This in vitro experimental study demonstrated that not only structural disruption, but also a single impact injury of the endplate without structural impairment could also initiate intervertebral disc degeneration (IDD), which might be mediated by activation of Piezo1 induced inflammation and abnormal energy metabolism of intervertebral disc cells.
Keywords: animal model, degeneration, endplate, energy metabolism, impact injury, inflammation, intervertebral disc, mechanical loading, piezo1, structure failure
Study Design.
In vitro experimental study.
Objective.
To establish an axial impact injury model of intervertebral disc (IVD) and to investigate if a single impact injury without endplate structural disruption could initiate intervertebral disc degeneration (IDD), and what is the roles of Piezo1 in this process.
Summary of Background Data.
Although IDD process has been confirmed to be associated with structural failures such as endplate fractures, whether a single impact injury of the endplates without structural disruption could initiate IDD remains controversial. Previous studies reported that Piezo1 mediated inflammation participated in the progression of IDD induced by mechanical stretch; however, the roles of Piezo1 in IVD impact injury remain unknown.
Methods.
Rats spinal segments were randomly assigned into Control, Low, and High Impact groups, which were subjected to pure axial impact loading using a custom-made apparatus, and cultured for 14 days. The degenerative process was investigated by using histomorphology, real-time Polymerase Chain Reaction(PCR), western-blot, immunofluorescence, and energy metabolism of IVD cell. The effects of Piezo1 were investigated by using siRNA transfection, real-time PCR, western-blot, and immunofluorescence.
Results.
The discs in both of the impact groups presented degenerative changes after 14 days, which showed significant up-regulation of Piezo1, NLRP3 inflammasome, the catabolic (MMP-9, MMP-13), and pro-inflammatory gene (IL-1β) expression than that of the control group (P < 0.05), accompanied by significantly increased release of ATP, lactate, nitric oxide (NO), and glucose consumption of IVD cells at first 7 days. Silencing Piezo1 reduced the activation of NLRP3 inflammasome and IL-1β expression in the nucleus pulposus induced by impact injury.
Conclusion.
It demonstrated that not only fracture of the endplate but also a single impact injury without structural impairment could also initiate IDD, which might be mediated by activation of Piezo1 induced inflammation and abnormal energy metabolism of IVD cells.
Level of Evidence: N/A.
BACKGROUND
Intervertebral disc degeneration (IDD) is strongly associated with low back pain (LBP),1,2 which accounts for approximately $100 billion in annual medical expenditure, lost wages, and decreased productivity in the United States.3 Degeneration of the disc is usually attributed to mechanical, aging, genetic, and nutritional factors, which is characterized by a decrease in proteoglycans, cell population, water content, and loss of disc height.4 The environmental and behavioral factors are also believed to influence the degenerative process.5
Since the intervertebral disc (IVD), which consists of the inner nucleus pulposus (NP) and the outer annulus fibrosus (AF), is the largest avascular cartilaginous tissue of the human body, its nutrition supply mainly depends on the diffusion from the surrounding AF and the adjacent cartilaginous endplates, which could be hindered by factors, such as endplate structural failure, calcification, and changes in proteoglycan content.6 It has been reported that IDD is associated with an aberrant cell-mediated response to structural failure, such as endplate fracture or vertebral burst fracture.7,8 However, whether a single impact injury of the endplate without structural disruption, which is commonly seen in the clinic,9 is sufficient to initiate IDD is still controversial.
Recently, Coste et al10 identified a novel mechanically activated (MA) cation channel known as the Piezo protein.11 It has been recognized as part of an evolutionarily conserved ion channel family of cation-permeable proteins involved in mechanotransduction that are closely related to the cytoskeleton. The Piezo family of vertebrate consists of Piezo1 and Piezo2, which are essential components of distinct mechanically activated cation channels. Researchers have reported that Piezo1 is widely expressed in the NP cells, and can be upregulated by mechanical stretch.12 It has been reported that Piezo 1 activation mediates degeneration-related inflammation in NP cells with the production of NOD-like receptor protein 3 (NLRP3) inflammasome.12 In addition, researchers have illustrated that abnormal compressive loading could affect IVD cell energy metabolism and disequilibrate the extracellular matrix (ECM) biosynthesis of NP, both of which are closely related to IDD.13–15 However, whether the impact injury of the endplate without structural disruption could activate Piezo1 and its role in IVD impact injury remains unknown.
Since a genuine in vivo impact injury model is ethically challenging, the availability of an experimental animal model that consistently reproduces the disease after impact injury would facilitate the investigation of post-traumatic degenerative processes. Although several animal trauma models have been established,16–19 the different nature of injury causes different emphasis on matrix remodeling, energy metabolism, and inflammation and hence poses the question of clinical relevance, in particular as none of them mimic the clinical situation.
In this study, our goal was to further evolve an in vitro impact injury model to investigate whether a single impact injury of the endplate without structural disruption could initiate IDD, and to determine the underlying mechanism. We hypothesized that, not only structural disruption but also a single impact injury of the endplate without structural impairment could initiate IDD which might be mediated by activation of Piezo1 induced inflammation and abnormal energy metabolism of IVD cells.
METHODS
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless stated otherwise. Full-organ IVD culture model and trauma induction. The animal study was also approved by the institutional review board and animal care committee of the Sun Yat-sen University (2014C-050).
This study was divided into two parts; the first part evolved an in vitro impact injury model of IDD and investigated the degenerative process by using histomorphology, real-time Polymerase Chain Reaction(PCR), western-blot, immunofluorescence, and energy metabolism of IVD cells. The second part investigated the effects of Piezo1 in the process of impact induced IDD by using siRNA transfection, real-time PCR, western-blot, and immunofluorescence analysis.
