Abstract
β-Adrenergic receptors (βARs) regulate normal and pathophysiological heart function through their impact on contractility. βARs are also regulators of immune function where they play a unique role depending on the disease condition and immune cell type. Emerging evidence suggests an important role for the β2AR subtype in regulating remodeling in the pathological heart; however, the importance of these responses has never been examined. In heart failure, catecholamines are elevated, leading to chronic βAR activation and contributing to the detrimental effects in the heart. We hypothesized that immune cell β2AR plays a critical role in the development of heart failure in response to chronic catecholamine elevations through their regulation of immune cell infiltration. To test this, chimeric mice were generated by performing bone marrow transplant (BMT) experiments using wild-type (WT) or β2AR knockout (KO) donors. WT and β2ARKO BMT mice were chronically administered the βAR agonist isoproterenol. Immune cell recruitment to the heart was examined by histology and flow cytometry. Numerous changes in immune cell recruitment were observed with isoproterenol administration in WT BMT mice including proinflammatory myeloid populations and lymphocytes with macrophages made up the majority of immune cells in the heart and which were absent in β2ARKO BMT animal. β2ARKO BMT mice had decreased cardiomyocyte death, hypertrophy, and interstitial fibrosis following isoproterenol treatment, culminating in improved function. These findings demonstrate an important role for immune cell β2AR expression in the heart’s response to chronically elevated catecholamines.
NEW & NOTEWORTHY Immune cell β2-adrenergic receptors (β2ARs) are important for proinflammatory macrophage infiltration to the heart in a chronic isoproterenol administration model of heart failure. Mice lacking immune cell β2AR have decreased immune cell infiltration to their heart, primarily proinflammatory macrophage populations. This decrease culminated to decreased cardiac injury with lessened cardiomyocyte death, decreased interstitial fibrosis and hypertrophy, and improved function demonstrating that β2AR regulation of immune responses plays an important role in the heart’s response to persistent βAR stimulation.
Listen to this article’s corresponding podcast at https://ajpheart.podbean.com/e/immune-cell-%ce%b22-adrenergic-receptor-in-the-heart/.
Keywords: β2-adrenergic receptor, cardiac, heart failure, inflammation
INTRODUCTION
Catecholamines are chronically elevated in heart failure (HF) and result in sustained β-adrenergic receptor (βAR) signaling, which adversely effects cardiac function through its impact on remodeling (1). A major contributing factor to cardiac remodeling are immune responses, which are activated in response to pathological stimuli (2). Although studies have focused on the role of βAR in the regulation of cardiac remodeling through their role in cardiomyocyte death (3), hypertrophy (4), and cardiac fibroblasts function (5), βARs are strong regulators of immune responses (6, 7), which is only starting to be appreciated in the context of the heart (8–10).
βAR signaling in the heart plays a critical role in the regulation of cardiac function both in the normal and diseased state (1). Although all three βAR subtypes are thought to be expressed on cardiomyocytes, the β1AR is the most highly expressed and plays an important role in regulating contractility through phosphorylation of contractile proteins and other mechanisms (11–13). βARs also play an important role during pathological conditions where there is enhanced sympathetic activity resulting in persistent βAR activation (14). Prolonged β1AR stimulation results in receptor downregulation, which contributes to declining contractile function, alterations in cellular survival and hypertrophy, and the progression of HF (15). βAR blockade has been used for decades to treat cardiovascular diseases including hypertension, congestive HF, and postmyocardial infarction (16, 17). β-Blockers are thought to be beneficial through their direct actions on cardiomyocyte-expressed βAR because of their influence in decreasing cardiac output and limiting cardiomyocyte death (18); however, our previous studies have demonstrated an important role for β2AR in regulating immune responses following cardiac injury (8–10).
