Objective:
The aim of this study was to evaluate the association between plasma aldosterone concentration (PAC) and renal impairment in patients with both hypertension and abnormal glucose metabolism (AGM).
Methods:
The longitudinal observational study included 2033 hypertensive individuals with AGM who did not have chronic kidney disease (CKD) at baseline. CKD was defined as estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73 m2 and/or positive proteinuria. Directed acyclic graphs and LASSO regression analyses were applied to identify adjusted sets. Cox proportional hazard models and linear regression were used to evaluate the association of PAC with CKD and its components including decreased renal function (DRF) and proteinuria. Mediation analysis was used to examine the role of blood pressure (BP) in the association between the two.
Results:
During total follow-up of 5951 person-years with a median follow-up of 31 months, 291 participants developed CKD. The incidence of CKD was increased with the elevation in tertile PAC. Multivariable Cox model showed that PAC was positively associated with increased CKD risk (hazard ratio = 1.76 for natural log-transformed PAC, P < 0.001), and with increased risk of DRF and proteinuria. SBP mediated 7.5–17.9% of the association between PAC and renal impairment. Overall results remained consistent and significant in sensitivity analysis by excluding those with suspicious primary aldosteronism, too short follow-up time and mineralocorticoid receptor antagonists use.
Conclusion:
Higher PAC was associated with increased CKD risk in patients with hypertension and AGM, even in the absence of suspicious primary aldosteronism. The results indicate PAC may serve as a potential therapeutic target in this population.
Keywords: aldosterone, chronic kidney disease, diabetes, hypertension
INTRODUCTION
Chronic kidney disease (CKD) has been shown to be a strong risk factor for cardio-cerebral vascular events, end-stage renal disease and death [1]. Prevalence of CKD has been increasing, largely due to an ongoing epidemic of metabolic disorders such as hypertension and abnormal glucose metabolism (AGM), both are known to increase the risk of CKD [2]. It is noteworthy that hypertension and diabetes mellitus have become leading causes of CKD in the elderly [3]. Recent studies show that the prevalence of CKD in patients with both hypertension and diabetes mellitus is higher than those with either one alone [4]. More than 40% diabetic patients in America and Japan are affected by kidney damage as well [5,6]. Also, 40% of CKD is associated with hypertension and diabetes mellitus among hospitalized patients in China [7]. Given the increasing burden of CKD, it is necessary to identify potential risk factors, as controlling classical factors such as blood pressure (BP) and glucose seems not to have achieved the goal of CKD prevention, especially in patients with both hypertension and AGM, a group at a high risk of CKD [8]. In this regard, aldosterone has become the focus of research.
Aldosterone is a steroid hormone produced by the adrenal glands and plays important roles in maintaining body fluid and homeostasis [9]. Aldosterone and mineralocorticoid receptor activation have been shown to contribute to the progression of CKD through haemodynamic and direct cellular actions [10,11]. Patients with primary aldosteronism have kidney impairment more frequently than essential hypertensive controls [12], and plasma aldosterone concentration (PAC) is closely associated with renal damage in these patients [13]. In recent years, several studies reported the association between PAC and renal damage in general population and often with inconsistent results [14–16]. Furthermore, studies conducted in general population have failed to eliminate the potential confounding of primary aldosteronism. In addition, few studies have specifically focused on patients with hypertension and AGM, a high-risk group for CKD.
Previous studies have shown that PAC is higher in patients with both hypertension and diabetes than in those with hypertension alone [17,18]. Except for increasing BP, aldosterone also induces electrolyte imbalance, inflammation, oxidative stress and fibrosis, which are associated with CKD. Therefore, we hypothesized that, in patients with both hypertension and AGM, PAC would be associated with CKD, even in the absence of primary aldosteronism. It would be beneficial to identify the association, as the prevalence of CKD remains high in this population and contributes to an increasing risk of adverse outcomes. To test this, we conducted longitudinal observation in patients with hypertension and AGM, and considered the potential confounding of primary aldosteronism.
MATERIALS AND METHODS
Study population
Individuals for the current study were patients referred by general practitioners and admitted to Hypertension Center of People's Hospital of Xinjiang Uygur Autonomous Region from January 2012 to May 2019. In the first stage, we screened patients who were aged 18 years or older, with hypertension and AGM and who also completed primary aldosteronism screening during hospitalization, using electronic medical record system. Patients with diagnosed secondary hypertension (primary aldosteronism diagnosed by confirmatory test, adrenal tumour, Cushing syndrome, pheochromocytoma, and polycystic ovary syndrome), history of cardiovascular events within last 3 months (including myocardial infarction, heart failure, stroke, unstable angina, coronary revascularization and coronary bypass operation) or malignant tumour were not included. In this stage, 2946 individuals were eligible. In the second stage, to evaluate the association between PAC and the incident CKD, we further excluded 410 individuals with CKD at baseline. Individuals who lacked baseline data (n = 77) were also excluded in this stage. In the third stage, a total of 2459 individuals were followed up for the interest of outcomes, and 426 individuals who did not have follow-up data available were excluded. Therefore, 2033 individuals were finally included in the statistical analysis. The present study was approved by Ethics Committee at People's Hospital of Xinjiang Uygur Autonomous Region. All participants or their legal representatives signed written consent forms.
Data collection
Baseline data were extracted from medical electronic system, including age, sex, BMI, BP, ethnicity (Han, Uyghur or others), marital status (married or single/separated), duration of hypertension and AGM, types of AGM, cigarette consumption (yes or no), alcohol intake (yes or no), fasting plasma glucose (FPG), total cholesterol (TC), triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), blood urea nitrogen (BUN), uric acid, serum creatinine (Scr), serum potassium and antihypertensive agents. Seated BP at the time of hospitalization was measured in the upper arm after patients rested quietly for 10 min at least with a mercury sphygmomanometer using international recommendations [19]. The mean value of two measurements was recorded and used for analysis.