Part 1
Animal Groups and Impact Loading
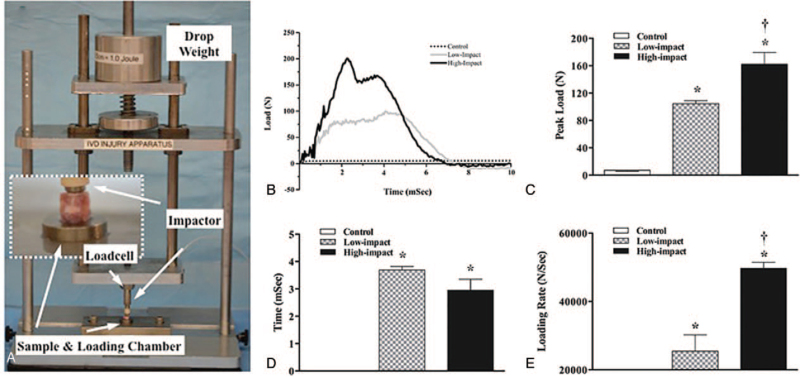
Thirty-five Sprague-Dawley rats (0.4–0.5 kg, male, 6 months old) obtained from Sun Yat-sen university animal facility were euthanatized with Barbiturate at a dose of 200 mg/kg via intra-peritoneal administration. Subsequently, 72 spinal segments (IVD/endplate with approximately 2 mm of the adjacent vertebral bodies) from L1/2 to L5/6 (3/animal) were isolated in less than four fours, and flushed with 0.9% NaCI containing 50 μL/mL penicillin. The segments were trimmed transversely by bone saw (IsoMet, Buehler, Lake Bluff, IL) with the cranial and caudal cutting planes parallel to each other at a height of 7.08 ± 0.21 mm (a distance in the cephalon-caudal direction), and perpendicular with respect to the cranial/caudal axis of the segment. The segments were then randomly assigned into three groups: Control (n = 24), Low-impact (12 J/cm3, n = 24), and High-impact (25 J/cm3, n = 24). The specimens were then subjected to single impact load using a custom-made apparatus we designed, which guaranteed axial load. The impact force and impact time were calculated and recorded using a piezoelectric loadcell (Kistler) (Figure 1A, Supplementary Figure 6). Pilot-experiment revealed 25 J/cm3 as the threshold energy for endplate failure, at which the endplate was expected to fracture in half of the specimens (the unit of the impact energy and the unit conversion was shown in the supplementary data). The height of each specimen was calculated as the vertical distance between the two vertebral cross sections, and was recorded before and after impact loading. Based on the pilot-experiment, more than 10% decrease of the height indicates endplate fracture, whereas less than 10% decrease indicates endplate intact.
Figure 1.
An ex vivo impact injury model for intervertebral disc degeneration (IVDD). A, The impact apparatus; B, impact load profile versus time, C, peak load, D, time to reach peak load, and E, loading rate in the Control, Low Impact, and High-impact groups (∗P < 0.05 vs. Control group; † P < 0.05 vs. Low Impact group).
Tissue Harvesting and Medium Collection
After impact, samples were washed with 0.9% NaCI containing 50 μL/mL penicillin for three times, and then cultured at 37 °C, 5% CO2 in DMEM (Dulbecco's Modified Eagle Medium, DMEM) with 2% fetal calf serum, 1% Pen/Strep, 50 mg/mL ascorbate-2-phosphate, and 0.1% Primocin. This culture conditions had been demonstrated in our pilot-experiment that it could maintain the segment viability over 14 days (Supplementary data, Figure 1). Half of the samples were collected at day 7, and the other half were collected at day 14. All the samples were dissected into two halves in the sagittal plane, one half was fixed for histology analysis, and other half, from which the NP cells were isolated and cultured in Dulbecco's Modification of Eagle's Medium (10–013-CVR; Corning, NY) containing 10% FBS (10099-141; Gibco, Australia) supplemented with 1% penicillin-streptomycin (SV30010; Hyclone, UT) at 37 °C with 5% CO2. The nucleus pulposus cells were taken for subsequent cell and molecular biological experiments.
The medium was changed and collected at the end of each day and used for ATP, lactate, nitric oxide (NO) release, and glucose consumption to evaluate the energy metabolism of IVD cells.
Histology
The specimens for histology were fixed with 4% paraformaldehyde for 24 hours at 4 °C, then transferred to a sealed vial containing a solution of 70% ethanol and decalcifying agent for at least 30 days. The specimens were then sequentially dehydrated, split down the mid-sagittal plane, embedded in paraffin for histological sectioning. Serial sections were cut in the transverse plane at 8 μm with a microtome (HM360, Microm International AG, Switzerland), and then stained with hematoxylin & eosin (H&E), and afranin O/fast green dyes (Fisher, Scientific, Pittsburgh, PA) using standard procedures and photographed under 40–200× magnification (Nikon Eclipse, Ti, Nikon, Tokyo, Japan). All the sections were imaged under bright field and cross-polarized light.
The scoring system that we developed based on our prior work20,21 was used to assess the disc degeneration (Supplementary data, Table 1). Histomorphometric assessment was performed by two orthopedic researchers (Z.L. and X.L.), who were blinded to the different treatments between groups. All histologic sections were reviewed 1 month after the first examination to determine the intraobserver reliability. The average score of the two measurements for each specimen was used for the statistical analysis.
Real-time PCR Analysis
Total RNA was extracted from the specimens using Trizol reagent (Ambion, Carllsbad, CA), followed by the RNeasy Mini Kit (Qiagen Inc., Duesseldorf, Germany). We selected eight genes (Aggrecan, Col2α1, Piezo1, NLRP3, IL-1β, MMP9, MMP13, TGF-β) to evaluate the degenerative changes16,22 (Supplementary data, Table 2). cDNA synthesis was performed as described previously.23 Gene expression was quantified by real-time PCR using the CFX96 Real-Time System (Bio-Rad, Hercules, CA). Data were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and expressed as fold change compared with the control group.
Western Blot
Western blotting was performed as described previously.24 The results were quantified using Image J analysis 1.46r (version Java 1.6.0–20; Media Cybernetics; Maryland, United States). The information of the antibodies were presented in supplementary files (Supplementary data, Tables 3 and 4).
Immunofluorescence
Immunofluorescence staining was performed according to the method previously reported.25 After stained with DAPI, the cells were examined with a confocal microscope (A1R; Nikon, Japan) and analyzed with ImageJ software (Rawak Software, Germany).
Energy Metabolism of IVD Cells
The energy metabolism of IVD cells after impact injury was investigated with quantification of ATP, lactate, NO, and glucose content.
