Abstract
BACKGROUND
Recent guidelines suggest that benzodiazepine premedication should be avoided in elderly patients, though with limited supporting evidence.
OBJECTIVE
We conducted a secondary analysis of the POSE data to explore the association of premedication in patients aged 80 years or older with 30-day mortality.
DESIGN
We used propensity score methods to perform a confounder-adjusted time-to-event analysis of the association between benzodiazepine premedication and 30-day mortality of the POSE study.
SETTING
POSE was conducted as a European multicentre prospective cohort study.
PATIENTS
Adults aged 80 years or older scheduled for surgical or nonsurgical intervention under anaesthesia.
RESULTS
A total of 9497 patients were analysed. One thousand five hundred and twenty-one patients received benzodiazepine premedication, 7936 patients received no benzodiazepine premedication, 30 received clonidine and 10 had missing premedication data. Inverse propensity-score-weighted log-rank analysis did not provide unambiguous evidence for an association between benzodiazepine premedication and 30-day mortality; median [range] P = 0.048 [0.044 to 0.078], estimated 30-day mortality rates 3.21% and 4.45% in benzodiazepine-premedicated and nonbenzodiazepine-premedicated patients, respectively. Inverse propensity-score-weighted Cox regression resulted in a hazard ratio of 0.71 (95% CI 0.49 to 1.04), pointing at a possible reduction of 30-day mortality in the benzodiazepine premedication group. Sensitivity analyses, which constituted subgroup, matched-pairs, and subclassification analyses, resulted in similar findings.
CONCLUSION
This secondary analysis of the POSE data did not find evidence for an unambiguous association between benzodiazepine premedication and 30-day mortality. Point estimates indicated a reduction of 30-day mortality in benzodiazepine-premedicated patients. The results presented here might be affected by unmeasured confounding factors, which could be addressed in a randomised trial.
TRIAL REGISTRATION
ClinicalTrials.gov Identifier: NCT03152734.
This article is accompanied by the following Editorial:
Columb MO, Longrois D, Hansen TG, Bruder N. Strike a pose: The POSE study poses some questions! Eur J Anaesthesiol 2022; 39:193–195.
Introduction
Surgery in patients aged 80 years and older is becoming more common. Risk factors, such as frailty and multimorbidity lead to enhanced mortality and morbidity.1,2 The Peri-interventional Outcome Study in the elderly (POSE) was designed to shed light on the peri-operative 30-day mortality rate in Europe. POSE was a prospective, multicentre cohort study of patients aged 80 years and older undergoing surgical or nonsurgical interventions under anaesthesia. In total, 9497 patients from 177 hospitals in 20 countries were analysed, and 388 deaths were observed. POSE revealed a postinterventional mortality rate of 4.2% (95% CI 3.8 to 4.7%).3 Secondary analyses of POSE assessed risk factors for mortality in the elderly, and these may open avenues for their analysis. Risk factors for 30-day mortality were analysed using a Cox regression model with 14 fixed effects and a random centre effect. Among others, the following risk factors for 30-day mortality were identified: multimorbidity, hazard ratio 1.87 (95% CI 1.26 to 2.78), frailty, hazard ratio 2.63 (95% CI 2.10 to 3.30), and limited mobility, hazard ratio 2.19 (95% CI 1.24 to 3.86).3
Of note, 1521 (16%) of the POSE study cohort received benzodiazepine premedication before intervention.3 Whilst benzodiazepine premedication is thought to reduce preoperative anxiety, it comes with an array of serious side effects, especially in the elderly. Side effects include dose-dependent sedation to respiratory depression, paradoxical reactions and antegrade amnesia, increased pneumonia rates and postoperative delirium.4–6 The latter is associated with an increased mortality rate.7 Recent guidelines suggest avoiding benzodiazepines for premedication in order to prevent postoperative delirium.8,9 Yet, these recommendations lack support from appropriate powered randomised controlled trials (Grade of Recommendation B).9 Of note, a recent study assessing more than 48 000 patients undergoing major noncardiac surgery in a large academic health system found that 65% of all patients aged 50 years or older received a benzodiazepine during anaesthesia care.10 The reason for the discrepancy between clinical practice and guidelines advising against benzodiazepine use in older surgical patients remains unanswered.10 A possible hypothesis for a beneficial effect of benzodiazepine premedication, offered by Jeon et al. in a randomised controlled trial, might be that midazolam premedication reduced entropy values, stabilized the cardiovascular system, and provided analgesia during the induction of anaesthesia.11
Surprisingly, the secondary analysis of POSE revealed that all-cause mortality was reduced up to day 30 in patients receiving benzodiazepine premedication (estimated hazard ratio, 0.58, unadjusted 95% CI 0.40 to 0.85; P = 0.02).3 Thus, the objective of this secondary POSE analysis was to investigate the effect of benzodiazepine premedication on the 30-day mortality applying propensity score analysis to adjust estimates for possible confounding and selection bias.
Methods
Study design, setting, and participants
POSE was a European multicentre, observational prospective cohort study. Patients were eligible if aged 80 years or older and undergoing surgical or nonsurgical intervention under anaesthesia. The study lasted from October 2017 to December 2018. Each centre recruited patients for 30 consecutive days within the study period. Interventions were classified as either surgical or nonsurgical, elective or nonelective, and in patient or outpatient. The primary outcome was all-cause mortality within 30 days after intervention and was presented by Kaplan–Meier curves with 95% CI. Risk factors for 30-day mortality were analysed using a Cox regression model with 14 fixed effects and a random centre effect. The full study protocol is available at www.pose-trial.org/study-documents/ and has been described previously.3 Mandatory research ethics board (REB) approval or a waiver was granted for each centre. Initial REB approval was received from the University Hospital RWTH Aachen, Germany (EK 162/17) on 18 September 2017. POSE was registered at ClinicalTrials.gov Identifier: NCT03152734.
