Abstract
BACKGROUND
Little is known about current clinical practice concerning peri-operative red blood cell transfusion in neonates and small infants. Guidelines suggest transfusions based on haemoglobin thresholds ranging from 8.5 to 12 g dl−1, distinguishing between children from birth to day 7 (week 1), from day 8 to day 14 (week 2) or from day 15 (≥week 3) onwards.
OBJECTIVE
To observe peri-operative red blood cell transfusion practice according to guidelines in relation to patient outcome.
DESIGN
A multicentre observational study.
SETTING
The NEonate-Children sTudy of Anaesthesia pRactice IN Europe (NECTARINE) trial recruited patients up to 60 weeks’ postmenstrual age undergoing anaesthesia for surgical or diagnostic procedures from 165 centres in 31 European countries between March 2016 and January 2017.
PATIENTS
The data included 5609 patients undergoing 6542 procedures. Inclusion criteria was a peri-operative red blood cell transfusion.
MAIN OUTCOME MEASURES
The primary endpoint was the haemoglobin level triggering a transfusion for neonates in week 1, week 2 and week 3. Secondary endpoints were transfusion volumes, ‘delta haemoglobin’ (preprocedure – transfusion-triggering) and 30-day and 90-day morbidity and mortality.
RESULTS
Peri-operative red blood cell transfusions were recorded during 447 procedures (6.9%). The median haemoglobin levels triggering a transfusion were 9.6 [IQR 8.7 to 10.9] g dl−1 for neonates in week 1, 9.6 [7.7 to 10.4] g dl−1 in week 2 and 8.0 [7.3 to 9.0] g dl−1 in week 3. The median transfusion volume was 17.1 [11.1 to 26.4] ml kg−1 with a median delta haemoglobin of 1.8 [0.0 to 3.6] g dl−1. Thirty-day morbidity was 47.8% with an overall mortality of 11.3%.
CONCLUSIONS
Results indicate lower transfusion-triggering haemoglobin thresholds in clinical practice than suggested by current guidelines. The high morbidity and mortality of this NECTARINE sub-cohort calls for investigative action and evidence-based guidelines addressing peri-operative red blood cell transfusions strategies.
TRIAL REGISTRATION
ClinicalTrials.gov, identifier: NCT02350348
KEY POINTS
Transfusion-triggering haemoglobin thresholds for neonates and small infants in clinical practice are lower than suggested by current guidelines.
There is a broad variability of transfusion-triggering haemoglobin thresholds in clinical practice.
The described sub-cohort had a high morbidity and mortality.
There is an urgent need for evidence-based guidelines addressing peri-operative red blood cell transfusion strategies for neonates and small infants.
Introduction
Neonates and small infants undergo physiological anaemia during their early days of life.1,2 Pre-operative anaemia is associated with increased morbidity and mortality.3 Acute surgical bleeding can cause severe anaemia with or without cardiovascular instability leading to an immediate need for red blood cell (RBC) transfusion.4 Massive transfusions of blood products exceeding 40 ml kg−1 administered within the first 24 h post trauma, are identified as a high risk factor for early in-hospital death among critically injured children.5 Potentially adverse events of RBC transfusions can be classified as infectious (bacterial, viral, parasitical or prion) or noninfectious risks.6,7 Infectious risks are considered to be extremely low in developed countries.7 Noninfectious risks include haemolytic transfusion reactions, acute volume, electrolyte and metabolic imbalances (e.g. transfusion associated circulatory overload) and immunomodulation (e.g. graft-versus-host disease; transfusion-related acute lung injury and alloimmunisation). Especially among premature infants, RBC transfusion may be associated with intraventricular haemorrhage, transfusion-associated necrotising enterocolitis, iron overload and retinopathy of prematurity.8 Furthermore, chronic anaemia in premature infants can have potential impact on delayed weight gain, impaired neurodevelopment and apnoea.1,9,10 Currently, there is no international consensus addressing peri-operative Hb transfusion thresholds for neonates and small infants. National patient blood management guidelines focusing on critically ill neonates -- perhaps the best comparable cohort -- suggest different Hb transfusion-triggering thresholds for neonates for their first, second week of life and from their third week of life.11–13 The rationale behind this classification is based on the consideration that neonates undergo physiological anaemia during the first few weeks of life. Preterm infants are especially prone to develop anaemia of prematurity.1,2
Considering the risks associated with RBC transfusion and in an effort to reduce transfusion-related morbidity and mortality, a clinically appropriate patient-based approach should be enforced in this vulnerable population.14–16
The recently published prospective cohort study, NEonate-Children sTudy of Anaesthesia pRactice IN Europe (NECTARINE),17–20 collected acute critical events and related interventions occurring peri-operatively among neonates and infants younger than 60 weeks postmenstrual age (PMA).17 The main critical events, low haemoglobin (Hb) levels and cardiovascular instability as judged by the anaesthesiologist in charge, constituted two of the primary endpoints, having resulted in an RBC transfusion requirement caused by the intervention concerned.18 Existing data revealed an overall prevalence for a peri-operative RBC transfusion in this cohort of 6.9% and was associated with an increased risk for morbidity, RR 2.41 (95% CI 2.055 to 2.817), and mortality, RR 4.52 (95% CI 3.087 to 6.61).