The adenosine-triphosphate (ATP) content in the culture media, which was used to evaluate ATP released from IVD cells, was measured using the Luciferin-luciferase method (Sigma). Lactate content in media was measured using the method previously reported.15 Nitric oxide (NO) secreted into the media for each sample was determined by using the Griess reaction.26 Glucose content in media was measured using a Cobas C System (Roche Diagnostics, Indianapolis, IN). Fresh DMEM was used as reference glucose concentration. The change of glucose concentration indicated the total glucose consumed by the IVD cells of each sample. The measurements were normalized to the data measured at day 0 (prior to impact loading).
Part 2
Animal Groups and Impact Loading
Ten spinal-segments were isolated from two Sprague-Dawley rats (0.4–0.5 kg, male, 6 months old), which were then assigned into two groups: Control (n = 4), Low-impact (12 J/cm3, n = 6). After suffered low impact injury, the nucleus pulposus cells were isolated and transfected with Piezo1-siRNA or scrambled sequence siRNA (siSS).
siRNA Transfection
siRNA transfection was used to silence the specific RNA Piezo1 in this study. The sequence of Piezo1-siRNA was as follows: Sense strand, 5′-CGGCCAACAUAAAGAACAUdTdT-3′, antisense strand, 5′-AUGUUCUUUAUGUUGGCCGdTdT-3′. According to the manufacturer's instructions, target gene-specific siRNAs (50 nM) were transfected with ribo FECT TMCP Transfection Kit (RiboBio Co., Ltd., Guangzhou, China) to the NP cells (See Figure 2 in the Supplement Digital Content), which were isolated from the spinal segments suffered impact injury. All the sequences were synthesized by RiboBio Co., Ltd.
Real-time PCR, Western Blot, and Immunofluorescence
The expression of IL-1β, NLRP3, MMP9, MMP13, aggrecan, and Col2α1 were assessed in the nucleus pulposus cells at 48 hours after siRNA transfection, by using real-time PCR, western-blot, immunofluorescence as shown in part 1.
Statistical Analysis
SPSS software (version 25.0; SPSS Inc., Chicago, IL) was used for univariate analysis of variance (ANOVA), and the least-significant difference (LSD) test was performed for the post-hoc comparisons. To assess inter- and intraobserver variation, we used the intraclass correlation coefficient (ICC) for average and single measurements. The agreement of intraclass correlation coefficient was rated as follows: 0 to 0.4, fair agreement; 0.41 to 0.60, moderate agreement; 0.61 to 0.8, substantial agreement, and 0.81 to 1.00, excellent agreement.27 Bland-Altman plots were generated to depict the consistency among the observers.28 Statistical significance was indicated at P < 0.05.
RESULTS
The impact time was less than 0.008 second for both the Low-impact and High-impact groups (Figure 1B, D). Significant higher Peak Load (162 ± 17 N) and Loading Rate (49,700 ± 1752 N/s) were found in the High-impact group than in the Low-impact group (105 ± 4 N and 25,430 ± 4753 N/s, respectively) as shown in Figure 1C, E.
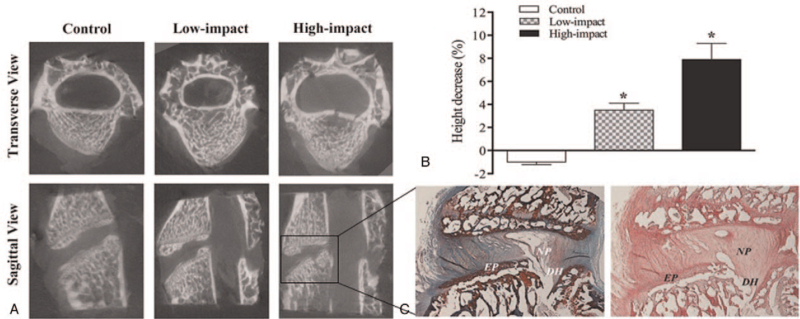
With the applied trauma-protocol, there were no specimens with endplate fracture in the Low-impact group. In the High-impact group, endplate fracture occurred in eight specimens, which has been confirmed by CT scan and histological evaluation (Figure 2A, C). After impact injury, the average height of specimens in the Low and High-impact groups decreased 3.49 ± 0.62% and 7.93 ± 1.34% respectively, both of which showed significant differences in comparison with the control group (P < 0.05, Figure 2B).
Figure 2.
Representative Micro-CT analysis for each group and the histological images of spinal segment with endplate fracture. A, The transverse and sagittal view of Micro-CT showed the endplate fracture of the spinal segment in the High-impact group; B, this graph showed the mean decrease of sample height between groups. The disc height in the High-impact group decreased significantly in comparison with that of the Control and Low-impact groups (∗P < 0.05 vs. Control and Low-impact groups); C, the representative H&E and safranin O staining of the vertebral endplate fracture in the High-impact group, combined with an osseous disc herniation. DH indicates disc height; EP, endplate; NP, nucleus pulposus.
Part 1
Evidence that a SINGLE impact Injury of Endplate without Structural Disruption Initiated Post-Traumatic Disc Degeneration
Histological Findings
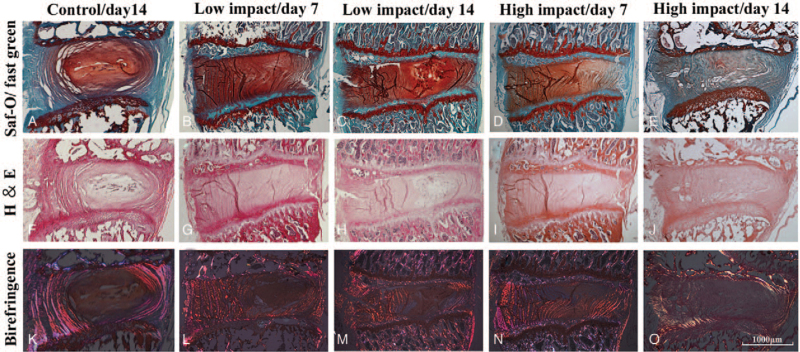
The intervertebral discs in the control group appeared normal. The NP contained abundant cells and was surrounded by large zones of acellular matrix, and the AF showed normal organization of fibrocartilage lamellae (Figure 3A, F, K).
Figure 3.
