OBJECTIVES
The aim of this study was to describe the 30-day mortality rate of patients aged 80 years and older undergoing surgical and nonsurgical procedures under anaesthesia in Europe and to identify risk factors associated with mortality.
DESIGN
A prospective cohort study.
SETTING
European multicentre study, performed from October 2017 to December 2018. Centres committed to a 30-day recruitment period within the study period.
PATIENTS
Nine thousand four hundred and ninety-seven consecutively recruited patients aged 80 years and older undergoing any kind of surgical or nonsurgical procedures under anaesthesia.
MAIN OUTCOME MEASURES
The primary outcome was all-cause mortality within 30 days after procedure described by Kaplan–Meier curves with 95% CI. Risk factors for 30-day mortality were analysed using a Cox regression model with 14 fixed effects and a random centre effect.
RESULTS
Data for 9497 patients (median age, 83.0 years; 52.8% women) from 177 academic and nonacademic hospitals in 20 countries were analysed. Patients presented with multimorbidity (77%), frailty (14%) and at least partial functional dependence (38%). The estimated 30-day mortality rate was 4.2% (95% CI 3.8 to 4.7). Among others, independent risk factors for 30-day mortality were multimorbidity, hazard ratio 1.87 (95% CI 1.26 to 2.78), frailty, hazard ratio 2.63 (95% CI 2.10 to 3.30), and limited mobility, hazard ratio 2.19 (95% CI 1.24 to 3.86). The majority of deaths (76%) occurred in hospital. Mortality risk for unplanned ICU admission was higher, hazard ratio 3.57 (95% CI 2.38 to 5.26) than for planned ICU admission, hazard ratio 1.92 (95% CI 1.47 to 2.50). Compared with other studies, the in-hospital complication rates of 17.4 and 3.9% after discharge were low. Admission to a unit with geriatric care within 30 days after the intervention was associated with a better survival within the first 10 days.
CONCLUSIONS
The estimated 30-day mortality rate of 4.2% was lower than expected in this vulnerable population.
TRIAL REGISTRATION
ClinicalTrials.gov Identifier: NCT03152734, https://clinicaltrials.gov.
This article is accompanied by the following Editorial:
Columb MO, Longrois D, Hansen TG, Bruder N. Strike a pose: The POSE study poses some questions! Eur J Anaesthesiol 2022; 39:193–195.
KEY POINTS
In this European prospective multicentre cohort study that included 9497 patients at least 80 years of age undergoing various interventions, the estimated 30-day mortality rate was unexpectedly low with 4.2%.
The majority of patients presented with multimorbidity. One-third had previously experienced at least one fall and were partially functionally dependent at admission, while approximately two-thirds presented with possible cognitive impairment and limited mobility.
Admission to a unit with geriatric care was associated with a better survival within the first 10 days postintervention.
Introduction
The WHO ‘Global strategy and action plan on ageing and health’ fosters research on older people in need of improvement.1 The population older than 80 years is expected to grow from 125 million by almost 3.5-fold to 434 million until 2050 worldwide.2 Likewise, the number of patients undergoing an array of surgical and nonsurgical procedures such as radiological, neuroradiological, cardiological or gastroenterological, with anaesthesia will increase. Furthermore, frailty, a multidimensional, dynamic and extreme consequence of the normal aging process, is seen as a serious global health burden.3,4 Multimorbidity, which peaks in older patients and frailty are associated with an increased risk of adverse outcomes and significant healthcare costs.3,5 Peri-operative mortality was stressed as one of the six core surgical indicators that should be assessed in all countries by 2030.6 In particular, the mortality within 30 days after a procedure is an important time frame representing the overall quality of care.7,8 Moreover, with 4.2 million postoperative deaths annually worldwide, postoperative mortality is ranked as the third most common cause of death globally.8
However, little is known about the 30-day mortality of patients aged 80 years and older undergoing any kind of interventions in Europe.9 We identified 11 studies in Europe including 3462 patients aged at least 80 years with an average postoperative 30-day mortality rate of 11.2% [range 5.3 to 33.3]. These studies predominantly focused on specific high-risk procedures such as cancer surgery,10 or small nonrepresentative patient populations, potentially overestimating postoperative mortality. Further, we aimed to gain information on postprocedural resource utilisation (ICU or a unit with geriatric support) in this population.
The Peri-interventional Outcome Study in the Elderly (POSE) was designed to provide essential data on the 30-day mortality of patients aged 80 years and older undergoing surgical and nonsurgical procedures under anaesthesia across Europe.
Materials and methods
Study design, setting and participants
POSE was a European multicentre, observational prospective cohort study. The full study protocol including protocol changes is presented in Supplementary Digital Content (SDC 2). Patients were eligible if aged at least 80 years and undergoing any kind of surgical or nonsurgical procedure such as radiological kyphoplasty or gastrointestinal stenting,11 under anaesthesia (performed by an anaesthetist). Furthermore, procedures without any intervention such as diagnostic computer tomography with sedation or solely anaesthetic interventions such as insertion of a central venous catheter were excluded. From October 2017 to December 2018, each centre recruited their patients during 30 self-selected consecutive days within the total study period. The follow-up period for each patient comprised 30 days after the procedure. Procedures were classified as either surgical or nonsurgical, elective or nonelective, and inpatient or outpatient. POSE aimed to recruit as many European countries and centres as possible using this convenient sampling strategy. Study centres (SDC 1) were invited to participate via the POSE website.12 Several anaesthesia societies (European, French, German and Swiss) endorsed POSE. A national co-ordinator was designated for each country and was in charge of national regulatory matters. Mandatory research ethics board (REB) approval or a waiver was granted at each centre. Patient or legal representative consent was sought as required according to respective national laws. Initial REB approval (EK 162/17) was granted to the University Hospital RWTH Aachen, Germany by the institutional REB of the University Hospital RWTH Aachen, Aachen, Germany on 18 August 2017. The study was registered with ClinicalTrials.gov (NCT03152734) and is reported in accordance with the STROBE statement.13
Variables and data
Patient data were collected on paper-based case report forms (SDC 3) and entered into an electronic database (OpenClinica, Boston, Massachusetts, USA) pseudonymised. In addition to automatic database completion, consistency and plausibility checks, and manual multilevel data validation were performed. Discrepancies were clarified with local investigators.