The sympathetic nervous system is an important regulator of immune responses (6, 7). βARs are expressed on virtually all cells of the immune system, whereas α1AR and α2AR are restricted to certain cellular populations and activation states (19). Although expression of all three βAR isoforms has been reported for various immune cell populations, the β2AR is the most highly and widely expressed isoform. β2AR is known to regulate a number of immune cell functions including hematopoiesis, homing, and maturation state in what is often a cell type-dependent and disease-specific manner (6). Although it is appreciated that β2AR can regulate immune responses, many of the studies have been confounding because of the use of cell lines and isolated cell that rapidly change phenotype in culture. Few studies have comprehensively examined the impact of β2AR in pathological states in vivo. Previous studies have shown β2AR regulates acute leukocyte recruitment to the heart following myocardial infarction through alterations in chemokine receptor expression (10) and cellular egress (9), demonstrating the importance of β2AR in regulating immune responses to the heart during injury. However, these studies failed to demonstrate the importance of β2AR regulation of immune responses to the pathological development of disease states. Furthermore, the role of β2AR in regulating B- and T-cell function has never been examined despite higher β2AR expression in these cell populations in comparison with myeloid populations.
Cell of the innate immune system, such as macrophages and neutrophils, plays an important role in clearing the injured tissue of dead cardiomyocytes and extracellular matrix debris and activating reparative cell populations such as lymphocytes and endothelial cells (20). Cells of the adaptive immune system, including T and B cells, become activated through antigen-dependent and -independent mechanisms and contribute to the resolution of inflammation and remodeling (21). However, dysregulation of these immune responses, either exacerbations or impairments, leads to aggravated cell death and maladaptive fibrotic responses culminating in worsened cardiac function (22). Although β2AR has been shown to regulate immune cell function in the context of ischemic injury (9, 10), a comprehensive examination of the role of β2AR in regulating immune cell infiltration to the heart and their importance to HF pathogenesis has not been examined. However, catecholamines are chronically elevated during HF and are thought to contribute to the disease pathogenesis (1, 23–25), and the infusion of endogenous catecholamines or βAR agonists are sufficient to produce many of the hallmarks of HF including cardiomyocyte death, hypertrophy, fibrosis, and dysfunction (26, 27). Since β2AR are known to be the strong regulators of immune function and inflammation plays a critical role in the progression of HF, we hypothesized that immune cell β2AR expression contributes to the detrimental actions of chronic βAR activation in the heart. To test this hypothesis, we used chimeric mice lacking β2AR on immune cells and chronically administered the βAR agonist isoproterenol. Hallmarks of HF were examined including hypertrophy, cardiomyocyte death, and fibrosis. The absence of β2AR on immune cell prevented isoproterenol-induced cardiac dysfunction. Flow cytometry to assess immune cell infiltration to the heart identified alterations in immune cell recruitment with immune cell β2AR knockout (KO). These studies demonstrate a critical role for β2AR regulation of immune responses in the progression of HF.
MATERIALS AND METHODS
Experimental Animals
Wild-type (WT) C57BL/6J mice (8–12 wk) and β2ARKO mice backcrossed (>5 generations) onto a C57BL/6J background were used in this study. Equal numbers of male and female mice were randomly assigned to a treatment group with the number of animals used per experimental condition listed in the figure legends. Mice were acclimated to housing at least 24 h before all experiments and housed in a temperature-controlled, humidity-controlled room with a diurnal cycle of 12-h:12-h light/dark. Mice were provided with food and water ad libitum. Mice were administered vehicle (sterile PBS with 0.001% ascorbic acid) or isoproterenol (30 mg/kg/day) for 1 wk via osmotic minipump (Alzet). Animal procedures were performed with approval by the Institutional Animal Care and Use Committee at the University of Missouri and in accordance to the National Institutes of Health’s Guidelines on the Use of Laboratory Animals.
Bone Marrow Transplant
Endogenous hematopoietic stem and progenitor cells were depleted from wild-type (WT) C57BL/6J mice (8–12 wk) by lethal irradiation delivered using a linear accelerator (950 rads). Donor bone marrow (BM) was isolated from WT C57BL/6J or β2ARKO mice and adoptively transferred (∼1 × 107 cells/mouse) by retro-orbital injection within 24 h of irradiation as previously described (9). BM was allowed to reconstitute for 1 mo before experimentation. Reconstitution was confirmed at the conclusion of the study for each mouse by reverse transcription quantitative PCR (RT-qPCR) analysis for ADRB2, the gene for β2AR, expression in BM.
Echocardiography
Two-dimensional transthoracic echocardiography was performed using a VisualSonics Vevo 2100 System with a 12-MHz probe on mice anesthetized with isoflurane (1.5%) as previously described (28). M-mode echocardiography was performed in the parasternal short-axis view at the level of the papillary muscle to assess several cardiac parameters including left ventricular (LV) volumes and internal diameters (ID) in systole (s) and diastole (d), wall thickness, LV fractional shortening, and ejection fraction.
