Background:
Clinically suspected and laboratory-confirmed bloodstream infections are frequent causes of morbidity and mortality during neonatal care. The most effective infection prevention and control interventions for neonates in low- and middle-income countries (LMIC) are unknown.
Aim:
To identify effective interventions in the prevention of hospital-acquired bloodstream infections in LMIC neonatal units.
Methods:
Medline, PUBMED, the Cochrane Database of Systematic Reviews, EMBASE and PsychInfo (January 2003 to October 2020) were searched to identify studies reporting single or bundled interventions for prevention of bloodstream infections in LMIC neonatal units.
Results:
Our initial search identified 5206 articles; following application of filters, 27 publications met the inclusion and Integrated Quality Criteria for the Review of Multiple Study Designs assessment criteria and were summarized in the final analysis. No studies were carried out in low-income countries, only 1 in Sub-Saharan Africa and just 2 in multiple countries. Of the 18 single-intervention studies, most targeted skin (n = 4) and gastrointestinal mucosal integrity (n = 5). Whereas emollient therapy and lactoferrin achieved significant reductions in proven neonatal infection, glutamine and mixed probiotics showed no benefit. Chlorhexidine gluconate for cord care and kangaroo mother care reduced infection in individual single-center studies. Of the 9 studies evaluating bundles, most focused on prevention of device-associated infections and achieved significant reductions in catheter- and ventilator-associated infections.
Conclusions:
There is a limited evidence base for the effectiveness of infection prevention and control interventions in LMIC neonatal units; bundled interventions targeting device-associated infections were most effective. More multisite studies with robust study designs are needed to inform infection prevention and control intervention strategies in low-resource neonatal units.
Keywords: Infection prevention and control, low-and-middle income countries, systematic review, neonatal infection, hospital-acquired infection
The World Health Organization estimates that bacterial infections cause ≈25% of the 2.8 million annual neonatal deaths and long-term neurodevelopmental disabilities in survivors.1 Hospital-acquired infection (HAI) is a major cause of neonatal morbidity and mortality with prevalence ratios in low- and middle-income countries (LMICs) 3–20× higher than high-income countries.2 Traditional definitions, applied in high-income countries, use a 72-hour cutoff to differentiate early- from late-onset infection: the former associated with vertical transmission of pathogens such as group B Streptococcus, the latter with horizontal transmission of hospital-acquired pathogens, often associated with prematurity and invasive procedures such as intravenous catheterization. However, particularly in LMICs, there is recognition that facility-based delivery is itself a risk for HAIs, with pathogens such as Klebsiella pneumoniae (previously associated with late-onset infection) commonly isolated in the first 24 hours of life.2,3 This observation informs the Strengthening the Reporting of Observational Studies in Epidemiology for Newborn Infection guidelines, which recommend recording the timing of symptom onset rather than the binary early/late-onset dicohotomy.1 It also raises questions about fundamental differences in the mechanisms of neonatal infections in LMICs, as compared with high-income countries. The leading neonatal pathogens are increasingly resistant to first- and second-line antimicrobials, with substantial resistance to commonly used agents including ampicillin (89% of Escherichia coli), ceftriaxone (49% of Klebsiella spp. isolates) and cloxacillin (40% of Staphylococcus aureus).3
In this context, effective, feasible and affordable interventions to enhance infection prevention and control (IPC) in LMIC neonatal units are critical to prevent both neonatal mortality and emerging antimicrobial resistance. However, even in high-income settings, implementing effective prevention measures is challenging, and a robust evidence base on what tools to use is limited. Randomized controlled trials are considered the gold standard for generating evidence in general. However, best practice procedures and quality improvement interventions must be contextual for maximum impact. As interventions are seldom identical across trial sites, patient-level randomization is often not possible. Trials within hospitals (randomizing wards for example) are at risk of bias due to movement between wards of staff and patients. Furthermore, matching hospitals for randomization can be complex.4
To address these methodologic challenges, new study designs, such as interrupted time series for cohorts and hospital-level stepped-wedge cluster randomization, have been adopted. In addition, qualitative research aiming at understanding behavior change is increasingly used to complement quantitative data.4 For neonates in LMICs, various HAI prevention strategies have been suggested but only studied in small and single-center studies. To date, the evidence base in these settings has not yet been systematically assessed. We set out to review a broad range of potential interventions (both single and bundled), aiming to reduce healthcare-associated infections, with a focus on bloodstream infections (BSIs) in LMIC neonatal units.
Methods
This systematic review was conducted in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statements of evaluations of healthcare interventions.5 We registered the search strategy on the international prospective register of systematic reviews (CRD42018112346 on International prospective register of systematic reviews; see Supplemental Digital Content, http://links.lww.com/INF/E517).
Search Strategy
We searched Medline, the Cochrane Database of Systematic Reviews, EMBASE and PsychInfo (January 1, 2003, to October 31, 2020) to identify studies reporting on the effectiveness of interventions to prevent infections in LMIC neonatal wards and neonatal intensive care units. We selected the year 2003 to reflect the rapid evolution and spread of resistant bacteria causing HAIs in the last 17 years. IPC interventions were defined as any intervention aiming to prevent the development of a healthcare-associated bacterial or fungal infection such as BSI, meningitis, laboratory-confirmed urinary tract infection or clinically suspected but culture-negative infections.
We limited results by age [neonates 0–27 days or 0–89 days if admitted on a neonatal ward or neonatal intensive care unit (NICU)], location (LMIC as defined by the 2021 World Bank classification6), language (articles written in English, German, French, Italian, Portuguese and Spanish were included) and by relevant filters as per exclusion criteria (for a full list of terms and filters, see Supplemental Digital Content, http://links.lww.com/INF/E517). Our primary outcome was the effect of the interventions on (1) incidence of infection or (2) attributable mortality, depending on study definitions. Fungal or bacterial hospital-acquired invasive infections in hospitalized neonates were the primary events for study. Secondary outcomes included impact on incidence of laboratory-confirmed urinary tract infection, thrombophlebitis, necrotizing enterocolitis (NEC), device-associated infections (clinically suspected or culture proven) and clinically suspected infection where laboratory cultures were negative or not available.
Inclusion Criteria
Studies were eligible for full-text review if conducted in hospitalized neonates, including neonatal ward and/or NICU settings, with a detailed description of the intervention. We included both single interventions [eg, probiotics, kangaroo mother care (KMC), breastfeeding, fluconazole prophylaxis] and bundled interventions (eg, vascular device care, hand hygiene and healthcare worker education combined). Studies conducted in several countries including both high-income countries and LMICs (as per the World Bank 2021 regions) could be included if possible, to extract data from the LMIC settings. Study designs included randomized controlled trials, controlled and noncontrolled before-after, controlled and noncontrolled interrupted time series and cohort studies.