Baseline characteristics and outcomes
All the baseline data collected and outcome measures are described in detail in the study protocol (SDC 2) and defined in the POSE glossary (SDC 4), as well as the statistical analysis plan (SAP) (SDC 5). In brief, apart from other patients’ characteristics, the comprehensive geriatric assessment comprised anaemia investigations, nutritional status, history of falls, functional dependency,14 the Mini-Cog,15 the timed ‘Up & Go’ (TUG) test16 and frailty.15,17 The POSE frailty assessment is based on the accumulation of deficits model.18 Frailty was scored as present if at least four of the following six markers were present: Mini-Cog score 3 points or less; albumin level 33 g l−1 or less; one fall in the last 6 months; haematocrit level less than 35%; partially or totally functionally dependent; and at least three comorbidities.15,17 Multimorbidity was defined as the presence of at least two of the assessed comorbidities according to the POSPOM19 and American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) risk calculators.14 The risk severity of the procedures was classified as described previously.20–22 Examples for minor, intermediate and major categories are presented in SDC 4. The procedures were classified as elective if scheduled in advance, as urgent if required within less than 48 h and as emergency if the patient's life or wellbeing was in direct jeopardy.
The primary outcome measure was ‘all cause mortality’ within 30 days after the procedure. All outcomes were documented at 30 days after the procedure. The secondary outcomes included in-hospital complications in compliance with the ACS-NSQIP,14 intervention related details, process measures such as admission to ICU or the use of a specific geriatric care model, and outcomes on day 30.
Bias
We attempted to minimise the risk of selection bias, aiming for generalisable results for the target population by consecutive enrolment of patients within each centre including legally incompetent and emergency patients. We assumed a negligible risk of detection bias due to the objective nature of our primary outcome variable. To avoid attrition bias after a failed first telephone follow-up, the study centres made at least one further attempt or contacted the patients’ next of kin or family physician. For most secondary outcomes, the risk of detection bias was controlled by clear a priori definitions and instructions in the POSE glossary (SDC 4). The majority of the data collected was routinely assessed within the hospital stay, further minimising the risk of attrition bias.
Sample size
According to the objective of this multicentre observational cohort study, the sample size calculation was explorative rather than rigorous. However, we propose that the sample size is reasonable to detect a 2% difference in mortality rate according to previously published rates. 23,24 For a preliminary estimate, rather than continuous variables, we used a χ2 test to detect a clinically relevant difference of 10 and 8% in event probabilities after 30 days between the levels of an arbitrary binary variable (5% significance level, 80% power). This resulted in 3313 patients per level and similar numbers resulted by using the log rank test. Accordingly, the total sample size was predicted to require approximately 7000. Thus, our actual sample size would be appropriate to establish the proposed rate difference for at least one risk factor.
Statistical analysis
The statistical analysis was performed according to the methods specified in the SAP, published on the POSE website before database lock (SDC 5). Deviations from the SAP in the statistical analyses are presented in the supplementary methods in SDC 1. Mean ± SD, median [Q1 to Q3], absolute and relative frequencies were used to summarise the data according to their characteristics.
Kaplan--Meier curves across stratified age groups (80 to 84 vs. 85 to 89 vs. ≥90 years) with 95% confidence intervals (95% CIs) were used to describe the mortality up to 30 days. The primary endpoint variable was analysed using a Cox regression model with 14 fixed effects and a random centre effect (frailty model with lognormal distribution) via multiple imputation. Five of the fixed effects were defined in advance as clinically important model building variables (age, sex, severity of intervention, urgency of intervention and frailty). The other nine fixed effects were selected from a set of nine candidate variables because they showed at least a moderate association with the primary endpoint in the corresponding Cox models with multiple imputation (median or pooled P value of at most 0.25). The proportional hazards assumption of all independent (candidate) variables was examined graphically using Schoenfeld residuals.
In two patients, the exact date of death could not be determined. The mean value between discharge date and follow-up date was therefore defined as the time of death. Using multiple imputation with 12 imputations, the full cohort was considered in the primary analysis. Missing values were imputed on the basis of all dependent and independent variables from the Cox regression model using the fully conditional specification method, as previously described.25 Estimated hazard ratios, 95% CIs and P values from the multiply imputed Cox models were combined using Rubin's rule. For categorical variables or interaction terms with more than two levels, the median type III P values were also reported.
Two sensitivity analyses were conducted, one based on the complete cases only, and one including three interaction effects based on clinical relevance (premedication with age and frailty, respectively, and anaesthesia technique with the severity of intervention). Administration of premedication before intervention was allocated to three categories: none, clonidine and benzodiazepine. Anaesthesia technique consisted of general anaesthesia, regional anaesthesia, sedation and a combination of at least two of them. All secondary endpoints were analysed descriptively. The nominal significance level was set as 5%. We did not adjust for multiple testing. All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Cary, North Carolina, USA) and R, version 3.5.1.26
Results
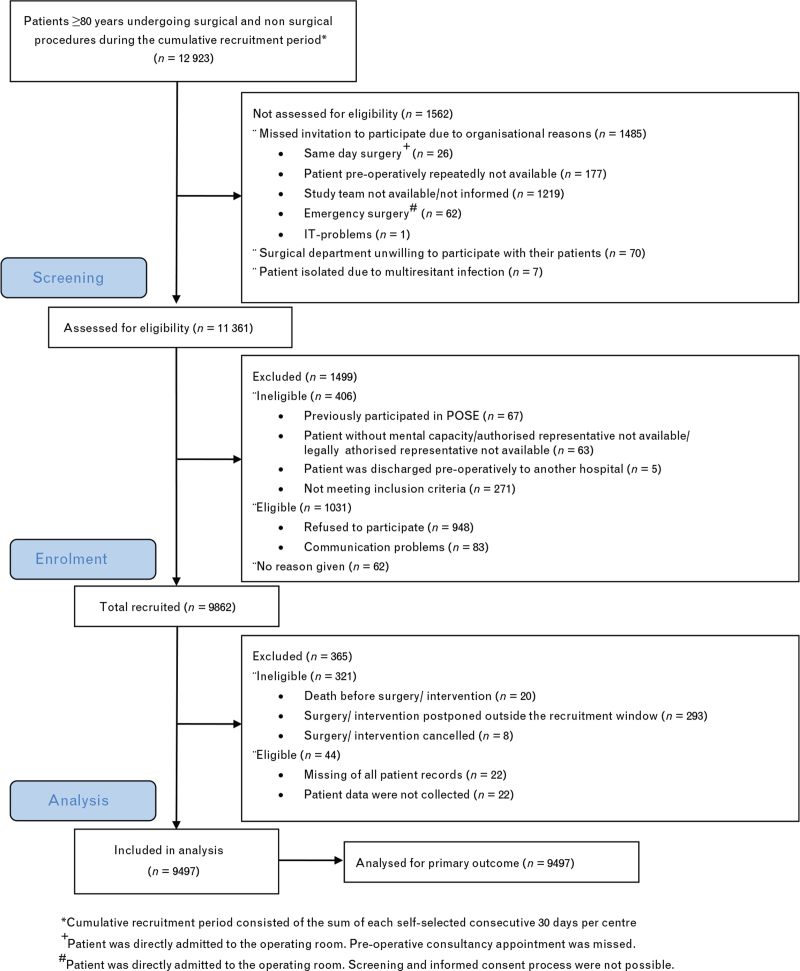
During the recruitment period, the 177 participating centres from 20 countries treated 12 923 patients at least 80 years of age undergoing procedures under anaesthesia. Of these, 9862 patients were recruited into the POSE study. Following exclusion of 365 patients, the data for 9497 patients were analysed (Fig. 1). The hospital characteristics are presented in Table S1 in SDC 1.