Bone Marrow-Derived Macrophages
Bone marrow-derived macrophages (BMDMs) were generated as previously described (9). In brief, BM was isolated from WT and β2ARKO mice and cultured for 7 days at 37°C in DMEM supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin-amphotericin B, and 10% L929 conditioned media.
Proliferation Assays
Bromodeoxyuridine (BrdU) incorporation was used to measure macrophage proliferation in BMDMs. Following 7 days of growth in L929 conditioned media as outlined in Bone Marrow-Derived Macrophages, media was changed to DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin-amphotericin B. Control BMDMs were treated with saline, whereas a separate group was treated with 1,000 U/mL macrophage colony stimulating factor (mCSF; R&D Systems, Cat. No. 416-ML). BrdU staining was performed 24 h after mCSF treatment as previously described (29). In brief, BrdU labeling solution was added to the culture media and cells were incubated for 2 h at 37°C. Labeling solution was removed and cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and treated with 2 M hydrochloric acid. Following blocking with bovine serum albumin, samples were incubated with an anti-BrdU antibody (10 µg/mL, R&D Systems, Cat. No. 87225) followed by an Alexa-488 secondary antibody. Nuclei were counterstained with DAPI. Coverslips were visualized in a blinded manner at ×20 magnification using a Nikon Eclipse microscope, and the percentage of BrdU-positive nuclei were counted by a blinded observer from 10 random fields/coverslips.
Histology and Immunohistochemistry
Excised hearts were fixed in 4% paraformaldehyde and paraffin embedded. Five micrometer sections were deparaffinized and stained for Masson’s trichrome (Thermo Fisher Scientific) according to the manufacturer’s protocol to detect fibrosis. Fluorescein-conjugated wheat germ agglutinin (WGA; 20 µg/mL; Sigma-Aldrich) was used to quantify cell size as previously described (30). Fluorescein-conjugated isolectin B4 (20 µg/mL; Vector Biolabs) was used to quantify capillary density. Hearts were visualized at ×20 magnification using a Nikon Eclipse microscope, and the area was quantified in ImageJ from 10 fields/heart.
Cell death was measured using an In Situ Cell Death Detection Kit, TMR Red (Roche Diagnostics) via TdT-mediated dUTP-X nick end-labeling (TUNEL) method as previously described (28). Deparaffinized sections were incubated with proteinase K, and DNA strands breaks were labeled according to the manufacturer’s instructions and as previously described (28). Sections were counterstained with troponin I (1:100; Cell Signaling, Cat. No. 4002) to identify cardiomyocytes. Positive staining was identified based on negative controls including no antibody staining/no terminal transferase, troponin I staining/no terminal transferase, and no antibody staining with TUNEL staining (Supplemental Fig. S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.15167790). DNase I (3 U/mL for 10 min) was used to induce DNA strand breaks for a positive control (Supplemental Fig. S1). Hearts were visualized at ×20 magnification using a Nikon Eclipse microscope, and the number of TUNEL-positive cardiomyocytes were calculated in relation to the number of DAPI-stained cardiomyocyte nuclei from 10 fields/heart.
Immunohistochemistry was performed on deparaffinized sections. Following citrate buffer antigen retrieval, hearts were blocked with 10% FBS in PBS and 0.3% H2O2 to prevent endogenous peroxide activity. Hearts were incubated with antibodies against CD45 (1:100; R&D Systems Cat. No. AF114). Washed slides were incubated with horseradish peroxidase (HRP)-conjugated anti-goat secondary antibody (1:1,000; Novex Cat. No. A15999). Stained hearts were developed using a DAB Substrate Kit (Thermo Fisher Scientific) and counterstained with hematoxylin to identify nuclei. Staining was visualized on a Nikon Eclipse microscope at ×20 magnification and analyzed using ImageJ from 10 fields/heart.
Fluorescence-Activated Cell Sorting
Immune cells were isolated from the thymus and spleen by manual digestion as previously described (8). Cells were isolated from the femurs of mice as previously described (9, 10). Circulating leukocytes were isolated from blood collected from mice using a heparinized syringe and centrifuged for 10 min at 800 g. The buffy coat layer was collected and washed two times with PBS before hypotonic red blood cell lysis using ammonium chloride-potassium (ACK) lysis buffer. Cells were washed two times with PBS followed by passage through a 70-µm cell strainer before flow cytometry analysis.