Exclusion Criteria
We excluded letters, opinion articles and reviews that did not report primary data. IPC interventions conducted during maternal care, in community-based settings and during outbreaks, were excluded. We also excluded studies conducted exclusively in high-income countries as per the World Bank 2021 regions.6 Interventions targeting viral infections (including HIV), infants older than 3 months or involving vaccination, diagnostic tools, infection prediction scores were excluded. We also excluded studies addressing IPC interventions on mixed neonatal/pediatric populations where extraction of neonatal data was not possible and where only abstracts were available despite contacting the corresponding author. Finally, we excluded studies where bacterial colonization as opposed to invasive infection was the outcome, if BSI was not also included.
Study Selection Process
The initial eligibility assessment of titles and abstracts identified by our search was conducted independently by F.C.F. and A.D. using the predetermined inclusion and exclusion criteria. Disagreements on eligibility were resolved by consensus, if needed by consulting a third party. The reference lists of all eligible publications were screened for cross-referencing. After finalizing articles for full-text review, 2 authors evaluated the quality of each eligible publication using the Integrated Quality Criteria for the Review of Multiple Study Designs (ICROMS) tool,7 with disagreements resolved as explained above. The ICROMS tool was designed to allow the systematic integration and assessment of differing study types including both quantitative and qualitative designs for reviews of public health interventions such as those targeting IPC.7 The ICROMS tool provides a list of quality criteria with a set of requirements specific for the study design. Studies are evaluated by a “decision matrix” where mandatory criteria must be met. The robustness of the study is measured by a score (see Tables, Supplemental Digital Content, http://links.lww.com/INF/E517, for criteria and scoring). To pass to the final analysis, studies must meet the minimum score and the mandatory ICROMS criteria, after duplicate review.
Data Abstraction
We extracted data using a standardized data collection form already independently piloted by F.C.F. and A.D. on a representative sample of studies. Study details collected on the form included author(s), year of publication, country or countries where the study was performed, study design, study time frame, setting (neonatal ward, NICU or both), intervention type, intervention details and effect. We grouped studies by intervention type: IPC bundles, catheter care, skin integrity and bacterial colonization (umbilical cord care, skin cleansing, emollients and/or massage), fluconazole prophylaxis, hand hygiene, KMC, rooming-in/parental involvement in neonatal care and gastrointestinal integrity (probiotics and feeding practices). Data synthesis involved the collation and tabulation of results by intervention type, summarizing the key interventions and their effectiveness in IPC for hospitalized neonates (using either relative risk, odds ratios or hazard ratios as reported by each study). We did not undertake a meta-analysis due to diversity of study type, interventions and outcomes; although all studies targeted reduction of neonatal infections, each study had different modes of action for the intervention and/or major differences in study design that precluded combining data.
Results
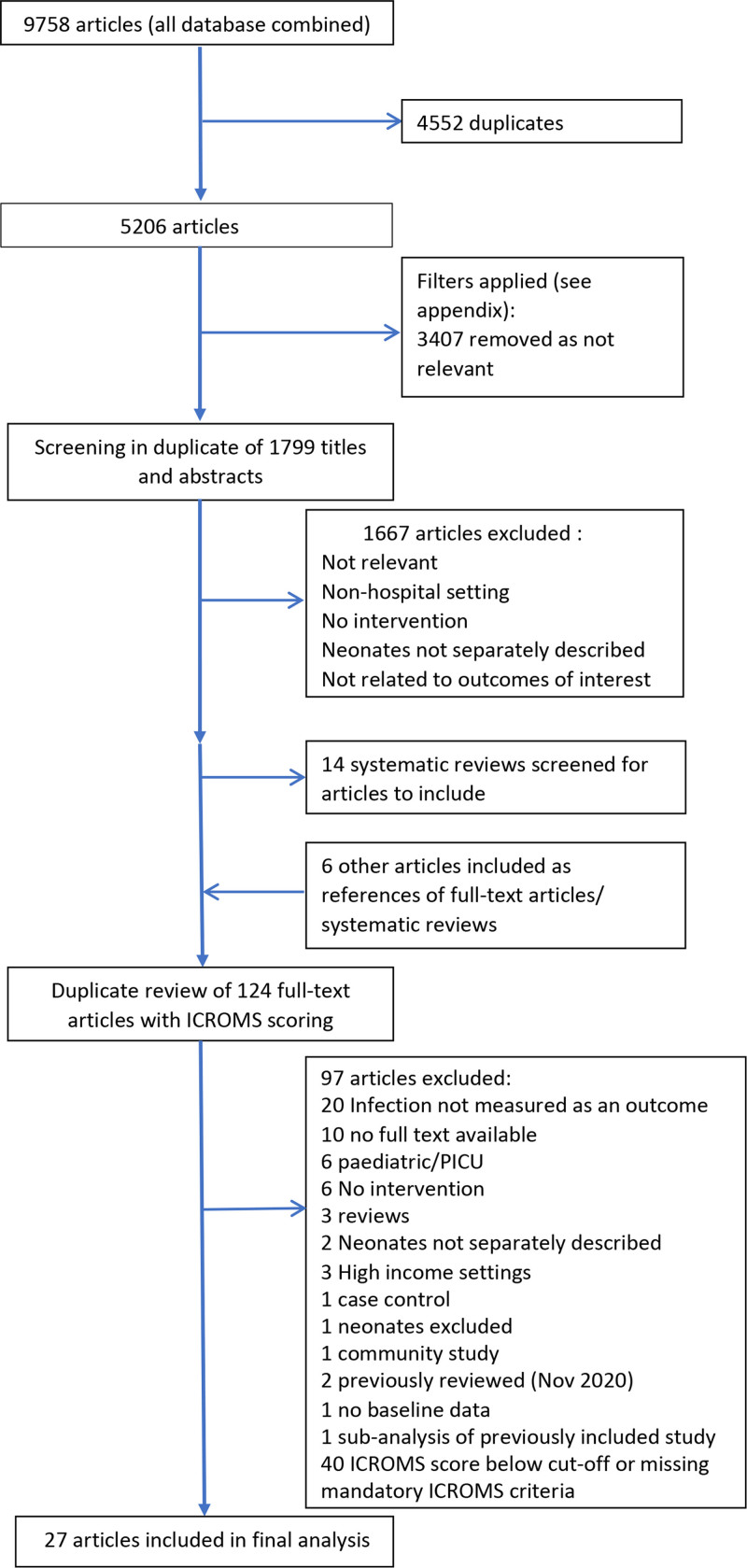
We identified 5206 articles on initial searching, after removal of duplicates (Fig. 1). Filter application (see Appendix, Supplemental Digital Content, http://links.lww.com/INF/E517) reduced this to 1799 titles and abstracts then reviewed independently by 2 study authors (F.C.F. and A.D.) for relevance. Of these, 124 were selected for full-text review in duplicate and ICROMS scoring, leading to another 97 exclusions and 27 selected for inclusion in the final review (Tables 1 and 2). Forty studies were excluded for either missing mandatory ICROMS criteria or ICROMS scores below the cutoff for the particular study design. Of the included studies, 8 were conducted in lower middle-income countries and 19 in upper middle-income countries (only 2 studies were multicountry). None were conducted in low-income countries. Including multisite studies and using the 2021 World Bank regions, 14 study sites were in Latin America/Caribbean, 14 in South-East Asia/Pacific, 5 in the Middle East/North Africa, 3 in Europe/Central Asia and 1 in Sub-Saharan Africa.6 Eighteen studies evaluated single interventions and 9 evaluated bundled interventions (two of which were conducted in multiple countries).