This secondary analysis was approved by the POSE Steering Committee, see https://pose-trial.org/secondary-analyses/. Data transfer agreement from the University Hospital RWTH Aachen to the Department of Medical Biometry, Informatics and Epidemiology, Faculty of Medicine, University of Bonn was established and signed accordingly.
Baseline data and outcome measures were described previously in the POSE study protocol, glossary and trial statistical analysis plan (www.pose-trial.org/study-documents).3 Premedication was assessed at visit 2 (intervention day) as ‘none’, ‘clonidine’ or ‘benzodiazepine’ premedication before intervention. Continuous variables are summarised using mean ± SD and median [range]. Categorical variables are summarised using absolute and relative frequencies.
Analysis approach
Propensity score analysis was used to control for confounding and selection bias. Briefly, the propensity score is defined as the probability of receiving the treatment of interest, here benzodiazepine premedication, conditional on a set of baseline covariates, for example, the confounding variables.12 It serves as a ‘balancing score’ in the sense that conditional on the propensity score, the distributions of the observed baseline covariates are expected to be similar between treated and untreated patients. Estimated values of the propensity score can be derived using logistic regression analysis (see below).
Here we used the propensity score to perform a confounder-adjusted time-to-event analysis of the association between benzodiazepine premedication and 30-day mortality. To this end, we implemented an inverse-propensity-score-weighted (IPW) approach.13 The IPW approach is designed to analyse the population average treatment effect (PATE), which for POSE corresponds to the effect of benzodiazepine premedication on 30-day mortality in patients sharing the baseline characteristics of the whole study population. The PATE was estimated by inverse-propensity-score-weighted Kaplan–Meier, log-rank, and Cox regression analysis.13 Technical details are outlined in the eMethods, Supplemental Digital Content 1.
Specification and validation of the propensity score model
We specified a multivariable logistic regression model with binary outcome variable ‘benzodiazepine premedication versus nonbenzodiazepine premedication’ to obtain estimates of the propensity score. The covariates chosen for this model included all covariates (except premedication) from the Cox regression model presented in Kowark et al., as these variables were shown to be related to 30-day mortality.3 Specifically, we included the covariates ‘age’, ‘sex’, ‘severity of intervention’, ‘urgency of intervention’, ‘frailty’, ‘interventional category’, ‘referring facility’, ‘transfusion of plasma’, ‘transfusion of platelets’, ‘transfusion of red blood cells’, ‘anaesthesia technique’, ‘multimorbidity’, and ‘limited mobility timed up & go test’. In addition, we included the covariates ‘chronic benzodiazepine medication’ and ‘Mini-Cog score ≤ 3 points’, as these would probably be related to benzodiazepine premedication.13 Unlike the Cox regression model in Kowark et al., our propensity score model did not include a (random) centre effect.3 This was because centre-specific treatment regimens allowed perfect prediction of benzodiazepine premedication in some of the study centres, implying that the inclusion of a centre effect would have destabilised the propensity score model.14 After model fitting, we checked the ability of the estimated propensity score to balance covariate distributions between the two premedication groups. This was done using standardised differences and mirror histograms.15
Handling of missing data
Preprocessing steps and handling of missing values were described previously.3 Missing values were replaced using a multiple imputation approach with 12 imputations. Imputations were based on all dependent and independent variables included in the Cox regression model.3 A separate propensity score model was fitted to each of the 12 imputed data sets. Estimated hazard ratios (hazard ratio, 95% CI) from Cox regression were combined using Rubin‘s rule. P values of log-rank tests were summarised using median [range].
Sensitivity analyses
Propensity score analysis may be affected by unmeasured confounding and by possible misspecification of the propensity score model. Therefore, in addition to the IPW analysis described above, we conducted five sensitivity analyses. For the first sensitivity analysis, we conducted IPW analyses in three subgroups defined by ‘Mini-Cog score ≤3 points’, ‘Mini-Cog score > 3 points’ and ‘surgical interventions’. In the second sensitivity analysis, we investigated the behaviour of the propensity score by repeatedly leaving out a randomly selected subset of three covariates from the logistic regression model and comparing the resulting IPW estimates. In the third sensitivity analysis, we performed a subclassification analysis using five subclasses defined by quintiles of the propensity score.13 In the fourth sensitivity analysis, we investigated the population average treatment effect for the treated (PATT), which, other than the PATE, corresponds to the effect of benzodiazepine premedication on 30-day mortality in the subcohort sharing the baseline characteristics of the benzodiazepine-premedicated patients. The PATT was analysed by a pairwise matching approach that evaluated 30-day mortality within matched pairs of benzodiazepine and nonbenzodiazepine-premedicated patients. Time-to-event analysis of the matched data was performed using stratified log-rank tests and Cox regression with a robust variance estimator. Several matching techniques, including caliper matching of the propensity scores and exact matching both with the propensity score and coarsened variables, were investigated and compared. Finally, in the fifth sensitivity analysis, we investigated whether similar results were obtained in the analysis of the outcome ‘major complication’ (yes/no), which was defined by either death within 30 days after intervention, any in-hospital complication according to the ACS NSQIP, or any complication after discharge. This outcome was analysed using inverse-propensity-score-weighted logistic regression. Details on the sensitivity analyses are provided in the eMethods, Supplemental Digital Content 1.
All statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, North Carolina, USA) and R, version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 9497 patients were analysed. In detail, 1521 patients received benzodiazepine premedication, 7936 patients received no benzodiazepine premedication, 30 patients received clonidine and 10 had missing data in the premedication variable (Table 1). Patients that received clonidine premedication (30 out of 9497 in each of the 12 imputed data sets) were excluded from further analysis because of the low numbers and because allocation to either the benzodiazepine or the nonbenzodiazepine premedication group was not justifiable. There were 388 observed deaths within 30 days after intervention. Altogether, 1348 patients (14.2%) had a follow-up time of less than 30 days. Baseline characteristics are presented in Table 1. The benzodiazepine premedicated group contained less urgent interventions, more otolaryngological interventions, less orthopaedic and nonsurgical interventions, more use of sedation as the main anaesthesia technique and slightly more with chronic benzodiazepine medication.
Table 1.
Patient, baseline and interventional characteristics
| Benzodiazepine premedicationa (n=1521)b | No benzodiazepine premedicationa (n=7936)b | |||
| Age (years) | 83.9 ± 3.5; 83 [80 to 100] | 84.3i | 84.4 ± 3.9; 83 [80 to 104] | 84.3 |
| Sex | ||||
| Male | 715 (47.0%) | 46.8%j | 3752 (47.3%) | 47.3% |
| Female | 806 (53.0%) | 53.2% | 4184 (52.7%) | 52.7% |
| Multimorbidityc | ||||
| No | 336 (22.1%) | 21.4% | 1817 (22.9%) | 22.6% |
| Yes | 1185 (77.9%) | 78.6% | 6119 (77.1%) | 77.4% |
| Limited mobility TUG testd | ||||
| Limited | 1037 (78.0%) | 77.6% | 5395 (77.0%) | 77.2% |
| Normal | 293 (22.0%) | 22.4% | 1612 (23.0%) | 22.8% |
| Missing | 191 | 929 | ||
| Mini-Cog score ≤3 pointse | ||||
| No | 608 (40.0%) | 42.4% | 3477 (43.8%) | 43.3% |
| Yes | 913 (60.0%) | 57.6% | 4459 (56.2%) | 56.7% |
| Frailtyf | ||||
| No | 1372 (90.2%) | 85.4% | 6752 (85.1%) | 85.9% |
| Yes | 149 (9.8%) | 14.6% | 1184 (14.9%) | 14.1% |
| Chronic benzodiazepine | ||||
| No | 1268 (83.4%) | 87.3% | 6973 (87.9%) | 87.2% |
| Yes | 253 (16.6%) | 12.7% | 958 (12.1%) | 12.8% |
| Referring facility | ||||
| Home | 1359 (89.4%) | 88.3% | 6825 (86%) | 86.6% |
| Other hospital | 23 (1.5%) | 1.8% | 159 (2%) | 1.9% |
| Rehabilitation | 3 (0.2%) | 0.5% | 57 (0.7%) | 0.6% |
| Nursing home | 64 (4.2%) | 6.5% | 604 (7.6%) | 7.1% |
| Other | 72 (4.7%) | 2.9% | 288 (3.6%) | 3.7% |
| Missing | 0 | 3 | ||
| Type of intervention | ||||
| Abdominal | 166 (10.9%) | 12.6% | 978 (12.3%) | 12.2% |
| Cardiovascular | 177 (11.6%) | 9.3% | 710 (9.0%) | 9.4% |
| ENT | 442 (29.1%) | 15.6% | 1151 (14.5%) | 16.6% |
| Gynaecological | 201 (13.2%) | 15.8% | 1229 (15.5%) | 15.2% |
| Interventional | 91 (6.0%) | 10.0% | 933 (11.8%) | 10.9% |
| Neurosurgery | 14 (0.9%) | 1.9% | 181 (2.3%) | 2.1% |
| Othopaedic | 381 (25.1%) | 30.8% | 2465 (31.1%) | 30.1% |
| Other surgery | 49 (3.2%) | 4.0% | 289 (3.6%) | 3.6% |
| Severity of intervention | ||||
| Minor | 311 (20.5%) | 19.1% | 1629 (20.5%) | 20.5% |
| Intermediate | 588 (38.7%) | 38.0% | 3012 (38.0%) | 38.0% |
| Major | 622 (40.9%) | 42.9% | 3295 (41.5%) | 41.5% |
| Urgency of intervention | ||||
| Elective | 1295 (85.1%) | 76.2% | 5852 (73.7%) | 75.5% |
| Urgent | 185 (12.2%) | 18.7% | 1648 (20.8%) | 19.4% |
| emergency | 41 (2.7%) | 5.1% | 436 (5.5%) | 5.1% |
| Anaesthesia technique | ||||
| Combinedg | 174 (11.4%) | 11.7% | 881 (11.1%) | 11.2% |
| General | 609 (40.0%) | 52.7% | 4420 (55.7) | 53.3% |
| Regionalh | 285 (18.7%) | 18.1% | 1340 (16.9%) | 17.2% |
| Sedation | 453 (29.8%) | 17.5% | 1295 (16.3%) | 18.3% |
| Transfusion of red blood cells | ||||
| No | 1407 (92.6%) | 94.0% | 7476 (94.2%) | 93.9% |
| Yes | 113 (7.4%) | 6.0% | 460 (5.8%) | 6.1% |
| Missing | 1 | 0 | ||
| Transfusion of plasma | ||||
| No | 1486 (97.8%) | 98.5% | 7829 (98.7%) | 98.5% |
| Yes | 34 (2.2%) | 1.5% | 107 (1.4%) | 1.5% |
| Missing | 1 | 0 | ||
| Transfusion of platelets | ||||
| No | 1501 (98.8%) | 99.4% | 7891 (99.4%) | 99.3% |
| Yes | 19 (1.3%) | 0.6% | 45 (0.6%) | 0.7% |
| Missing | 1 | 0 | ||
Data are presented as n (%), mean ± SD or median [range]. ENT, Ear nose and throat; TUG, Timed Up and Go test.