Immune cells were isolated from the heart by enzymatic digestion as previously described (8). In brief, hearts were isolated, flushed with HBSS to remove the blood, and manually digested into ∼1-mm3 pieces. Heart pieces were enzymatically digested in collagenase II (150 U/mL; Worthington Biochemical) and trypsin (0.6 mg/mL; Worthington Biochemical) at 37°C with agitation. Following digestion, myocyte and nonmyocyte fractions were separated by centrifugation at 8 g for 5 min. The nonmyocyte containing supernatant was passed through a 70-µm cell strainer before flow cytometry analysis.
Cells were stained in 1% FBS in PBS for 30 min at 4°C with the following antibodies: LIVE/DEAD Fixalbe Aqua Dead Cell Stain Kit (1:40, Invitrogen, Cat. No. L34957), CCR2-APC (1:100; BioLegend, Cat. No.160103), CD3-PE-Cy7 (1:100; BioLegend, Cat. No. 100220), CD4-PE (1:100; BD Biosciences, Cat. No. 553049), CD8-FITC (1:100; BD Biosciences, Cat. No. 553030), CD11b-FITC (1:200; BioLegend, Cat. No. 101206), CD19-BV650 (1:100; BD Biosciences, Cat. No. 563235), CD45-BV480 (1:100; BD Biosciences, Cat. No. 746429), CD45-FITC (1:100; BD Biosciences, Cat. No. 553080), CX3CR1-Alexa 647 (1:100; BioLegend, Cat. No. 149003), Ly-6C-APC-Cy7 (1:100; BioLegend, Cat. No. 128026), and Ly-6G-BV421 (1:200; BD Biosciences, Cat. No. 562737) as previously described (8). Positive staining was identified based on single antibody controls, which were performed for all antibodies on all tissues examined. Fluorescence minus one controls were included performed on splenic samples to validate cell staining. Isotype control were also performed on splenic samples using APC rat IgG2b (κ isotype, 1:100, BioLegend, Cat. No. 400611), FITC rat IgG2b (κ isotype, 1:100, BioLegend, Cat. No. 400633), APC-H7 rat IgG2b (κ isotype, 1:100, BD Biosciences, Cat. No. 560200), BUV480 rat IgG2b (κ isotype, 1:100, BD Biosciences, Cat. No. 565652), BV421 rat IgG2a (κ isotype, 1:200, BD Biosciences, Cat. No. 562602), BV650 rat IgG2a (κ isotype, 1:100, BD Biosciences, Cat. No. 563236), PE-Cy7 rat IgG2b (κ isotype, 1:100, BioLegend, Cat. No. 400617), Alexa Fluor 647 rat IgG2a (κ isotype, 1:100, BioLegend, Cat. No. 400234), and PE rat IgG2a (κ isotype, 1:100, BioLegend, Cat. No. 400507). Following staining, cells were washed twice with PBS and analyzed by flow cytometry using a BD LSRFortessa X-20. Analysis was performed using FlowJo software. Viable cells expressing CD45 were considered immune cells and further dividing using the following gating strategy: proinflammatory monocytes (CD45+Ly-6Chigh), neutrophils (CD45+Ly-6Ghigh), macrophages (CD45+CD11b+), T cells (CD45+CD3+), or B cells (CD45+CD19+) (Supplemental Fig. S2). Macrophages were further characterized as being proinflammatory like (CD45+CD11b+CCR2high) or reparative (CD45+CD11b+CX3CR1high). T cells were further divided into cytotoxic T cells (CD45+CD3+CD8+) or helper T cells (CD45+CD3+CD4+).
Cardiac Macrophage Isolation
Hearts were manually and enzymatically digested as described in Fluorescence-Activated Cell Sorting to separate the cardiomyocyte and nonmyocyte fractions. Macrophages were separated from the nonmyocyte portion by positive selection using anti-CD11b magnetic particles (BD Biosciences, Cat. No. 558013) according to the manufacturer’s protocol.