FIGURE 1.
Search strategy for the identification and selection of publications reporting the effectiveness of interventions to prevent infections in neonatal wards and intensive care (January 2003–October 2020).
Table 1.
Studies Reaching Integrated Quality Criteria for the Review of Multiple Study Designs Criteria for Inclusion Describing Single Interventions for the Prevention of Hospital-acquired Neonatal Bloodstream Infections and Clinically Suspected Infection in Low-resource Settings (January 2003–2020)
| Author | Study Design* | Country | Population/Setting | Sample Size | Intervention type | Intervention | Outcome | Key Findings |
|---|---|---|---|---|---|---|---|---|
| Akin et al8 | RCT | Turkey | Preterm <32 weeks’ gestation or <1500-g birth weight neonates admitted to 1 NNU | 50 | Probiotics/feeding | Oral lactoferrin 200 mg/d vs. placebo | Episodes of culture-proven nosocomial infection and NEC | Reduction in infection in intervention vs. control: 4.4 vs. 17.3/1000 patient-days; P = 0.007 |
| No episodes of NEC in either group | ||||||||
| Kaur and Gathwala9 | RCT | India | Neonates <2000-g birth weight admitted to 1 NNU | 130 | Probiotics/feeding | Oral bovine lactoferrin vs. placebo (80–142 mg/kg/d) | Incidence of the first episode of culture-proven LOS (bacterial or fungal), probable infection, any LOS, infection-attributable mortality | Reduction in LOS in intervention vs. placebo: 2/63 (3.2%) vs. 9/67 (13.4%); risk ratio, 0.211; 95% CI 0.044–1.019; P = 0.036 |
| Reduction of infection-attributable mortality in intervention: 0/63 (0%) vs. 5/67 (7.5%); P = 0.027 | ||||||||
| Li et al11 | RCT | China | Neonates <37 weeks’ gestation and <2000-g birth weight admitted to 1 NNU | 53 | Probiotics/feeding | Parenteral glutamine supplementation vs. none | Growth and development, tolerance to oral feeding, nosocomial infection | Nonsignificant reduction in nosocomial infection in intervention vs. control: 10% vs. 16%; P = 0.518 |
| Ochoa et al10 | RCT | Peru | Neonates 500–2500 g in 3 NNUs | 190 | Probiotics/feeding | Oral bovine lactoferrin (200 mg/kg) vs. placebo | Incidence of the first episode of LOS (culture proven or clinical), frequency of culture-proven LOS, incidence of NEC, length of stay, overall mortality, infection-related mortality, other adverse events, treatment intolerance | Infection incidence: 12/95 (12.6%) vs. 21/95 (22.1%) in interventions vs. control; P = 0.085. |
| Subsequent subgroup analysis: significant reduction in infection in <1500 g | ||||||||
| Wang et al12 | RCT | China | Term neonates admitted to NNU | 100 | Probiotics/feeding | Administration of mixed probiotic (L. casei, L. acidophilus, Bacillus subtilis, E. faecalis) vs. placebo | Nosocomial pneumonia, nosocomial infection (culture proven), multiple organ dysfunction syndrome, NEC, diarrhea | Nonsignificant reduction in infection in intervention vs. control: 4% vs. 2%; P = 0.4 |
| Similar in NEC: 4% vs. 8%; P = 0.47 | ||||||||
| Significant reduction in nosocomial pneumonia: 16% vs. 36%; P = 0.023 | ||||||||
| Darmstadt et al13 | RCT | Egypt | 1 NICU, neonates <34 weeks’ GA and <72 h of life | 103 | Emollient therapy | Sunflower seed oil (n = 51) vs. usual care (minimal use of emollients, n = 52) | Incidence of culture-proven infection, skin condition score, mortality from infection | Significant reduction in nosocomial infections with sunflower oil vs. controls (adjusted IRR 0.46; 95% CI 0.26–0.81; P = 0.007). No difference in mortality due to infection (adjusted odds ratio, 0.72; 95% CI 0.39–1.34). Significantly improved skin condition scores in the intervention group. |
| Darmstadt et al14 | RCT | Bangladesh | 1 NNU, neonates <33 weeks’ GA and <72 h of life | 497 | Emollient therapy | Sunflower seed oil (n = 159), aquaphor (n = 157), usual care (n = 181) | Incidence of culture-proven nosocomial BSI | Significant decrease in nosocomial infections with sunflower oil vs. controls (adjusted IRR 0.59; 95% CI 0.37–0.96; P = 0.032). Aquaphor: nonsignificant decrease (adjusted IRR 0.60; 95% CI 0.35–1.03; P = 0.065). |
| Erdemir et al16 | RCT | Turkey | 1 NICU, neonates <34 weeks’ GA and ≤24 h of life | 197 | Emollient therapy | Aquaphor emollient vs. routine care (none) | Incidence of neonatal infection, skin colonization, incidence of UTI | No difference in incidence of infection as a one-off outcome (41/100 vs. 43/97 intervention vs. controls, P = 0.63) or culture-proven infection [23/100 (intervention group) vs. 19/97 (controls); P = 0.42] |
| Salam et al15 | RCT | Pakistan | 1 NICU, neonates 26–37 weeks’ gestation | 258 | Emollient therapy | Daily topical application of coconut oil vs. no intervention | Incidence of HAI, weight gain, skin condition, mortality at 28 d of life | Significant reduction in culture-proven infection in intervention vs. controls (9/128 vs. 27/130), adjusted hazard of HAI 6.0 (95% CI 2.3–16) in controls; incidence of HAI 40 vs. 219/1000 patient-days in the intervention group vs. controls. Improved weight gain and skin condition in the intervention group, no impact on mortality or duration of admission. |