Measurements of plasma aldosterone concentration, plasma renin activity and aldosterone:renin ratio
Hospitalized hypertensive individuals were first performed a preliminary screening test for primary aldosteronism under standardized conditions. That is, withdrawal of interfering drugs for 4–6 weeks or replacement with slow-release verapamil and/or α1-adrenergic antagonists was done to minimize the interference with measurement of PAC and PRA, as described in previous studies from the centre [20]. Patients with ARR at least 20 ng/dl per ng/ml per h together with PAC at least 12 ng/dl were suspected of having primary aldosteronism and recommended to undergo a saline infusion test (SIT) for identifying primary aldosteronism, and a postinfusion PAC more than 10 ng/dl was considered to have primary aldosteronism [21]. PAC was measured by radioimmunoassay using a commercially available kit (Beckman Coulter Inc., Brea, California, USA), and the intra-assay and inter-assay coefficients of variation were 4.3 and 9.2%, respectively. PRA was measured using a radioimmunoassay kit from Northern Biotechnology Institutes, and the intra-assay and inter-assay coefficients of variation were 9.2 and 12.5%, respectively. ARR was calculated by dividing PAC by PRA.
Definition of diseases
Hypertension was defined as SBP at least 140 mmHg and/or DBP at least 90 mmHg, or under antihypertensive therapy. AGM included impaired fasting glucose (IFG), impaired glucose tolerance (IGT) and diagnosed diabetes mellitus. IFG was defined if a FPG ranged from 6.1 to less than 7.0 mmol/l, whereas 2-h glucose was less than 7.8 mmol/l; IGT was defined if 2-h glucose ranged from 7.8 to less than 11.0 mmol/l; diabetes mellitus was defined if there was a previous confirmed diagnosis, or FPG was at least 7.0 mmol/l, or 2-h glucose was at least 11.1 mmol/l.
Estimated glomerular filtration rate (eGFR) was calculated by the simplified modification of diet in renal disease (MDRD) equation on the basis of data from Chinese adults [22]. Decreased renal function (DRF) was defined as eGFR less than 60 ml/min per 1.73 m2. Urine protein was determined using urine dipstick results, and a positive of proteinuria was defined as urine protein at least 1+. CKD was defined as having an eGFR less than 60 ml/min per 1.73 m2 or the presence of proteinuria.
Follow-up and outcome
The outcome for the current study was a new-onset CKD and its components (DRF and proteinuria) during follow up. Follow-up data were obtained using annual health check-ups or hospital readmission. Examination time of 3 months or more after baseline was considered to be a valid data. If a participant experienced the outcomes more than once during follow-up, only the first outcome was used for analysis. For those without CKD events during follow-up, the data of the last follow-up were included in the analysis.
Statistical analyses
Continuous variables were presented as mean ± standard deviation or median (interquartile range) and compared between groups using analysis of variance (ANOVA) or nonparametric Kruskal--Wallis H test. Categorical variables are summarized as number and percentage and compared between groups using Pearson chi-square test. To investigate the independent relationship between PAC and CKD, we first used directed acyclic graphs (DAGs) by the program DAGitty for drawing and analysed causal diagrams, to identify suitable minimally sufficient adjustment sets [23]. A minimally sufficient adjustment set consists of the smallest number of variables needed to account for confounding factors. We also used the least absolute shrinkage and selection operator (LASSO) regression to obtain another adjusted set. LASSO regression was often used to screen multidimensional variables; some variables were eliminated because they were not associated with CKD or because they had strongly collinear with other variables [24].
The cumulative incidence of CKD was estimated using the Kaplan--Meier method and compared using the log-rank test. Cox proportional hazard regression models were constructed to determine the independent predictive value of PAC for CKD, by considering three adjusted sets derived from DAGs (Model 1 and Model 2) and LASSO regression (Model 3), as well as a full-adjusted model. As it was not clear if PAC had a linear association with CKD, we treated PAC as both continuous and tertile variable. PAC was also natural log-transformed (ln PAC) due to skewed distributions. Hazard ratios for CKD development were calculated for tertile PAC, per 1, 5, 10 unit of PAC and per 1 unit of ln PAC. Multiple linear regression was used to evaluate the association between ln PAC and eGFR considered potential confounders, and tolerance and variance inflation factor (VIF) were used for collinearity testing (Collinearity was considered when tolerance <0.1 or VIF >10). In addition, mediation analysis with 5000 bootstrap samples was applied to examine the proportion of mediation by BP in the relationship between PAC and renal impairment.
We used abovementioned methods to evaluate the association of PAC with CKD and its components including DRF and proteinuria. Sensitivity analyses were performed to further determine the relationship by excluding individuals with suspicious primary aldosteronism (defined as ARR ≥20 and PAC ≥12), with follow-up time less than 12 months, and with treatment of mineralocorticoid receptor antagonists (MRA) at baseline for each analytic set. Statistical analyses were performed using SPSS version 20.0 for Windows (SPSS Inc., Chicago, Illinois, USA) and R version 4.0.3 (R Foundation, Vienna, Austria).
RESULTS
Characteristics of the study population
A total of 2033 participants were analysed. The mean age and BMI were 55.5 ± 11.1 years and 28.1 ± 3.9 kg/m2, respectively. Eight hundred and eighty-four (43.5%) were women, 592 (29.1%) were smokers and 539 (26.5%) were drinkers. Mean SBP and DBP levels were 148.5 ± 21.2 and 87.9 ± 14.8 mmHg, respectively. Baseline eGFR was 118.2 ± 30.0 ml/min per 1.73 m2. The median (interquartile range) values were 13.6 (11.6–19.8) ng/dl for PAC, 1.4 (0.5–2.6) ng/ml per h for PRA and 11.1 (5.9–25.9) ng/dl per ng/ml per h for ARR. Mean SBP, DBP and serum creatinine were increased significantly with elevated PAC tertile, whereas eGFR was decreased significantly with increased PAC. Details of baseline characteristics across PAC tertile are summarized in Table 1.
TABLE 1.