Reverse Transcription Quantitative PCR
cDNA was synthesized from BM or BMDM samples using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). RT-qPCR was performed with PowerUP SYBR Master Mix (Applied Biosystems) in triplicate for each sample using the primer sequences listed in Supplemental Table 1. Data were analyzed using the Applied Biosystems Comparative CT Method (ΔΔCT). Gene expression was normalized to translationally controlled tumor protein 1 (TPT-1) and expressed as 2−ΔΔCT with min/max indicated for range.
Statistical Analysis
Presented data are expressed as means ± SE. Statistical analyses were performed using a one-way ANOVA with Tukey’s multiple comparison test or a two-way repeated-measures ANOVA with Bonferroni posttest as appropriate using Prism 7 software (GraphPad Software; San Diego, CA) with a P value < 0.05 being considered significant. The n values are indicated in the figure legends. No statistical differences were observed between male and female animals with the exception of vessel density; thus, all data are a combination of equal numbers of both sexes within each treatment group, unless otherwise indicated.
RESULTS
Immune Responses in WT and β2-Adrenergic Receptor Knockout Bone Marrow-Transplanted Mice with Chronic Isoproterenol Administration
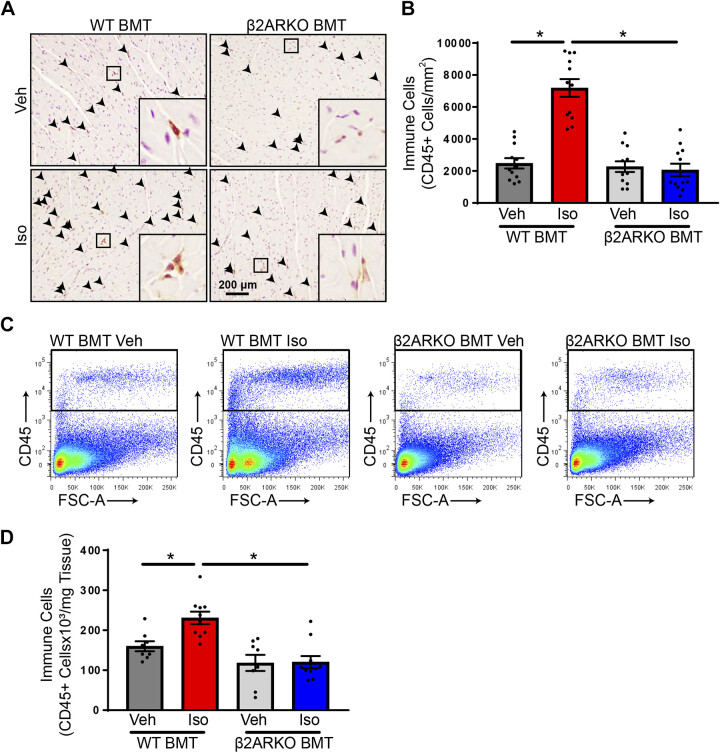
To determine whether chronic isoproterenol induces immune responses and the importance of immune cell β2AR in detrimental cardiac effects of prolonged βAR activation, WT mice were lethally irradiated and received WT or β2ARKO bone marrow transplant (BMT) to generate immune cell-specific mice. Following BM reconstitution, mice were infused with vehicle or isoproterenol for 1 wk. BM reconstitution was confirmed by ADRB2 gene expression in the BM and demonstrated a lack of β2AR in the BM of β2ARKO BMT mice with a minor downregulation of ADRB2 with isoproterenol administration in WT BMT animals (Supplemental Fig. S3). To determine the impact of immune cell infiltration into the heart following chronic isoproterenol infusion, WT and β2ARKO BMT mice were administered vehicle or isoproterenol for 1 wk. Hearts were stained for the CD45, a marker of cells of the hematopoietic lineage. Isoproterenol promoted a substantial recruitment of CD45+ cells to the heart in WT BMT animals when investigated by histology (Fig. 1, A and B) or flow cytometry (Fig. 1, C and D). No differences were observed between WT and β2ARKO BMT sham animals; however, immune cell infiltration following isoproterenol administration was severely blunted in β2ARKO BMT animals, which was confirmed by flow cytometry (Fig. 1, A–D).
Figure 1.