Representative hematoxylin & eosin (H&E) and safranin O/fast green stained sagittal sections of spinal segments under (A–J) brightfield and (K–O) polarized light (×200) in different groups. A, F, K, The intervertebral disc (IVD) in the control group showed that the nucleus pulposus contained abundant cells and surrounded by large zones of acellular matrix, and the annulus fibrosus showed normal organization of fibrocartilage lamellae. B, G, L, D, I, N, The discs in the Low and High-impact groups at day 7 after injury showed no significant degenerative changes, although the nucleus pulposus contained less cells and the annulus fibrosus showed less organization of fibrocartilage lamellae. C, H, M, E, J, O, The discs in the Low and High-impact groups at day 14 after impact injury showed significant degenerative changes, where the nucleus pulposus comprised relatively few cells (C, H, E, J), with less deeply stained proteoglycans, and the collagen fibers formed a wavy arrangement in the annulus fibrosus (M, O).
At day 7, the discs in the Low and High-impact groups showed no significant degenerative changes (Figure 3B, G, L, D, I, N). However, at day 14, the discs in both the Low and High-impact groups showed significant degenerative changes, where the NP comprised relatively few cells (Figure 3C, E, H, J), with less deeply stained proteoglycans (Figure 3C, E) relative to the control group. The AF showed less organized fibrocartilage lamellae, as compared with the control group, and the collagen fibers formed a wavy arrangement (Figure 3M, O). Both of the histological scores in the Low and High-impact groups were significantly higher than that of the control group (P < 0.05, see Figure 3 in the supplementary file). There were no significant difference in the histological score between the Low and High-impact groups (P > 0.05, see Figure 2 in the supplementary file). The intralclass correlation coefficient was 0.883 for a single measurement (95% confidence interval range: 0.8836–0.912) and 0.958 for average measurements (95% confidence interval range: 0.939–0.971), which showed strong agreement (see Figure 4 in the Supplementary Digital Content).
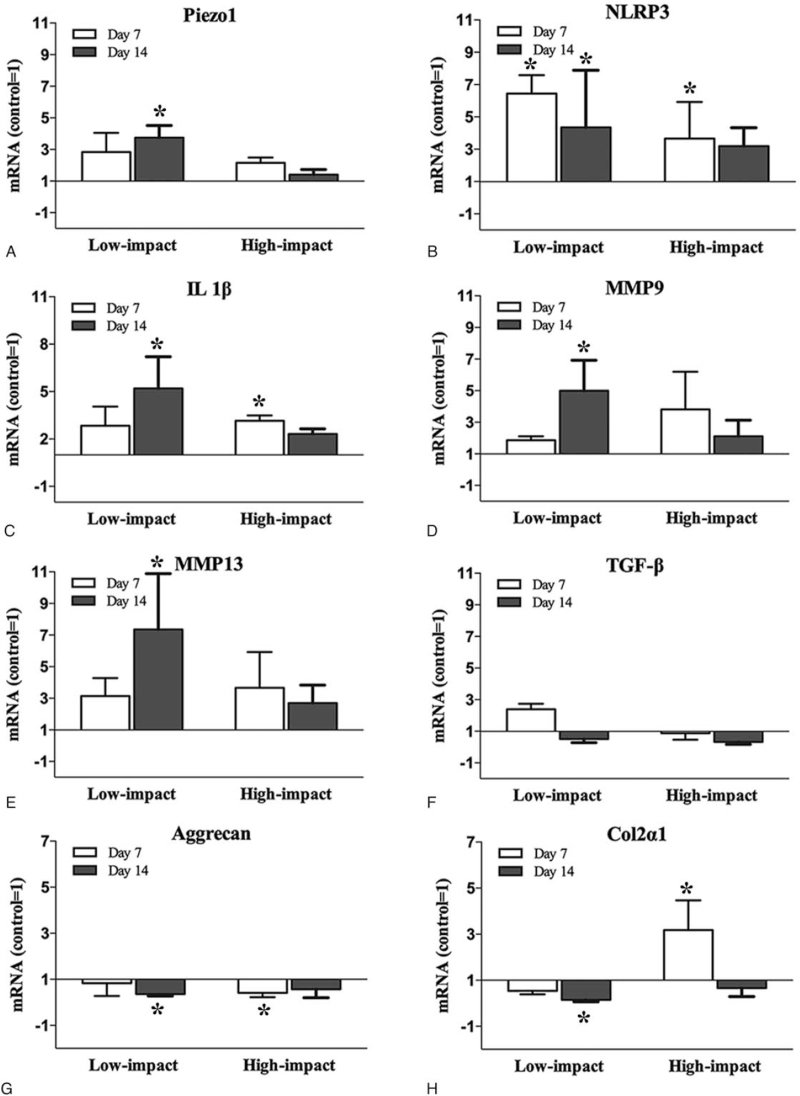
Real-time PCR
The pro-inflammatory gene transcription, IL-1β was strongly upregulated in the Low-impact group at day 14 and in the High-impact group at day 7 (P < 0.05, Figure 4C), followed by the catabolic (MMP-9, MMP-13) genes transcription upregulated, especially in the Low-impact group at day 14 (P < 0.05, Figure 4D, E). For the anabolic (col2α1, aggrecan, TGF-β) genes, TGF-β was upregulated in the Low-impact group at day 7, but reversed to down-regulation at day 14 (Figure 4F). In the High-impact group, TGF-β was downregulated at day 7 and 14, although there were no significant differences compared with the control group (Figure 4F). Both the Low and High-impact injury caused a down-regulation of aggrecan at day 7 and 14, especially in the Low-impact group at day 14 and in the High-impact group at day 7 (P < 0.05, Figure 4G). Although strongly upregulated at day 7 in the High-impact group, the gene expression of col2α1 reversed to down-regulation at day 14 (Figure 4H). In the Low-impact group, the col2α1 was significantly downregulated at day 14 compared with the control level (Figure 4H).
Figure 4.