Baseline characteristics of study population across plasma aldosterone concentration tertile
| PAC tertile (ng/dl) | ||||
| Characteristics | T1 (n = 677) ≤12.16 | T2 (n = 677) 12.17–17.16 | T3 (n = 679) ≥ 17.17 | P |
| Age (years) | 56.8 ± 11.2 | 55.9 ± 10.7 | 53.9 ± 11.1 | < 0.001 |
| Women, n (%) | 310 (45.8) | 299 (44.2) | 275 (40.5) | 0.132 |
| BMI (kg/m2) | 27.6 ± 3.8 | 28.3 ± 4.0 | 28.2 ± 3.9 | 0.079 |
| Ethnicity, n (%) | ||||
| Han | 385 (56.9) | 414 (61.2) | 416 (61.3) | 0.164 |
| Uyghur | 200 (29.5) | 170 (25.1) | 162 (23.8) | |
| Others | 92 (13.6) | 93 (13.7) | 101 (14.9) | |
| Marital status, n (%) | ||||
| Married | 650 (96.0) | 656 (96.9) | 660 (97.2) | 0.444 |
| Single/separated | 27 (4.0) | 21 (3.1) | 19 (2.8) | |
| Duration of HTN (year) | 5 (2–11) | 7 (2–13) | 8 (3–13) | 0.007 |
| SBP (mmHg) | 146.7 ± 21.0 | 147.7 ± 20.2 | 151.1 ± 22.2 | < 0.001 |
| DBP (mmHg) | 86.3 ± 14.5 | 86.8 ± 13.8 | 90.6 ± 15.7 | < 0.001 |
| AGM types, n (%) | ||||
| Impaired fasting glucose | 70 (10.3) | 89 (13.1) | 78 (11.5) | 0.577 |
| Impaired glucose tolerance | 208 (30.7) | 197 (29.1) | 210 (30.9) | |
| Diabetes mellitus | 399 (58.9) | 391 (57.8) | 391 (57.6) | |
| FPG (mmol/l) | 6.4 ± 2.5 | 6.2 ± 2.1 | 6.0 ± 2.2 | 0.020 |
| Smoking, n (%) | 197 (29.1) | 183 (27.0) | 212 (31.2) | 0.236 |
| Alcohol drinking, n (%) | 172 (25.4) | 181 (26.7) | 186 (27.4) | 0.700 |
| Total cholesterol (mmol/l) | 4.34 ± 1.04 | 4.49 ± 1.23 | 4.48 ± 1.03 | 0.030 |
| Triglyceride (mmol/l) | 2.06 ± 2.00 | 2.24 ± 2.12 | 2.15 ± 1.68 | 0.207 |
| HDL-C (mmol/l) | 0.97 ± 0.22 | 0.98 ± 0.25 | 0.97 ± 0.24 | 0.563 |
| LDL-C (mmol/l) | 2.57 ± 0.84 | 2.63 ± 0.85 | 2.67 ± 0.89 | 0.119 |
| Blood urea nitrogen (mmol/l) | 5.13 ± 1.46 | 5.20 ± 1.40 | 4.99 ± 1.33 | 0.015 |
| Uric acid (μmol/l) | 317.2 ± 87.2 | 338.7 ± 80.7 | 340.8 ± 85.9 | < 0.001 |
| Serum potassium (mmol/l) | 3.69 ± 0.28 | 3.69 ± 0.26 | 3.66 ± 0.31 | 0.114 |
| Serum creatinine (mmol/l) | 64.6 ± 15.4 | 65.8 ± 15.3 | 67.8 ± 16.2 | 0.001 |
| Baseline eGFR (ml/min per 1.73 m2) | 120.5 ± 31.3 | 118.2 ± 30.1 | 115.8 ± 28.4 | 0.017 |
| PAC (ng/dl) | 11.0 (8.3–11.6) | 13.6 (12.9–15.3) | 22.8 (19.8–27.5) | < 0.001 |
| PRA (ng/mL per h) | 1.0 (0.4–2.1) | 1.2 (0.5–2.5) | 2.0 (0.8–3.2) | < 0.001 |
| ARR | 9.7 (4.7–22.5) | 11.1 (5.6–27.1) | 12.5 (7.4–29.1) | < 0.001 |
| Antihypertensive agents | ||||
| ACEI/ARB | 393 (58.1) | 389 (57.5) | 393 (57.9) | 0.975 |
| CCB | 544 (80.4) | 554 (81.8) | 579 (85.3) | 0.050 |
| Beta-blocker | 155 (22.9) | 147 (21.7) | 141 (20.8) | 0.636 |
| Diuretics | 205 (30.3) | 229 (33.8) | 282 (41.5) | < 0.001 |
Data are presented as means ± standard deviation or number (percentage) unless otherwise noted. ACEI, angiotensin-converting-enzyme inhibitors; AGM, abnormal glucose metabolism; ARB, angiotensin receptor blockers; ARR, aldosterone to renin activity ratio; CCB, calcium channel blockers; DRF, decreased renal function; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; PAC, plasma aldosterone concentration; PRA, plasma renin activity.
During the total follow-up of 5951 person-years with a median follow-up of 31 (interquartile range: 18–51) months, 291 patients subsequently developed CKD, with an incidence rate of 48.9/1000 person-years. The incidence rate of DRF and proteinuria were 17.9/1000 person-years and 41.8/1000 person-years, respectively.
Selection of adjusted sets
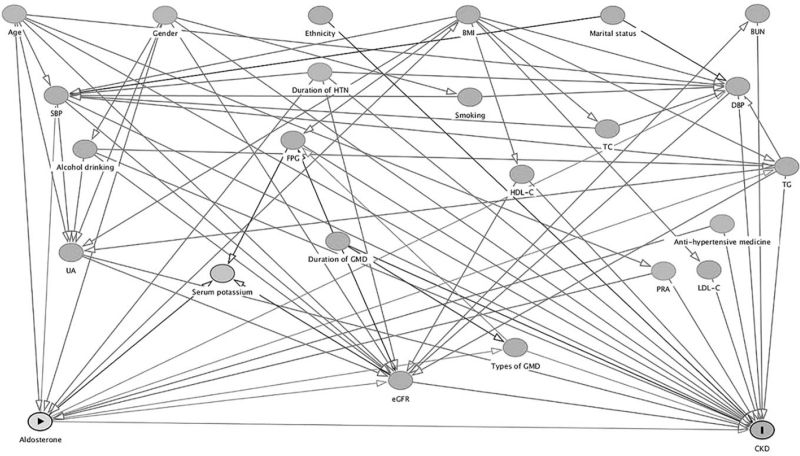
Figure 1 shows the results of DAGs describing association between PAC and CKD, based on literature reports and empirical confirmability. Two minimal sufficient adjustment sets were identified:
FIGURE 1.