Immune cell β2AR impacts recruitment with isoproterenol infusion. A: representative CD45 staining (brown) from WT and β2ARKO BMT hearts from mice treated with vehicle or isoproterenol (Iso). Sections were counterstained with hematoxylin (purple) to identify nuclei. B: the number of CD45-positive cells were quantified and normalized to LV area. n = 12 animals, two-way ANOVA, *P < 0.05. Representative flow cytometry scatterplots (C) and quantification (D) of CD45 flow cytometry from hearts of WT or β2ARKO BMT mice treated with vehicle or isoproterenol. n = 8 for vehicle treatments, n = 10 for isoproterenol treatments, two-way ANOVA, *P < 0.05. β2AR, β2-adrenergic receptor; β2ARKO BMT, β2-adrenergic receptor knockout bone marrow transplant; LV, left ventricular; Veh, vehicle; WT, wild-type; FSC-A, forward scatter area.
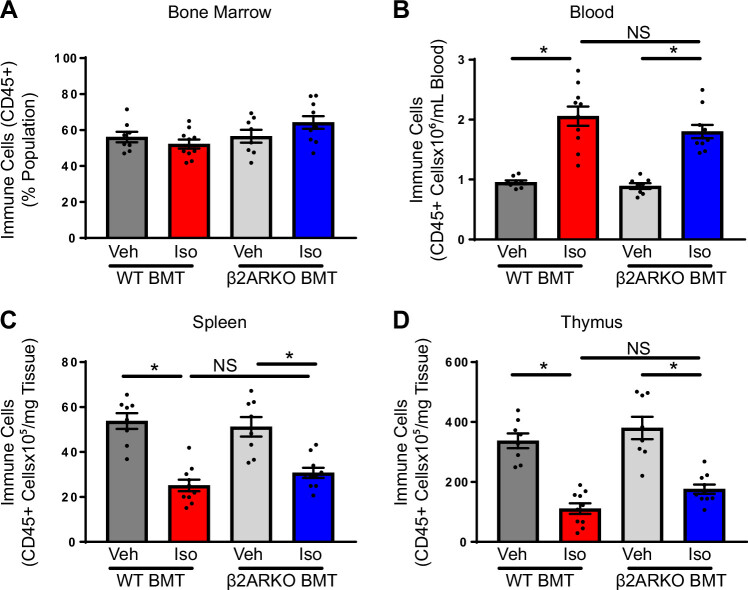
To determine the systemic immune response, myeloid and lymphoid cell populations were examined in BM, the site of stem and progenitor cells. There were no alterations in the number of CD45+ cells between all groups, indicating no difference in the number of hematopoietic stem cells (Supplemental Fig. S2A). Subsets of immune populations were examined and unchanged between WT and β2ARKO BMT groups (Supplemental Fig. S4, A–E).
Immune populations generated in the BM are released into the blood where they travel to other sites for storage, maturation, and to exert their biological function (31). Thus, immune cell populations were examined in the blood of WT and β2ARKO BMT mice treated with vehicle or isoproterenol. No differences were observed between vehicle-treated WT and β2ARKO BMT animals (Fig. 2B) in accordance with previously published findings (9). Circulating levels of immune (CD45+) cells were elevated with isoproterenol treatment in both WT and β2ARKO BMT groups (Fig. 2B). Although there were increases in a number of circulating immune cells with isoproterenol including Ly-6Chi monocytes, neutrophils, T cells, and B cells, no changes in immune cell subpopulations were observed between WT and β2ARKO BMT mice in the presence or absence of isoproterenol (Supplemental Fig. S5, A–D).
Figure 2.
The involvement of immune cell β2AR in regulating systemic immune responses. Flow cytometry analysis for BM (A), blood (B), spleen (C), and thymus (D) from WT and β2ARKO BMT hearts from mice treated with vehicle or isoproterenol. n = 8 for vehicle treatments, n = 10 for isoproterenol treatments, two-way ANOVA, *P < 0.05. β2AR, β2-adrenergic receptor; β2ARKO BMT, β2-adrenergic receptor knockout bone marrow transplant; BM, bone marrow; Iso, isoproterenol; NS, not significant; Veh, vehicle; WT, wild-type.