Previous studies have focused mainly on children with a wide range of ages. There is a paucity of data pertaining to peri-operative RBC transfusion practice among neonates and small infants. This study seeks to characterise current peri-operative Hb transfusion-triggering thresholds, to compare these findings to patient blood management guidelines, and lastly to describe RBC transfusion associated outcomes in this large European cohort.
Materials and methods
Study design
Detailed study design and data collection for the NECTARINE cohort have been described previously.17,18 In brief, NECTARINE is a European multicentre, prospective, observational cohort study funded by the European Society of Anaesthesiology and Intensive Care (ESAIC) through the Clinical Trial Network grant. Peri-operative anaesthesia management data on children aged from birth to 60 weeks PMA was collected in 165 European centres. The ESAIC selected participating centres through a ‘call for centre’. Each country was represented by a national co-ordinator, and all participating centres obtained ethical approval in accordance with local or national requirements (approval forms available online: https://www.esaic.org/research/clinical-trial-network/completed-trials/nectarine/). Following ethics approval of all participating centres, patients were recruited over a 3-month period at each centre, with overall recruitment between 1 March 2016 and 31 January 2017. The study was registered (ClinicalTrials.gov, NCT02350348). This manuscript adheres to the applicable Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (www.strobe-statement.org).
Participants
All neonates and infants up to 60 weeks of age PMA undergoing anaesthesia for surgical or nonsurgical procedures, in the operating room, paediatric or neonatal ICU or diagnostic suite were eligible for inclusion. In the present cohort, all patients receiving peri-operative RBC transfusion were included.
Variables
Children were observed for the occurrence of any peri-operative medical interventions triggered by a predefined list of critical events. In summary, these critical events included difficult airway management, poor peripheral oxygen saturation (SpO2) and/or arterial oxygen saturation (SaO2), end-tidal carbon dioxide (ETCO2) and/or arterial or venous CO2 derangement, hypo or hyperglycaemia, and/or hypo or hypernatraemia, cardiovascular instability, hypo or hyperthermia, impaired brain oxygenation as measured by near-infrared spectroscopy (NIRS) and low Hb levels as judged by the anaesthesiologist in charge.17,18 For each procedure, more than one critical event could be recorded. In the critical event, ‘low Hb level’ and/or ‘cardiovascular instability’, the selected specific intervention could be RBC transfusion. The transfusion-triggering Hb threshold and the RBC transfusion volume were recorded. As per the NECTARINE protocol, previous neonatal medical history, cyanotic congenital heart defects and pre-operative physical status including American Society of Anaesthesiology Physical Status (ASA) at the time of anaesthesia were also documented. All the children included were followed up 30 days after the procedure for morbidity and mortality. Morbidity considered cardiovascular, respiratory, central nervous or surgical complications, renal impairment and liver failure. A patient might accumulate multiple morbidity complications. A second follow-up 90 days after the procedure for in- and out-of-hospital mortality was also conducted. Detailed definitions pertaining to patient demography, medical history and general anaesthesia related parameters are contained in the study protocol.17
Outcomes
To compare these findings to existing patient blood management guidelines, the primary endpoint was defined as the transfusion-triggering Hb thresholds levels in grams per decilitre (g dl−1) for children in the first week of life (‘Group 1’), the second week of life (‘Group 2’) and the third week of life (‘Group 3’). Secondary endpoints were the RBC transfusion volume in millilitres per kilogram body weight (ml kg−1), ‘delta-Haemoglobin (ΔHb) (median preprocedure Hb – median transfusion triggering Hb) in g dl−1, relationship between PMA and transfusion trigger and 30-day and 90-day morbidity and mortality of those children transfused.
Statistical methods
The study size determination for NECTARINE was based on the estimation of approximately 5000 patients in order to obtain 462 critical events, assuming that the expected percentage of severe peri-operative critical events is approximately 11% and the drop-out rate is 15%. An a priori statistical analysis plan for the primary and secondary analysis was defined in the initial protocol which is accessible online.17
Continuous variables are summarised as medians with their first and third quartiles (Q1 and Q3, respectively), while absolute frequencies and percentages are presented for categorical variables.
Statistical analyses were performed using R v4.0.5. [R Core Team (2021); R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria] with the ‘tidyverse’ and the stats packages.21 Ordinary least squares regression lines were fitted to model the relationship between transfusion-triggering Hb and PMA. We performed a receiver operating characteristic analysis to find a threshold for transfusion volume (ml kg−1) above which the probability of morbidity and mortality was greater than below this given threshold.