Directed acyclic graph of causal assumptions. Nodes represent variables and arrows represent causal associations. Yellow-coloured and blue-coloured nodes indicates exposure and outcome, respectively. Gray-coloured nodes represent possible confounding factors. AGM, abnormal glucose metabolism; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; PRA, plasma renin activity; TC, total cholesterol; TG, triglyceride; UA, uric acid.
-
(1)
Age, sex, alcohol intake, BMI, duration of AGM, types of AGM, duration of hypertension, antihypertensive agents, SBP, DBP, PRA, triglyceride, HDL-C, uric acid and baseline eGFR (model 1).
-
(2)
Age, sex, alcohol intake, duration of AGM, types of AGM, duration of hypertension, antihypertensive agents, SBP, DBP, FPG, PRA, triglyceride, HDL-C, LDL-C, uric acid and baseline eGFR (model 2).
LASSO regression identified nearly two-third factors related CKD and is used as another adjusted set:
-
(3)
Age, sex, ethnicity, marital status, cigarette consumption, duration of hypertension, antihypertensive agents, duration of AGM, types of AGM, BMI, SBP, FPG, TC, triglyceride, HDL-C, uric acid, PRA and baseline eGFR (model 3).
Association of plasma aldosterone concentration with chronic kidney disease
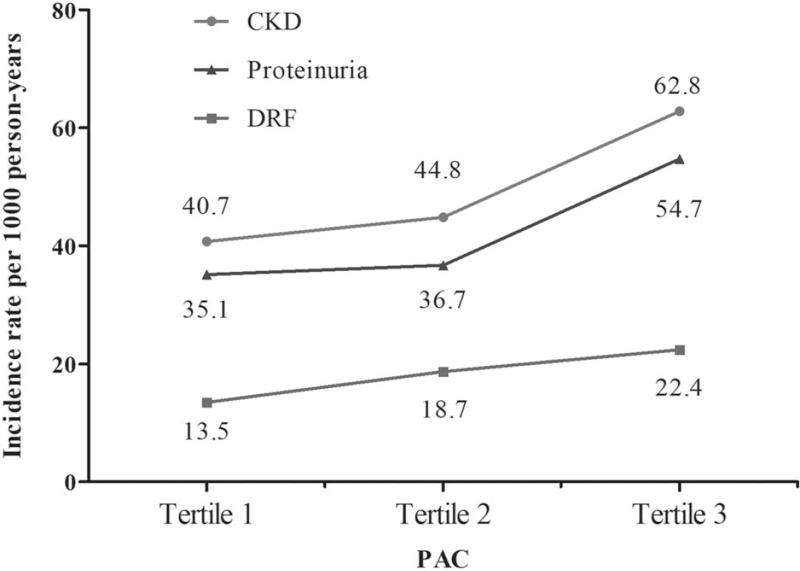
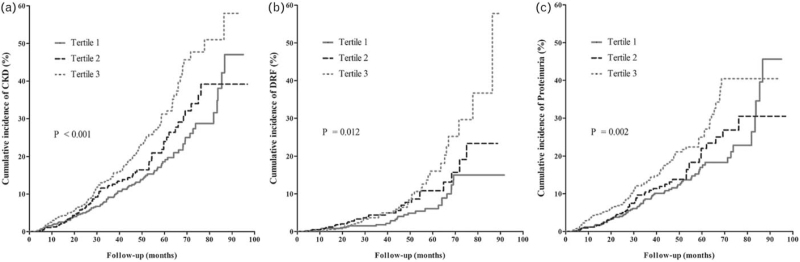
The incidence rate of CKD was increased with elevation in PAC, with the highest rate of 62.8/1000 person-years in the third tertile (Fig. 2). The Kaplan--Meier curve for cumulative incidence of CKD is shown in Fig. 3a. Unadjusted Cox regression showed that PAC had a positive association with CKD. After adjustment of potential confounders in three different models and a full-adjusted model, the association remained consistent and significant (Table 2). Individuals in the third tertile of PAC had nearly 70% increased risk of developing CKD compared with those in the first tertile. Sensitivity analysis by excluding individuals with suspicious primary aldosteronism showed augmented consistent results, with a stronger association between PAC and CKD (hazard ratio = 2.20 and 2.10 for tertile-3 and ln PAC, both P < 0.001) (Table S1). Also, after excluding individuals with follow-up less than 12 months or with MRA treatment at baseline, there was still significant association between PAC and CKD (Table S2 and S3). In the mediation analysis, we observed that SBP, but not DBP, had significant mediation effect on the association of PAC with CKD, by a mediated effect of 8.8% (P = 0.027).
FIGURE 2.
Incidence rate of chronic kidney disease, decreased renal function and proteinuria across tertile plasma aldosterone concentration. CKD, chronic kidney disease; DRF, decreased renal function; PAC, plasma aldosterone concentration.
FIGURE 3.
Kaplan--Meier curve of cumulative incidence of CKD (a), DRF (b) and proteinuria (c) across tertile PAC. P value was generated based on the log-rank test. CKD, chronic kidney disease; DRF, decreased renal function; PAC, plasma aldosterone concentration.
TABLE 2.