| Gathwala et al17 | RCT | India | 1 NICU, neonates ≤32 GA ≤1500 g | 140 | Chlorhexidine gluconate for cord care | Daily application of 2.5% CHG (n = 70) to the umbilical cord vs. “dry” cord care (n = 70) | Time to cord separation (primary outcome). Incidence of culture-proven neonatal infection, probable neonatal infection, meningitis, umbilical infection (secondary outcomes) | Significantly fewer episodes of culture-proven infection (2 vs. 15; P = 0.02; absolute risk, 21% vs. 3%; absolute risk reduction, 19%; CIs not shown), in interventions vs. control; borderline significantly greater episodes of probable infection (10 vs. 3; P = 0.052) interventions vs. controls. Significant reduction in time to cord separation in the intervention group (mean 9 vs. 10 d; P = 0.02) |
| Gupta et al18 | RCT | India | 1 pediatric ward, neonates <24 h of life | 140 | Chlorhexidine gluconate for skin cleansing | Daily application of 0.25% CHG to the whole body (n = 70) vs. tepid water (n = 70) | Incidence of culture-proven HA-neonatal infection (cultures taken on days 1, 3 and 7 of life) | 6/168 (3.6%) blood culture samples positive in the intervention group vs. 12/175 (6.9%) in the controls (P = 0.195) |
| Boo and Jamli19 | RCT | Malaysia | Stable neonates <1500 g birth weight admitted to 1 NNU | 126 | KMC | Intermittent skin to skin contact for minimum 1 h/d (n = 62) vs. standard care (n = 64) | Weight gain, occipitofrontal circumference, breastfeeding. Infection and NEC as secondary outcomes | No significant difference in culture-proven infection: 2/64 neonates (intervention group) vs. 1/64 (controls, P = 1.0) |
| No episodes of NEC in either group. | ||||||||
| Charpak et al20 | RCT | Colombia | Stable, neonates, birthweight <2000 g admitted to 1 NNU | 746 | KMC | Continuous KMC (n = 382) vs. traditional management (n = 364) | Growth and mortality to 40/41 weeks corrected gestational age. Severe infection requiring systemic antibiotics and nosocomial infections secondary outcomes | Similar numbers of infectious episodes 49/382 (intervention) vs. 44/364 (controls) but more mild/moderate infectious episodes (7% interventions vs 3% controls), absolute figures not given. Reduction in nosocomial infections: 8% vs. 4% in interventions/controls (P = 0.026) absolute figures not given |
| Li et al23 | NCBA | China | Stable neonates >1500 g in 1 NNU | 1446 | Rooming-in | Neonates moved to Room-in from NICU (n = 1018) vs. those eligible to move but staying in NICU (n = 428). 629 admitted directly to Room-in | Mortality, growth, duration of admission. secondary outcomes: nosocomial infection and NEC (unclear how defined) | No difference in nosocomial infection: 100/1081 vs. 48/428 in interventions vs. controls; P = 0.41; fewer neonates in the intervention group with NEC: 7/1081 vs. 8/428 (P = 0.04). |
| Reduction in mortality: 2% vs. 0%; P < 0.001 | ||||||||
| Parikh et al25 | RCT | India | Preterm neonates <1500 g admitted to 1 tertiary NNU | 120 | Fluconazole prophylaxis | Fluconazole prophylaxis within the first 3 d to day 28 or discharge/death if sooner (n = 60) vs. placebo (n = 60) | Fungal colonization and invasive fungal infection cultures taken on days 1–3, 7, 14, 21 and 28 | No reduction in invasive candida infection detected by blood cultures: 16/60 episodes vs. 15/60 in intervention vs. control; P = 0.835; of note, 30/31 of invasive species were non-albicans species. |
| Barrera et al21 | Cohort | Colombia | All neonates admitted to 1 NNU | 6655 | Hand hygiene | Introduction of ABHR dispensers; initial education; daily surveillance, quarterly feedback | HAI, CLABSI, VAP and UTI as per CDC definitions | 1260 patients with HAI, 724/1848 episodes confirmed by culture. Trend in reduction of Methicillin-resistant Staphylococcus aureus, 2.2–0.6 infections/1000 patient-days in from 2001–2005, −30%, P = 0.001 |
| No trend in reduction of Acinetobacter baumannii (0.6–0.2/1000 patient-days; P value not given) Significant increase in use of alcohol-based hand rub | ||||||||
| Mendes and Procianoy22 | RCT | Brazil | 1 NICU, all neonates ≤32 weeks’ GA and 750–1500 g | 104 | Massage therapy | Massage therapy (tactile-kinesthetic stimulation, n = 52) vs. no intervention (n = 52) | Primary outcome: length of NNU stay; secondary outcomes: weight gain, time to enteral feeds, time to oral feeds, incidence of LOS (clinically and blood culture confirmed), incidence of NEC (clinical and radiologic confirmation) | Lower incidence LOS in intervention vs. controls (5/46 vs. 18/47; P = 0.005); 8 vs. 22 pathogens identified in cultures (unclear how many cultures had multiple pathogens). |
| Barría et al24 | RCT | Chile | “high-risk” neonates admitted to 1 NNU | 74 | Intravenous catheterization | Peripherally inserted central catheters (n = 37) vs. standard peripheral intravenous catheters (n = 37) | Length of neonatal intensive care unit stay and incidence of infection and phlebitis. | No difference in incidence of suspected infection between groups: 14/37 vs. 8/37; P = 0.127 |
| Or culture-proven infection: 1/37 vs. 2/37; P = 0.53. Reduction in phlebitis: 4/37 vs. 15/37; P = 0.007. no difference in the length of stay: median, 20 vs. 17 d in intervention/control groups; P = 0.158 |
ABHR indicates alcohol-based hand rub; CA-BSI, catheter-associated blood stream infections; CBA, controlled before and after; CLABSI, central line–associated bloodstream infection; CVC, central venous catheters; ITS, interrupted time series; IRR, incident rate ratio; LOS, late-onset infection; NCBA, noncontrolled before and after.
Table 2.