The genes transcription of (A) Piezo1, (B) NLRP3, (C) IL-1β, (D) MMP9, (E) MMP13, (F) TGF-β, (G) Aggrecan, and (H) Col2α1 in the nucleus pulposus cells of the discs in different groups. A, B, Both the Low and High-impact injury caused an up-regulation of the Piezo1 and NLRP3 genes transcription in comparison with the control group, especially in the Low-impact group at day 7 and 14 (P < 0.05). C, The pro-inflammatory (IL-1β) gene transcription was also strongly upregulated in both the Low and High-impact group, especially in the High-impact group at day 7 and in the Low- impact group at day 14 (P < 0.05). D, E, The catabolic (MMP-9, MMP-13) genes transcription were upregulated after impact injury in comparison with the control group, especially in the Low-impact group at day 14 (P < 0.05). F, No significant differences were found in the gene expression of TGF-β between the control and impact injury groups. G, H, The aggrecan and Col2α1 genes transcription were downregulated in both the Low and High-impact groups in comparison with the control group, except at day 7 in the High-impact group.
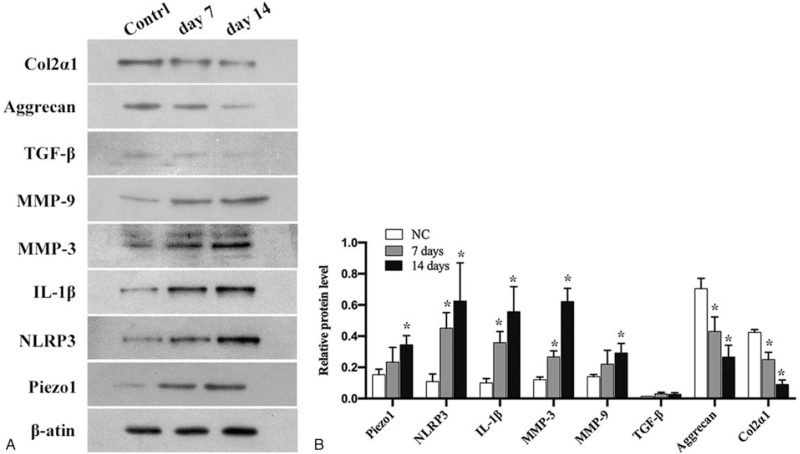
Western Blot
Because endplate fracture was found in 10 specimens in the High-impact group, we did not perform the western blotting analysis for the High-impact group due to the limited protein extracted. In the Low-impact group, as shown in Figure 5, the protein expression of IL-1β, MMP3, and MMP9 in NP cells increased time dependently, especially of the IL-1β and MMP3, which showed a significant increase in the gray value ratio at both day 7 and 14 ( P < 0.05, Figure 6A, B). There was no significant changes in the protein expression of TGF-β at different time point; however, significant decrease in the protein level of aggrecan and col2α1 were detected at both day 7 and 14 (P < 0.05, Figure 6A, B).
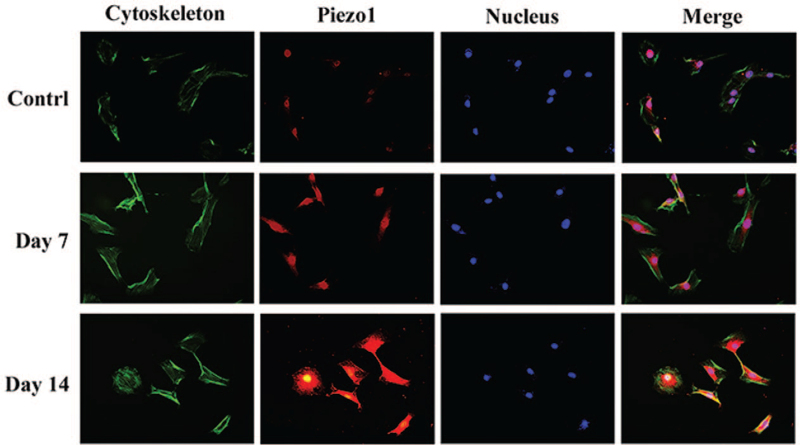
Figure 5.
The immunofluorescence staining of Piezo1 in the nucleus pulposus cells of the Low-impact group. The results showed that the level of Piezo1 protein expression increased in the Low-impact group at day 7 and day 14.
Figure 6.
The results of Western-blot analysis and the relative expression of Piezo1, NLRP3, IL-1β, MMP9, MMP13, TGF-β, Aggrecan, and Col2α1 in the nucleus pulposus cells of the Low-impact group. A, B, The protein expression of Piezo1, NLRP3, IL-1β, MMP3, and MMP9 in NP cells increased significantly and time dependently, accompanied by significantly decreased expression of Aggrecan and Col2α1(∗P < 0.05 vs. Control group).
The Energy Metabolism of IVD Cells After Impact Injury
Both the Low and High impact injury increased ATP release, lactate, and NO production, and glucose consumption of IVD cells relative to the control conditions, especially at the first 7 days, reaching significance when compared with the control group (P < 0.05); however, all of them then decreased slowly at the second 7 days, with no significant difference compared with the control group (P > 0.05, see Figure 5 in the supplementary file).
Expression of Piezo1 and NLRP3 Inflammasome Was Upregulated in the NP Cells After Impact Injury
The messenger RNA (mRNA) expression of Piezo1 was upregulated in both the Low and High- impact groups, especially in the Low-impact group at day 14 (P < 0.05, Figure 4A). The NLRP3 gene transcription was also found strongly upregulated in the Low-impact group at day 7 and 14, and in the High-impact group at day 7 (P < 0.05, Figure 4B). The protein expression of Piezo1 and NLRP3 showed significant increase in the gray value ratio both at day 7 and 14 (P < 0.05, Figure 6A, B).
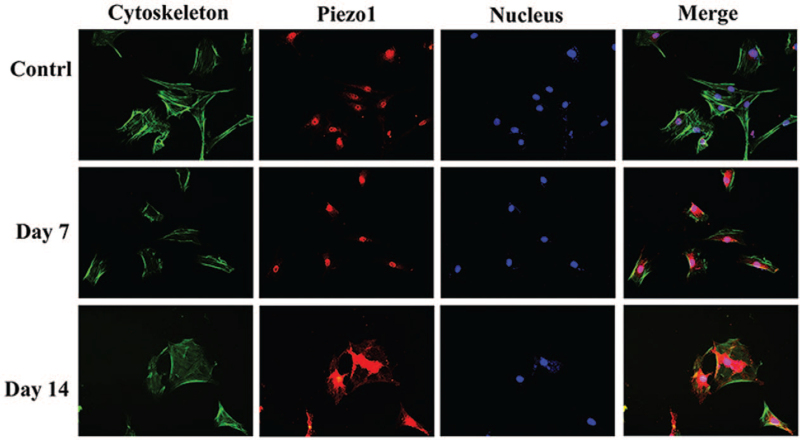
The immunofluorescence staining showed that Piezo1 localized in the cell membrane and nucleus of the rat NP cells. The results further verified that the level of Piezo 1 protein expression increased in both Low and High-impact groups at day 7 and 14 (Figures 5 and 7).