Results
Participants
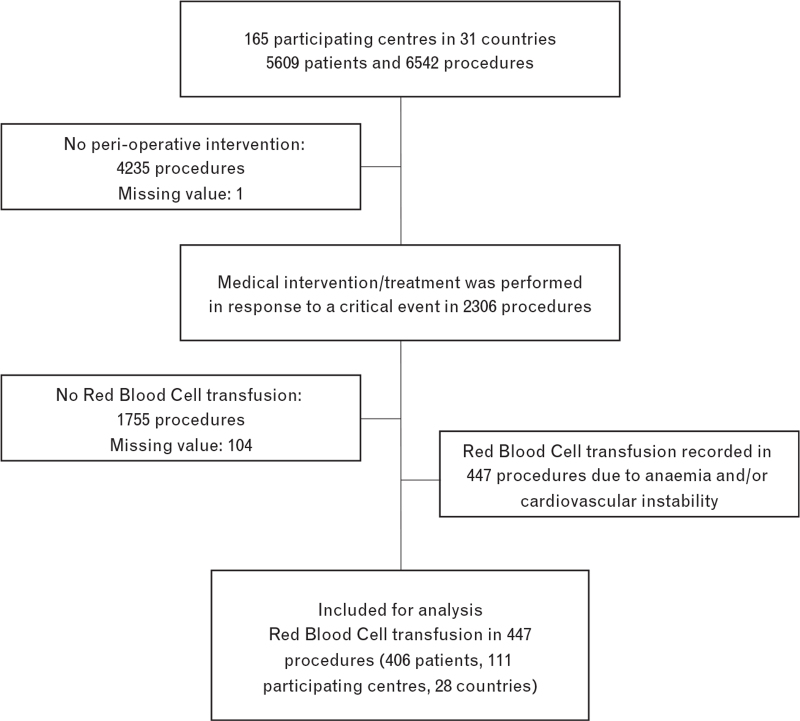
Peri-anaesthetic data included 5609 patients undergoing 6542 procedures, between 1 March 2016 and 31 January 2017, in the 165 centres of the NECTARINE network. At least one intervention for a critical event was recorded in 2306 of 6437 procedures, yielding 447 RBC transfusions in 406 patients (6.9%, 95% CI 6.3 to 7.6). Medical conditions warranting RBC transfusion were listed as follows: In 218 procedures, intra-operative anaemia was the sole reason leading to RBC transfusion, in 113 procedures RBC transfusion was solely due to cardiovascular instability and in 116 procedures based on both intra-operative anaemia and cardiovascular instability. Figure 1 displays the flow-chart of the patients included and analysed (Fig. 1).
Fig. 1.
Flow-chart of included/analysed patients.
The median age in days [first to third quartile] of the included children at the time of transfusion was 33.0 [9.0 to 79.0] days, while the median PMA was 40.0 [37.0 to 47.0] weeks. One hundred and six patients (23.7%) were younger than 37 weeks PMA at the time of inclusion and 213 (47.7%) were born prematurely. The cohort had a slight male predominance (57.5%, n = 257). A majority of the patients were classified as ASA at least 3 (80.3%, n = 359). When stratified according to type of procedure, RBC transfusions occurred in 66.2% during noncardiac surgery (52.3% during visceral surgery and 10.2% during neurosurgery) and in 30.4% during cardiac surgery. RBC transfusions were carried out for nonsurgical procedures during noncardiac catheterisation in 2.0%, and during cardiac catheterisation in 1.3% of the interventions. The sub-cohort of transfused children contained 30 with cyanotic congenital heart defects (7.4%), who underwent 54 procedures (12.1%). Table 1 summarises patient demography, transfusion-related data, type of procedures and other critical events for the 447 procedures with RBC transfusion (Table 1).
Table 1.
Number of peri-operative red blood cell transfusion, demography, transfusion-related data, type of procedure and critical events
| Variables | Week 1 ‘Group 1’ n = 84 | Week 2 ‘Group 2’ n = 52 | Week ≥3 ‘Group 3’ n = 311 | Total cohort n = 447 |
| Gestational age at birth, weeks | 37.0 [34.0 to 39.0] | 37.0 [28.0 to 39.0] | 37.0 [30.0 to 39.0] | 37.0 [31.0 to 39.0] |
| Birth weight, kg | 2.9 [2.2 to 3.5] | 2.8 [1.0 to 3.2] | 2.7 [1.3 to 3.2] | 2.8 [1.4 to 3.3] |
| Age on day of transfusion, days | 3 [1 to 5] | 9 [8 to 11] | 61 [30 to 98] | 33 [19 to 79] |
| Weight on day of transfusion, kg | 2.9 [2.2 to 3.5] | 2.8 [0.9 to 3.3] | 3.6 [2.6 to 4.6] | 3.2 [2.3 to 4.2] |
| Sex, male | 54 (64.3) | 28 (53.8) | 175 (56.3) | 257 (57.5) |
| ASA physical status ≥3 | 73 (86.9) | 48 (92.4) | 238 (76.5) | 359 (80.3) |
| Cyanotic congenital heart defect | 10 (11.9) | 10 (19.2) | 34 (10.9) | 54 (12.1) |
| RBC transfusion related dataa | ||||
| Preprocedure Hb, g dl−1 | 13.9 [12.0 to 16.0] | 12.6 [10.6 to 14.6] | 10.4 [9.1 to 11.8] | 11.1 [9.5 to 12.9] |