The spleen is an important site for the storage of leukocytes, extramedullary hematopoiesis, and the activation of adaptive immune responses. To examine the changes in the cellular composition of the spleen in WT and β2ARKO BMT mice with vehicle and isoproterenol treatment, flow cytometry was performed to examine specific immune cell makers. Similar to what has previously been observed, β2ARKO BMT vehicle-treated animals trended toward splenomegaly as indicated by the spleen weight (SW) to tibia length (TL) ratio compared with their WT BMT counterparts, which was decreased with isoproterenol (Supplemental Fig. S6A). The number of immune cells was unchanged between vehicle-treated WT and β2ARKO BMT mice, which was significantly decreased with isoproterenol in both groups (Fig. 2C). However, the decrease in the number of immune cells was to a lesser level in β2ARKO BMT mice compared with WT BMT controls (Fig. 2C). No significant alterations were observed between any group on examination of immune cell subpopulations (Supplemental Fig. S6, B–F).
The thymus is instrumental in T-cell maturation. Thymus size did not differ between vehicle-treated WT and β2ARKO BMT animals and both had a similar decrease in size with chronic isoproterenol administration (Supplemental Fig. S7A). In correspondence, no differences in immune cell numbers were observed in the thymus of vehicle-treated WT and β2ARKO BMT mice and there was a decrease in thymic CD45-positive cell populations in both groups with isoproterenol (Fig. 2D). In accordance to what is known, the majority of the cells in the thymus were T cells with very small populations of cells of the monocytic lineage, neutrophils, and B cells. Reductions in T-cell populations were observed in both WT and β2ARKO BMT mice with isoproterenol treatment with no differences between genotypes (Supplemental Fig. S7, B–E).
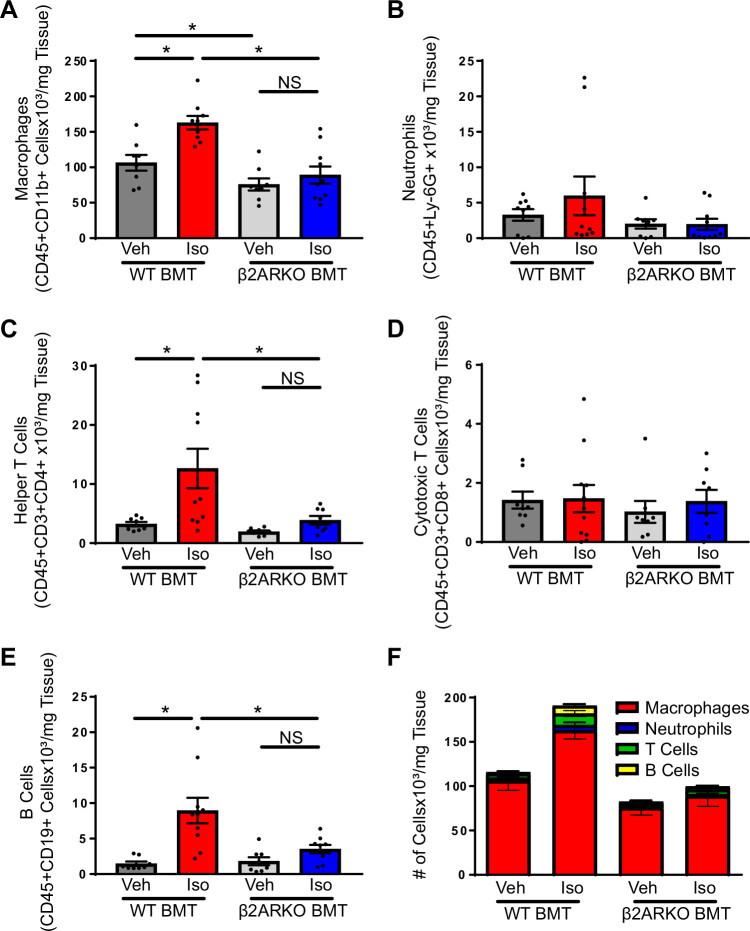
To characterize the immune cell types recruited to the heart following chronic isoproterenol administration, flow cytometry was performed on immune cells isolated from WT and β2ARKO BMT immune hearts following vehicle or isoproterenol examining recruitment of macrophages (Fig. 3A; CD45+CD11b+), neutrophils (Fig. 3B; CD45+Ly-6Ghigh), helper T cells (Fig. 3C; CD45+CD3+CD4+), cytotoxic T cells (Fig. 3D; CD45+CD3+CD8+), and B cells (Fig. 3E; CD45+CD19+). Several immune cell types were recruited to the WT BMT isoproterenol-treated mouse hearts including macrophages (Fig. 3A), helper T cells (Fig. 3C), and B cells (Fig. 3E), which were all diminished in β2ARKO BMT animals (Fig. 3, A, C, and E). Other cell populations including cytotoxic T cells (Fig. 3D) and neutrophils (Fig. 3B) were unaltered between groups. The largest population of cells in the heart in both vehicle and isoproterenol-treated animals were macrophages with other myeloid cell and lymphocyte cell populations making up a much small portion of the immune cells in the heart (Fig. 3F).