Figure 7.
The immunofluorescence staining of Piezo1 in the nucleus pulposus cells of the High-impact group. The results also showed that the level of Piezo 1 protein expression increased in the High-impact group at day 7 and day 14.
Part 2
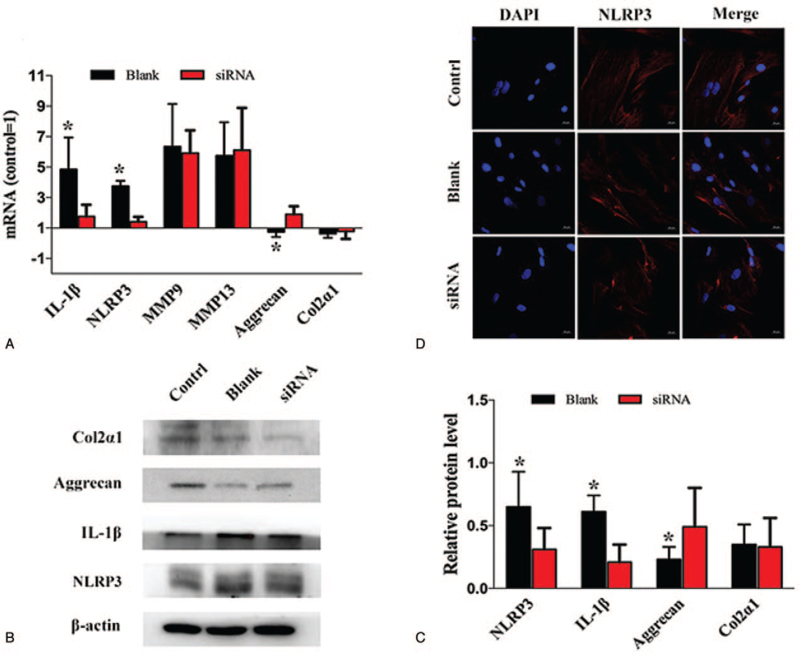
Evidence that Silencing Piezo1 Reduced the Activation of NLRP3 Inflammasome Induced by Impact Injury
At 48 hours after silencing Piezo1, the mRNA expression of IL-1β, NLRP3 in the NP cells were significantly downregulated, accompanied by significant upregulation of aggrecan. However, there were no significant differences in the mRNA expression of Col2α1, MMP9, and MMP13 (Figure 8A).
Figure 8.
Silencing Piezo1 reduced the activation of NLRP3 inflammasome induced by impact injury. A–C, The mRNA and protein expression of IL-1β, NLRP3 in the nucleus pulposus cells exposed to Piezo1-siRNA were significantly downregulated, accompanied by significant upregulation of aggrecan. D, The results of immunofluorescence staining showed that the expression of NLRP3 was downregulated in the nucleus pulposus cells by silencing Piezo1.
The protein expression of IL-1β and NLRP3 were also abolished by silencing Piezo1, followed by upregulated protein expression of aggrecan; however, the protein expression of Col2α1 was not upregulated by silencing Piezo1 (Figure 8B, C).
The immunofluorescence staining further verified that the level of NLRP3 protein expression was downregulated by silencing Piezo1 (Figure 8D).
DISCUSSION
In this study, the results showed that even a single impact injury without structural disruption could initiate IDD. Further experiments revealed that Piezo1 and pro-inflammatory genes transcription were significantly upregulated combined with the abnormal energy metabolism of IVD cells in the degenerative process; Silencing Piezo1 could reduce the activation of inflammation and delay the degenerative process. These results verified our hypotheses that not only structural disruption but also a single impact injury of the endplate without structural impairment could initiate IDD which might be mediated by activation of Piezo1 induced inflammation and abnormal energy metabolism of IVD cells. To our knowledge, this is the first study to demonstrate the effects of impact injury without endplate structural failure on the IDD.
Whether a single impact injury without structural disruption, which represents a frequent trauma during a lifetime, is sufficient to initiate IDD is still controversial. Researchers investigated the degenerative process of the rabbit spinal segments suffered impact loading and demonstrated that fracture of vertebral endplate, but not equienergetic loading promoted IDD.22 In this study, we designed a dropped-weight apparatus, which could induce impact injury in precise axial direction. This design could more mimic the secondary impact loading in the clinical situation compared with the apparatus with steel ball falling in previous studies.19,22 The histomorphological findings showed that, even in the Low-impact group without endplate structural failure, the proteoglycans in nucleus pulposus decreased significantly and the fibrocartilage lamellae showed less organized compared with the control group at day 14. These changes were consistent with the previous studies showing loss of proteoglycans and less organized collagens during the initial phase of disc degeneration.29–31
Previous studies demonstrated that the catabolic MMPs (matrix metalloproteinase) and pro-inflammatory (such as IL-1β, TNF-α, etc.) gene transcriptions were significantly upregulated in the posttraumatic IDD19,22,32–34; however, they did not track the biological responses on protein level. In this study, we found that both the expressions of catabolic (MMP-9, MMP-13) and pro-inflammatory (IL-1β) genes were significantly upregulated in the IVDs suffered impact injury without endplate structural failure, which has also been confirmed on protein level. This further demonstrated that not only structural disruption but also a single impact injury of the endplate without structural impairment could initiate post-traumatic IDD. It has been reported that, the activity of MMPs is not limited to matrix cleavage; they also modulate the inflammatory response,35 which may explain why degenerative discs were also sensitive to pro-inflammatory stimuli.36
Sun et al12 reported that Piezo1 activated NLRP3 inflammasome in the nucleus pulposus cells that suffered mechanical stretch; however, the roles of Piezo1 in the degenerated discs induced by impact injury remain unclear. In this study, we found that the mRNA and protein expression of Piezo1 and NLRP3 were significantly upregulated in the NP cells of the IVDs that suffered impact injury, and silencing Piezo1 reduced the activation of NLRP3 inflammasome induced by impact injury. Moreover, the results of mRNA and protein expression demonstrated that the pro-inflammatory (IL-1β) gene was also reduced by silencing Piezo1 followed by up regulated expression of aggrecan; this results suggested that Piezo1 might play an important role in the process of impact injury induced IDD by activating NLRP3 and pro-inflammatory (IL-1β) gene. However, Leddy et al37 confirmed that IL-1α-mediated inflammatory signaling articular chondrocytes upregulated Piezo1 gene expression and function, and demonstrated that inflammatory signaling sensitized Piezo1 mechanotransduction in as a pathogenic feed-forward mechanism in osteoarthritis. Thus, although silencing Piezo1 reduced the IL-1β gene expression in this study, further studies are required to investigate the interaction between Piezo1 and inflammatory signaling in the process of IDD induced by impact injury.