Thirty patients that received clonidine and 10 patients with missing data in the premedication variable are excluded from the table.
Percentages may not total 100 because of rounding.
Multimorbidity was defined as the presence of at least two of the assessed comorbidities.
Limited mobility was defined as Timed Up and Go test performed in more than 12 s.
Mini-Cog screening tool to detect cognitive impairment or dementia: 3 or less cognitive impairment according to Robinson et al.
Frailty was classified as present, if at least four of the following six markers were present: Mini-Cog total score of 3 or less points; albumin level of 33 g l−1 or less; >1 fall in the last 6 months; haematocrit level of less than 35%; preoperative functional status is partially dependent or totally dependent; and at least three comorbidities present (according to Robinson et al.14 and Oresanya et al.16).
Regional anaesthesia constitutes the epidural, spinal or other regional anaesthesia technique.
Combined anaesthesia is defined as a combination of at least two of the three categories: general anaesthesia, sedation or regional anaesthesia.
Inverse-propensity-score weighted mean.
Effective relative frequency defined as the proportion on the inverse-propensity-score weights for each category in the benzodiazepine and nonbenzodiazepine premedication groups.
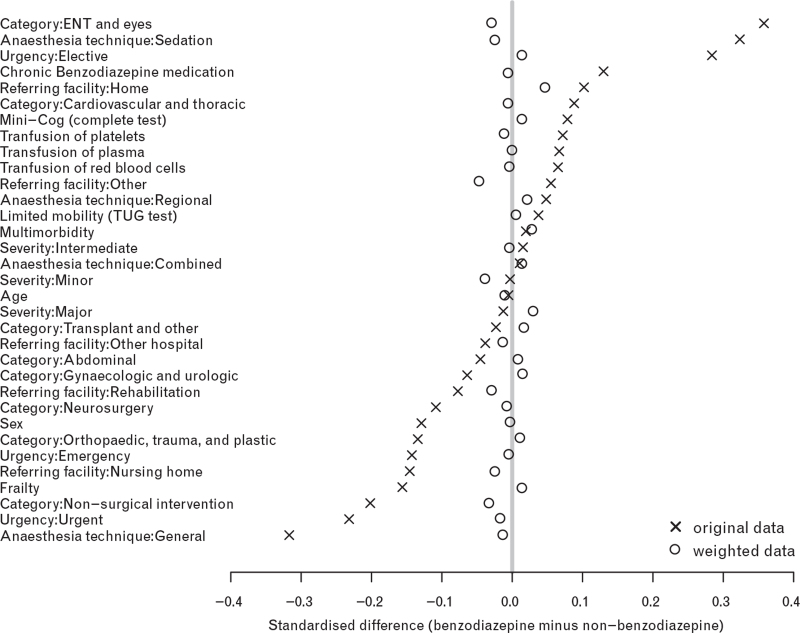
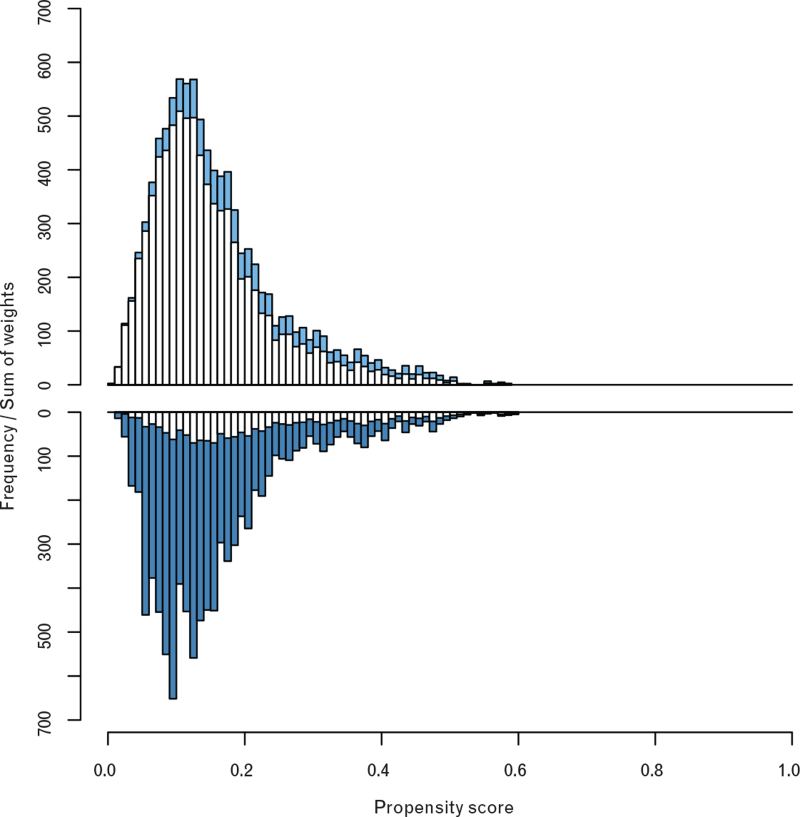
Imputation 1 (Fig. 1) presents standardised differences of the covariates that were included in the propensity score model. Fig. 1 shows that standardised differences were below 10% after propensity score adjustment, indicating a very good ability of the propensity score model to balance covariate distributions between the two premedication groups.12 Results obtained from imputations 2 to 12 were similar (data not shown). The mirror histogram presented in Fig. 2 (imputation 1) suggests a satisfactory overlap of the two premedication groups with respect to the distribution of the propensity score values (see white bars in Fig. 2). Again, results obtained from imputations 2 to 12 were similar (data not shown).