Bundled Interventions for the Prevention of Hospital-Acquired Neonatal Bloodstream Infections and Clinically Suspected Infection in Low-resource Settings (January 2003 to September 2018)
| Author | Study Design | Country | Population/Setting | Sample Size | Bundle Elements | Outcome(s) | Key Findings |
|---|---|---|---|---|---|---|---|
| Azab et al26 | NCBA | Egypt | 1 NICU, all NICU admissions with duration of invasive ventilation >48 h | 62 vs. 81 | VAP prevention bundle + routine IPC measures: head-of-bed elevation, hand hygiene, sterile suctioning, strict indications for intubation, reintubation and suctioning, ventilator circuit change if visibly soiled or malfunctioning, mouth care, daily evaluation for readiness for extubation, sedation vacations | VAP episodes per 1000 mechanical ventilator days | VAP rate reduced from 36.4 to 23 episodes/1000 MV days (RR 0.565; 95% CI 0.408–0.782; P = 0.0006) and reduced MV days/case in the postintervention period (21.50 ± 7.6 to 10.36 ± 5.2 d; P = 0.0001). Trend toward reduction in NICU LOS (23.9 ± 10.3 to 22.8 ± 9.6 d; P = 0.56) and overall mortality (25%–17.3%; P = 0.215) |
| Length of stay in NICU | |||||||
| Overall mortality | |||||||
| Gilbert et al32 | NC ITS | Brazil | 5 NNUs, all admissions <1500 g | 679 vs. 563 | Nurse training package, including IPC measures | Mortality in VLBW neonates (primary outcome) | Despite improvement in nurses’ knowledge and practices, there was no change in survival (pre-training, 80%; post-training, 78.2%), severe ROP (1.6 vs. 2.8%), late-onset infection (11.3 vs. 12.3 cases/1000 infant days) or other outcomes |
| Incidence of late-onset infection, NEC and other secondary outcomes | |||||||
| Gill et al31 | NCBA | Philippines | 2 NICUs, all admissions between 2003 and 2004. | phase 1, 925; phase 2, 902 | Bundle with blood culture quality improvement, provision of alcohol hand rub, infection and HH surveillance, education, case discussions, infection control checklists | Proportion of neonates newly colonized with resistant pathogens. Secondary outcomes included bacteremia rates, cumulative mortality in NICU and hand hygiene compliance rates | Rates of colonization with drug-resistant pathogens and rates of infection did not change significantly. Staff hand hygiene compliance improved compared with the control period (NICU1: RR 1.3; 95% CI 1.1–1.5; NICU2: RR 1.6; 95% CI 1.4–2.0). Overall mortality decreased (NICU1: RR 0.5; 95% CI 0.4–0.6; NICU2: RR 0.8; 95% CI 0.7–0.9) |
| Leng et al33 | Cohort | China | 1 NNU, consecutive outborn neonates <1500 g | 86 vs. 86 | Hypothermia prevention bundle including standardized transport procedures, skilled transfer teams, process reviews with feedback | Axillary temperature on arrival (primary outcome) | Mean delivery room and NICU admission temperatures rose from 35.5 to 36.1 °C and from 34.6 to 36.2 °C (P < 0.01), with significantly decreased mortality (P < 0.02). There was no difference in the incidence of NEC and infection following implementation of the intervention. |
| Description of rates of NEC, early- and late-onset neonatal infection | |||||||
| Mwananyanda et al34 | Cohort | Zambia | All admissions to 1 NNU | 2669: 852 baseline, 268 implementation, 1549 intervention evaluation | IPC training, text message reminders, alcohol hand rub, enhanced environmental cleaning and weekly bathing of neonates ≥1.5 kg with 2% chlorhexidine gluconate | Mortality primary outcome, HAI, BSI secondary outcomes | Absolute mean monthly mortality reduction, –9% (95% CI –11 to –7); overall relative mortality risk reduction, 21% (RR 0.79; 95% CI 0.76–0.83) |
| Incidence rate ratio of suspected infection (0.48–0.65) and pathogen-identified (0.28–0.62) decreased for all weight groups except <1 kg suspected infection (1.38; P = 0.53; P values for others, all <0.001) | |||||||
| Resende et al30 | NCBA | Brazil | All admissions to 1 NNU | 251 | Catheter bundle: surveillance, feedback of CA-BSI; education, training, posters, hand hygiene; full-barrier precautions during CVC insertion; chlorhexidine skin cleaning; avoiding femoral site; removing unnecessary catheters | BSI rates pre/post-intervention | Reduction in culture-proven CA-BSI incidence pre/post-intervention (32% vs. 20%; 24 vs. 15 per 1000 catheter days; P = 0.04) |
| Rosenthal et al27 | NCBA | Argentina, Colombia, El Salvador, India, Mexico, Morocco, Peru, Turkey Philippines, Tunisia | 10 NICUs, all admissions to NICU | 1237 vs. 5592 | VAP bundle with active surveillance, HH, readiness to wean assessment, oral antiseptics, noninvasive ventilation, orotracheal intubation, management of ventilation circuits | VAP rates | The VAP rate declined from 17.8/1000 MV days to 12.0/1000 MV days; RR 0.67, 95% CI 0.50–0.91; a 33% reduction in VAP rate |
| Rosenthal et al29 | NCBA | El Salvador, Mexico, Philippines, Tunisia | 4 NICUs, all admissions to NICU | 374 vs. 1867 | CLABSI prevention bundle: IPC interventions; education; outcome + process surveillance, feedback of CLABSI rates, performance feedback of IPC practices | CLABSI rates | CLABSI rate decreased by 55%, from 21.4/1000 CL-days in phase 1 to 9.7/1000 CL-days in phase 2 (rate ratio, 0.45; 95% CI 0.33–0.63) |
| Zhou et al28 | NCBA | China | 1 NICU, all admissions with duration of invasive ventilation >48 h and at least 5 d NICU stay | 106 vs. 169 vs. 216 | Bundle: HH, waste disposal, patient isolation, ventilator disinfection, education, rational antibiotic use | VAP rates | VAP rate decreased from 48.84/1000 MV days to 25.73/1000 MV days in phase 2 and 18.50/1000 MV days in phase 3 (P < 0.001). Overall mortality rate decreased from 14.0% in phase 1 to 2.9% in phase 2 and 2.7% in phase 3 (P < 0.000) |
| Overall mortality |
ABHR indicates alcohol-based hand rub; CA-BSI, catheter-associated blood stream infection; CBA, controlled before and after; CVC, central venous catheter; HH, hand hygiene; ITS, interrupted time series; LOS, late-onset infection; MV, mechanical ventilation; NCBA, noncontrolled before and after; ICU, neonatal intensive care unit; RCT, randomized controlled trial.
Single-Intervention Studies
Of the single interventions (Table 1), probiotics/feeding interventions were the most commonly evaluated (5), followed by emollients (4), chlorhexidine cord cleansing (2) and KMC (2).
Three of the 5 probiotic/feeding interventions evaluated oral bovine lactoferrin versus placebo in a total of 370 neonates with birth weights <2500 g.8–10 Varying bovine lactoferrin dosage (from 80 to 200 mg/kg/day) and weight/gestational age thresholds made data incomparable and meta-analysis inappropriate. Two studies showed reduction in HAI in the intervention groups, one documenting 4.4 infections per 1000 patient-days in the intervention arm versus 17.3 (P = 0.007), the other finding a risk ratio of 0.211 (95% CIs, 0.044–1.019; P = 0.036), in those receiving the intervention versus placebo.8,9 Two studies evaluated enteral supplements but neither reduced infection incidence [parenteral glutamine supplementation (P = 0.518)11 or mixed probiotic administration (P = 0.4)12].