| Transfusion-triggering Hb, g dl−1 | 9.6 [8.7 to 10.9] | 9.6 [7.7 to 10.4] | 8.0 [7.3 to 9.0] | 8.4 [7.5 to 9.6] |
| RBC transfusion volume, ml kg−1 | 16.7 [12.8 to 21.5] | 20.0 [12.5 to 30.2] | 17.1 [10.9 to 26.4] | 17.1 [11.1 to 26.4] |
| Delta Hb, g dl−1 | 2.4 [0.8 to 5.3] | 1.3 [0.1 to 4.2] | 1.6 [0.0 to 3.2] | 1.8 [0.0 to 3.6] |
| Prevalence for RBC transfusion | 84/817 (10.3) | 52/374 (13.9) | 311/5246 (5.9) | 447/6437 (6.9) |
| Type of procedure | ||||
| Surgical, noncardiacd | 63 (75.0) | 28 (46.2) | 205 (65.9) | 296 (66.2) |
| Gastrointestinal surgery | 48 (57.1) | 25 (48.1) | 153 (51.7) | 226 (52.3) |
| Neurosurgery | 9 (10.7) | 2 (3.8) | 33 (11.1) | 44 (10.2) |
| Thoracic surgery | 1 (1.2) | 1 (1.9) | 9 (3.0) | 11 (2.5) |
| Other surgerye | 5 (6.0) | 1 (1.9) | 13 (4.4) | 19 (4.5) |
| Specification missing | 0 (0.0) | 0 (0.0) | 15 (4.8) | 15 (3.4) |
| Surgical, cardiac | 21 (25.0) | 24 (53.8) | 91 (29.3) | 136 (30.4) |
| Nonsurgical, no cardiac catheterisation | 0 (0.0) | 0 (0.0) | 9 (2.9) | 9 (2.0) |
| Nonsurgical, cardiac catheterisation | 0 (0.0) | 0 (0.0) | 6 (1.9) | 6 (1.3) |
| Critical event for interventionc | ||||
| Cardiovascular instability | 76 (90.5) | 43 (82.7) | 216 (69.5) | 335 (74.9) |
| Impaired oxygenation | 22 (26.2) | 20 (38.5) | 79 (25.4) | 121 (27.1) |
| Hypo or hypercapnia | 21 (25.0) | 10 (19.2) | 76 (24.4) | 107 (23.9) |
| Body temperature | 21 (25.0) | 16 (30.8) | 55 (17.7) | 92 (20.6) |
| Impaired brain oxygenationb | 18 (21.4) | 14 (26.9) | 47 (15.1) | 79 (17.7) |
| Metabolic disturbance | 16 (19.0) | 6 (11.5) | 48 (15.4) | 70 (15.7) |
| Difficult airway management | 3 (3.6) | 2 (3.8) | 19 (6.1) | 24 (5.4) |
Values are numbers (%) for categorical or median [IQR] for continuous variables.
ASA, American Society of Anesthesiology; Hb, haemoglobin; RBC, red blood cell.
Numbers of missing data: Preprocedure Hb n = 11 (2.5%), Transfusion-triggering Hb n = 122 (27.3%), Delta Hb n = 128 (28.6%).
A child might have had multiple procedures at different times.
Near-infrared spectroscopy (NIRS) not available in 47.2% of total cases.
Multiple events for interventions were recorded during one procedure.
One procedure could contain different types of surgery.
Including procedures for genitourinary, orthopaedic, ear nose throat, dermatology and ophthalmology surgery.
Primary endpoint
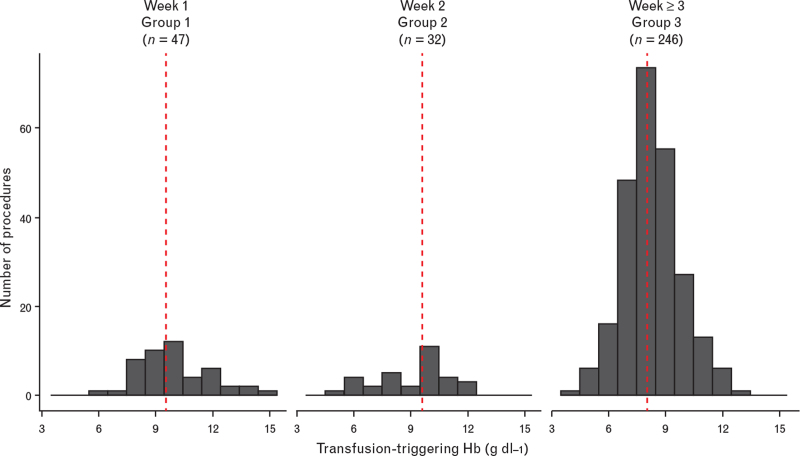
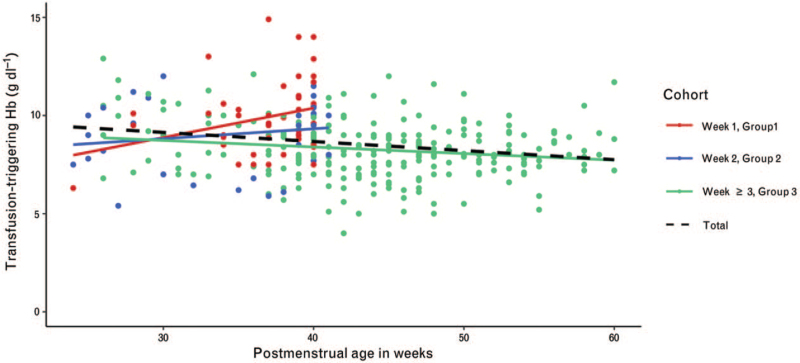
Figure 2 depicts the distribution of Hb transfusion-triggering thresholds for the entire cohort. The transfusion-triggering Hb thresholds, displayed as median [first to third quartile], for neonates was 9.6 [8.7 to 10.9] g dl−1 in group 1, 9.6 [7.7 to 10.4] g dl−1 and 8.0 [7.3 to 9.0] g dl−1 for groups 2 and 3, respectively. Figure 3 displays the Hb transfusion-triggering histogram for the three different age groups. A weak and nonsignificant correlation was found between transfusion-triggering Hb levels and PMA for the three different groups (r = 0.27, 0.16 and -0.18 for the first, second and as of their third week, respectively).