Although researchers have confirmed that static or dynamic mechanical loading could affect the energy metabolism of IVD cells, whether and how the impact injury of endplate influences the energy metabolism of IVD cells remains unclear. In this study, we found that the total ATP content, lactate production, and glucose consumption increased significantly during the first 7 days after impact injury, although tending to fall back slightly at the second 7 days. This suggests that the glycolysis in the IVD cells might be promoted by impact injury. Interestingly, although previous research suggested that intracellular ATP of NP cells might be mainly produced via mitochondrial respiration instead of glycolysis,12,25 increased NO production detected in our study suggested that ATP production in IVD cells via mitochondrial respiration might be inhibited by impact injury. However, we did not separate the NP and AF cells when evaluating the data. Thus, the effects of impact injury on the energy metabolism of NP cells require further study to investigate.
Several limitations of the study should be clarified. Firstly, there are some biomechanical and anatomic differences between the spine of the rats and that of the humans; however, the objective of this study was to evolve an in vitro impact injury model of IVD and to investigate the roles of a single impact injury of the endplates without structural disruption on the process of IDD. This presented model established by axial impact loading could accurately mimics the loading conditions in patients suffering endplate trauma. In addition, drawing conclusions from animal models as well as from in vitro studies is always problematic. The increased gene transcription as demonstrated by quantitative PCR and western blot, may not essentially induce a functional gene product, as mechanisms such as post-transcriptional modifications or gene silencing may interfere. Finally, due to the limited number of samples in this study, we did not focus on the difference in the gene expression between the discs with intact endplates and those with endplate fractures. Further studies are warranted to verify the results of this study.
Taken together, an in vitro IDD model induced by impact injury has been developed and applied to demonstrate that, not only structural disruption but also a single impact injury of the endplate without structural impairment can initiate disc degenerative changes, which might be mediated by activation of Piezo1 induced inflammation and abnormal energy metabolism of IVD cells. This may provide some clues for us to investigate the initiation of disc degeneration after axial impact injury of endplates.
CONCLUSIONS
This study demonstrated that, not only structural disruption but also a single impact injury of the endplate without structural impairment could initiate post-traumatic IDD, which might be mediated by activation of Piezo1 induced inflammation and abnormal energy metabolism of IVD cells.
Key Points
A single impact injury (12 J/cm3) on rats endplates can initiate intervertebral disc degeneration (IDD) concomitant with up-regulation of pro-inflammatory (IL-1β) and the catabolic (MMP-9, MMP-13) genes transcription at 14 days after injury.
Not only structural disruption but also a single impact injury of the endplate without structural impairment could also initiate IDD.
The process of IDD induced by impact injury might be mediated by activation of Piezo1 induced inflammation and abnormal energy metabolism of the disc cells.
Supplementary Material
Acknowledgment
The authors thank Wenfang Chen for her assistance with pathological evaluation and Steffen Ringgaard for technical assistance with T1ρ imaging. They thank Mingmei for technical assistance of sample embedding for histology. They especially thank Pro. Rui Sun, who is a senior biostatistician in our research center, for statistical consultation.
Footnotes
Z.S., X.Z., and S.L. contributed equally to this work.
The manuscript submitted does not contain information about medical device(s)/drug(s).
National Natural Science Foundation of China (No. 81972135), Qingdao Outstanding Health Professional Development Fund, Funds for Dongguan Social Science and Technology Development (No. 201950715001202) and Sanming Project of Medicine in Shenzhen (No. SZSM201911002) funds were received in support of this work.
No relevant financial activities outside the submitted work.
Supplemental digital content is available for this article.
