Background:
Antimicrobial stewardship (AMS) is central to the World Health Organisation Global Action Plan against antimicrobial resistance (AMR). If antibiotics are used without restraint, morbidity and mortality from AMR will continue to increase. In resource-rich settings, AMS can safely reduce antibiotic consumption. However, for children in low- and middle-income countries (LMIC), the impact of different AMS interventions is unknown.
Aim:
To determine the impact of different AMS interventions on antibiotic use and clinical and microbiologic outcomes in children in LMIC.
Methods:
MEDLINE, Embase and PubMed were searched for studies of AMS interventions in pediatric population in LMIC settings. Controlled trials, controlled before-and-after studies and interrupted time series studies were included. Outcomes assessed were antibiotic use, multidrug-resistant organism (MDRO) rates, clinical outcomes and cost.
Results:
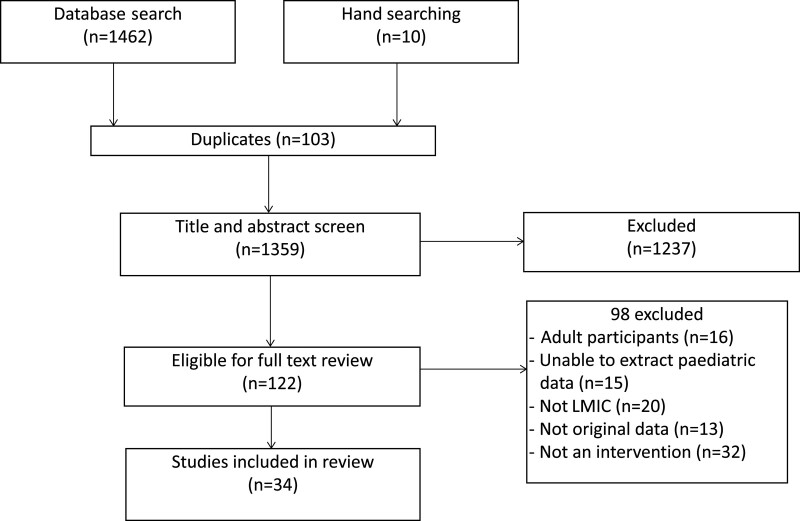
Of 1462 studies, 34 met inclusion criteria including a total population of >5,000,000 in 17 countries. Twenty were in inpatients, 2 in ED, 10 in OPD and 2 in both. Seven studies were randomized controlled trials. All types of interventions reported a positive impact on antibiotic prescribing. AMS bundles with education, and clinical decision tools appeared more effective than guidelines alone. AMS interventions resulted in significantly decreased clinical infections (4/4 studies) and clinical failure (2/2) and reduced MDRO colonization rate (4/4). There was no concomitant increase in mortality (4/4 studies) or length of stay (2/2).
Conclusion:
Multiple effective strategies exist to reduce antibiotic consumption in LMIC. However, marked heterogeneity limit conclusions regarding the most effective approach, particularly regarding clinical outcomes. Overall, AMS strategies are important tools in the reduction of MDRO-related morbidity in children in LMIC.
Keywords: antimicrobial stewardship, low-middle income, prescribing, antibiotic
Antimicrobial resistance (AMR) poses a huge threat to global health, and effective antibiotics to treat multidrug-resistant organisms (MDRO) are limited, particularly in low- and middle-income countries (LMICs). Half of deaths in neonates attributable to resistant pathogens occur in just 5 LMICs.1 Access to antibiotics is a continuing problem in many LMIC settings, and more deaths are attributed to limited and delayed access than to infections with resistant organisms.1 However, antibiotic consumption has rapidly increased in countries such as China and India, with a shift toward broad-spectrum antibiotic use.1 Consequently, the World Health Organization (WHO) has outlined a global action plan to combat AMR. A key objective includes monitoring and optimization of antimicrobial use at national and local levels.2
Antimicrobial stewardship (AMS) interventions are effective in increasing adherence to antibiotic policy, reducing antibiotic duration and reducing infection rates with MDROs and Clostridium difficile.3,4 However, the large majority of studies are from high-income countries, so findings may not be applicable to LMIC settings. A consensus has been published on core necessary elements for hospital AMS programs (ASPs) which could be applied to both high- and low/middle-income settings, and the WHO has developed a toolkit for ASPs in LMIC.5,6 However, specific guidelines are lacking for ASPs in children.
Implementing ASPs for children in LMIC has specific challenges. Infections are the leading cause of death in children under 5 years old in LMIC, particularly in neonates, so the impetus to prescribe antibiotics is high.1,7 Limited access to microbiology laboratory services, and specifically antibiotic susceptibility testing, means that broad-spectrum antibiotics have been used frequently, with low incentive to change in these high-mortality settings.8 Although poor access to antibiotics is a tangible problem in LMIC, the irony is that untargeted prescribing has worsened the problem by increasing AMR, leading to antibiotics becoming less effective.1 There has been one previous systematic review of AMS in children.9 Only 1 of the 9 studies was in an LMIC, and outpatients and neonatal intensive care units (NICUs) were excluded. The only review of AMS interventions in LMIC was limited to hospital settings and did not consider pediatrics specifically.10
This systematic review aimed to determine, for children accessing hospital and/or community healthcare in LMIC, the impact of different AMS interventions on antibiotic use, clinical outcomes, AMR and cost.
METHODS
This systematic review of the impact of AMS interventions in children in LMIC followed PRISMA guidelines. The review protocol was registered at the PROSPERO International Prospective Register of Systematic Reviews (available at http://www.crd.york.ac.uk): Registration number CRD42020153776. The search terms are outlined in Supplemental Digital Content 1, http://links.lww.com/INF/E516.