Fig. 2.
Histogram of the transfusion-triggering haemoglobin (Hb) thresholds in gram per decilitre (g dl−1) classified by week of life
Fig. 3.
Regression line of the transfusion-triggering haemoglobin (Hb) in g dl−1 classified by week of life in relation to postmenstrual age (weeks)
Secondary endpoints
The median preprocedure Hb level was 13.9 [12.0 to 16.0] g dl−1 and the ΔHb level was 2.4 [0.8 to 5.3] for patients in group 1 (18.8%), which was higher than the other two groups. The median transfusion volume was 16.7 [12.8 to 21.5] ml kg−1 being lowest in the first group.
Group 2 was the smallest group in the cohort (11.6%). This group had a median preprocedure Hb level of 12.6 [10.6 to 14.6] g dl−1 and a ΔHb level of 1.3 [0.1 to 4.2]. The lowest ΔHb was also found in group 2, yielding a median transfusion volume of 20.0 [12.5 to 30.2] ml kg−1, higher than in both of the two other groups.
Finally, group 3 was the largest of the cohort (69.6%). The group's median preprocedure Hb was 10.4 [9.1 to 11.8] with a median ΔHb of 1.6 [0.0 to 3.2] g dl−1. The preprocedure Hb was lower than the two other groups. The median transfusion volume in this group was 17.1 [10.9 to 26.4] ml kg−1.
Follow-up
Table 2 summarises the 30-day and 90-day follow-up data. The overall 30-day complication rate was 47.8% (194/406), representing an incidence of 51.2% (95% CI 46.0 to 56.3).
Table 2.
Morbidity, status and mortality for children with peri-operative red blood cell transfusion
| Variables | Week 1 ‘Group 1’ n = 78 | Week 2 ‘Group 2’ n = 44 | Week ≥3 ‘Group 3’ n = 284 | Total cohort n = 406 |
| Morbidity at day 30a | ||||
| Overall morbidity RBC | 46 (59.0) | 28 (63.6) | 120 (42.3) | 194 (47.8) |
| Cardiovascular complication | 26 (56.5) | 25 (89.3) | 65 (54.2) | 116 (59.8) |
| Respiratory complication | 24 (52.2) | 18 (64.3) | 67 (55.8) | 109 (56.2) |
| Surgical complication | 20 (43.5) | 9 (32.1) | 57 (47.5) | 86 (44.3) |
| Brain / CNS complication | 6 (13.0) | 4 (14.3) | 29 (24.2) | 39 (20.1) |
| Renal insufficiency | 9 (19.6) | 5 (17.9) | 25 (20.8) | 39 (20.1) |
| Liver failure | 5 (10.9) | 2 (7.1) | 13 (10.8) | 20 (10.3) |
| Overall morbidity NECTARINEb | 132 (22.0) | 81 (29.6) | 495 (11.3) | 708 (13.5) |
| Patient status at day 30 | ||||
| Discharged to home | 29 (37.2) | 16 (36.4) | 143 (50.4) | 188 (46.3) |
| Remained in hospital | 18 (23.1) | 4 (9.1) | 48 (16.9) | 70 (17.2) |
| Discharged to another hospital | 6 (7.7.) | 1 (2.3) | 24 (8.5) | 31 (7.6) |
| Remained in ICU | 16 (20.5) | 13 (29.5) | 32 (11.3) | 61 (15.0) |
| Missing | 1 (1.3) | 3 (6.8) | 11 (3.9) | 15 (3.7) |
| Patient status at day 90 | ||||
| Discharged to home | 42 (53.8) | 18 (40.9) | 177 (62.6) | 237 (58.4) |
| Still in hospital | 11 (14.1) | 13 (29.5) | 25 (8.8) | 49 (12.1) |
| Missing | 23 (29.5) | 13 (29.5) | 79 (27.8) | 115 (28.3) |
| Mortality | ||||
| Overall mortality RBC | 10 (12.8) | 7 (15.9) | 29 (10.2) | 46 (11.3) |
| Death by day 30 | 8 (80) | 7 (100) | 26 (89.7) | 41 (89.1) |
| Death between day 30 and day 90 | 2 (20) | 0 (0.0) | 3 (10.3) | 5 (10.9) |
| Overall mortality NECTARINEb | 16 (2.7) | 15 (5.5) | 72 (1.6) | 103 (2.0) |
Values are numbers (%).