For emollients, one group conducted 2 studies using sunflower seed oil in 103 Egyptian and 497 Bangladeshi neonates <72 hours of age, born at <34 or <33 weeks’ gestational age, respectively.13,14 Both studies found that sunflower seed oil massage was associated with a significant decrease in the adjusted incidence rate ratio (aIRR, adjusted for weight on admission, gestational age and sex) of culture-proven BSI than control (aIRR 0.46; 95% CI 0.26–0.81 and aIRR 0.59; 95% CI 0.37–0.96). Notably, the Bangladeshi study showed no difference in the rate of clinically suspected infection triggering taking of blood cultures or antibiotic treatment rates between groups, although culture-proven BSI decreased in the intervention arm. Topical coconut oil was used in a Pakistani study in 270 neonates (26–34 weeks gestational age), first in the neonatal unit (NNU) and then at home.15 Neonates randomized to the control arm had an increased risk of hospital-acquired BSI (adjusted hazard ratio, 6.0; 95% CI 2.3–16). A Turkish study of 197 preterm neonates (<34 weeks’ gestation and <24 hours old) found no difference in mortality, incidence of culture-proven or clinically suspected infection in patients randomized to receive aquaphor emollient versus standard skin care.16
Two studies from India examined the impact of topical application of chlorhexidine gluconate; one in 140 neonates ≥32 weeks’ gestational age and ≥1500 g using chlorhexidine 2.5% to clean the umbilical stump; the other in 140 neonates comparing whole-body cleansing with chlorhexidine 0.25% versus tepid water.17,18 The first demonstrated a significant decrease in culture-proven BSI with chlorhexidine cord care (2 vs. 15; P = 0.02; absolute risk, 21% vs. 3%; absolute risk reduction, 19%; CIs not shown), although clinically suspected infections increased in intervention versus control subjects (Table 1).17 The second study found a nonsignificant decrease in blood culture positivity with whole-body cleansing (6/168 blood cultures positive in the intervention group vs. 12/175; P = 0.195), possibly owing to a small sample size and that blood cultures were taken at set intervals regardless of clinical indication.18
Studies on KMC were carried out in Colombia and Malaysia, in 746 neonates <2000 g and 126 neonates <1500 g, respectively.19,20 These studies evaluated substantially different KMC interventions (≈24 hours per day of KMC vs. ≥1 hour per day of KMC; Table 1). The Colombian study found similar numbers of infectious episodes [49/382 (intervention) vs. 44/364 (controls)], although they describe a milder phenotype in the intervention arm and a reduction in nosocomial infections (8% vs. 4% in interventions/controls; P = 0.026; absolute figures not given), without a clear distinction of the definition of “nosocomial” versus other infections. In the Malaysian study, there were 2/64 infections in the intervention group versus 1/64 controls (P = 1.0).
A large cohort study in Colombia (6655 neonates) evaluating a hand hygiene intervention (alcohol-based hand rub dispensers, daily surveillance and quarterly feedback) found a decreased incidence density of neonatal methicillin-resistant Staphylococcus aureus BSIs (from 2.2 to 0.6 per 1000 patient-days; P = 0.01), although no decrease in Acinetobacter baumannii21 (0.6–0.2 per 1000 patient-days; P not given).
A small Brazilian study of massage therapy versus no intervention (n = 104) reported lower incidence of late-onset infections in the intervention versus control groups.22
No study evaluating “rooming-in” (defined as continuous presence of parent caregivers in the neonatal unit23), peripherally inserted central catheters versus standard intravenous catheters24 and fluconazole prophylaxis25 found differences in infection rates between the study arms (Table 1).
Bundled Interventions
Five of the 9 studies reporting the impact of IPC bundles (Table 2) focused on preventing device-associated infection.26–30 One small, single-center study in an Egyptian NICU achieved significant reduction in ventilator-associated pneumonia (VAP) rates and mechanical ventilation days, with a trend toward reduction in NICU length of stay and overall mortality.26 A multicountry study in 10 NICUs demonstrated significant reduction in VAP rates (RR 0.67; 95% CI 0.50–0.91), after implementation of a multimodal strategy including hand hygiene, oral antiseptics, ventilator circuit management and enhanced infection surveillance.27 A tertiary hospital, 50-bed NICU in China, significantly reduced VAP rates, as well as overall mortality following implementation of a bundle including hand hygiene, ventilator disinfection, education and rational antibiotic use.28
Two studies targeted prevention of central line–associated BSI. A multicountry study in 4 NICUs demonstrated significant reduction in central line–associated BSI rates following a multimodal intervention strategy including education, enhanced process and outcome surveillance and staff feedback (rate ratio, 0.45; 95% CI 0.33–0.63).29 A single-center Brazilian NICU significantly reduced central line–associated BSI rates (24 vs. 15 per 1000 catheter days; P = 0.04) following implementation of a bundle including education, hand hygiene, chlorhexidine gluconate skin preparation and removal of unnecessary catheters.30
The first of two studies utilizing education/training interventions was a noncontrolled “before-after” study conducted in 2 NICUs in the Philippines. The bundle focused on quality improvement in blood culture collection, hand hygiene compliance, use of infection control checklists and staff education. Although there was no change in the primary outcome (proportion of neonates newly colonized with resistant pathogens) or in the secondary outcome of bacteremia, the study achieved improved hand hygiene compliance rates and reduction in overall mortality.31 A Brazilian study in 5 neonatal units conducted an interrupted time series analysis following introduction of a nurse training package including IPC measures. Despite improvement in nurses’ knowledge and practices, there was no change in mortality or rates of hospital-acquired BSI (11.3 vs. 12.3 cases/1000 infant days).32
A single-center cohort study at a large, academic center NICU in China enrolled outborn neonates <1500 g to assess the impact of a hypothermia prevention bundle on admission temperature, rates of NEC and neonatal infection. Mean axillary temperature on arrival increased, and overall mortality rates decreased significantly; however, there was no difference in either NEC or infection incidence following the intervention.33
A recent, large cohort study in a Zambian neonatal unit evaluated the impact of IPC training, text message reminders for staff, hand hygiene promotion with alcohol-based hand rub, enhanced environmental cleaning and weekly whole-body bathing of neonates ≥1.5 kg with 2% chlorhexidine gluconate. The bundle achieved significant reduction in overall mortality, clinically suspected infection and culture-proven BSI for all birth weight groups except those <1 kg.34 In a subsequent subanalysis of the intervention group data, chlorhexidine gluconate bathing reduced the hazard rate of BSI among inborn babies ≥1.5 kg by a factor of 0.58 (P = 0.10; 95% CI 0.31–1.11).35
Discussion
Although infection is the most frequent complication of hospitalization in LMIC neonates, the most effective IPC interventions remain unknown. We, therefore, conducted a systematic review of published studies describing the impact of various IPC interventions on healthcare-associated infection rates in LMIC NNUs. We identified 27 eligible publications that assessed single (n = 18) and bundled IPC interventions (n = 9). None were carried out in low-income countries, only 1 in Sub-Saharan Africa and just 2 had sites in multiple countries. We found considerable heterogeneity of study design, analysis and outcomes selected, as well as diversity in the modes of infection prevention targeted (skin and gastrointestinal mucosal integrity, promotion of normal flora acquisition and reduction of bacterial pathogen colonization). The evidence base we have identified for the effectiveness of IPC interventions in LMIC neonatal units is limited but appears most promising for bundled interventions targeting device-associated infections.