Search Strategy
MEDLINE, EMBASE, and PUBMED databases (inception to February 2020) were searched for original studies, and the Cochrane Database was searched for systematic reviews. Additional records were identified through reference checking. Participants were children 0–18 years of age in inpatient, ED or outpatient settings. LMICs were defined based on gross national income per capita as published by the World Bank.12
AMS interventions reviewed were those that constitute one of the core or supplemental strategies in an ASP defined according to the Infectious Diseases Society of America and Society for Healthcare Epidemiology of America9: audit and feedback, formulary restriction and preauthorisation, education, guidelines and clinical pathways and antimicrobial cycling. Clinical decision support (point-of-care [POC] test or decision support algorithm) was also included as the latter may be feasible to institute in LMIC. Interventions of interest were those that targeted healthcare professionals, but if studies also targeted parents they were included.
Inclusion Criteria
Studies were included for full-text review if they were randomized or nonrandomized controlled trials, before-after studies or interrupted time series. Studies could be of single or bundled interventions. There needed to be one or more of the following outcomes: antibiotic prescription rate and appropriateness (compliance with antibiotic guidelines and reduction in use of nontargeted antibiotics, decision to treat, total duration3); clinical outcomes (infection, clinical failure, morbidity/mortality and length of hospital stay); microbiologic outcomes (reduction in resistant bacterial colonization or infection); and cost.
Exclusion Criteria
Exclusion criteria were studies solely related to antifungal, antimalarial or antiretroviral medication, studies without outcome measures and studies with adults where pediatric data could not be evaluated separately. Case series, editorials and reviews without primary data were excluded.
Study Selection Process
Two reviewers independently conducted the database searches and reviewed the title and/or abstract of each study. Full text was reviewed by the authors if articles appeared to meet study inclusion, and any discrepancy was discussed and resolved between the reviewers
Data Extraction
Data were extracted independently and compiled in a PRISMA format table, including: study design, setting, number of participants, population, intervention group, comparison group, outcomes and risk of bias (Supplemental Digital Content, http://links.lww.com/INF/E516). Studies were grouped by intervention type. A meta-analysis was not done as the disparity in study design, interventions and outcomes prevented combining of data from different studies. Study bias was assessed using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.13
RESULTS
Thirty-four studies met inclusion criteria with total population of >5,000,000 children in 17 countries; China (14 studies),14–28 Argentina (2),29,30 Bangladesh (2),31,32 India (2),33,34 Tanzania (2),35,36 Brazil,37 Cuba,38 Indonesia,39 Iran,40 Kenya,41 Korea,42 Peru,43 Pakistan,44 Russia,45 Serbia,46 Turkey47 and Vietnam48 (Table 1, see Table 1, Supplemental Digital Content 2, http://links.lww.com/INF/E516).
Table 1.
Study and Type of Outcomes Assessed (Primary Outcome Unless Stated Secondary, 20)
| Study Type Author, Year |
Antibiotic Consumption | Antibiotic Type | Antibiotic Indication | Antibiotic Prescribing Rate | Cost | MDRO Isolation | Mortality (All Cause) |
Other Clinical Outcome |
|---|---|---|---|---|---|---|---|---|
| Implementation of AMS Bundle, n = 16 | ||||||||
| Zou et al, 201528 | ||||||||
| Wei et al, 201719 | ||||||||
| Ding et al, 200814 | ||||||||
| Wei et al, 201721 | ||||||||
| Wei et al, 201920 | ||||||||
| Zhang et al, 201825 | ||||||||
| Zhang et al, 201826 | ||||||||
| Lu et al, 201917 | 20 | 20 | 20 | |||||
| Kalaba et al, 201846 | 20 | |||||||
| Ruvinsky et al, 201430 | ||||||||
| Opondo et al, 201141 | ||||||||
| González Ochoa et al, 199638 | ||||||||
| Haque et al, 201744 | 20 | |||||||
| Sultana, 201732 | ||||||||
| Chowdhury et al, 201831 | ||||||||
| Murni et al, 201539 | 20 | 20 | ||||||
| Calil et al, 200137 | ||||||||
| Guideline or policy implementation, n = 7 | ||||||||
| Zhang et al, 200818 | 20 | |||||||
| Zhang et al, 200824 | ||||||||
| Liang et al, 201416 | ||||||||
| Jinka et al, 201733 | 20 | 20 | 20 | |||||
| Berild et al, 200845 | 20 | 20 | ||||||
| Lee et al, 200742 | 20 | |||||||
| Murki et al, 200934 | 20 | |||||||
| Clinical decision tool, n = 7 | ||||||||
| Shao et al, 201536 | ||||||||
| Keitel et al, 201735 | 20 | 20 | ||||||
| Do et al, 201648 | 20 | 20 | ||||||
| Bucher et al, 201243 | ||||||||
| Ozkaya et al, 200947 | ||||||||
| Torres et al, 201429 | 20 | 20 | ||||||
| Wu et al, 201722 | 20 | |||||||
| Antibiotic restriction and financial disincentive, n = 2 | ||||||||
| Gong et al, 201615 | ||||||||
| Xu et al, 201923 | ||||||||
| Audit and feedback on restricted antibiotics, n = 1 | ||||||||
| Rahbarimanesh et al, 201940 | ||||||||
| Total outcomes | 14 | 10 | 11 | 10 | 9 | 8 | 8 | 11 |
The majority of study designs were uncontrolled before-after series (17)14,22–24,26–28,30,31,34,37–39,42,44,46 or RCTs (7).21,29,35,38,41,43,48 Additionally, there were 3 uncontrolled interrupted time series,15,17,33 3 controlled before-after studies,16,19,45 2 non-RCTs,36,47 2 studies assessing long-term follow-up20 and cost-effectiveness of an RCT.25 The majority of studies were at medium (12) or high (16) risk of bias (Table 2).
Table 2.