Cohort is defined as the last transfusion procedure for each child.
A child could accumulate multiple morbidity complications.
If a child had one procedure containing RBC transfusion and an additional procedure without RBC transfusion, the follow-up data (morbidity and mortality) contribute to both groups.
The overall mortality rate for children receiving RBC transfusions was 11.3% (46/332), representing an incidence of 13.9% (95% CI 10.3 to 18.0).
The highest complication rate and mortality was observed in group 2, that is among children that were transfused during their second week of life. A receiver operating characteristic analysis was unable to identify a threshold for transfusion volume (ml kg−1) influencing morbidity and mortality.
Discussion
The current sub-analysis of peri-operative RBC transfusions among neonates and small infants of the NECTARINE cohort, a prospective European multicentre observational study, revealed a large variability for transfusion-triggering Hb thresholds. Hb transfusion thresholds were lower than current guidelines recommend. Furthermore, patients exposed to peri-operative RBC transfusions in this analysis had an almost four-fold increased morbidity and an up to five-fold increased mortality compared with patients who were not exposed to RBC transfusions.
Currently, there is no international consensus regarding peri-operative Hb transfusion thresholds for neonates and small infants and a general paucity of tangible recommendations. Existing patient blood management guidelines focussing on critically ill neonates, perhaps the best comparable cohort, suggest RBC transfusion thresholds to be based on a patient's age. Recommendations for transfusion-triggering Hb thresholds during the first week of life (group 1) range between Hb 10.0 and 13.0 g dl−1, between Hb 9.5 and 12.5 g dl−1 during second week of life (group 2) and between Hb 8.5 and 11.0 g dl−1 from their third week of life (group 3).11–13 The rationale behind this classification is based on the consideration that after birth neonates undergo physiological anaemia during the first few weeks of life. Preterm infants are especially prone to develop anaemia of prematurity.1,2
Surprisingly, the transfusion triggers in this analysis were found to be lower than suggested by the existing guidelines.11–13 A survey published in 2018 addressing current clinical practice in Germany emphasises this existing discrepancy.22 A 2011 Cochrane review analysed four randomised controlled studies comparing low versus high Hb thresholds in very premature infants requiring high levels of respiratory support. No differences were found in morbidity and mortality between patients subjected to low versus high Hb thresholds. Low Hb thresholds were defined as a Hb level of less than 11.5 g dl−1 during the first week, less than 10.0 g dl−1 for the second week and less than 8.5 g dl−1 as of the third week of life.23 The interpretation of these findings should be approached with caution, as the studies mentioned analysed neonatal ICU populations with birth weight 1500 g or less, or infants 32 weeks or less gestational age and were not specific to peri-operative management. The restrictive transfusion practice did not have a significant impact on death or major morbidities at first hospital discharge or at follow-up. However, the review lacked conclusive clarity relative to well tolerated lower Hb level transfusion thresholds as well as the benefits of maintaining a higher Hb level for the observed population. This stands in contrast to our analysis which revealed that current European RBC transfusion practice for neonates and infants is conducted at much lower Hb levels than recommended by the above-mentioned Cochrane review.23
Red blood cell transfusion-triggering physiological parameters
A simple and practical consideration rarely mentioned in guidelines is that RBC transfusion is also commonly triggered by a host of factors including clinical presentation. A patient's Hb level is only one of many objective laboratory parameters that can be readily obtained peri-operatively by way of point-of-care testing such as lactate or oxygen extraction ratio. Critical peri-operative events including cardiovascular instability, poor global oxygen delivery (DO2) or high oxygen consumption (VO2) need to be recognised and treated immediately. When making a decision whether to transfuse or not, clinicians should consider the oxygen extraction ratio and foetal Hb presence (HbF), as HbF has a higher affinity to oxygen than haemoglobin A. An increased VO2 to maintain peripheral blood oxygen saturation may indicate the need for RBC transfusion. Assessing the degree of perfusion impairment can also be done by measuring tissue and/or organ perfusion.24,25 Continuous real-time organ perfusion in neonates, especially brain and gut perfusion, is commonly assessed with the help of noninvasive NIRS. Unfortunately, NIRS values were not considered in this analysis, as only half of the patients included in this cohort had undergone anaesthesia in the presence of NIRS monitoring. For this reason, NIRS perfusion data did not contribute consistently to the decision to transfuse. Impaired tissue oxygenation due to a decrease in DO2 or an increase in VO2, may lead to acidaemia due to increased lactate production. In such situations, a patient-based approach should be promoted rather than rigidly sticking to a number-based threshold designed to fit the general population in question. Thus, it may be imperative to transfuse some children well before the lower limits of recommended Hb levels are reached.