Fig. 1.
Flow of participants according to the STROBE guideline13
The patients’ baseline characteristics are presented in Table 1. Multimorbidity was present in 7334 (77%) of 9497 patients. Our specific pre-interventional geriatric assessment revealed frailty in 1336 (14.1%) of 9497 patients. About one-third had previously experienced at least one fall and were partially functionally dependent at admission, while about two-thirds presented with possible cognitive impairment (Mini-Cog score of ≤3)15 and limited mobility. Other comorbid conditions according to the ACS-NSQIP, laboratory values and chronic pre-operative medication are presented in Table S2 and S3 in SDC 1. Interventional characteristics are presented in Table 2. Most patients (80%) underwent major and intermediately severe interventions, whereas 7176 (75.6%) were elective. Nonsurgical procedures were performed in 1026 (10.8%) of 9497 patients.
Table 1.
Patient demographics and baseline characteristics
| All patients (n = 9497) | |
| Age, median [IQR], years | 83.0 [81.0 to 86.0] |
| Age, mean ± SD, years | 84.3 ± 3.8 |
| Sex, No. (%) | |
| Male | 4485 (47.2) |
| Female | 5012 (52.8) |
| Current smokera, No. (%) | 540 (5.7) |
| Heighta, median [IQR], cm | 165.0 [158.0 to 170.0] |
| Heighta, mean ± SD, cm | 164.6 ± 9.2 |
| Weighta, median [IQR], kg | 70.0 [60.0 to 80.0] |
| Weighta, mean ± SD, kg | 70.4 ± 13.5 |
| ASA categorya, median [IQR] | 3.0 [2.0 to 3.0] |
| ASA categorya, No. (%) | |
| 1 | 170 (1.8) |
| 2 | 3499 (36.9) |
| 3 | 5106 (53.8) |
| 4 | 692 (7.3) |
| 5 | 23 (0.2) |
| Comorbidity, No. (%) | |
| Hypertension requiring medicationa | 7090 (74.7) |
| Cardiac rhythm disorder | 3000 (31.6) |
| Ischaemic heart disease | 2464 (26.0) |
| Cancer | 2271 (23.9) |
| Chronic heart failure or cardiomyopathy | 2109 (22.2) |
| Diabetes | 1947 (20.5) |
| Chronic renal failure | 1673 (17.6) |
| Other cognitive complaints | 1322 (13.9) |
| Cerebrovascular disease | 1246 (13.1) |
| Mild cognitive impairment | 1077 (11.3) |
| Peripheral vascular disease | 1056 (11.1) |
| Chronic obstructive pulmonary disease | 961 (10.1) |
| Dementia | 756 (8.0) |
| Chronic respiratory failure | 390 (4.1) |
| Hemiplegia | 231 (2.4) |
| Chronic alcohol abuse | 143 (1.5) |
| Transplanted organ(s) | 12 (0.1) |
| Multimorbidityb, No. (%) | 7334 (77.2) |
| Frailtyc, No. (%) | 1336 (14.1) |
| History of falls during the last 6 monthsa,d, No. (%) | |
| None | 6426 (68.3) |
| Once | 1805 (19.2) |
| More than once | 1181 (12.6) |
| Unintentional weight loss of ≥4.5 kg in the last yeara, No. (%) | 1714 (18.3) |
| Mini-Cog (complete test)a,d,e | |
| Total score, median [IQR] | 3.0 [1.0 to 5.0] |
| 0 points (profound cognitive dysfunction), No. (%) | 1392 (15.4) |
| ≤3 points, (cognitive impairment)15 No. (%) | 5393 (59.6) |
| 5 points, (normal cognition), No. (%) | 2303 (25.4) |
| Mini to Cog (recall of three words)a,d,e | |
| Total score, median [IQR] | 2.0 [1.0 to 3.0] |
| Mini to Cog (clock draw points)a,e | |
| Total score, median [IQR] | 0 [0.0 to 2.0] |
| Functional statusa | |
| Independent, No. (%) | 5845 (61.6) |
| Partially dependent, No. (%) | 2903 (30.6) |
| Totally dependent, No. (%) | 743 (7.8) |
| Limited mobility according to the TUG testa,f, No. (%) | 6461 (77.2) |
| Referring facilitya | |
| Home, No. (%) | 8220 (86.6) |
| Nursing home, No. (%) | 670 (7.1) |
| Other Hospital, No. (%) | 184 (1.9) |
| Other, No. (%) | 360 (3.8) |
| Rehabilitation facility, No. (%) | 60 (0.6) |
ASA, American Society of Anesthesiologists; IQR, interquartile range; SD, standard deviation; TUG, Timed Up and Go test.
Missing data: Current smoker, n = 14; height, n = 146; weight, n = 97; ASA category, n = 7; history of falls, n = 85; unintentional weight loss, n = 104; Mini-Cog (complete test), n = 443; Mini-Cog (recall), n = 373; Mini-Cog (clock drawing), n = 442; functional status, n = 6; limited mobility (TUG), n = 1125; referring facility, n = 3; hypertension, n = 1.
Multimorbidity was defined as the presence of at least two of the assessed comorbidities.
Frailty was classified as present, if at least four of the following six markers were present: Mini-Cog total score of ≤3 points; albumin level of ≤33 g l−1; >1 fall in the last 6 months; haematocrit level of <35%; pre-operative functional status is partially dependent or totally dependent; and ≥3 comorbidities present (according to Robinson et al.15 and Oresanya et al.17).
Percentages may not total 100 because of rounding.
Mini-Cog screening tool to detect cognitive impairment or dementia: 0 = profound cognitive dysfunction, ≤3 = cognitive impairment according to Robinson et al., 5 = normal cognition.
Limited mobility was defined as Timed Up and Go test performed in >12 s.
Table 2.