Risk of Bias Assessment of AMS Intervention Studies by Type in Children in LMICs13
| Study Type | Risk of Bias Criteria* | Overall Risk† | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | A | B | C | D | E | F | G | H | I | J | K | L | M | N | |
| Interrupted time series | |||||||||||||||
| Gong et al, 201615 | U | L | L | L | H | L | L | NA | NA | NA | NA | NA | NA | L | Medium |
| Jinka et al, 201733 | U | L | L | L | L | U | L | NA | NA | NA | NA | NA | NA | L | Medium |
| Lu et al, 201917 | U | L | L | L | L | U | L | NA | NA | NA | NA | NA | NA | L | Medium |
| Rahbarimanesh et al, 201940 | H | L | L | L | L | H | H | NA | NA | NA | NA | NA | NA | H | High |
| Cluster RCT | |||||||||||||||
| Opondo et al, 201141 | NA | NA | NA | NA | NA | U | L | H | L | U | U | L | L | High | |
| González Ochoa et al, 199638 | NA | NA | NA | NA | NA | U | L | H | L | L | H | H | L | H | High |
| Wei et al, 201721 | NA | NA | NA | NA | NA | L | L | L | L | L | L | L | L | L | Low |
| Wei et al, 201920 | NA | NA | NA | NA | NA | L | U | L | L | L | L | L | L | L | Medium |
| Case-control/controlled and uncontrolledU before-after studies | |||||||||||||||
| Liang et al, 201416 | NA | NA | NA | NA | NA | H | L | H | H | U | U | L | L | L | High |
| Berild et al, 200845 | NA | NA | NA | NA | NA | U | U | H | H | L | U | L | H | L | High |
| Wei et al, 201719 | NA | NA | NA | NA | NA | U | L | H | H | H | H | L | L | L | High |
| Murki et alU, 200934 | NA | NA | NA | NA | NA | H | U | H | H | L | L | H | U | H | High |
| Chowdhury et alU, 201831 | NA | NA | NA | NA | NA | L | L | H | H | L | L | L | L | L | Medium |
| SultanaU, 201732 | NA | NA | NA | NA | NA | U | U | H | H | L | H | U | U | L | High |
| Wu et alU, 201722 | NA | NA | NA | NA | NA | L | L | H | H | L | L | L | L | L | Medium |
| Murni et alU, 201539 | NA | NA | NA | NA | NA | L | L | H | H | L | L | L | L | L | Medium |
| Ruvinsky et alU, 201430 | NA | NA | NA | NA | NA | U | L | H | H | U | H | L | L | H | High |
| Calil et alU, 200137 | NA | NA | NA | NA | NA | L | U | H | H | L | L | L | L | L | Medium |
| Lee et alU, 200742 | NA | NA | NA | NA | NA | L | L | H | H | L | L | L | L | L | Medium |
| Xu et alU, 201923 | NA | NA | NA | NA | NA | L | L | H | H | L | L | L | L | L | Medium |
| Zhang et alU, 200824 | NA | NA | NA | NA | NA | L | L | H | H | U | L | L | L | U | High |
| Zhang et alU, 200828 | NA | NA | NA | NA | NA | L | L | H | H | U | L | L | L | U | High |
| Zou et alU, 201528 | NA | NA | NA | NA | NA | U | L | H | H | L | L | L | L | L | Medium |
| Ding et alU, 200814 | NA | NA | NA | NA | NA | U | L | H | H | L | L | L | L | L | Medium |
| Kalaba et alU, 201846 | NA | NA | NA | NA | NA | H | L | H | H | U | U | L | L | H | High |
| Zhang et alU, 201826 | NA | NA | NA | NA | NA | U | H | H | H | L | L | L | L | L | High |
| RCT | |||||||||||||||
| Bucher et al, 201243 | NA | NA | NA | NA | NA | U | L | U | U | U | L | L | H | L | High |
| Shao et al, 201536 | NA | NA | NA | NA | NA | L | L | L | L | L | H | L | L | L | Medium |
| Keitel et al, 201735 | NA | NA | NA | NA | NA | L | L | L | L | L | L | L | L | L | Low |
| Do et al, 201648 | NA | NA | NA | NA | NA | L | L | L | L | L | L | L | L | L | Low |
| Torres et al, 201429 | NA | NA | NA | NA | NA | L | L | L | L | U | L | L | H | L | Medium |
| Non-RCT | |||||||||||||||
| Ozkaya et al, 200947 | NA | NA | NA | NA | NA | L | L | U | U | U | L | U | H | High | |
| Haque et al, 201744 | NA | NA | NA | NA | NA | L | L | L | L | L | L | L | L | L | Low |
*Criteria: A: intervention independent of other changes, B: shape of intervention prespecified, C: intervention unlikely to affect data collection, D: knowledge of allocated interventions adequately prevented during study, E: seasonality taken into account, F: incomplete outcome data adequately addressed, G: study free from selective outcome reporting, H: random sequence generation, I: allocation concealment, J: baseline outcome measures similar, K: baseline characteristics similar, L: any blinding reported, M: study protected against contamination, N: other risk of bias.
†The risk of bias was considered low if all criteria were scored as low, medium if 1 or 2 criteria were scored as unclear or high and high if >2 criteria were scored as unclear or hig.
Risk of bias criteria: H, high risk of bias; L, low risk of bias; NA, not applicable to this study design; U, unclear risk of bias.
Fourteen studies were of AMS bundles14,17,19–21,26,28,30–32,37–39,41,44,46 (multiple interventions applied at once, further described below), 2 of which also included infection prevention and control measures.37,39 The remaining interventions were: a clinical decision tool (7),22,29,35,36,43,47,48 implementation of a guideline (7),16,24,27,33,34,42,45 financial disincentives for antibiotic prescribing (2),15,23 audit and feedback (1)40 and cost-effectiveness (1).25
Settings in which ASP were implemented varied between inpatient wards (13 studies),22,24,26,28,30,32,39–42,45,46 outpatient clinics (11),16,19,21,23,27,29,31,35,36,38,48 intensive care (NICU 417,33,34,37 and PICU 314,30,44) combined inpatient and outpatient (2)15,23 and ED (2).43,47 The outcomes measured were markedly heterogeneous (see Table, Supplemental Digital Content 3, http://links.lww.com/INF/E516).
