, Born J, Nature Neuroscience. 22, 1598 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Xie L et al. , Science. 342, 373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eide PK, Vinje V, Pripp AH, Mardal K-A, Ringstad G, Brain. 144, 863 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Iliff JJ et al. , Science Translational Medicine. 4, 147ra111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holth JK et al. , Science. 363, 880 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fultz NE et al. , Science. 366, 628 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber F, Dan Y, Nature. 538, 51 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Amzica F, Steriade M, Neurology. 49, 952 (1997). [DOI] [PubMed] [Google Scholar]
- 12.Achermann P, Borbely A, Neuroscience. 81, 213 (1997). [DOI] [PubMed] [Google Scholar]
- 13.Cash SS et al. , Science. 324, 1084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis LD et al. , eLife. 7, e33250 (2018).30095069 [Google Scholar]
- 15.Iber C, Ancoli-Israel S, Chesson AL, Quan SF, The AASM Manual for the Scoring of Sleep and Associated Events (American Academy of Sleep Medicine, Westchester, Illinois, ed. 1, 2007). [Google Scholar]
- 16.Vyazovskiy VV et al. , Nature. 472, 443 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nir Y et al. , Neuron. 70, 153 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funk CM, Honjoh S, Rodriguez AV, Cirelli C, Tononi G, Current Biology. 26, 396 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakker ENTP, Naessens DMP, VanBavel E, Exp Physiol. 104, 1013 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mestre H, Mori Y, Nedergaard M, Trends Neurosci. 43, 458 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal N, Carare RO, Front Neurol. 11, 611485 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shokri-Kojori E et al. , Proc Natl Acad Sci USA. 115, 4483 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hablitz LM et al. , Sci Adv. 5, eaav5447 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feinberg DA, Mark AS, Radiology. 163, 793 (1987). [DOI] [PubMed] [Google Scholar]
- 25.Iadecola C, Neuron. 96, 17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mateo C, Knutsen PM, Tsai PS, Shih AY, Kleinfeld D, Neuron. 96, 936 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turner KL, Gheres KW, Proctor EA, Drew PJ, eLife. 9, e62071 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colrain IM, Sleep. 28, 255 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Özbay PS et al. , Commun Biol. 2, 421 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Picchioni D, bioRxiv (2021), doi: 10.1101/2021.05.04.442672. [DOI] [Google Scholar]
- 31.Berridge CW, Schmeichel BE, España RA, Sleep Medicine Reviews. 16, 187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peppiatt CM, Howarth C, Mobbs P, Attwell D, Nature. 443, 700 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H-C, bioRxiv, (2021), doi: 10.1101/2021.03.29.437406. [DOI] [Google Scholar]
- 34.Dreha-Kulaczewski S et al. , J Neurosci. 35, 2485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Veluw SJ et al. , Neuron. 105, 549 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diem AK, Carare RO, Weller RO, Bressloff NW, PLoS ONE. 13, e0205276 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kedarasetti RT et al. , Fluids Barriers CNS, 1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding F et al. , Science. 352, 550 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Özcan GG, Lim S, Leighton PL, Allison WT, Rihel J, eLife. 9, e53995 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besedovsky L, Lange T, Haack M, Physiol. Rev 99, 1325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ulv Larsen SM et al. , Plos Biol. 18, e3000623 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C, Holtzman DM, Neuropsychopharmacology. 45, 104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mander BA et al. , Nature Neuroscience. 16, 357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winer JR et al. , Curr. Biol 30, 4291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kisler K, Nelson AR, Montagne A, Zlokovic BV, Nat Rev Neurosci. 18, 419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han F et al. , Plos Biol. 19, e3001233 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV, Sleep. 27, 1255 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Bergel A, Deffieux T, Demené C, Tanter M, Cohen I, Nature Communications. 9, 5364 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai X et al. , Proc Natl Acad Sci USA. 117, 668 (2020).31848247 [Google Scholar]
- 50.Hablitz LM et al. , Nature Communications. 11, 4411 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benca RM, Obermeyer WH, Thisted RA, Gillin JC, Arch. Gen. Psychiatry 49, 651 (1992). [DOI] [PubMed] [Google Scholar]