A 2017 study conducted in children aged 0 to 15 years reported peri-operative cardiovascular instability in 1.2% of the patients with 13.2% of them requiring blood products.26 Compared with our analysis, the documented mortality of 5.4% of this latter study was less than half the mortality found in our study. This underscores the finding that neonates and small infants receiving peri-operative RBC transfusions are at an increased risk for morbidity and mortality compared with older children. Patients receiving RBC transfusions during the second week of life showed the highest morbidity and mortality. This finding coincided with number of properties specific to this group when compared with the entire cohort: Group 2 contained children with a larger portion of neonates with young gestational age (i.e. first quartile), the highest proportion of ASA at least 3, the highest proportion of cardiac surgery and received the highest total RBC transfusion volumes. Patients with these characteristics may be considered at risk for poor outcome following RBC transfusions. The high ASA score among patients in group 2 would indicate that these patients were intrinsically more vulnerable and might have shown increased morbidity and mortality irrespective of RBC transfusions.
This study does not permit a clear indication whether the reported mortality was a consequence of RBC transfusions or if the RBC transfusions were the result of the patients’ underlying clinical conditions and thus not directly related to the ‘reported mortality’. Our findings should encourage future randomised controlled trials to investigate such questions that this study was unable to answer. Whether the current European clinical practice of undercutting the transfusion-triggering Hb threshold recommendations is justified remains a point of debate.
An important finding of this study is that peri-operative RBC transfusion is associated with increased morbidity and mortality and should therefore be administered judiciously. In the absence of impaired tissue oxygenation, a haemodynamically stable neonate or infant is unlikely to require RBC transfusion even when presenting with Hb levels that are lower than the currently recommended Hb thresholds. This point is, in part, supported by the finding that morbidity and mortality for group 1 (neonates who were transfused earlier in life), was not higher than group 2 (neonates who were transfused somewhat later in life), despite group 2 having a lower ΔHb than group 1.
Limitations
This study has several limitations. Due to the observational design of the study, the heterogeneous distribution and size of the groups, and the relatively small number of patients within the groups, interpretation of these data needs to consider the descriptive nature of several findings. The current study contained also considerable heterogeneity relative to the procedures involved, patient demography and transfusion volumes, which may have affected patient outcome. The data we present do not distinguish between cardiac and noncardiac procedures or patients with cyanotic congenital heart defects. We are unable to report blood loss or even estimated blood loss per procedure, as this had not been documented in the original study data. As an approximation, we calculated the ΔHb, which may also have been influenced by dilution caused by intravenous fluids administered peri-operatively. Finally, Hb levels after RBC transfusion were not recorded.
Conclusion
This study reports on RBC transfusions in neonates and infants in their early life undergoing anaesthesia in 31 different European countries. It describes lower transfusion-triggering Hb thresholds in clinical practice than suggested by current guidelines. The question of whether the current European clinical practice of undercutting the current transfusion-triggering Hb threshold recommendations is justified remains controversial. The considerable morbidity and mortality in this patient population underscores the clinical relevance of this topic. The gap between clinical practice and national recommendations calls for urgent action on behalf of National and Regional Societies to improve our understanding of safe RBC transfusion practice and patient blood management in this vulnerable population. Best practice guidelines should be strengthened and supported with evidence stemming from large, multicentre, randomised controlled studies stratifying for both gestational age and selected procedures. There is an urgent need to determine ‘age appropriate’ peri-operative transfusion-triggering Hb thresholds relative to any associated morbidity and mortality.
Supplementary Material
Acknowledgements relating to this article
The authors acknowledge all participating centres and staff for contributing to success of the NECTARINE study. We thank the ESAIC research team for providing the necessary infrastructure to conduct this trial and their assistance in identifying the national study coordinating investigators, and their assistance in liaising with local investigators regarding the ethics review process and the inclusion period and monitoring the data entry and cleaning. We thank Dr Angela Pistorio, Senior Statistician at Istituto Giannina Gaslini, Genoa, Italy, for the initial contribution in finalising the study protocol, the cleaning process and the statistical analysis plan.
Assistance with the study: none.
Financial support and sponsorship: European Society of Anaesthesiology and Intensive Care Medicine (ESAIC) and Clinical Trial Network (CTN). The Association of Paediatric Anaesthetists of Great Britain and Ireland (APAGBI) funded the study for the follow-up of patients enrolled in the UK.
Conflicts of interest: none.
Presentation: none.
Individual names are given in the list of collaborators in the supplementary information.
Published online 29 November 2021
Supplemental digital content is available for this article.