Antibiotic Prescribing Results According to Intervention
AMS Bundles
Sixteen studies assessed effectiveness of the implementation of an AMS bundle, 6 of which were from China and 14 of which had a medium/high risk of bias. AMS bundles as a minimum included policy or guideline implementation plus education. Some also included audit and feedback, an AMS committee, restrictions for overprescribing, antibiotic resistance reporting, prior authorization, point of prescription interventions (automatic stop after 48 hours), and parental education. Outcomes assessed were heterogeneous (Table 1).
Five studies targeted antibiotic prescribing for specific conditions: patients with intussusception after air enema reduction,28 respiratory tract infection20,21,31,38 and acute nonbloody diarrhea.41 When applied to specific conditions, all AMS bundles substantially reduced inappropriate antibiotic prescribing,20,21,28,38,41 with 4 of 5 statistically significant.41
In inpatient wards and PICU, AMS bundles significantly reduced overall antibiotic consumption (except for one study in PICU, although empiric use reduced14) and antibiotic resistance rates,14,26 and increased adherence to guidelines.20 An AMS bundle applied in a PICU in China resulted in significant reduction in use of the third generation cephalosporins from 53% to 17% (P < 0.01), increased use of beta-lactam/beta-lactamase-inhibitors (BL/BLIs) from 4% to 44% (P < 0.01), and no change in carbapenem use.14 In addition, there was a reduction in bacterial resistance in Pseudomonas aeruginosa and Enterobacteriaceae). When applied in NICUs, AMS bundles led to reduction in empiric and overall antibiotic use17,33,34 and resistance rates,17,34,37 without increase in late-onset sepsis or mortality.17
One outpatient study followed up 14 of 25 primary healthcare facilities 12 months after completing an RCT of an AMS bundle to reduce antibiotics in acute URTI.20 Compared with the preintervention rate of 84%, antibiotic prescribing 6 months postimplementing the intervention was 37% (P ≤ 0.01), and at 18 months 54% (P ≤ 0.01) (ie, some rebound), compared with control facilities where it remained stable at approximately 75%.
Two of the AMS bundle studies additionally included infection prevention and control interventions. One from Indonesia found a reduction in HAI from 23% to 9% (P < 0.01) in addition to significant reductions in inappropriate prescriptions and all-cause mortality.39 The other in an NICU in Brazil showed a reduction in multidrug-resistant (MDR) Enterobacter cloacae colonization and all MDRO infections.37
Antibiotic Guidelines or Policy
Seven studies assessed the effectiveness of guidelines, and 5 had a high risk of bias. Three from China implemented the national guidelines16,18,24 and the others implemented local guidelines/policy.33,34,42,45 Combined, the number of participants studied was large, including multiple different inpatient specialty units, and the follow-up period of assessment of intervention was 12–24 months.
Guideline/policy implementation alone significantly reduced antibiotic consumption in only 2 studies16,45 and in 1 of 3 hospitals in another study.24 While not all studies showed reduction in this specific measure, there were frequently reductions in other antibiotic measures, such as proportion of neonates in an Indian NICU treated with antibiotics (58%–46%, P < 0.001) and use of third generation cephalosporins (41%–7%, P < 0.01).33 There were other impacts as well, such as significant reduction in ESBL rates (47%–25%, P = 0.04), also found after guideline implementation in a Korean hospital which resulted in significant reduction in ESBL Klebsiella pneumoniae(64%–26%; P < 0.01).42
Clinical Decision Tools
Seven studies evaluated the impact of a clinical decision tool or algorithm, 4 of which had a low risk of bias. Two controlled trials from Tanzania used a clinical algorithm36 and an electronic POC test35 to assess acute febrile illness. The new ALMANACH algorithm was compared with standard practice and resulted in a significantly lower prescribing rate on day 0 (15% vs. 84%, P < 0.001) and a higher percentage of cure on day 7 (97% vs. 92%, P < 0.001).36 Although the reduction in prescribing rate in the ALMANACH arm looks striking, it is worth noting that the 2 arms recruited in 2 different primary care facilities where there were differences in baseline bacterial infection rate. Subsequently, an RCT compared ePOCT, an algorithm using clinical signs plus POC tests (eg, malaria, hemoglobin and oximetry), to ALMANACH. Clinical failure on day 7 in ePOCT was 2.3% versus 4.1% in ALMANACH (P = 0.005) with significantly reduced antibiotic prescribing of 12% versus 30% (P < 0.001).35 In a smaller RCT from Argentina, a clinical score was assessed for outpatients 3–60 months of age with nonsevere pneumonia, finding that antibiotic prescriptions were reduced from 87% to 47% with no increase in poor outcomes (hospitalization, PICU and death) at day 10.29
POC tests alone without clinical components have been subject to a few studies. Use of CRP,38 a fecal rotavirus plus fecal leucocyte POC test for acute diarrhea in <5-year-olds,43 and an influenza POC test for mild influenza-like illness in 3- to 14-year-olds47 reduced antibiotic prescriptions by 14% (P < 0.001), 21% (P = 0.03) and 32% (P = 0.01), respectively. In contrast, measuring procalcitonin at day 1 and repeated during admission, resulted in no difference in prescribing rate or antibiotic duration.22
Financial Disincentive
Two studies from China assessed the impact of financial disincentive, rewarding prescription of desired antibiotics and/or penalizing nondesired antibiotics. Outpatient prescriptions reduced by 59% (P ≤ 0.01), and in inpatients prescriptions reduced by 15% with preauthorization alone, and a further 28% after adding “financially punished” audit and feedback (P < 0.001).15 Another study implemented incentive/disincentive per prescription and found a sustained decrease in antibiotic usage year on year over a 7-year period and reduction in MDRO colonization rate.23 No clinical outcomes were assessed in either study.