Procedure characteristics
| All patients (n = 9497)a | |
| Severity of the procedure, n (%) | |
| Major | 3938 (41.5) |
| Intermediate | 3612 (38.0) |
| Minor | 1947 (20.5) |
| Urgency of the intervention, n (%) | |
| Elective | 7176 (75.6) |
| Urgent | 1842 (19.4) |
| Emergency | 479 (5.0) |
| Category of intervention, n (%) | |
| Orthopaedic, trauma, and plastic | 2860 (30.1) |
| ENT and Opthalmic | 1594 (16.8) |
| Gynaecologic and urological | 1437 (15.1) |
| Abdominal | 1149 (12.1) |
| Nonsurgical procedureb | 1026 (10.8) |
| Cardiovascular and thoracic | 896 (9.4) |
| Other | 338 (3.6) |
| Neurosurgery | 196 (2.1) |
| Transplant | 1 (0.0) |
| Planned kind of procedure, n (%) | |
| Inpatient intervention | 7562 (79.6) |
| Outpatient intervention | 1935 (20.4) |
| Premedication before intervention, n (%)c | |
| None | 7936 (83.7) |
| Benzodiazepine | 1521 (16.0) |
| Clonidine | 30 (0.3) |
| Anaesthesia technique, n (%) | |
| General | 5052 (53.2) |
| Sedation | 1755 (18.5) |
| Regionald | 1628 (17.1) |
| Combinede | 1062 (11.2) |
| Duration of anaesthesia, median [IQR], minc | 90.0 [48.0 to 142.0] |
| Duration of anaesthesia, mean (SD), minc | 109.8 (89.1) |
| Use of any advanced intra-operative monitoring, n (%)c | 3336 (35.1) |
| Intra-arterial blood pressure measurement | 1675 (17.6) |
| Anaesthesia depth monitoring device | 1443 (15.2) |
| Other | 577 (6.1) |
| Central venous pressure | 517 (5.4) |
| Near-infrared spectroscopy | 389 (4.1) |
| Transoesophageal echocardiogram | 190 (2.0) |
| Cardiac output | 117 (1.2) |
| Pulmonary artery catheter | 54 (0.6) |
| Transfusion of plasma during surgeryc | 141 (1.5) |
| Transfusion of platelets during surgeryc | 64 (0.7) |
| Transfusion of red blood cells during surgeryc | 575 (6.1) |
| Use and completion of a safe surgery checklist (e.g. WHO-safe surgery checklist)c | |
| Yes | 7079 (74.7) |
| No | 2402 (25.3) |
| Extubation at the end of surgeryf | |
| Yes | 4997 (91.0) |
| No | 496 (9.0) |
ENT, Ear nose and throat; IQR, interquartile range, SD, standard deviation; WHO, World Health Organisation.
Percentages may not total 100 because of rounding.
Examples for nonsurgical interventions: radiological such as kyphoplasty, neuroradiological, cardiological or gastroenterological such as gastrointestinal stenting.
Missing data: premedication before intervention, n = 10; duration of anaesthesia, n = 22; use of any advanced intra-operative monitoring, n = 2; transfusion of plasma, platelets or red blood cells during surgery, respectively, n = 1; use and completion of a safe surgery checklist, n = 16.
Regional anaesthesia comprises the epidural, spinal or other regional anaesthesia technique.
Combined anaesthesia is defined as a combination of at least two of the three categories: general anaesthesia, sedation, or regional anaesthesia.
Applicable cases/ missing data: extubation at the end of surgery, n = 5493/n = 2.
Description of 30-day mortality
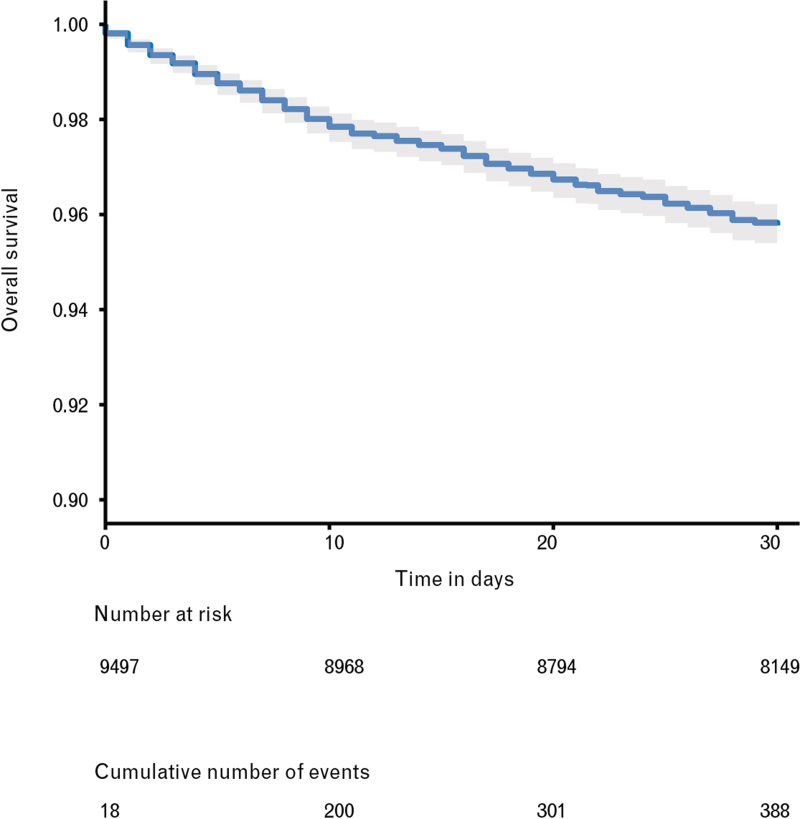
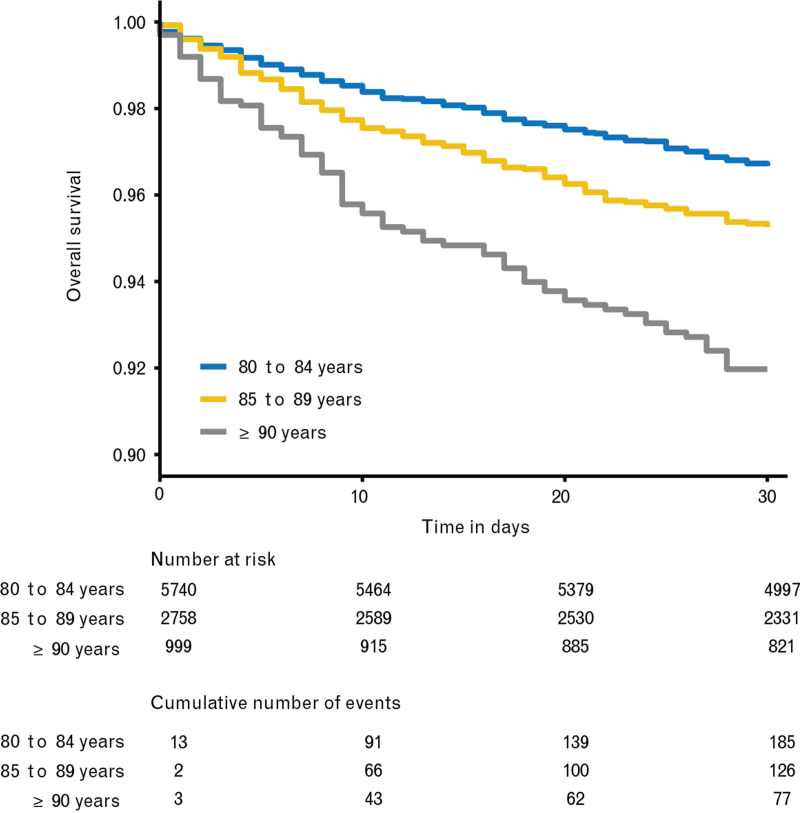
By day 30 after the procedure, 388 deaths were observed among the 9497 patients, of which 93 occurred after hospital discharge (Table 3). The Kaplan--Meier estimate of 30-day mortality was 4.2% (95% CI 3.8 to 4.7) (Fig. 2) and the Kaplan--Meier curves stratified by age category showed that mortality increased with age (Fig. 3).