Fig. 1.
Standardised differences of the covariates included in the propensity score model
Standardised mean differences between the benzodiazepine-premedicated and nonbenzodiazepine-premedicated patients obtained from imputed data set 1. The crosses refer to the standardised differences in the original data set; the circles refer to the inverse-propensity-score-weighted standardised differences (see eMethods for the definition). The grey vertical line refers to a zero difference between the (weighted) means of the benzodiazepine-premedicated and nonbenzodiazepine-premedicated patients. Overall, the small distances between the circles and the reference line (all <10%) indicate a favourable balancing property of the fitted propensity score model.
Fig. 2.
Distribution of the propensity score values
Histogram of the estimated propensity scores of the benzodiazepine-premedicated patients (lower panel), and nonbenzodiazepine-premedicated patients (upper panel) obtained from imputed data set #1. The heights of the blue bars equal the sums of the inverse-propensity-score weights (see eMethods for the definition) of the benzodiazepine-premedicated (dark blue) and nonbenzodiazepine-premedicated (light blue) patients in the respective strata. The similarity of the two blue histograms suggests a satisfactory overlap of the two premedication groups with respect to the distribution of the propensity score values. This overlap is required for the IPW analysis to draw credible inferences about the treatment effect for the whole study cohort.
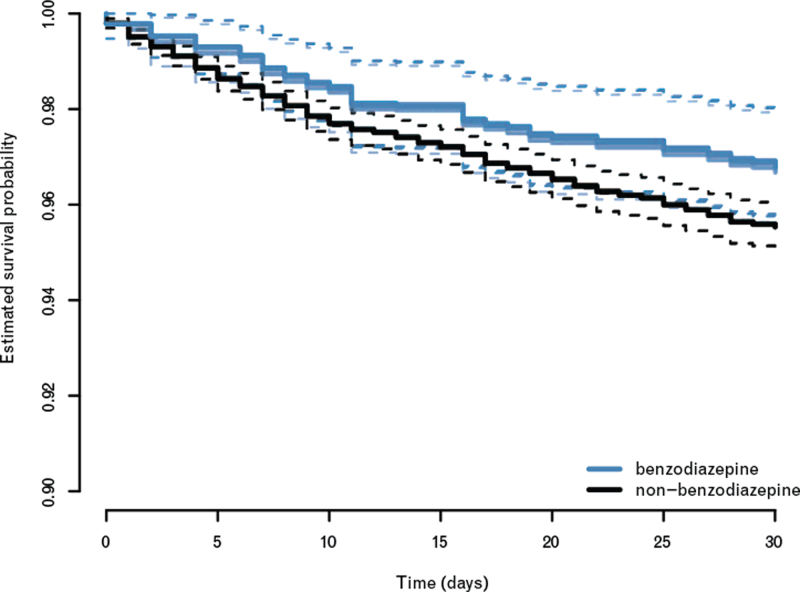
Inverse-propensity-score-weighted log-rank analysis did not provide unambiguous evidence for an association between benzodiazepine premedication and 30-day mortality, median P = 0.048 [0.044 to 0.078]. The 30-day survival probabilities in the benzodiazepine and nonbenzodiazepine premedicated patients were 96.79 and 95.54%, respectively, when averaged across the 12 imputations (Fig. 3). Accordingly, the respective 30-day mortality rates were estimated to be 3.21% and 4.46%. Inverse-propensity-score-weighted Cox regression resulted in a hazard ratio of 0.71 (95% CI, 0.49 to 1.04). This result confirmed the results obtained from log-rank analysis, as it did not provide unambiguous evidence for a reduced 30-day mortality in the benzodiazepine premedication group.
Fig. 3.
Estimated survival probabilities in the benzodiazepine-premedicated and nonbenzodiazepine-premedicated patients
Inverse-propensity-score-weighted Kaplan–Meier survival curves obtained from the 12 imputed data sets. The black lines refer to the estimates for the nonbenzodiazepine-premedicated patients; the blue lines refer to the respective estimates for the benzodiazepine-premedicated patients. The dashed lines indicate pointwise 95% confidence bands.