Audit and Feedback
Audit and immediate feedback on meropenem and vancomycin prescriptions resulted in significant reduction in use of these antibiotics compared with the preintervention period: meropenem 10% to 1.5% (P < 0.05) and vancomycin 36% to 4% (P < 0.05). There was also a significant reduction in mortality from 28% to 6% (P = 0.001) with significantly fewer positive blood cultures during the intervention period (24% to 4%) (P = 0.01).40
Results According to Outcome
Thirteen studies evaluated clinical outcomes after implementing an AMS intervention17,22,29,33–36,39,40,42,44,45,48 (Table 1). In 7, this was a primary outcome, and of these only 4 were low to medium risk of bias.
Infections and Clinical Failure
In 2 studies that implemented an AMS bundle including infection control, there was a significant reduction in HAI from 23% to 9% (P < 0.001)39 and reduction of nosocomial infections due to MDRO from 18 to 2 cases per year (no P value).37 A further AMS bundle implemented in a large NICU reduced late-onset sepsis from 11% to 7% (P = 0.01).17 An audit and feedback strategy to manage selected antibiotics resulted in a reduction in bacteremia from 24% to 4% (P = 0.001).40 Introducing a clinical algorithm and measuring clinical outcomes at day 7, there was a reduction in clinical failure from 4.1% to 2.3% (P = 0.005) in one study,35 and an increase in proportion of cure at day 7 from 92% to 97% (P < 0.001) in another that also used a POCT.36 Use of POC CRP in primary care for children with acute respiratory infection reduced immediate antibiotic use without resulting in increased hospitalization from severe infection.48
MDRO Colonization or Infection
Eight studies evaluated reduction in MDRO colonization and/or infection from implementation of an AMS intervention.14,17,23,26,34,37,42,46 In 6, this was a primary outcome14,23,26,37,42 and all were medium to high risk of bias. Differences in reporting included whether bacteria were colonizing, causing invasive infection, both or unspecified; and whether MDROs were multiple specified bacteria, single bacterial types or unspecified, making summarizing MDRO outcomes difficult. Overall, in the 4 studies measuring MDRO colonization, all showed significant reduction ranging from 29% to 66%, including MRSA, VRE, ESBL-producing and carbapenem-resistant Enterobacteriaceae.17,23,26,37 In the 4 studies measuring MDRO invasive infection, 3 showed reductions ranging from 22% to 89%, including unspecified MDRO and ESBL-producing Gram-negative bacteria.34,37,42 In the fourth study, there was a significant reduction of 44% overall in MDRO, but no reduction in CRE.17
Most commonly, when studies assessed the impact on multiple specific MDRO, they found reductions in all of them, for example, implementation of an AMS bundle found reductions in all of carbapenem-resistant P. aeruginosa and ESBL-producing Escherichia coli and K. pneumoniae.14 The only divergence from this was in another study implementing an AMS bundle, in which there was a 96% reduction in MRSA colonization and/or infection but no change in ESBL-producing E. coli.46
Mortality
Eleven studies measured mortality as an outcome, the majority as a secondary outcome. Only two showed a reduction in mortality—implementation of an AMS bundle resulted in a reduction from 10% to 8% (P < 0.05), most likely related to a reduction in HAI,39 and audit and feedback reduced all-cause mortality from 28% to 6% (P = 0.001), likely related to reduction in bacteremia.40 In the majority, the salient finding was that better management of antibiotics did not increase mortality, through either AMS bundles,17,34,44 guidelines33,42,44,45 or decision tools.35,48 This was shown in different settings, including NICU,17,33,34 PICU,44 general inpatients,45 outpatients29,35 and primary care.48
Inappropriate Prescribing
Appropriateness of prescribing was measured in 4 studies, and all involved implementation of AMS bundles. Two studies showed a significant reduction in overall inappropriateness compared with preintervention (43% decreased to 21%)39 or the control group (53% vs. 77%).41 In contrast, one study showed no significant reduction in inpatient inappropriateness (51% to 42%),19 but because all 3 studies introduced AMS bundles, it is impossible to know which components may have contributed to this difference. Additionally, one study showed a specific increase in appropriateness of ceasing antibiotics within 48 hours in culture-negative sepsis from 6% to 45% (P < 0.0001).44
Length of Stay
Length of stay in hospital was a secondary outcome in 4 studies. Two very different studies—one implementing a broad AMS bundle in NICU17 and one specific use of procalcitonin in LRTI22—found no change in median length of stay, remaining at 7 and 10 days, respectively. In contrast, a study specifically addressing meropenem and vancomycin use in PICU with a much higher baseline length of stay of 23 days, showed a significant reduction to 16 days (P = 0.02).40
Cost-effectiveness
There was one cost-effectiveness study from China of an AMS bundle applied to 2- to 14-year-old outpatients with URTI (from a previous RCT21).25 Incremental cost-effectiveness ratios were measured using costs of consultation (time cost of doctor), prescription monitoring process, peer-review meetings (time cost of participants) and medication costs. They found an incremental cost of USD0.03 per percentage point reduction in antibiotic prescribing in addition to a USD390 upfront cost per healthcare facility and concluded that the AMS bundle was “close to cost neutral.”
Seven other studies reported on antibiotic expenditure pre and post AMS intervention. Four found a significant reduction in expenditure ranging from 10% to 75%,14–16,44 while one reported a reduction in cost per DDD without p values46: ceftriaxone had an 85% reduction and meropenem a 56% reduction in cost per DDD between 2010 and 2014. One study found a 16% reduction in average cost per patient on the gastroenterology ward with introduction of a locally developed guideline, but 38% increase on the respiratory ward (no P values provided).45 A final study found no difference in the cost of prescriptions from introducing an AMS bundle, despite reduction in antibiotic use.21
DISCUSSION
AMR poses an increasing threat to global health. This systematic review of AMS interventions in pediatric healthcare settings in LMIC shows that any type of AMS intervention can reduce antibiotic consumption, with more consistent reduction resulting from clinical decision tools and enabling strategies than guideline implementation alone. The variability in outcome measures limits the ability to summarize clinical, AMR and cost outcomes. However, most of these were improved significantly by AMS interventions, most likely due to the poor baseline position in many LMICs.