Table 3.
Secondary outcomes
| All patients (n = 9497)a | |
| n (%)e | |
| In-hospital outcomes | |
| Hospital length of stay, median [IQR], days | 3.0 [1.0 to 8.0) |
| Hospital length of stay, mean ± SD, days | 5.9 ± 7.3 |
| ICU length of stay, median [IQR], daysb | 2.0 [1.0 to 4.0] |
| ICU length of stay, mean ± SD, daysb | 3.6 ± 5.5 |
| Admission to ICU immediately after procedure | |
| Yes | 1657 (17.5) |
| No | 7839 (82.6) |
| Planned admission to ICU immediately after procedured | 1508 (15.9) |
| Unplanned admission to ICU immediately after procedured | 149 (1.6) |
| Unplanned ICU admission at any time-point after intervention until day 30c | 387 (4.1) |
| Admission to a unit with geriatric support immediately after procedurec | |
| Yes | 679 (7.2) |
| No | 8817 (92.9) |
| Admission to a unit with geriatric support at any time-point after procedure until day 30c | 1030 (10.9) |
| Discharge to postacute care according to the ACS-NSQIPc | 2026 (21.3) |
| At least one in-hospital complication according to the ACS-NSQIPc | 1650 (17.4) |
| Return to the operating room | 369 (3.9) |
| Urinary tract infection | 338 (3.6) |
| Pneumonia | 335 (3.5) |
| Acute kidney injury | 317 (3.3) |
| Cardiac arrest | 222 (2.3) |
| Systemic sepsis | 209 (2.2) |
| Superficial incisional surgical site infection | 167 (1.8) |
| Ventilator dependency >48 h | 148 (1.6) |
| Deep incisional surgical site infection | 132 (1.4) |
| Wound dehiscence | 124 (1.3) |
| Myocardial infarction | 83 (0.9) |
| Unplanned intubation | 74 (0.8) |
| Stroke | 52 (0.6) |
| Organ space surgical site infection | 48 (0.5) |
| Pulmonary embolism | 37 (0.4) |
| Deep vein thrombosis | 33 (0.4) |
| Venous thromboembolism/blood clot | 27 (0.3) |
| Discharge destinationd | |
| Home | 6797 (76.5) |
| Rehabilitation facility | 938 (10.6) |
| Nursing home | 690 (7.8) |
| Other hospital | 363 (4.1) |
| Other | 100 (1.1) |
| 30-day follow-up outcomes | |
| Survival status | |
| Patient was discharged and alive | 8359 (88.0) |
| Patient was discharged and lost to follow-up | 442 (4.7) |
| Patient was still at ward and alive | 308 (3.2) |
| Patient died in-hospital | 295 (3.1) |
| Patient died after discharge | 93 (1.0) |
| At least one of the following complications after discharged | 335 (3.9) |
| Pulmonary complications after discharge | 152 (1.8) |
| Cardiac complications after discharge | 106 (1.2) |
| Acute kidney injury after discharge | 99 (1.1) |
| Stroke after discharge | 34 (0.4) |
| Functional statusd | |
| Independent | 4270 (49.9) |
| Partially dependent | 3084 (36.0) |
| Totally dependent | 1205 (14.1) |
| Brief screen for cognitive impairment, 3-item delayed recall partd | |
| Total correct words, median [IQR] | 2.0 [1.0 to 3.0] |
| 0 correct words | 1584 (20.2) |
| 1 correct word | 868 (11.1) |
| 2 correct words | 1864 (23.8) |
| 3 correct words | 3517 (44.9) |
ACS-NSQIP, American College of Surgeons National Surgical Quality Improvement Program; IQR, interquartile range; SD, standard deviation.
Percentages may not total 100 because of rounding.
Referring to the total number of patients (n = 1796) admitted to ICU at any time-point within 30 postprocedure days.
Missing data: Admission to ICU immediately after intervention, n = 1; unplanned ICU admission at any time-point until day 30, n = 3; admission to a unit with geriatric support immediately after intervention, n = 1; admission to a unit with geriatric support at any time-point until day 30, n = 3; discharge to postacute care, n = 4; any in-hospital complications according to the ACS-NSQIP, n = 4.
Applicable cases/ missing data: Admission to ICU directly after procedure, n = 1657/ n = 0; discharge destination n = 8894/ n = 6; complications after discharge, n = 8694/ n = 200; functional status on day 30, n = 9109/ n = 550; brief screen for cognitive impairment, n = 9109/ n = 1276.
If not otherwise stated.
Fig. 2.
Survival in the entire cohort
Kaplan--Meier survival curve for the entire cohort until day 30 after the intervention.
Fig. 3.
Survival stratified by age
Kaplan--Meier survival curves stratified by age groups until day 30 after the intervention.
Additional Kaplan--Meier curves of the survival stratified by ICU admission showed greatest mortality for patients with unplanned admissions (Fig. S1 in SDC 1). Patients with an admission to a unit with geriatric care within 30 days showed better survival within the first 10 days after the procedure (Fig S2 in SDC 1).
Analysis of 30-day mortality
All 14 fixed effects besides anaesthesia technique were identified as independent risk factors for mortality (P < 0.05) in the Cox model with multiply imputed data (n = 9497) (Table 4 and Table S4 in SDC 1).
Table 4.