Sensitivity analyses
Results from the first sensitivity analysis (subgroup analyses) are presented in Table 2. Although hazard ratios pointed in the same direction as in the analysis of the complete data set, there were strong differences between patients with ‘Mini-Cog score >3 points’ (hazard ratio = 0.35, 95% CI 0.13 to 0.97, median P = 0.02, 30-day survival probabilities 99% and 97.19%) and patients with ‘Mini-Cog score ≤ 3 points’ (hazard ratio = 0.86, 95% CI 0.57 to 1.29, median P = 0.41, 30-day survival probabilities 95.05% and 94.28%). In the subgroup of patients with a surgical intervention, estimates were similar to the analysis of the complete data set (hazard ratio = 0.69, 95% CI 0.47 to 1.01, median P = 0.03, 30-day survival probabilities 96.77% and 95.36%). In the second sensitivity analysis (stability of the propensity score model, analysed using 100 random subsamples of the covariate set), variations in the inverse-propensity-score-weighted estimates did not contradict the analysis of the complete data set (hazard ratio range 0.59 to 0.75, 95% CI lower bound range 0.41 to 0.51, 95% CI upper bound range 0.86 to 1.08, Table 2). In the third sensitivity analysis (subclassification model with random centre effects, defined by quintiles of the propensity score), a stronger effect than in the inverse-propensity-score weighted analysis was found (pooled hazard ratio = 0.56, 95% CI 0.38 to 0.84, Table 2). Regarding the fourth sensitivity analysis, that of the PATT using pairwise matching, HR estimates obtained from Cox regression were 0.64 (95% CI, 0.39 to 1.03); 0.73 (95% CI, 0.41 to 1.33), and 0.90 (95% CI 0.41 to 1.97), respectively for caliper matching on the propensity score values, exact matching on the propensity score values, and exact matching on coarsened variables (see Table 2, which also includes the respective estimates of the 30-day survival probabilities). Estimates of the PATT pointed in the same direction as the estimate of the PATE obtained from inverse-propensity-score-weighted analysis. Results obtained from the fifth sensitivity analysis (inverse-propensity-score-weighted logistic regression analysis of the outcome ‘major complication’; yes/no) also agreed with the PATE estimates of 30-day mortality, pooled odds ratio estimate = 0.94 (95% CI 0.89 to 1.00), median P value from logistic regression = 0.047 [0.035 to 0.069], Table 2.
Table 2.
Results of the sensitivity analyses
| Analysis | Description | Hazard ratio (95% CI) | P value of log-rank test/logistic regression median [range] | 30-day survival (95% CI) |
| 1 | MiniCog score >3 (n = 4093, deaths = 101) | 0.3502 (0.1264 to 0.9699) | 0.0193 [0.0183 to 0.0632] | Nonbenzodiazepine: 0.9719 (0.9666 to 0.9775) benzodiazepine: 0.9900 (0.9803 to 0.9998) |
| MiniCog score <= 3 (n=5374, deaths = 286) | 0.8598 (0.5711 to 1.2943) | 0.4140 [0.3968 to 0.4233] | Nonbenzodiazepine: 0.9428 (0.9360 to 0.9497) benzodiazepine 0.9505 (0.9319 to 0.9695) | |
| Surgical interventions (n=8441, deaths = 358) | 0.6870 (0.4669 to 1.0110) | 0.0343 [0.0316 to 0.0369] | Nonbenzodiazepine: 0.9536 (0.9487 to 0.9586) benzodiazepine: 0.9677 (0.9560 to 0.9796) | |
| 2 | Stability of the propensity score model (100 random subsamples) (n=9467, deaths = 388) | Range: 0.5933 to 0.7455 Lower limit (0.4095 to 0.5135) Upper limit (0.8598 to 1.0823) | — | — |
| 3 | Subclassification with random centre effect (n=9467, deaths = 388) | 0.5630 (0.3765 to 0.8424) | — | — |
| 4 | Caliper matching on the propensity score values (n= 972 to 2988, deaths = 82 to 100) | 0.6376 (0.3930 to 1.0344) | 0.0560 [0.0016 to 0.2100] | Nonbenzodiazepine: 0.9621 (0.9509 to 0.9708) benzodiazepine: 0.9757 (0.9663 to 0.9825) |
| Exact matching on the propensity score values (n=2444 to 2532, deaths = 65 to 90) | 0.7342 (0.4054 to 1.3299) | 0.1758 [0.0137 to 0.6115] | Nonbenzodiazepine: 0.9637 (0.9516 to 0.9729) benzodiazepine: 0.9735 (0.9627 to 0.9811) | |
| Coarsened exact matching (n=1452 to 1480, deaths = 27 to 29) | 0.9018 (0.4134 to 1.9672) | 0.8474 [0.3532 to 1.0000] | Nonbenzodiazepine: 0.9797 (0.9666 to 0.9877) benzodiazepine: 0.9817 (0.9687 to 0.9893) | |
| 5 | Major complication (n=9308, complications = 3315) | 0.9411 (0.8854 to 1.0004) | 0.0469 [0.0352 to 0.0694] | — |
Hazard ratios with 95% confidence intervals from Cox regression are presented. P values from log-rank tests/logistic regression (if applicable), and estimated 30-day survival probabilities with 95% confidence intervals (if applicable). For each sub-analysis, the sample size and number of deaths is indicated in the second column. Pairwise matching (sensitivity analysis 4) resulted in strongly reduced sample sizes (n = 1452 to 2988). There were 189 missing values in the outcome ‘major complication’ (no death + no in-hospital complication + missing complication after discharge: 186; no death + no complication after discharge + missing in-hospital complication: 2; no death + missing in-hospital complication + missing complication after discharge: 1).