There are a number of differences between the AMS interventions in LMIC in this review and those in children in HIC.9,49 In HIC, the majority of AMS studies include audit and feedback39; however, only 7 of 32 in this review used this. This is likely because this strategy is very labor intensive without access to an electronic medical record, a luxury not available in most LMIC healthcare settings. Another review found that use of locally developed empiric guidelines was much more common in HIC.49 This is likely because of access to microbiologic culture and susceptibility testing, without which it is impossible to develop local antibiograms.50 While national/international guidelines can be effective in reducing antibiotic consumption, without accounting for local resistance patterns, they may represent a blunter tool against the development of AMR. In contrast, clinical decision tools have been subjected to more study in LMIC than HIC,49 and this review suggests they are highly effective in reducing antibiotic use. Care should therefore be taken not to presume that AMS interventions that are successful in HIC are automatically applicable to LMIC.
The importance of outcomes of AMS may also differ between LMIC and HIC. In LMIC, where infections are the leading cause of under-5 mortality, it is critical to know whether interventions that reduce antibiotic prescribing result in increased morbidity or mortality. Only a minority of studies addressed mortality or clinical failure and most were not powered for these outcomes and/or had a high risk of bias. In addition, only 6 studies included NICU or PICU, where there are high rates of broad-spectrum antibiotic prescribing16,51 and resistance in LMIC.1 For evidence to impact on sustained practice,52 mortality and behavioral change in these high prescribing settings both need to be addressed.
The impact on MDRO colonization and infection was investigated more frequently than has been reported for children in HIC, where only one study found no change with the AMS intervention.47 In the 8 studies identified in this review, 3 in ICU, MDRO rates were significantly reduced in all. While this likely reflects high baseline rates of MDRO in LMIC,1 this very fact means this outcome is important. In 2 studies, MDRO remained low several years after implementation of the intervention.23,37 Surveillance to ensure gains are sustained depends on access to ongoing antibiotic susceptibility testing.
That there was only one cost-effectiveness study identified25 highlights a major gap in studies of pediatric healthcare in LMIC where scarce resources frequently result in lack of oversight of broad-spectrum antibiotic prescribing. Simple, potentially inexpensive interventions may offer the greatest cost savings, as has been shown for the management of childhood pneumonia and neonatal infection.53 Without high-quality cost-effectiveness analyses to inform broader health economic benefits of reducing morbidity and mortality, necessary rationing of resources in LMIC settings may mean that AMS is avoided.
The WHO recommends that ASPs should be implemented in a stepwise manner, build on existing structures and reporting, maximize teamwork, and encourage champions and clinical staff to participate: “start small and keep it simple and doable.”6 The findings in this review provide evidence to support this approach: ASPs that included education of clinical staff as part of the AMS bundle were successful in reducing prescribing rate, and those that were adapted to existing structures, such as clinical decision algorithms, were successfully implemented and achieved positive results. The WHO approach could be coupled with the suggestion to identify conditions with frequent antibiotic use to prioritize targets of ASPs.54
A limitation of this review was the low quality of many of the included studies which limits the ability to determine which interventions are the most successful. Adult reviews excluded a large proportion of studies due to bias limitations of uncontrolled before-after studies. However, these may be the only feasible option for LMIC with high volumes of prescriptions, for example, in hospitals in China, or with limited access to funding. Therefore, these studies were included as important pragmatic examples in this review. Ideally, if an RCT is not possible, studies should use a controlled interrupted time series methodology to confidently determine which interventions are most successful.55 Beyond a simple measure of antibiotic consumption, many included studies did not evaluate appropriateness of antibiotic use including route and duration of antibiotics for specific infections.56
CONCLUSION
Controlling excessive antibiotic use is an important tool in the effort to combat rising AMR with its consequent impact on morbidity and mortality. It is important to put this in the context of pressures in LMIC of high childhood mortality from infection and frequent antibiotic shortages, by tailoring interventions and outcome measures appropriately.57 This means incorporating the review findings of the success of AMS bundles and clinical decision tools into existing systems.58 It also means that key outcomes of reduction of HAI and MDRO infection should be prioritized in studies above the easier measure of antibiotic consumption, as only addressing these comprehensively in a broad public health approach will reduce mortality from MDRO in a cost-effective and sustainable way.
FIGURE 1.
Studies included in the review of AMS in children in LMIC.
Supplementary Material
Footnotes
P.A.B. was in part supported by a Medical Research Futures Fund Australia Investigator Grant and a Melbourne Campus Clinician Scientist Fellowship, Melbourne, Australia. The Clinical Paediatrics research group, MCRI, was in part supported by the Victorian Government infrastructure support program.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com)
REFERENCES
- 1.Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387:168–175. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Global Action Plan on Antimicrobial Resistance. World Health Organization; 2015. [DOI] [PubMed] [Google Scholar]
- 3.Davey P, Marwick CA, Scott CL, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017;2:CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990–1001. [DOI] [PubMed] [Google Scholar]
- 5.Pulcini C, Binda F, Lamkang AS, et al. Developing core elements and checklist items for global hospital antimicrobial stewardship programmes: a consensus approach. Clin Microbiol Infect. 2019;25:20–25. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Antimicrobial Stewardship Programmes in Health-care Facilities in Low- and Middle-income Countries. A Practical Toolkit. World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO.; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha SK, Schrag SJ, El Arifeen S, et al. Causes and incidence of community-acquired serious infections among young children in south Asia (ANISA): an observational cohort study. Lancet. 2018;392:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox JA, Vlieghe E, Mendelson M, et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect. 2017;23:812–818. [DOI] [PubMed] [Google Scholar]
- 9.Araujo da Silva AR, Albernaz de Almeida Dias DC, Marques AF, et al. Role of antimicrobial stewardship programmes in children: a systematic review. J Hosp Infect. 2018;99:117–123. [DOI] [PubMed] [Google Scholar]
- 10.Van Dijck C, Vlieghe E, Cox JA. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: a systematic review. Bull World Health Organ. 2018;96:266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wellcome Institute. Low- and middle-income countries. 2020. Available at: https://wellcome.ac.uk/grant-funding/guidance/low-and-middle-income-countries. Accessed November 1, 2020.