Multivariable Cox regression for all-cause mortality until day 30
| Model with multiply imputed data | ||
| Estimated HR (95% CI) | P | |
| Independent variable | ||
| Age | 1.04 (1.01 to 1.06) | 0.003 |
| Sex (male) | 1.42 (1.14 to 1.76) | 0.001 |
| Severity of procedure | NA | 0.02 |
| Major vs. Minor | 1.56 (1.05 to 2.33) | 0.03 |
| Intermediate vs. Minor | 1.18 (0.79 to 1.77) | 0.41 |
| Urgency of procedure | NA | <0.001 |
| Urgent vs. Elective | 2.17 (1.66 to 2.84) | <0.001 |
| Emergency vs. Elective | 4.17 (3.09 to 5.64) | <0.001 |
| Frailtya | 2.63 (2.10 to 3.30) | <0.001 |
| Procedure category | NA | <0.001 |
| Referring facility | NA | <0.001 |
| Transfusion of plasma | 2.08 (1.35 to 3.19) | <0.001 |
| Transfusion of platelets | 2.12 (1.16 to 3.85) | 0.01 |
| Transfusion of red blood cells | 1.92 (1.42 to 2.58) | <0.001 |
| Anaesthesia techniqueb | NA | 0.28 |
| Multimorbidityc | 1.87 (1.26 to 2.78) | 0.002 |
| Premedication | NA | 0.02 |
| None vs. Clonidine | 1.39 (0.19 to 10.20) | 0.74 |
| None vs. Benzodiazepine | 1.71 (1.18 to 2.49) | 0.005 |
| Limited mobility TUG testd | 2.19 (1.24 to 3.86) | 0.007 |
All pairwise comparisons are presented in Supplementary Table S4 in SDC 1.
CI, confidence interval; HR, hazard ratio; NA, not applicable; TUG, Timed Up and Go test.
Frailty was classified as present, if at least four of the following six markers were present: Mini Cog total score of ≤3 points; albumin level of ≤33 g l−1; >1 fall in the last 6 months; haematocrit level of <35%; pre-operative functional status is partially dependent or totally dependent; and ≥3 comorbidities present (according to Robinson et al.15 and Oresanya et al.17
Anaesthesia techniques were categorised in four groups as general anaesthesia, regional anaesthesia (comprising epidural, spinal and other regional), sedation or a combination of any of the three previous categories.
Multimorbidity was defined as the presence of at least two of the assessed comorbidities.
Limited mobility was defined as Timed Up and Go test performed in >12 s.
Emergency surgery had a higher mortality risk than elective surgery, hazard ratio 4.17 (95% CI 3.09 to 5.64); the risk of death increased if the patients were either frail, hazard ratio 2.63 (95% CI 2.10 to 3.30), showed limited mobility by the TUG, hazard ratio 2.19 (1.24 to 3.86), were multimorbid, hazard ratio 1.87 (95% CI 1.26 to 2.78), or male, hazard ratio 1.42 (95% CI 1.14 to 1.76). For every 5 years of age difference, the mortality risk increased by a factor of 1.22, calculated from hazard ratio per year 1.04 (95% CI 1.01 to 1.06). The mortality risk was increased for major compared with minor interventions, hazard ratio 1.56 (95% CI 1.05 to 2.33).
Sensitivity analysis of 30-day mortality
Of 8365 patients with complete data, 330 died within 30 days, and the estimated effects were comparable to our primary analysis with multiple imputed data. However, significant effects were abolished for the following variables: severity of procedure, transfusion of platelets and premedication; the point estimates of the hazard ratios were similar.
The sensitivity analysis accounting for the predefined clinical interactions revealed no statistically significant interaction effects (Table S5 in SDC 1). An additional sensitivity analysis of the variable ‘Admission to ICU’ as a further risk factor (Table S6 in SDC 1) confirmed the results of the main model. Furthermore, an unplanned ICU admission compared with no admission showed a significantly higher mortality risk with a hazard ratio of 3.57 (CI 2.38 to 5.26) than a planned ICU admission, hazard ratio 1.92 (CI 1.47 to 2.50).
Secondary outcome variables
The secondary outcomes are presented in Table 3 and Table S7 in SDC 1. At least one in-hospital complication defined by ACS-NSQIP14 was detected among 1650 (17.4%) of 9493 patients. The median hospital length of stay was 3.0 days [1.0 to 8.0]. The median stay in ICU of patients admitted to ICU within 30 days of intervention (n = 1796) was 2.0 days [1.0 to 4.0]. Immediate postinterventional admission to ICU occurred in 1657 (17.5%) of 9496 patients for whom 1508 (91.0%) admissions had been planned. A total of 387 (4.1%) of 9494 patients had an unplanned admission to the ICU within 30 days after intervention. Direct postinterventional admission to a unit with geriatric support occurred among 679 (7.2%) of 9496 patients, whereas an admission at any time-point within 30 days of procedure occurred for 10.9%. The 30-day follow-up showed that 4270 (49.9%), 3084 (36.0%) and 1205 (14.1%) of the 8559 patients were functionally independent, partially dependent and totally dependent, respectively. At least one of four predefined serious complications (pulmonary, cardiac, renal and stroke) occurred in 335 (3.9%) of 8694 patients after hospital discharge, with serious pulmonary complications being the most frequent complications (1.8%).
Discussion
POSE is the largest European prospective multicentre cohort study thus far, involving 9497 patients aged at least 80 years undergoing surgical and nonsurgical procedures. POSE revealed an estimated 30-day mortality rate of 4.2%, which was lower than expected for this vulnerable patient population. The majority of patients were multimorbid, cognitively and functionally impaired, and underwent major, elective in-patient procedures. Particularly striking is the contrast of this POSE mortality rate to the rate of 8.2% in the large ACS-NSQIP register-based, noncardiac surgery study with patients of similar age.23 Interestingly, the mortality in the large European EuSOS study was only slightly lower (4.0%), though EuSOS involved younger patients with a mean age of 56.7 ± 18.5 years and excluded higher-risk surgery such as cardiac surgery and neurosurgery.22 One reason for our low mortality rate might be different inclusion criteria, as other studies have either excluded day-case surgery22 or minor surgery,23 both of which are supposed to be associated with a better outcome. Although POSE involved 10.8% nonsurgical procedures, which are associated with less mortality and morbidity in elderly patients,11 our data did not show differences in mortality risks for surgical and nonsurgical patients.