Discussion
Our inverse-propensity-score-weighted log-rank analysis of the POSE data did not show an unambiguous association between benzodiazepine premedication and 30-day mortality. However, point estimates consistently indicated a reduced 30-day mortality in benzodiazepine-premedicated patients. This finding is novel and in contrast to recent guidelines suggesting benzodiazepine premedication could increase 30-day survival.7,8
It should be noted that premedication groups in our study population differed considerably with respect to the distribution of possible confounder variables, implying that inverse-propensity-score-weighting may not have fully balanced the groups as in a randomised design. Thus far, benzodiazepines for premedication have been given mainly for its anxiolytic effects in relation to overall patient satisfaction. However, a recently conducted randomised, placebo-controlled study in France that included 1062 elective surgical patients aged less than 70 years (mean 50 years) showed no difference in regard to the patient satisfaction between three groups receiving 2.5 mg lorazepam, placebo and no premedication.15 Time to extubation and early postoperative recovery were both significantly prolonged, though worse in the lorazepam group than in the control or placebo-group. Only 24% of the patients showed an increased pre-operative anxiety level and the subgroup analysis of these patients did not reveal a difference in regard to the overall patient satisfaction,15 for example, a retrospective analysis showed higher discomfort and pain in premedicated patients.16
Previous attempts to investigate the effects of premedication with alpha-2 adrenergic agonists, such as clonidine, mivazerol and dexmedetomidine in high-risk patients who underwent high-risk surgery revealed that prophylactic alpha-2 adrenergic agonists generally do not prevent peri-operative death or major cardiac complications. For noncardiac surgery, there is moderate-to-high-quality evidence that these agents do not prevent death, myocardial infarction or stroke. Conversely, there is evidence of moderate quality suggesting that these agents have important adverse effects, namely increased risks of hypotension and bradycardia. For cardiac surgery, there is evidence of moderate quality that alpha-2 adrenergic agonists have no effect on the risk of mortality or myocardial infarction, and that they increase the risk of bradycardia. The quality of evidence was too inadequate to draw conclusions regarding the effects of alpha-2 agonists on stroke or hypotension during cardiac surgery.17 Therefore, reasoning by analogy is not in favour of such a strong effect of a single intervention of benzodiazepine on 30-day survival. Furthermore experimental ‘evidence’ is not consistent with the results of the data obtained here.6
The cognitive assessment (as reflected by the Mini-Cog score) revealed a major impact on outcome. This could be an important management modifier as it could have direct practical consequences for the anaesthetic team. The need for cognitive assessment is underlined by the updated guideline ‘Preoperative evaluation of adults undergoing elective noncardiac surgery’ from the European Society of Anaesthesiology and Intensive Care Medicine, which recommends the preoperative evaluation of cognitive function in the geriatric patient.18
There is a need for a large randomised controlled trial to clarify the evidence for or against the use of preoperative benzodiazepine in elderly patients. At present, a multicentre, randomised, placebo controlled trial is being carried out to assess patient satisfaction after premedication in elderly patients. In total 614 patients aged between 65 and 80 years undergoing elective surgery with general anaesthesia have been randomised to receive either 3.75 mg midazolam or placebo.19 This will be, to the best of our knowledge, the first randomised controlled trial to assess the effect of benzodiazepine premedication in elderly patients. At present, patient inclusion has been successfully completed and data analysis is ongoing (ClinicalTrials.gov, NCT03052660).
Limitations
POSE was designed to describe the 30-day mortality rate of patients aged 80 years and older undergoing interventions under anaesthesia. The primary outcome was all-cause mortality within 30 days. POSE enrolled patients consecutively in order to minimise selection bias. The objective nature of the primary outcome and the multiple attempts to obtain follow-up data reduced the risk of detection and attrition bias. However ten missing data values for the premedication before intervention occurred in 9497 patients. Premedication data were assessed on day of intervention as follows: ‘premedication before intervention: none, clonidine or benzodiazepine’. Consequently, a major limitation is the lack of data on type, time schedule (benzodiazepine on-site premedication is not comparable to a preoperative drug intake) and dose of benzodiazepine premedication. In line with the outcome definition and analysis strategy for the main study3, we did not analyse the length of stay in patients discharged alive. This aspect could be investigated using, for example, a cause-specific approach treating ‘discharge alive’ as a competing event for in-hospital death. Further increased transfusion treatments in the midazolam premedication group could also indicate a confounder treatment bias in that these patients may have been selected for more aggressive clinical management. Finally, POSE is an observational study with nonrandom assignment of benzodiazepine and nonbenzodiazepine premedication.
Considerations on propensity score adjustment
The results presented here are based on the statistical framework of propensity score adjustment. This framework is useful for reducing bias in the estimation of treatment effects in observational studies, as it balances the distribution of covariates between the treatment and the control groups, here benzodiazepine premedication vs. nonbenzodiazepine premedication.10 Consequently, propensity score adjustment tries to mimic randomised treatment assignment in a controlled trial, allowing for direct comparisons of the respective outcomes, here log-rank analysis of 30-day mortality. Although Fig. 1 showed that our propensity score model possesses very favourable balancing properties, it must be noted that the approach is not without limitations. Most importantly, as pointed out by Stuart, propensity-score-adjusted estimations rely on the assumption of ignorability, which states that there are ‘no unobserved differences between the treatment and control groups, conditional on the observed covariates’.13 As a consequence, propensity-score-adjusted estimation will only yield unbiased results if all variables that are related to both treatment assignment and the outcome have been recorded and included in the propensity score model. In case of POSE, data collection was prospectively planned and a large number of potential confounder variables had been already identified before the beginning of the study. In particular, all variables that were previously shown to be associated with the outcome were included in the propensity score model. Still, by the nature of the observational design of POSE, unmeasured confounding and a potentially misspecified propensity score model cannot be ruled out. With regard to the effect of possibly unmeasured confounding, one may additionally calculate the E value, which represents ‘the minimum strength of both the confounder associations that must be present, above and beyond the measured covariates, for an unmeasured confounder to explain away an association’.20 In case of the inverse-propensity-score-weighted estimate obtained in this study (hazard ratio = 0.71), the E value is calculated as E = 2.17, with a lower confidence limit of 1 (since the 95% CI for the hazard ratio 0.49 to 1.04 includes the value 1). Hence, any unmeasured confounder associated with both benzodiazepine premedication and 30-day mortality by 2.17-fold risk or hazard could explain away the observed association, whereas weaker confounding could not. For the lower confidence limit, any unmeasured confounder could explain away the observed effect.