- 12.Dellit TH, Owens RC, McGowan JE, Jr, et al. ; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. [DOI] [PubMed] [Google Scholar]
- 13.Cochrane Effective Practice and Organisation of Care Suggested risk of bias criteria for EPOC reviews. EPOC Reources for review authors. 2017. Available at: https://epoc.cochrane.org/resources/epoc-resources-review-authors. Accessed November 1, 2020.
- 14.Ding H, Yang Y, Wei J, et al. Influencing the use of antibiotics in a Chinese pediatric intensive care unit. Pharm World Sci. 2008;30:787–793. [DOI] [PubMed] [Google Scholar]
- 15.Gong S, Qiu X, Song Y, et al. Effect of financially punished audit and feedback in a pediatric setting in China, within an antimicrobial stewardship program, and as part of an International Accreditation Process. Front Public Health. 2016;4:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang X, Xia T, Zhang X, et al. Governance structure reform and antibiotics prescription in community health centres in Shenzhen, China. Fam Pract. 2014;31:311–318. [DOI] [PubMed] [Google Scholar]
- 17.Lu C, Liu Q, Yuan H, et al. Implementation of the smart use of antibiotics program to reduce unnecessary antibiotic use in a neonatal ICU: a prospective interrupted time-series study in a developing country. Crit Care Med. 2019;47:e1–e7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Shen X, Wang Y, et al. Outpatient antibiotic use and assessment of antibiotic guidelines in Chinese children’s hospitals. Eur J Clin Pharmacol. 2008;64:821–828. [DOI] [PubMed] [Google Scholar]
- 19.Wei X, Yin J, Walley JD, et al. Impact of China’s essential medicines scheme and zero-mark-up policy on antibiotic prescriptions in county hospitals: a mixed methods study. Trop Med Int Health. 2017;22:1166–1174. [DOI] [PubMed] [Google Scholar]
- 20.Wei X, Zhang Z, Hicks JP, et al. Long-term outcomes of an educational intervention to reduce antibiotic prescribing for childhood upper respiratory tract infections in rural China: follow-up of a cluster-randomised controlled trial. PLoS Med. 2019;16:e1002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei X, Zhang Z, Walley JD, et al. Effect of a training and educational intervention for physicians and caregivers on antibiotic prescribing for upper respiratory tract infections in children at primary care facilities in rural China: a cluster-randomised controlled trial. Lancet Glob Health. 2017;5:e1258–e1267. [DOI] [PubMed] [Google Scholar]
- 22.Wu G, Wu G, Wu S, et al. Comparison of Procalcitonin Guidance-Administered Antibiotics with Standard Guidelines on Antibiotic Therapy in Children with Lower Respiratory Tract Infections: a retrospective study in China. Med Princ Pract. 2017;26:316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu YL, Hu LM, Xie ZZ, et al. Impact of antimicrobial stewardship program on antimicrobial usage and detection rate of multidrug-resistant gram-negative bacteria. Zhonghua Er Ke Za Zhi. 2019;57:553–558. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Shen X, Wang Y, et al. Antibiotic use in five children’s hospitals during 2002-2006: the impact of antibiotic guidelines issued by the Chinese Ministry of Health. Pharmacoepidemiol Drug Saf. 2008;17:306–311. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Dawkins B, Hicks JP, et al. Cost-effectiveness analysis of a multi-dimensional intervention to reduce inappropriate antibiotic prescribing for children with upper respiratory tract infections in China. Trop Med Int Health. 2018;23:1092–1100. [DOI] [PubMed] [Google Scholar]
- 26.Zhang ZG, Chen F, Chen JZ. Introducing an antibiotic stewardship program in a pediatric center in China. World J Pediatr. 2018;14:274–279. [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Chen X, Wang Y, et al. Outpatient antibiotic use and assessment of antibiotic guidelines in Chinese children’s hospitals. Eur J Clin Pharmacol. 2008;64:821–828. [DOI] [PubMed] [Google Scholar]
- 28.Zou W, Zhang Y, Ye W, et al. Reducing antibiotic use for young children with intussusception following successful air enema reduction. PLoS ONE. 2015;10:e0142999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres FA, Pasarelli I, Cutri A, et al. Impact assessment of a decision rule for using antibiotics in pneumonia: a randomized trial. Pediatr Pulmonol. 2014;49:701–706. [DOI] [PubMed] [Google Scholar]
- 30.Ruvinsky S, Mónaco A, Pérez G, et al. Effectiveness of a program to improve antibiotic use in children hospitalized in a children’s tertiary care facility in Argentina. Arch Argent Pediatr. 2014;112:124–131. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury F, Sturm-Ramirez K, Mamun AA, et al. Effectiveness of an educational intervention to improve antibiotic dispensing practices for acute respiratory illness among drug sellers in pharmacies, a pilot study in Bangladesh. BMC Health Serv Res. 2018;18:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sultana SR, MS. Dynamic online antimicrobial guideline with stewardship program: impact on antimicrobial prescribing. Bangladesh J Pharmacol. 2017;12:364–370. [Google Scholar]
- 33.Jinka DR, Gandra S, Alvarez-Uria G, et al. Impact of antibiotic policy on antibiotic consumption in a neonatal Intensive Care Unit in India. Indian Pediatr. 2017;54:739–741. [DOI] [PubMed] [Google Scholar]
- 34.Murki S, Jonnala S, Mohammed F, et al. Restriction of cephalosporins and control of extended spectrum beta-lactamase producing gram negative bacteria in a neonatal intensive care unit. Indian Pediatr. 2010;47:785–788. [DOI] [PubMed] [Google Scholar]