Nevertheless, procedures with low Operative Stress Scores are associated with higher postoperative mortality if the patients are frail and thus frailty should also be assessed in patients undergoing minor risk procedures.27 To our knowledge, POSE is the first European study that included a comprehensive pre-intervention evaluation of specific geriatric domains in patients aged at least 80 years, such as cognition, function, nutrition, comorbidities and frailty. This is an important step in offering optimal strategies and shared decision-making.1,3,4,9,17,27 The weighted average prevalence of frailty is estimated to be 10 to 11%.3,5 Our data showed a slightly higher frailty rate of 14.1%, which might be attributed to the heterogeneous definitions of frailty. One shortcoming of the POSE frailty definition is that missing values for haematocrit and albumin and the use of a cut-off of >117 instead of ≥1 for the falls may have underestimated the frailty rate in POSE. Interestingly, 38% of these patients were at least partially dependent, and about two-thirds presented with limited mobility and possible cognitive impairment, which is somewhat inconsistent with the apparent low frailty rate, which may be attributable to the frailty definition we used. In particular, the TUG result was not considered as a frailty marker in this study. Yet, frailty was identified as a significant risk factor (hazard ratio 2.63) for postoperative mortality, in line with previous reports.3,17,27 The POSE assessment using the TUG test and the level of independence14 could easily be implemented for most patients in routine clinical practice. TUG was previously strongly associated with institutionalisation, morbidity and mortality,17,28 and POSE revealed a hazard ratio of 2.19 for 30-day mortality. However, our applied cut off value of more than 12 s for prolonged TUG might have overestimated limited mobility. Maintenance of functional independence including prevention of falls is a desirable goal to foster older people's autonomy.1,4,5 In POSE, comparable to WHO data,5 31.8% of patients presented with falls during the previous 6 months and dependency for activities of daily living increased until day 30. Furthermore, about 60% of our patients already presented with possible cognitive impairment at admission, which might be associated with increased postoperative mortality and needs to be taken into account for patient tailored information, treatments, and decision making.17
POSE identified further independent risk variables for 30-day mortality, such as age, male sex, comorbidities and severity and urgency of intervention, which are in line with other investigations.22,27,29,30 The majority of patients presented with a decline in physiological capacities and underwent elective in-patient interventions. Thus, the implementation of pre-interventional optimisation strategies17 regarding treatment of the components of frailty such as medical, nutritional, functional and cognitive conditions,17 would be beneficial, timely and feasible. However, the identification of the clinically most relevant component has to be evaluated in future interventional studies.
Postinterventional complications are one important cause for postoperative mortality apart from other factors such as the patient's morbidity and the in-hospital ‘failure to rescue rate’.29
POSE revealed a similar complication rate of 17.4% in hospital and 3.9% after discharge, compared with the 20% complication rate in a large ACS-NSQIP study in patients over 80 years.23 So far, the most frequently reported complications for patients aged more than 80 years associated with in-hospital mortalities were bleeding, followed by unplanned intubation and septic shock.29 Although we have not explicitly assessed the mortality attributable to such complications in the POSE study, they may be unlikely factors considering the low rates of sepsis (2.2%) and unplanned intubations (0.8%).
Compared with the ISOS study that analysed patients with a mean age of 55 years, the in-hospital complication rate was 16.8%,21 making the rate of 17.4% in POSE rather low. Nevertheless, 15.9% of the POSE patients were routinely admitted to ICU immediately after intervention, which was higher than in the EuSOS and ISOS studies with 5.5 and 9.7%, respectively.21,22 The difference might be attributed to the older age of the POSE cohort with higher rates of planned ICU admissions, though the value of this latter approach remains controversial.21 Only 1.6% of the patients experienced unplanned admissions to the ICU immediately after the procedure, which is associated with a significantly higher mortality rate in patients aged at least 80 years.31 This was confirmed in our sensitivity analysis of the ICU admissions. Nevertheless, 76% of deaths in the POSE study occurred before hospital discharge, suggesting that improved measures for early detection of clinical deterioration and more effective pathways are needed.21 In addition, in POSE, only 10.9% patients were admitted to a unit with geriatric support within 30 days after the procedure, despite the value of peri-interventional specialised geriatric care models being widely accepted.1,4,5 Lower postoperative mortality, higher-quality care, shorter hospital stays and lower costs have been associated with the use of acute geriatric wards.5,32 Our descriptive analysis of admissions to geriatric care confirmed a benefit for survival within the first 10 postintervention days. Interestingly, only 3.9% of the discharged patients experienced serious complications (pulmonary, cardiac, renal and stroke). Nevertheless, about 25% of deaths occurred after hospital discharge, underlining the need for continued monitoring of elderly patients after discharge.
The strength of POSE is the prospective and consecutive inclusion of patients, even patients lacking mental capacity who are frequently excluded in studies.1 Clear definitions and the collection of mostly routinely available data resulted in reliable data with low rates of missing values. Altogether, reduced selection and attrition bias increased the validity of POSE.13 However, we cannot exclude a possible effect of the loss to follow-up rate of 4.7% on our primary outcome estimate, though rates less than 5% are considered low.33 The risk in POSE that some centres contributed very few patients was addressed by modelling a random centre effect in our statistical analysis. However, as all centres voluntarily registered for participation, we cannot exclude an effect of selection bias at the hospital level.
The case-mix, interventional volume and available workforce of the participating hospitals might have influenced the outcomes.6 Also, the majority of the participating hospitals in POSE were either tertiary or academic secondary hospitals. Similar to other studies,8 causal factors for death cannot be derived from POSE. In addition, POSE did not aim to compare these results to the general mortality of the elderly European population not undergoing procedures.
POSE revealed a lower than expected 30-day mortality rate of 4.2%, in patients aged at least 80 years in Europe, which might, among other factors, represent quite well tolerated anaesthesia in this vulnerable population. POSE has identified several mortality-related risk factors. POSE highlights the importance of peri-interventional infrastructure and services for older patients, and patient-centred research targeting individualised, flexible treatment approaches. The implementation of prehabilitation programmes,17 establishment of peri-interventional geriatric expertise and telemedical approaches34 for surveillance after discharge might be considered. Further studies should address individuals’ needs and analyse relevant patient-reported outcomes.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: we gratefully acknowledge Prof. Soo Borson (Professor Emerita, University of Washington, Seattle, Washington, USA), the developer of the Mini-Cog screening tool, for her great support with the use of the Mini-Cog tool in our POSE study. Further, we thank Mr. Monroe Coburn for his valuable English language support and Mr. Marian Ohligs for his excellent support with the OpenClinica database. Only Mr. Ohligs listed in this section received compensation for his work.
Financial support and sponsorship: this study was supported by the European Society of Anaesthesiology and Intensive Care (ESAIC) as an ESAIC Research Group. This comprised the advertising of the POSE study and POSE meetings on the annual Euroanaesthesia congress, the indirect use of the ESAIC members contact lists and the financial support for holding three steering committee meetings at the ESAIC Secretariat in Brussels.
The funder had no role in the study design or in the collection, analysis, interpretation of data, writing of the report or decision to submit the article for publication.
POSE-Study group. All contributing co-authors and collaborators are listed in the Supplementary Digital Content 1.