In summary, the obtained data are novel and hypothesis generating. At present, data from a large randomised, placebo-controlled trial assessing benzodiazepine premedication in elderly patients are expected, and should be taken into consideration before rolling out possible new randomised trials.19 Then, if favourable, and to ultimately confirm our results and address the issue of unmeasured confounding, a controlled trial with randomised assignment of benzodiazepine premedication would be needed.
Conclusion
This secondary analysis of the observational POSE study did not provide unambiguous evidence for an association between benzodiazepine premedication and 30-day mortality in elderly patients. However, point estimates indicated a reduced mortality hazard in benzodiazepine-premedicated patients. This reduction was also seen in several sensitivity analyses, which included subgroup analyses of surgical interventions and cognitively impaired patients. The results presented here might be affected by unmeasured confounding, which could be addressed in a randomised trial.
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: POSE Study group collaborators substantially contributed to the patient recruitment and acquisition and processing of data.
Financial support and sponsorship: this study was supported by the European Society of Anaesthesiology and Intensive Care (ESAIC) as an ESAIC Research Group. This constituted the advertising of the POSE study and POSE meetings on the annual Euroanaesthesia congress, the indirect use of the ESAIC members contact lists, and the financial support for holding of three steering committee meetings at the ESAIC Secretariat in Brussels.
Conflicts of interest: AK, MB, MC, MS and RR report grants from ESAIC, during the conduct of the study
Presentation: none.
AK and MB equally contributed as first authors.
MS and MC equally contributed as last authors.
POSE-Study group.
Published online 25 November 2021
All collaborators are listed in Supplementary Digital Content 1.
Supplemental digital content is available for this article.
References
- 1.Hamel MB, Henderson WG, Khuri SF, et al. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc 2005; 53:424–429. [DOI] [PubMed] [Google Scholar]
- 2.Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet 2019; 394:1376–1386. [DOI] [PubMed] [Google Scholar]
- 3.POSE-Study group. Peri-interventional outcome study in the elderly (POSE) in Europe. Eur J Anaesthesiol 2022; 39:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinbroum AA, Szold O, Ogorek D, et al. The midazolam-induced paradox phenomenon is reversible by flumazenil. Epidemiology, patient characteristics and review of the literature. Eur J Anaesthesiol 2001; 18:789–797. [DOI] [PubMed] [Google Scholar]
- 5.Obiora E, Hubbard R, Sanders RD, et al. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax 2013; 68:163–170. [DOI] [PubMed] [Google Scholar]
- 6.Sanders RD, Godlee A, Fujimori T, et al. Benzodiazepine augmented γ-amino-butyric acid signaling increases mortality from pneumonia in mice∗. Crit Care Med 2013; 41:1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. The Lancet 2014; 383:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc 2015; 63:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldecoa C, Betteli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol 2017; 34:192–214. [DOI] [PubMed] [Google Scholar]
- 10.Lei VJ, Navathe AS, Seki SM, et al. Perioperative benzodiazepine administration among older surgical patients. Br J Anaesth 2021; 127:e69–e71. [DOI] [PubMed] [Google Scholar]
- 11.Jeon S, Lee HJ, Do W, et al. Randomized controlled trial assessing the effectiveness of midazolam premedication as an anxiolytic, analgesic, sedative, and hemodynamic stabilizer. Medicine (Baltimore) 2018; 97:e12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014; 33:1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci 2010; 25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garrido MM, Kelley AS, Paris J, et al. Methods for constructing and assessing propensity scores. Health Serv Res 2014; 49:1701–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurice-Szamburski A, Auquier P, Viarre-Oreal V, et al. PremedX Study Investigators. Effect of sedative premedication on patient experience after general anesthesia: a randomized clinical trial. JAMA 2015; 313:916–925. [DOI] [PubMed] [Google Scholar]
- 16.Auquier P, Pernoud N, Bruder N, et al. Development and validation of a perioperative satisfaction questionnaire. Anesthesiology 2005; 102:1116–1123. [DOI] [PubMed] [Google Scholar]
- 17.Duncan D, Sankar A, Beattie WS, et al. Alpha-2 adrenergic agonists for the prevention of cardiac complications among adults undergoing surgery. Cochrane Database Syst Rev 2018; 3:CD004126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Hert S, Staender S, Fritsch G, et al. Preoperative evaluation of adults undergoing elective noncardiac surgery. Updated guideline from the European Society of Anaesthesiology. Eur J Anaesthsiol 2018; 35:407–465. [DOI] [PubMed] [Google Scholar]
- 19.Kowark A, Rossaint R, Keszei AP, et al. I-PROMOTE study group. Impact of preoperative midazolam on outcome of elderly patients (I-PROMOTE): study protocol for a multicentre randomized controlled trial. Trials 2019; 20:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-Value. Ann Intern Med 2017; 167:268–274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.