Association between plasma aldosterone concentration and chronic kidney disease in multivariate Cox regression models
| Unadjusted | Model 1 | Model 2 | Model 3 | Full-adjusted model | ||||||
| PAC | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Tertile 1 | – | – | – | – | – | – | – | – | – | – |
| Tertile 2 | 1.25 (0.93–1.69) | 0.141 | 1.20 (0.89–1.63) | 0.233 | 1.23 (0.91–1.66) | 0.187 | 1.23 (0.91–1.67) | 0.178 | 1.24 (0.91–1.68) | 0.176 |
| Tertile 3 | 1.75 (1.33–2.31) | < 0.001 | 1.65 (1.24–2.20) | 0.001 | 1.69 (1.26–2.26) | < 0.001 | 1.67 (1.25–2.24) | 0.001 | 1.71 (1.27–2.29) | < 0.001 |
| P for trend | < 0.001 | 0.001 | < 0.001 | 0.001 | < 0.001 | |||||
| Per 1 unit increase | 1.03 (1.02–1.04) | < 0.001 | 1.03 (1.01–1.04) | < 0.001 | 1.03 (1.01–1.04) | < 0.001 | 1.03 (1.01–1.04) | < 0.001 | 1.03 (1.01–1.04) | < 0.001 |
| Per 5 unit increase | 1.16 (1.10–1.23) | < 0.001 | 1.14 (1.07–1.22) | < 0.001 | 1.14 (1.07–1.22) | < 0.001 | 1.14 (1.07–1.21) | < 0.001 | 1.14 (1.07–1.22) | < 0.001 |
| Per 10 unit increase | 1.35 (1.20–1.52) | < 0.001 | 1.30 (1.15–1.48) | < 0.001 | 1.30 (1.15–1.48) | < 0.001 | 1.30 (1.14–1.47) | < 0.001 | 1.30 (1.15–1.48) | < 0.001 |
| Ln PAC | 1.82 (1.44–2.31) | < 0.001 | 1.73 (1.34–2.22) | < 0.001 | 1.75 (1.36–2.25) | < 0.001 | 1.74 (1.35–2.24) | < 0.001 | 1.76 (1.37–2.27) | < 0.001 |
Results were shown as hazard ratio (95% confidence interval) derived from Cox proportional hazard models adjusted confounders identified by DAGs and LASSO regression. Model 1 adjusted the first minimal sufficient adjustment set; model 2 adjusted the second minimal sufficient adjustment set; model 3 adjusted variables derived from LASSO selection. The full-adjusted model adjusted for all variables including age, gender, ethnicity, marital status, smoking, alcohol drinking, BMI, SBP, DBP, FPG, TC, TG, HDL-C, LDL-C, baseline eGFR, duration of hypertension, duration of AGM, antihypertensive drugs, types of AGM, BUN, uric acid, PRA, serum potassium.
Association of plasma aldosterone concentration with estimated glomerular filtration rate and decreased renal function
The incidence rate of DRF was increased with elevated PAC (Fig. 2), and Kaplan--Meier curve showed that the cumulative incidence of DRF was higher in patients with PAC in second and third tertiles, compared with first tertile (P = 0.012; Fig. 3b). Cox regression adjusted confounders showed significant associations between PAC and DRF (Table 3). Compared with the first tertile, patients in the second and third tertiles of PAC had more than 70 and 80% increased risk of developing DRF, respectively. The ln PAC was also significantly associated with DRF (adjusted hazard ratio ∼1.90, P < 0.01). Moreover, results of multivariate linear regression revealed that per 1-unit increase of ln PAC induced nearly 6 unit decrease of eGFR (Table 4). SBP mediated 17.9% of the association between PAC and DRF (P = 0.007). Sensitivity analyses showed that there were still consistent significant association between PAC and DRF, excepted for the analysis excluding subjects with MRA treatment at baseline, although with consistent trend but only marginal significance (Table S4 to S6).
TABLE 3.
Association between plasma aldosterone concentration and decreased renal function in multivariate Cox regression models
| Unadjusted | Model 1 | Model 2 | Model 3 | Full-adjusted model | ||||||
| PAC | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Tertile 1 | – | – | – | – | – | – | – | – | – | – |
| Tertile 2 | 1.76 (1.04–2.95) | 0.034 | 1.56 (0.92–2.65) | 0.101 | 1.69 (0.99–2.87) | 0.055 | 1.72 (1.00–2.93) | 0.048 | 1.72 (1.00–2.94) | 0.049 |
| Tertile 3 | 2.09 (1.27–3.43) | 0.004 | 1.86 (1.10–3.15) | 0.020 | 1.95 (1.15–3.30) | 0.014 | 1.81 (1.07, 3.08) | 0.027 | 1.87 (1.09–3.19) | 0.022 |
| P for trend | 0.004 | 0.020 | 0.014 | 0.028 | 0.022 | |||||
| Per 1 unit increase | 1.03 (1.01–1.05) | 0.001 | 1.03 (1.01–1.05) | 0.008 | 1.03 (1.01–1.05) | 0.005 | 1.03 (1.01–1.05) | 0.016 | 1.03 (1.01–1.05) | 0.013 |
| Per 5 unit increase | 1.18 (1.07–1.30) | 0.001 | 1.16 (1.04–1.29) | 0.008 | 1.17 (1.05–1.30) | 0.005 | 1.14 (1.03–1.27) | 0.016 | 1.16 (1.03–1.29) | 0.013 |
| Per 10 unit increase | 1.38 (1.14–1.68) | 0.001 | 1.34 (1.08–1.67) | 0.008 | 1.36 (1.10–1.69) | 0.005 | 1.30 (1.05–1.61) | 0.015 | 1.33 (1.06–1.68) | 0.013 |
| Ln PAC | 1.93 (1.29–2.87) | 0.001 | 1.86 (1.19–2.89) | 0.006 | 1.97 (1.26–, 3.07) | 0.003 | 1.82 (1.17–2.83) | 0.007 | 1.90 (1.20–3.00) | 0.006 |
Results were shown as hazard ratio (95% confidence interval) derived from Cox proportional hazard models adjusted confounders identified by DAGs and LASSO regression. Model 1 adjusted the first minimal sufficient adjustment set; model 2 adjusted the second minimal sufficient adjustment set; model 3 adjusted variables derived from LASSO selection. The full-adjusted model adjusted for all variables including age, gender, ethnicity, marital status, smoking, alcohol drinking, BMI, SBP, DBP, FPG, TC, TG, HDL-C, LDL-C, baseline eGFR, duration of hypertension, duration of AGM, antihypertensive drugs, types of AGM, BUN, uric acid, PRA, serum potassium.