Limitations of this review include the paucity of published research on neonatal IPC from LMIC, the lack of multicenter studies or large sample sizes and the failure to use optimal study interventional study designs. Although we endeavored to be as inclusive as possible in our search terms, we only searched 4 databases and in 6 languages, so it is possible that we missed some relevant studies. It was not appropriate to do meta-analyses due to heterogeneity of both interventions and outcomes. Most studies were carried out in tertiary or academic neonatal units, which further limits the generalizability of the findings. Of note, although our initial search captured a large number of potentially eligible studies, full-text review led to 40/120 (33%) papers being excluded due to not including mandatory criteria required by ICROMS or having a low score for study design/analysis quality. Thus, some geographic areas were not well represented, in particular, Sub-Saharan Africa with only one study included.34 This highlights the challenges for clinicians in LMIC settings to identify and implement contextually appropriate evidence-based guidelines. It also demonstrates the difficulties of designing and analyzing high-quality IPC studies where facility, laboratory and statistical support may be lacking.
IPC studies are notoriously complex to design and implement, with issues of contamination between arms, the need for large-scale randomization (eg, cluster randomization of hospitals) and use of study designs unfamiliar to many academic clinicians, for example, interrupted time series analysis. IPC interventions also frequently involve behavior change, which does not lend itself to RCT evaluation. In recognition of the importance of evaluating effective behavior change in interventions in fields such as IPC, the UK Medical Research Council has developed guidance on how these studies should be designed and implemented.36 Similarly, the ICROMS score was developed to allow the inclusion of studies such as controlled before-after studies, noncontrolled before-after studies and qualitative studies in assessing evidence, the exclusion of which from standard systematic reviews undermines their potential contribution to the evidence base.7
A major challenge in selecting the primary end point for neonatal IPC studies is the very low yield of blood cultures (the current gold standard for confirmation of BSI) in both high- and low-income settings. This necessitates recruitment of large numbers of neonates to conclusively demonstrate an intervention’s impact, which is often particularly challenging in LMIC owing to budgetary and logistic constraints. Sensitive and specific neonatal infection diagnostic tools that are accessible and affordable in LMIC settings are needed. In addition, standardized and validated definitions for clinically suspected, culture-negative neonatal infections are required, to allow for comparison of findings across study sites. Use of multiple study outcomes (proven infection, clinically suspected infection and mortality) may complicate interpretation of findings, particularly where the results are discrepant.14 Until there is consensus on definitions of clinically suspected neonatal infection, particularly in settings where cultures have limited availability, the issue of quantifying reduction in infection rates will persist.
Despite these inherent limitations in the available data, end point definitions and study methodologies used, we have conducted the first systematic review of IPC interventions for LMIC NNUs. We used a robust search strategy, long inclusion time frame and ICROMS quality assessment to ensure we have identified all relevant and rigorously conducted research on this topic.
Among the single-intervention studies, emollient therapy (sunflower oil) in low-birth-weight babies had the strongest evidence supporting its use, demonstrating reduced healthcare-associated infection rates in both studies.13,14 There was also evidence to support the use of oral bovine lactoferrin, although the studies were small and there was inconsistency in dosage used. This finding is echoed in a recent Cochrane review of studies in high- and low-resource settings, which concluded there was low-certainty evidence that lactoferrin supplementation could reduce late-onset sepsis, though not NEC or all-cause mortality.37 Contrary to another previous Cochrane review, we did not find strong evidence for KMC as an intervention to reduce BSI in LMICs—only 2 studies fulfilled the ICROMS criteria and only 1 had some evidence of impact on BSI.20,38 For studies that analyzed the impact of bundled interventions, the strongest evidence was generated from studies aiming to prevent device-associated infection. Bundles incorporating other interventions (education, infection surveillance with feedback, hand hygiene promotion and chlorhexidine gluconate bathing) were also effective, but the evidence was generated from single-center or small studies.
Particular areas that appear promising for future research on neonatal IPC in LMIC are the use of chlorhexidine gluconate body washing and/or emollient therapy. Bundles that target neonatal BSI (the most common neonatal HAI) should be developed, utilizing lessons learned from the success of bundles targeting device-associated infections. The ideal bundled intervention should target all portals of entry for pathogenic bacteria causing neonatal BSI. It could include avoidance of hospitalization and/or invasive procedures, promotion of mucosal integrity (gut and skin), promotion of colonization with normal flora and reduced colonization with pathogenic bacteria.
Future studies in LMICs should utilize multinational collaborations, standardize definitions (or at least clearly elucidate what criteria have been used) and use robust study designs, for example, individual randomized or cluster-randomized controlled trials and interrupted time series analysis to generate evidence for IPC interventions that can be adopted in neonatal practice. Wherever possible, guidelines such as Strengthening the Reporting of Observational Studies in Epidemiology for Newborn Infection should be followed to allow for future comparisons between studies.1
Conclusions
There is a limited evidence base for IPC interventions in LMIC neonatal units. Overall, bundled interventions targeting prevention of device-associated infection are supported by the strongest evidence to date. More multisite studies using standardized neonatal infection definitions and robust study designs are needed to inform IPC interventions for use in low-resource neonatal units.
Supplementary Material
Footnotes
F.C.F. is supported by the Academy of Medical Sciences, the funders of the Starter Grant for Clinical Lecturers scheme and UCL Great Ormond Street NIHR Biomedical Research Centre. A.D. is supported by the Fogarty International Center of the National Institutes of Health, Emerging Global Leader Award Number K43-TW010682.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com)
References
- 1.Fitchett EJA, Seale AC, Vergnano S, et al. ; SPRING (Strengthening Publications Reporting Infection in Newborns Globally) Group. Strengthening the Reporting of Observational Studies in Epidemiology for Newborn Infection (STROBE-NI): an extension of the STROBE statement for neonatal infection research. Lancet Infect Dis. 2016;16:e202–e213. [DOI] [PubMed] [Google Scholar]
- 2.Zaidi AK, Huskins WC, Thaver D, et al. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;365:1175–1188. [DOI] [PubMed] [Google Scholar]
- 3.Okomo U, Akpalu ENK, Le Doare K, et al. Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect Dis. 2019;19:1219–1234. [DOI] [PubMed] [Google Scholar]
- 4.Gould DJ, Creedon S, Jeanes A, et al. Impact of observing hand hygiene in practice and research: a methodological reconsideration. J Hosp Infect. 2017;95:169–174. [DOI] [PubMed] [Google Scholar]
- 5.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Bank. World Bank Country and Lending Groups. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed November 2020.