References
- 1.Deyo RA, Weinstein JN. Low back pain. N Engl J Med 2001; 344:363. [DOI] [PubMed] [Google Scholar]
- 2.Andersson GB. Epidemiological features of chronic low back pain. Lancet 1999; 354:581–585. [DOI] [PubMed] [Google Scholar]
- 3.Battié MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am 2006; 88:S3–S9. [DOI] [PubMed] [Google Scholar]
- 4.Ariga K, Yonenobu K, Nakase T, et al. Mechanical stress-induced apoptosis of endplate chondrocytes in organ-cultured mouse intervertebral discs: an ex vivo study. Spine (Phila Pa 1976) 2003; 28:1528–1533. [PubMed] [Google Scholar]
- 5.Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage 2015; 23:1057–1070. [DOI] [PubMed] [Google Scholar]
- 6.Grunhagen T, Shirazi-Adl A, Fairbank JC, et al. Intervertebral disk nutrition: a review of factors influencing concentrations of nutrients and metabolites. Orthop Clin North Am 2011; 42:465–477. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Videman T, Battié MC. Morphometrics and lesions of vertebral end plates are associated with lumbar disc degeneration: evidence from cadaveric spines. J Bone Joint Surg Am 2013; 95:e26. [DOI] [PubMed] [Google Scholar]
- 8.Cinotti G, Della Rocca C, Romeo S, et al. Degenerative changes of porcine intervertebral disc induced by vertebral endplate injuries. Spine (Phila Pa 1976) 2005; 30:174–180. [DOI] [PubMed] [Google Scholar]
- 9.Mekkodathil A, El-Menyar A, Kanbar A, et al. Epidemiological and clinical characteristics of fall-related injuries: a retrospective study. BMC Public Health 2020; 20:1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coste B, Xiao B, Santos JS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012; 483:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Zhou Y, Yadav PS, et al. Piezo1/2 mediate mechanotransduction essential for bone formation through concerted activation of NFAT-YAP1-ß-catenin. Elife 2020; 9:e52779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun Y, Leng P, Song M, et al. Piezo1 activates the NLRP3 inflammasome in nucleus pulposus cell-mediated by Ca2+/NF-κB pathway. Int Immunopharmacol 2020; 85:106681. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Gonzales S, Levene H, et al. Energy metabolism of intervertebral disc under mechanical loading. J Orthop Res 2013; 31:1733–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desmoulin GT, Pradhan V, Milner TE. Mechanical aspects of intervertebral disc injury and implications on biomechanics. Spine (Phila Pa 1976) 2020; 45:E457–E464. [DOI] [PubMed] [Google Scholar]
- 15.Navone SE, Peroglio M, Guarnaccia L, et al. Mechanical loading of intervertebral disc modulates microglia proliferation, activation, and chemotaxis. Osteoarthritis Cartilage 2018; 26:978–987. [DOI] [PubMed] [Google Scholar]
- 16.Ao X, Wang L, Shao Y, et al. Development and characterization of a novel bipedal standing mouse model of intervertebral disc and facet joint degeneration. Clin Orthop Relat Res 2019; 477:1492–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashinsky BG, Gullbrand SE, Bonnevie ED, et al. Multiscale and multimodal structure-function analysis of intervertebral disc degeneration in a rabbit model. Osteoarthritis Cartilage 2019; 27:1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh K, Masuda K, An HS. Animal models for human disc degeneration. Spine J 2005; 5:267S–279S. [DOI] [PubMed] [Google Scholar]
- 19.Dudli S, Haschtmann D, Ferguson SJ. Persistent degenerative changes in the intervertebral disc after burst fracture in an in vitro model mimicking physiological post-traumatic conditions. Eur Spine J 2015; 24:1901–1908. [DOI] [PubMed] [Google Scholar]
- 20.Wei F, Zhong R, Wang L, et al. Pingyangmycin-induced in vivo lumbar disc degeneration model of rhesus monkeys. Spine (Phila Pa 1976) 2015; 40:E199–E210. [DOI] [PubMed] [Google Scholar]
- 21.Wei F, Zhong R, Pan X, et al. Computed tomography guided subendplate injection of pingyangmycin for a novel rabbit model of slowly progressive disc degeneration. Spine J 2019; 19:e6–e18. [DOI] [PubMed] [Google Scholar]
- 22.Dudli S, Haschtmann D, Ferguson SJ. Fracture of the vertebral endplates, but not equienergetic impact load, promotes disc degeneration in vitro. J Orthop Res 2012; 30:809–816. [DOI] [PubMed] [Google Scholar]
- 23.Haschtmann D, Stoyanov JV, Ettinger L, et al. Establishment of a novel intervertebral disc/endplate culture model: analysis of an ex vivo in vitro whole-organ rabbit culture system. Spine (Phila Pa 1976) 2006; 31:2918–2925. [DOI] [PubMed] [Google Scholar]
- 24.Liao Z, Luo R, Li G, et al. Exosomes from mesenchymal stem cells modulate endoplasmic reticulum stress to protect against nucleus pulposus cell death and ameliorate intervertebral disc degeneration in vivo. Theranostics 2019; 9:4084–4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Zhang W, Deng M, et al. CircGLCE alleviates intervertebral disc degeneration by regulating apoptosis and matrix degradation through the targeting of miR-587/STAP1. Aging (Albany NY) 2020; 12:21971–21991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu GZ, Ishihara H, Osada R, et al. Nitric oxide mediates the change of proteoglycan synthesis in the human lumbar intervertebral disc in response to hydrostatic pressure. Spine (Phila Pa 1976) 2001; 26:134–141. [DOI] [PubMed] [Google Scholar]
- 27.Shrout PE. Measurement reliability and agreement in psychiatry. Stat Methods Med Res 1998; 7:301–317. [DOI] [PubMed] [Google Scholar]
- 28.Stelzeneder D, Welsch GH, Kovács BK, et al. Quantitative T2 evaluation at 3.0T com- pared to morphological grading of the lum- bar intervertebral disc: a standardized eval- uation approach in patients with low back pain. Eur J Radiol 2012; 81:324–330. [DOI] [PubMed] [Google Scholar]
- 29.Zhang C, Smith MP, Zhou GK, et al. Phlpp1 is associated with human intervertebral disc degeneration and its deficiency promotes healing after needle puncture injury in mice. Cell Death Dis 2019; 10:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson DG, Tannoury C. Molecular pathogenic factors in symptomatic disc degeneration. Spine J 2005; 5:260S–266S. [DOI] [PubMed] [Google Scholar]
- 31.Silagi ES, Shapiro IM, Risbud MV. Glycosaminoglycan synthesis in the nucleus pulposus: dysregulation and the pathogenesis of disc degeneration. Matrix Biol 2018; 71–72:368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Chen N, Du Z, et al. Bioinformatics analysis integrating metabolomics of M6A RNA microarray in intervertebral disc degeneration. Epigenomics 2020; 12:1419–1441. [DOI] [PubMed] [Google Scholar]
- 33.Dudli S, Ferguson SJ, Haschtmann D. Severity and pattern of post-traumatic intervertebral disc degeneration depend on the type of injury. Spine J 2014; 14:1256–1264. [DOI] [PubMed] [Google Scholar]
- 34.Handa T, Ishihara H, Ohshima H, et al. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine (Phila Pa 1976) 1997; 22:1085–1091. [DOI] [PubMed] [Google Scholar]
- 35.Gao C, Ning B, Sang C, et al. Rapamycin prevents the intervertebral disc degeneration via inhibiting differentiation and senescence of annulus fibrosus cells. Aging (Albany NY) 2018; 10:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, He F, Chen Z, et al. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging (Albany NY) 2019; 11:10499–10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leddy HA, Liu F, McNulty AL, et al. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc Natl Acad Sci U S A 2021; 118:e2001611118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.