TABLE 4.
Association of ln plasma aldosterone concentration with estimated glomerular filtration rate in multivariate linear regression models
| Model | B coefficients (95% CI) | P | Minimum Tolerance | Maximum VIF |
| Crude | –7.71 (–11.70, –3.73) | < 0.001 | – | – |
| Model 1 | –6.06 (–9.87, –2.25) | 0.002 | 0.419 | 2.384 |
| Model 2 | –6.20 (–10.02, –2.38) | 0.001 | 0.420 | 2.380 |
| Model 3 | –6.26 (–10.08, –2.43) | 0.001 | 0.600 | 1.668 |
| Full-adjusted model | –6.18 (–10.01, –2.34) | 0.002 | 0.416 | 2.406 |
Results derived from linear regression models adjusted confounders identified by DAGs and LASSO regression. Model 1 adjusted the first minimal sufficient adjustment set; model 2 adjusted the second minimal sufficient adjustment set; model 3 adjusted variables derived from LASSO selection. The full-adjusted model adjusted for all variables including age, gender, ethnicity, marital status, smoking, alcohol drinking, BMI, SBP, DBP, FPG, TG, HDL-C, LDL-C, baseline eGFR, duration of hypertension, duration of AGM, antihypertensive drugs, types of AGM, BUN, uric acid, PRA, serum potassium. VIF, variance inflation factor.
Association of plasma aldosterone concentration with proteinuria
In regard to proteinuria, the incidence rates were 35.1 and 54.7/1000 person-years in the first and third tertiles PAC, respectively (Fig. 2). Similar trend of cumulative incidence in proteinuria was also observed (Fig. 3c). Multivariate Cox regression revealed that increase in tertile PAC was positively associated with an increased risk of proteinuria (P for trend <0.01; Table 5). Per 1-unit increase in ln PAC had nearly 70% increased risk of developing proteinuria. Mediation analysis showed that SBP mediated 7.5% of the association between PAC and proteinuria (P = 0.038). Sensitivity by excluding individuals with suspicious primary aldosteronism, follow-up less than 12 months or with MRA treatment did not change the results (Table S7 to S9).
TABLE 5.
Association between plasma aldosterone concentration and proteinuria in multivariate Cox regression models
| Unadjusted | Model 1 | Model 2 | Model 3 | Full-adjusted model | ||||||
| PAC | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Tertile 1 | – | – | – | – | – | – | – | – | – | – |
| Tertile 2 | 1.16 (0.81–1.66) | 0.422 | 1.09 (0.76–1.57) | 0.631 | 1.12 (0.78–1.62) | 0.533 | 1.12 (0.78–1.62) | 0.541 | 1.12 (0.78–1.62) | 0.544 |
| Tertile 3 | 1.72 (1.25–2.38) | 0.001 | 1.58 (1.12–2.22) | 0.009 | 1.62 (1.15–2.29) | 0.006 | 1.61 (1.14–2.27) | 0.007 | 1.66 (1.17–2.35) | 0.004 |
| P for trend | 0.001 | 0.008 | 0.005 | 0.006 | 0.004 | |||||
| Per 1 unit increase | 1.03 (1.02–1.05) | < 0.001 | 1.03 (1.01–1.04) | 0.001 | 1.03 (1.01–1.04) | 0.001 | 1.03 (1.01–1.04) | 0.001 | 1.03 (1.01–1.05) | < 0.001 |
| Per 5 unit increase | 1.18 (1.10–1.27) | < 0.001 | 1.15 (1.06–1.24) | 0.001 | 1.15 (1.06–1.24) | 0.001 | 1.15 (1.06, 1.24) | 0.001 | 1.16 (1.07–1.25) | < 0.001 |
| Per 10 unit increase | 1.39 (1.20–1.62) | < 0.001 | 1.31 (1.12–1.54) | 0.001 | 1.32 (1.12–1.54) | 0.001 | 1.31 (1.12–1.54) | 0.001 | 1.34 (1.14–1.57) | < 0.001 |
| Ln PAC | 1.82 (1.37–2.43) | < 0.001 | 1.67 (1.23–2.26) | 0.001 | 1.69 (1.25–2.29) | 0.001 | 1.69 (1.25–2.30) | 0.001 | 1.75 (1.28–2.39) | < 0.001 |
Results were showed as hazard ratio (95% confidence interval) derived from Cox proportional hazard models adjusted confounders identified by DAGs and LASSO regression. Model 1 adjusted the first minimal sufficient adjustment set; model 2 adjusted the second minimal sufficient adjustment set; model 3 adjusted variables derived from LASSO selection. The full-adjusted model adjusted for all variables including age, gender, ethnicity, marital status, smoking, alcohol drinking, BMI, SBP, DBP, FPG, TC, TG, HDL-C, LDL-C, baseline eGFR, duration of hypertension, duration of AGM, antihypertensive drugs, types of AGM, BUN, uric acid, PRA, serum potassium.
DISCUSSION
The present study, with longitudinal design, observes that PAC is positively and independently associated with the increased risk of CKD in patients with both hypertension and AGM, even in the absence of primary aldosteronism. Positive association is also observed for PAC with DRF and proteinuria, reflecting existence of both functional and structural renal damage in this specific population. The effect of PAC on renal impairment is even stronger in the analyses excluding suspicious primary aldosteronism. SBP mediated slight effects on the association between the two.
Recently, increasing studies have focused on the effects of aldosterone on the kidney in humans. Renal damage is more common in primary aldosteronism patients than in patients with essential hypertension [12], and PAC is positively associated with renal damage, suggesting aldosterone may have a more direct effect on the kidney [13]. Furthermore, in support of the current results, aldosterone has been demonstrated to predict microalbuminuria, and shows a significant inverse association with eGFR in general community population [16,25–27]. In the present study, PAC impairs renal function and structure, and increases the risk of CKD in non-primary aldosteronism patients with hypertension and AGM. The results could be an important extension of this research field and may be valuable for the prevention and treatment of CKD in this high-risk population, as controlling traditional risk factors such as BP seemingly failed to prevent CKD. One of the main effects of aldosterone is to raise BP level, and it was supposed that aldosterone-induced volume expansion serves as an important pathway for CKD pathogenesis. However, our mediation analysis shows that SBP mediates only 7.5–17.9% of the association, suggesting that the direct effects of aldosterone on the kidney cannot be ignored. In this regard, MRA treatment may have potential benefits. A meta-analysis of randomized controlled trials suggested that add-on spironolactone reduces urinary albumin in hypertensives with diabetes mellitus [28]. Recently, Minakuchi et al. report that eplerenone, a selective MRA, can be an effective strategy in preventing CKD progression, especially in those with high PAC [29].