- 35.Keitel K, Kagoro F, Samaka J, et al. A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): a randomized, controlled non-inferiority trial. PLoS Med. 2017;14:e1002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shao AF, Rambaud-Althaus C, Samaka J, et al. New Algorithm for Managing Childhood Illness Using Mobile Technology (ALMANACH): a Controlled Non-Inferiority Study on Clinical Outcome and Antibiotic Use in Tanzania. PLoS One. 2015;10:e0132316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calil R, Marba ST, von Nowakonski A, et al. Reduction in colonization and nosocomial infection by multiresistant bacteria in a neonatal unit after institution of educational measures and restriction in the use of cephalosporins. Am J Infect Control. 2001;29:133–138. [DOI] [PubMed] [Google Scholar]
- 38.González Ochoa E, Armas Pérez L, Bravo González JR, et al. Prescription of antibiotics for mild acute respiratory infections in children. Bull Pan Am Health Organ. 1996;30:106–117. [PubMed] [Google Scholar]
- 39.Murni IK, Duke T, Kinney S, et al. Reducing hospital-acquired infections and improving the rational use of antibiotics in a developing country: an effectiveness study. Arch Dis Child. 2015;100:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahbarimanesh A, Mojtahedi SY, Sadeghi P, et al. Antimicrobial stewardship program (ASP): an effective implementing technique for the therapy efficiency of meropenem and vancomycin antibiotics in Iranian pediatric patients. Ann Clin Microbiol Antimicrob. 2019;18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Opondo C, Ayieko P, Ntoburi S, et al. Effect of a multi-faceted quality improvement intervention on inappropriate antibiotic use in children with non-bloody diarrhoea admitted to district hospitals in Kenya. BMC Pediatr. 2011;11:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J, Pai H, Kim YK, et al. Control of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a children’s hospital by changing antimicrobial agent usage policy. J Antimicrob Chemother. 2007;60:629–637. [DOI] [PubMed] [Google Scholar]
- 43.Bucher A, Rivara G, Briceño D, et al. Use of a rapid rotavirus test in prescription of antibiotics in acute diarrhea in pediatrics: an observational, randomized, controlled study. Rev Gastroenterol Peru. 2012;32:11–15. [PubMed] [Google Scholar]
- 44.Haque A, Hussain K, Ibrahim R, et al. Impact of pharmacist-led antibiotic stewardship program in a PICU of low/middle-income country. BMJ Open Qual. 2018;7:e000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berild D, Abrahamsen TG, Andresen S, et al. A controlled intervention study to improve antibiotic use in a Russian paediatric hospital. Int J Antimicrob Agents. 2008;31:478–483. [DOI] [PubMed] [Google Scholar]
- 46.Kalaba M, Kosutic J, Godman B, et al. Experience with developing antibiotic stewardship programs in Serbia: potential model for other Balkan countries? J Comp Eff Res. 2018;7:247–258. [DOI] [PubMed] [Google Scholar]
- 47.Ozkaya E, Cambaz N, Coşkun Y, et al. The effect of rapid diagnostic testing for influenza on the reduction of antibiotic use in paediatric emergency department. Acta Paediatr. 2009;98:1589–1592. [DOI] [PubMed] [Google Scholar]
- 48.Do NT, Ta NT, Tran NT, et al. Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: a randomised controlled trial. Lancet Glob Health. 2016;4:e633–e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fay MJ, Bryant PA. Antimicrobial stewardship in children: where to from here? J Paediatr Child Health. 2020;56:1504–1507. [DOI] [PubMed] [Google Scholar]
- 50.McKnight J, Maina M, Zosi M, et al. Evaluating hospital performance in antibiotic stewardship to guide action at national and local levels in a lower-middle income setting. Glob Health Action. 2019;12(sup1):1761657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osowicki J, Gwee A, Noronha J, et al. Australia-wide point prevalence survey of antibiotic prescribing in neonatal units: how much and how good? Pediatr Inf Dis J 2015;34:e185–90. [DOI] [PubMed] [Google Scholar]
- 52.Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA. 2014;312:2569–2570. [DOI] [PubMed] [Google Scholar]
- 53.Stenberg K, Watts R, Bertram MY, et al. Cost-effectiveness of interventions to improve maternal, newborn and child health outcomes: a WHO-CHOICE Analysis for Eastern Sub-Saharan Africa and South-East Asia [published online ahead of print March 17, 2021]. Int J Health Policy Manag. doi: 10.34172/ijhpm.2021.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerber JS, Kronman MP, Ross RK, et al. Identifying targets for antimicrobial stewardship in children’s hospitals. Infect Control Hosp Epidemiol. 2013;34:1252–1258. [DOI] [PubMed] [Google Scholar]
- 55.de Kraker MEA, Abbas M, Huttner B, et al. Good epidemiological practice: a narrative review of appropriate scientific methods to evaluate the impact of antimicrobial stewardship interventions. Clin Microbiol Infect. 2017;23:819–825. [DOI] [PubMed] [Google Scholar]
- 56.McMullan BJ, Andresen D, Blyth CC, et al. ; ANZPID-ASAP Group. Antibiotic duration and timing of the switch from intravenous to oral route for bacterial infections in children: systematic review and guidelines. Lancet Infect Dis. 2016;16:e139–e152. [DOI] [PubMed] [Google Scholar]
- 57.INDEPTH. Abacus Project. 2017. Available at: http://www.indepth-network.org/projects/abacus. Accessed February 1, 2020.
- 58.Brink AJ, Messina AP, Feldman C, et al. Antimicrobial stewardship across 47 South African hospitals: an implementation study. Lancet Infect Dis. 2016;16:1017–1025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.