Conflicts of interest: all authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare the following: AK, RR and MC report grants from ESAIC, during the conduct of the study. RDH reports personal fees from Bayer and Regeneron, outside the submitted work. SR reports personal fees from Edwards Lifesciences, Nordic Pharma, Orion Pharma and Vifor Pharma; nonfinancial support from Air Liquide; and grants from FWO, outside the submitted work. WB report grants from STARS, WearIt4Health/Interreg, Fresenius/B. Braun and other financial support from ESA Research Committee, outside the submitted work. MM reports nonfinancial support from Pfizer Serbia and personal fees from Astellas Pharma, outside the submitted work; and World Federation of Societies of Anaesthesiologists (WFSA), Council member and Chair of the Education Committee. SM report grants from the French Government and Health ministry, during the conduct of the study. MC reports personal fees from Baxter Healthcare and Orion Pharma, and grants and personal fees from Air Liquide Santé International, outside the submitted work. T.S. declares to be a board member of the Perioperative Medicine Clinical Trials Network, UK. OD reports personal fees from Medtronic, outside the submitted work. MLG reports personal fees from Fisher and Paykel, and other financial support for an airway management workshop from Medtronic, outside the submitted work. All other authors declare no competing interests.
Presentation: none.
POSE-Study group. All contributing co-authors and collaborators are listed in the Supplementary Digital Content 1.
Published online 18 November 2021
Supplemental digital content is available for this article.
Contributor Information
Collaborators: POSE-Study group
References
- 1.WHO. Global strategy and action plan on ageing and health. 2017. https://www.who.int/ageing/WHO-GSAP-2017.pdf?ua=1 [Accessed 20 April 2021]. [Google Scholar]
- 2.WHO. Aging and health. https://www.who.int/en/news-room/fact-sheets/detail/ageing-and-health [Accessed 20 April 2021]. [Google Scholar]
- 3.Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet 2019; 394:1365–1375. [DOI] [PubMed] [Google Scholar]
- 4.Dent E, Martin FC, Bergman H, et al. Management of frailty: opportunities, challenges, and future directions. Lancet 2019; 394:1376–1386. [DOI] [PubMed] [Google Scholar]
- 5.WHO. World report on aging and health. 2015. https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf?sequence=1 [Accessed 02 May 2021]. [Google Scholar]
- 6.Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 2015; 386:569–624. [DOI] [PubMed] [Google Scholar]
- 7.Ariyaratnam R, Palmqvist CL, Hider P, et al. Toward a standard approach to measurement and reporting of perioperative mortality rate as a global indicator for surgery. Surgery 2015; 158:17–26. [DOI] [PubMed] [Google Scholar]
- 8.Nepogodiev D, Martin J, Biccard B, et al. National Institute for Health Research Global Health Research Unit on Global S. Global burden of postoperative death. Lancet 2019; 393:401. [DOI] [PubMed] [Google Scholar]
- 9.De Hert S, Staender S, Fritsch G, et al. Preoperative evaluation of adults undergoing elective noncardiac surgery: updated guideline from the European Society of Anaesthesiology. Eur J Anaesthesiol 2018; 35:407–465. [DOI] [PubMed] [Google Scholar]
- 10.Widdison AL, Barnett SW, Betambeau N. The impact of age on outcome after surgery for colorectal adenocarcinoma. Ann R Coll Surg Engl 2011; 93:445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsanos K, Ahmad F, Dourado R, et al. Interventional radiology in the elderly. Clin Interv Aging 2009; 4:1–15. [PMC free article] [PubMed] [Google Scholar]
- 12.Kowark A. Peri-interventional outcome study in the elderly. 2017. https://pose-trial.org [Accessed 12 May 2021]. [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 14.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg 2013; 217:833–842.e1-e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg 2009; 250:449–455. [DOI] [PubMed] [Google Scholar]
- 16.Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39:142–148. [DOI] [PubMed] [Google Scholar]
- 17.Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA 2014; 311:2110–2120. [DOI] [PubMed] [Google Scholar]
- 18.National Institute on Ageing. We can’t address what we don’t measure consistently. Building consensus on frailty in Canada. 2018. https://www.cfn-nce.ca/wp-content/uploads/2018/10/cfn-nia-frailty-paper-2018-09-24.pdf [Accessed 09 January 2021]. [Google Scholar]
- 19.Le Manach Y, Collins G, Rodseth R, et al. Preoperative Score to Predict Postoperative Mortality (POSPOM): derivation and validation. Anesthesiology 2016; 124:570–579. [DOI] [PubMed] [Google Scholar]
- 20.Biccard BM, Madiba TE, Kluyts HL, et al. Perioperative patient outcomes in the African Surgical Outcomes Study: a 7-day prospective observational cohort study. Lancet 2018; 391:1589–1598. [DOI] [PubMed] [Google Scholar]
- 21.International Surgical Outcomes Study group. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle- and high-income countries. Br J Anaesth 2016; 117:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet 2012; 380:1059–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamel MB, Henderson WG, Khuri SF, Daley J. Surgical outcomes for patients aged 80 and older: morbidity and mortality from major noncardiac surgery. J Am Geriatr Soc 2005; 53:424–429. [DOI] [PubMed] [Google Scholar]
- 24.Verweij NM, Schiphorst AH, Maas HA, et al. [Colorectal cancer surgery in the oldest Dutch patients: retrospective analysis of two national databases covering 2011 and 2012]. Ned Tijdschr Geneeskd 2016; 160:D517. [PubMed] [Google Scholar]
- 25.Van Buuren S, Brand JP, Groothuis-Oudshoorn CG, Rubin DB. Fully conditional specification in multivariate imputation. J Stat Comput Simulat 2006; 76:1049–1064. [Google Scholar]
- 26.R Core Team. R Foundation for Statistical Computing. R: a language and environment for statistical computing. 2019. https://www.R-project.org/ [Accessed 28 February 2020]. [Google Scholar]
- 27.Shinall MC, Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg 2019; 155:e194620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson TN, Wallace JI, Wu DS, et al. Accumulated frailty characteristics predict postoperative discharge institutionalization in the geriatric patient. J Am Coll Surg 2011; 213:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freundlich RE, Maile MD, Sferra JJ, et al. Complications associated with mortality in the National Surgical Quality Improvement Program Database. Anesth Analg 2018; 127:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glance LG, Lustik SJ, Hannan EL, et al. The Surgical Mortality Probability Model: derivation and validation of a simple risk prediction rule for noncardiac surgery. Ann Surg 2012; 255:696–702. [DOI] [PubMed] [Google Scholar]
- 31.Guidet B, de Lange DW, Boumendil A, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med 2020; 46:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moyet J, Deschasse G, Marquant B, et al. Which is the optimal orthogeriatric care model to prevent mortality of elderly subjects post hip fractures? A systematic review and meta-analysis based on current clinical practice. Int Orthop 2019; 43:1449–1454. [DOI] [PubMed] [Google Scholar]
- 33.Fewtrell MS, Kennedy K, Singhal A, et al. How much loss to follow-up is acceptable in long-term randomised trials and prospective studies? Arch Dis Child 2008; 93:458–461. [DOI] [PubMed] [Google Scholar]
- 34.Williams AM, Bhatti UF, Alam HB, Nikolian VC. The role of telemedicine in postoperative care. Mhealth 2018; 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.