The PAC level in this study is higher than that of the Japanese and Framingham Offspring study [14,25]. Differences in dietary habits, such as salt intake, may play a role. In addition, the differences in the study population may be another reason to be considered. Individuals included in this study were all hypertensives with AGM, and it has been shown that PAC in this population is higher than that in the general population or those with hypertension alone [17,18]. As expected, PAC is associated with renal damage in this population. Experimental studies showed that aldosterone increases renal vascular resistance and glomerular capillary pressure, resulting in proteinuria and renal damage [30]. It has become clear that aldosterone leads to cellular damage through different pathophysiologic mechanisms independent haemodynamic changes, resulting in organs impairment. Studies on hypertensive rats revealed that aldosterone/salt induces intrarenal vascular inflammation, glomerular sclerosis, and tubular damage, through the pathway of reactive oxygen species and mitogen-activated protein kinase [31,32]. Liang et al.[33] reported that aldosterone induces direct glomerular injury, and eplerenone administration provides protection against podocyte apoptosis. These studies also support the present results that PAC increases CKD risk through haemodynamic and direct mechanisms.
The present study has several strengths. First, we evaluated the association between PAC and CKD with longitudinal design and a reliable method for aldosterone measurement. Second, our study was consisted of a sample of individuals at high-risk for CKD, and the results may contribute to the prevention and treatment of CKD. Last, DAGs and LASSO regression were used to identify adjustment sets, and a full-adjusted model was also applied, which makes the results more convincing. Several limitations warrant discussion. First, we failed to evaluate other therapy at baseline, such as glucose and lipid-lowering drugs, and the biomarkers of FPG and blood lipid can only be a partial indicator of disease status. Confounding of medication changes or other interventions added during follow-up should also be considered in future study. In addition, we also lacked 24-h urinary sodium to evaluate the influence of dietary sodium intake on aldosterone. Second, SIT may have false negatives and result in some primary aldosteronism patients being included in this study, and thus biased the results. However, we further excluded individuals with suspicious primary aldosteronism and those with MRA treatment at baseline as sensitivity analyses, and yield similar results. Third, serum creatinine and urinary protein were measured only one-time, and proteinuria was examined through qualitative, but not quantitative, methods. Fourth, the study was conducted in a single centre, although conducted in a regional centre for hypertension with patients of large age range and ethnic groups. Fifth, the portion of lost to follow-up may potentially bias the results. To minimize the bias, we combined rehospitalization and annual health check-up data for reducing the loss of follow-up rate (17.3% in this study), and there was no significant difference between included subjects and lost follow-up individuals for most baseline information.
In conclusion, higher PAC is associated with an increased risk of CKD, including renal functional and structural impairment, in hypertensive individuals with AGM, even in the absence of primary aldosteronism. Therefore, aldosterone may be a candidate therapeutic targets for CKD prevention in this high-risk population.
ACKNOWLEDGEMENTS
The authors thank the staff and participants involved in this study for their critical contributions.
The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
This research was supported by National Key R&D Program of China (grant number: 2018YFC1311503).
Conflicts of interest
The authors have declared that no conflict of interest exists.
Supplementary Material
Footnotes
Abbreviations: AGM, abnormal glucose metabolism; ARR, aldosterone to renin ratio; BP, blood pressure; BUN, blood urea nitrogen; CKD, chronic kidney disease; DAGs, directed acyclic graphs; DM, diabetes mellitus; DRF, decreased renal function; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; LASSO, least absolute shrinkage and selection operator; LDL-C, low density lipoprotein cholesterol; MRA, mineralocorticoid receptor antagonists; PA, primary aldosteronism; PAC, plasma aldosterone concentration; PRA, plasma renin activity; Scr, serum creatinine; SIT, saline infusion test; TC, total cholesterol; VIF, variance inflation factor
Supplemental digital content is available for this article.