- 7.Zingg W, Castro-Sanchez E, Secci FV, et al. Innovative tools for quality assessment: integrated quality criteria for review of multiple study designs (ICROMS). Public Health. 2016;133:19–37. [DOI] [PubMed] [Google Scholar]
- 8.Akin IM, Atasay B, Dogu F, et al. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am J Perinatol. 2014;31:1111–1120. [DOI] [PubMed] [Google Scholar]
- 9.Kaur G, Gathwala G. Efficacy of bovine lactoferrin supplementation in preventing late-onset sepsis in low birth weight neonates: a randomized placebo-controlled clinical trial. J Trop Pediatr. 2015;61:370–376. [DOI] [PubMed] [Google Scholar]
- 10.Ochoa TJ, Zegarra J, Cam L, et al. ; NEOLACTO Research Group. Randomized controlled trial of lactoferrin for prevention of sepsis in peruvian neonates less than 2500 g. Pediatr Infect Dis J. 2015;34:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li ZH, Wang DH, Dong M. Effect of parenteral glutamine supplementation in premature infants. Chin Med J (Engl). 2007;120:140–144. [PubMed] [Google Scholar]
- 12.Wang Y, Gao L, Zhang YH, et al. Efficacy of probiotic therapy in full-term infants with critical illness. Asia Pac J Clin Nutr. 2014;23:575–580. [DOI] [PubMed] [Google Scholar]
- 13.Darmstadt GL, Badrawi N, Law PA, et al. Topically applied sunflower seed oil prevents invasive bacterial infections in preterm infants in Egypt: a randomized, controlled clinical trial. Pediatr Infect Dis J. 2004;23:719–725. [DOI] [PubMed] [Google Scholar]
- 14.Darmstadt GL, Saha SK, Ahmed AS, et al. Effect of topical treatment with skin barrier-enhancing emollients on nosocomial infections in preterm infants in Bangladesh: a randomised controlled trial. Lancet. 2005;365:1039–1045. [DOI] [PubMed] [Google Scholar]
- 15.Salam RA, Darmstadt GL, Bhutta ZA. Effect of emollient therapy on clinical outcomes in preterm neonates in Pakistan: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2015;100:F210–F215. [DOI] [PubMed] [Google Scholar]
- 16.Erdemir A, Kahramaner Z, Yuksel Y, et al. The effect of topical ointment on neonatal sepsis in preterm infants. J Matern Fetal Neonatal Med. 2015;28:33–36. [DOI] [PubMed] [Google Scholar]
- 17.Gathwala G, Sharma D, Bhakhri Bk. Effect of topical application of chlorhexidine for umbilical cord care in comparison with conventional dry cord care on the risk of neonatal sepsis: a randomized controlled trial. J Trop Pediatr. 2013;59:209–213. [DOI] [PubMed] [Google Scholar]
- 18.Gupta B, Vaswani ND, Sharma D, et al. Evaluation of efficacy of skin cleansing with chlorhexidine in prevention of neonatal nosocomial sepsis - a randomized controlled trial. J Matern Fetal Neonatal Med. 2016;29:242–247. [DOI] [PubMed] [Google Scholar]
- 19.Boo NY, Jamli FM. Short duration of skin-to-skin contact: effects on growth and breastfeeding. J Paediatr Child Health. 2007;43:831–836. [DOI] [PubMed] [Google Scholar]
- 20.Charpak N, Ruiz-Peláez JG, Figueroa de C Z, et al. Kangaroo mother versus traditional care for newborn infants </=2000 grams: a randomized, controlled trial. Pediatrics. 1997;100:682–688. [DOI] [PubMed] [Google Scholar]
- 21.Barrera L, Zingg W, Mendez F, et al. Effectiveness of a hand hygiene promotion strategy using alcohol-based handrub in 6 intensive care units in Colombia. Am J Infect Control. 2011;39:633–639. [DOI] [PubMed] [Google Scholar]
- 22.Mendes EW, Procianoy RS. Massage therapy reduces hospital stay and occurrence of late-onset sepsis in very preterm neonates. J Perinatol. 2008;28:815–820. [DOI] [PubMed] [Google Scholar]
- 23.Li XY, Lee S, Yu HF, et al. Breaking down barriers: enabling care-by-parent in neonatal intensive care units in China. World J Pediatr. 2017;13:144–151. [DOI] [PubMed] [Google Scholar]
- 24.Barría RM, Lorca P, Muñoz S. Randomized controlled trial of vascular access in newborns in the neonatal intensive care unit. J Obstet Gynecol Neonatal Nurs. 2007;36:450–456. [DOI] [PubMed] [Google Scholar]
- 25.Parikh TB, Nanavati RN, Patankar CV, et al. Fluconazole prophylaxis against fungal colonization and invasive fungal infection in very low birth weight infants. Indian Pediatr. 2007;44:830–837. [PubMed] [Google Scholar]
- 26.Azab SF, Sherbiny HS, Saleh SH, et al. Reducing ventilator-associated pneumonia in neonatal intensive care unit using “VAP prevention Bundle”: a cohort study. BMC Infect Dis. 2015;15:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenthal VD, Rodríguez-Calderón ME, Rodríguez-Ferrer M, et al. Findings of the International Nosocomial Infection Control Consortium (INICC), Part II: impact of a multidimensional strategy to reduce ventilator-associated pneumonia in neonatal intensive care units in 10 developing countries. Infect Control Hosp Epidemiol. 2012;33:704–710. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Q, Lee SK, Jiang SY, et al. Efficacy of an infection control program in reducing ventilator-associated pneumonia in a Chinese neonatal intensive care unit. Am J Infect Control. 2013;41:1059–1064. [DOI] [PubMed] [Google Scholar]
- 29.Rosenthal VD, Dueñas L, Sobreyra-Oropeza M, et al. Findings of the International Nosocomial Infection Control Consortium (INICC), part III: effectiveness of a multidimensional infection control approach to reduce central line-associated bloodstream infections in the neonatal intensive care units of 4 developing countries. Infect Control Hosp Epidemiol. 2013;34:229–237. [DOI] [PubMed] [Google Scholar]
- 30.Resende DS, Ó JM, Brito Dv, et al. Reduction of catheter-associated bloodstream infections through procedures in newborn babies admitted in a university hospital intensive care unit in Brazil. Rev Soc Bras Med Trop. 2011;44:731–734. [DOI] [PubMed] [Google Scholar]
- 31.Gill CJ, Mantaring JB, Macleod WB, et al. Impact of enhanced infection control at 2 neonatal intensive care units in the Philippines. Clin Infect Dis. 2009;48:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert C, Darlow B, Zin A, et al. Educating neonatal nurses in Brazil: a before-and-after study with interrupted time series analysis. Neonatology. 2014;106:201–208. [DOI] [PubMed] [Google Scholar]
- 33.Leng H, Wang H, Lin B, et al. Reducing transitional hypothermia in outborn very low birth weight infants. Neonatology. 2016;109:31–36. [DOI] [PubMed] [Google Scholar]
- 34.Mwananyanda L, Pierre C, Mwansa J, et al. Preventing bloodstream infections and death in Zambian neonates: impact of a low-cost infection control bundle. Clin Infect Dis. 2019;69:1360–1367. [DOI] [PubMed] [Google Scholar]
- 35.Westling T, Cowden C, Mwananyanda L, et al. Impact of chlorhexidine baths on suspected sepsis and bloodstream infections in hospitalized neonates in Zambia. Int J Infect Dis. 2020;96:54–60. [DOI] [PubMed] [Google Scholar]
- 36.Craig P, Dieppe P, Macintyre S, et al. ; Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2020;3:CD007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Conde-Agudelo A, Diaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016;2016:CD002771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.