References
- 1.Colombatti R, Sainati L, Trevisanuto D. Anemia and transfusion in the neonate. Semin Fetal Neonatal Med 2016; 21:2–9. [DOI] [PubMed] [Google Scholar]
- 2.Saito-Benz M, Flanagan P, Berry MJ. Management of anaemia in preterm infants. Br J Haematol 2020; 188:354–366. [DOI] [PubMed] [Google Scholar]
- 3.Goobie SM, Faraoni D, Zurakowski D, DiNardo JA. Association of preoperative anemia with postoperative mortality in neonates. JAMA Pediatr 2016; 170:855–862. [DOI] [PubMed] [Google Scholar]
- 4.Keung CY, Smith KR, Savoia HF, Davidson AJ. An audit of transfusion of red blood cell units in pediatric anesthesia. Paediatr Anaesth 2009; 19:320–328. [DOI] [PubMed] [Google Scholar]
- 5.Neff LP, Cannon JW, Morrison JJ, et al. Clearly defining pediatric massive transfusion: cutting through the fog and friction with combat data. J Trauma Acute Care Surg 2015; 78:22–28. discussion 28–29. [DOI] [PubMed] [Google Scholar]
- 6.Lavoie J. Blood transfusion risks and alternative strategies in pediatric patients. Paediatr Anaesth 2011; 21:14–24. [DOI] [PubMed] [Google Scholar]
- 7.Ree IMC, Lopriore E. Updates in neonatal hematology: causes, risk factors, and management of anemia and thrombocytopenia. Hematol Oncol Clin North Am 2019; 33:521–532. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham KE, Okolo FC, Baker R, et al. Red blood cell transfusion in premature infants leads to worse necrotizing enterocolitis outcomes. J Surg Res 2017; 213:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zagol K, Lake DE, Vergales B, et al. Anemia, apnea of prematurity, and blood transfusions. J Pediatr 2012; 161:417–421.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zonnenberg IA, Vermeulen RJ, Rohaan MW, et al. Severe neonatal anaemia, MRI findings and neurodevelopmental outcome. Neonatology 2016; 109:282–288. [DOI] [PubMed] [Google Scholar]
- 11.New HV, Berryman J, Bolton-Maggs PH, et al. Guidelines on transfusion for fetuses, neonates and older children. Br J Haematol 2016; 175:784–828. [DOI] [PubMed] [Google Scholar]
- 12.Canadian Blood Services. Clinical Guide to Transfusion - Neonatal and Pediatric Transfusion (Chapter 13). 2017; https://professionaleducation.blood.ca/en/neonatal-and-pediatric-transfusionhttps://professionaleducation.blood.ca/en/neonatal-and-pediatric-transfusion [Accessed 20 November 2021]. [Google Scholar]
- 13.National Blood Authority (NBA) (2016). Patient Blood Management Guidelines: Module 6 – Neonatal and Paediatrics. NBA, Canberra, Australia. [Google Scholar]
- 14.Lopriore E. Updates in red blood cell and platelet transfusions in preterm neonates. Am J Perinatol 2019; 36:S37–S40. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics 2012; 129:529–540. [DOI] [PubMed] [Google Scholar]
- 16.Heeger LE, Counsilman CE, Bekker V, et al. Restrictive guideline for red blood cell transfusions in preterm neonates: effect of a protocol change. Vox Sang 2019; 114:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Society of Anaethesia and Intensive Care (ESAIC). Study protocol NECTARINE. https://www.esaic.org/research/clinical-trial-network/completed-trials/nectarine/. [Accessed 20 November 2021]. [Google Scholar]
- 18.Disma N, Leva B, Dowell J, et al. Assessing anaesthesia practice in the vulnerable age group: NECTARINE: a European prospective multicentre observational study. Eur J Anaesthesiol 2016; 33:233–235. [DOI] [PubMed] [Google Scholar]
- 19.Disma N, Veyckemans F, Virag K, et al. Morbidity and mortality after anaesthesia in early life: results of the European prospective multicentre observational study, neonate and children audit of anaesthesia practice in Europe (NECTARINE). Br J Anaesth 2021; 126:1157–1172. [DOI] [PubMed] [Google Scholar]
- 20.Disma N, Virag K, Riva T, et al. Difficult tracheal intubation in neonates and infants. NEonate and Children audiT of Anaesthesia pRactice IN Europe (NECTARINE): a prospective European multicentre observational study. Br J Anaesth 2021; 126:1173–1181. [DOI] [PubMed] [Google Scholar]
- 21.Wickham H, Averick M, Bryan J, et al. Welcome to the Tidyverse. J Open Source Softw 2019; 4:1686. [Google Scholar]
- 22.Wittenmeier E, Troeber C, Zier U, et al. Red blood cell transfusion in perioperative pediatric anesthesia: a survey of current practice in Germany. Transfusion 2018; 58:1597–1605. [DOI] [PubMed] [Google Scholar]
- 23.Whyte R, Kirpalani H. Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Database Syst Rev 2011; CD000512. [DOI] [PubMed] [Google Scholar]
- 24.Howarth C, Banerjee J, Aladangady N. Red blood cell transfusion in preterm infants: current evidence and controversies. Neonatology 2018; 114:7–16. [DOI] [PubMed] [Google Scholar]
- 25.Seidel D, Blaser A, Gebauer C, et al. Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants. J Perinatol 2013; 33:282–287. [DOI] [PubMed] [Google Scholar]
- 26.Habre W, Disma N, Virag K, et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): a prospective multicentre observational study in 261 hospitals in Europe. Lancet Respir Med 2017; 5:412–425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.