REFERENCES
- 1.Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011; 80:572–586. [DOI] [PubMed] [Google Scholar]
- 2.Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol 2019; 1165:3–15. [DOI] [PubMed] [Google Scholar]
- 3.Cao Y, Li W, Yang G, Liu Y, Li X. Diabetes and hypertension have become leading causes of CKD in Chinese elderly patients: a comparison between 1990-1991 and 2009-2010. Int Urol Nephrol 2012; 44:1269–1276. [DOI] [PubMed] [Google Scholar]
- 4.Tannor EK, Sarfo FS, Mobula LM, Sarfo-Kantanka O, Adu-Gyamfi R, Plange-Rhule J. Prevalence and predictors of chronic kidney disease among Ghanaian patients with hypertension and diabetes mellitus: a multicenter cross-sectional study. J Clin Hypertens (Greenwich) 2019; 21:1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laliberté F, Bookhart BK, Vekeman F, Corral M, Duh MS, Bailey RA, et al. Direct all-cause healthcare costs associated with chronic kidney disease in patients with diabetes and hypertension: a managed care perspective. J Manag Care Pharm 2009; 15:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohta M, Babazono T, Uchigata Y, Iwamoto Y. Comparison of the prevalence of chronic kidney disease in Japanese patients with Type 1 and Type 2 diabetes. Diabet Med 2010; 27:1017–1023. [DOI] [PubMed] [Google Scholar]
- 7.Huang YM, Xu D, Long J, Shi Y, Zhang L, Wang H, et al. Spectrum of chronic kidney disease in China: a national study based on hospitalized patients from 2010 to 2015. Nephrology (Carlton) 2019; 24:725–736. [DOI] [PubMed] [Google Scholar]
- 8.Fourkiotis VG, Hanslik G, Hanusch F, Lepenies J, Quinkler M. Aldosterone and the kidney. Horm Metab Res 2012; 44:194–201. [DOI] [PubMed] [Google Scholar]
- 9.Hattangady NG, Olala LO, Bollag WB, Rainey WE. Acute and chronic regulation of aldosterone production. Mol Cell Endocrinol 2012; 350:151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 1996; 98:1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Vecchio L, Procaccio M, Viganò S, Cusi D. Mechanisms of disease: the role of aldosterone in kidney damage and clinical benefits of its blockade. Nat Clin Pract Nephrol 2007; 3:42–49. [DOI] [PubMed] [Google Scholar]
- 12.Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, et al. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension 2006; 48:232–238. [DOI] [PubMed] [Google Scholar]
- 13.Kawashima A, Sone M, Inagaki N, Takeda Y, Itoh H, Kurihara I, et al. Renal impairment is closely associated with plasma aldosterone concentration in patients with primary aldosteronism. Eur J Endocrinol 2019; 181:339–350. [DOI] [PubMed] [Google Scholar]
- 14.Terata S, Kikuya M, Satoh M, Ohkubo T, Hashimoto T, Hara A, et al. Plasma renin activity and the aldosterone-to-renin ratio are associated with the development of chronic kidney disease: the Ohasama Study. J Hypertens 2012; 30:1632–1638. [DOI] [PubMed] [Google Scholar]
- 15.Sim JJ, Shi J, Calara F, Rasgon S, Jacobsen S, Kalantar-Zadeh K. Association of plasma renin activity and aldosterone-renin ratio with prevalence of chronic kidney disease: the Kaiser Permanente Southern California cohort. J Hypertens 2011; 29:2226–2235. [DOI] [PubMed] [Google Scholar]
- 16.Buglioni A, Cannone V, Sangaralingham SJ, Heublein DM, Scott CG, Bailey KR, et al. Aldosterone predicts cardiovascular, renal, and metabolic disease in the general community: a 4-year follow-up. J Am Heart Assoc 2015; 4:e002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karashima S, Yoneda T, Kometani M, Ohe M, Mori S, Sawamura T, et al. Angiotensin II receptor blocker combined with eplerenone or hydrochlorothiazide for hypertensive patients with diabetes mellitus. Clin Exp Hypertens 2016; 38:565–570. [DOI] [PubMed] [Google Scholar]
- 18.Li N, Wang M, Wang H, Zhang D, Wang X, Zu F, et al. Prevalence of primary aldosteronism in hypertensive subjects with hyperglycemia. Clin Exp Hypertens 2013; 35:175–182. [DOI] [PubMed] [Google Scholar]
- 19.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, et al. Human blood pressure determination by sphygmomanometry. Circulation 1993; 88:2460–2470. [DOI] [PubMed] [Google Scholar]
- 20.Luo Q, Li NF, Yao XG, Zhang DL, Abulikemu SF, Chang GJ, et al. Potential effects of age on screening for primary aldosteronism. J Hum Hypertens 2016; 30:53–61. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Luo Q, Tuersun T, Wang G, Wu T, Zhang D, et al. Higher prevalence of metabolic disorders in patients with bilateral primary aldosteronism than unilateral primary aldosteronism. Clin Endocrinol (Oxf) 2021; 94:3–11. [DOI] [PubMed] [Google Scholar]
- 22.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17:2937–2944. [DOI] [PubMed] [Google Scholar]
- 23.Knüppel S, Stang A. DAG program: identifying minimal sufficient adjustment sets. Epidemiology 2010; 21:159. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Sillanpää MJ. Overview of LASSO-related penalized regression methods for quantitative trait mapping and genomic selection. Theor Appl Genet 2012; 125:419–435. [DOI] [PubMed] [Google Scholar]
- 25.Fox CS, Gona P, Larson MG, Selhub J, Tofler G, Hwang SJ, et al. A multimarker approach to predict incident CKD and microalbuminuria. J Am Soc Nephrol 2010; 21:2143–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buglioni A, Cannone V, Cataliotti A, Sangaralingham SJ, Heublein DM, Scott CG, et al. Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension 2015; 65:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannemann A, Rettig R, Dittmann K, Völzke H, Endlich K, Nauck M, et al. Aldosterone and glomerular filtration: observations in the general population. BMC Nephrol 2014; 15:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin M, Heizati M, Wang L, Nurula M, Yang Z, Wang Z, et al. A systematic review and meta-analysis of effects of spironolactone on blood pressure, glucose, lipids, renal function, fibrosis and inflammation in patients with hypertension and diabetes. Blood Press 2021; 30:145–153. [DOI] [PubMed] [Google Scholar]
- 29.Minakuchi H, Wakino S, Urai H, Kurokochi A, Hasegawa K, Kanda T, et al. The effect of aldosterone and aldosterone blockade on the progression of chronic kidney disease: a randomized placebo-controlled clinical trial. Sci Rep 2020; 10:16626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arima S, Kohagura K, Xu HL, Sugawara A, Abe T, Satoh F, et al. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol 2003; 14:2255–2263. [DOI] [PubMed] [Google Scholar]
- 31.Blasi ER, Rocha R, Rudolph AE, Blomme EA, Polly ML, McMahon EG. Aldosterone/salt induces renal inflammation and fibrosis in hypertensive rats. Kidney Int 2003; 63:1791–1800. [DOI] [PubMed] [Google Scholar]
- 32.Nishiyama A, Yao L, Nagai Y, Miyata K, Yoshizumi M, Kagami S, et al. Possible contributions of reactive oxygen species and mitogen-activated protein kinase to renal injury in aldosterone/salt-induced hypertensive rats. Hypertension 2004; 43:841–848. [DOI] [PubMed] [Google Scholar]
- 33.Liang W, Chen C, Shi J, Ren Z, Hu F, van Goor H, et al. Disparate effects of eplerenone, amlodipine and telmisartan on podocyte injury in aldosterone-infused rats. Nephrol Dial Transplant 2011; 26:789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.