Figure 3.
The identity of immune cell subpopulations in the heart. Immune cells isolated from WT and β2ARKO BMT hearts underwent flow cytometry analysis to identify macrophages (A; CD45+CD11b+), neutrophils (B; CD45+Ly-6Ghigh), helper T cells (C; CD45+CD3+CD4+), cytotoxic T cells (D; CD45+CD3+CD8+), and B cells (E; CD45+CD19+). n = 8 for vehicle treatments, n = 10 for isoproterenol treatments, two-way ANOVA, *P < 0.05. F: the relative number of immune cell subpopulations from WT and β2ARKO BMT with vehicle or isoproterenol treatment. β2ARKO BMT, β2-adrenergic receptor knockout bone marrow transplant; Iso, isoproterenol; NS, not significant; Veh, vehicle; WT, wild-type.
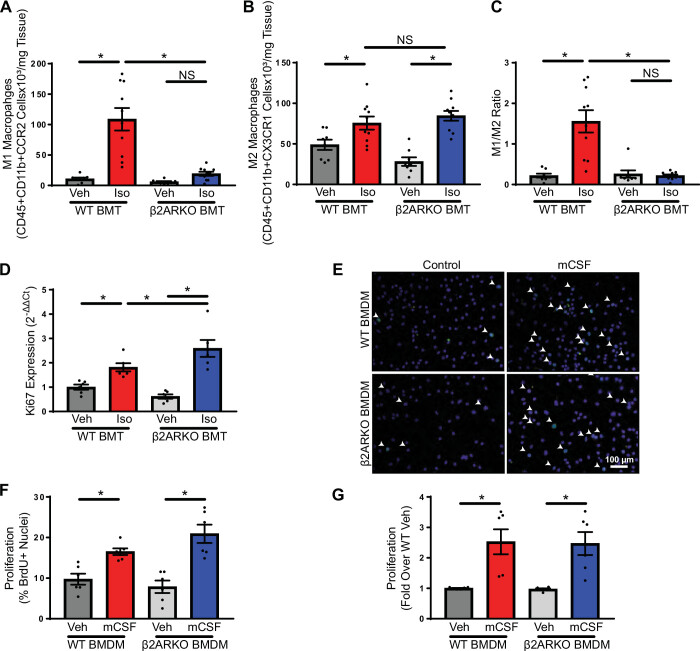
Because of the abundance of macrophages in both the vehicle and isoproterenol-treated heart, the macrophage phenotype was investigated. There was an increased number of proinflammatory macrophages in WT BMT with isoproterenol treatment compared with vehicle, which did not occur in β2ARKO BMT mice (Fig. 4A). Interestingly, similar increases in reparative macrophages occurred with isoproterenol treatment in both WT and β2ARKO BMT mice, but proinflammatory macrophages were substantially increased with isoproterenol only in WT BMT animals. This was also true of proinflammatory monocytes, which were increased with isoproterenol treatment in WT BMT mice, but not in β2ARKO BMT animals (Fig. 4B). The M1/M2 ratio, which reflects an imbalance in macrophage polarization and is often used as an indicator of inflammatory state, was increased with isoproterenol treatment in WT BMT mice, which was abolished in β2ARKO BMT animals (Fig. 4C).
Figure 4.
Macrophage phenotype with immune cell β2ARKO. Flow cytometry of macrophage populations from hearts of WT and β2ARKO BMT mice administered vehicle or isoproterenol. M1-like macrophages were identified as being CD45+CD11b+CCR2high (A), whereas M2-like macrophages were characterized by CD45+CD11b+CX3CR1+ (B), and the ratio of M1 to M2 cells was calculated (C). n = 8 for vehicle treatments, n = 10 for isoproterenol treatments, two-way ANOVA, *P < 0.05. D: Ki67 transcript expression was measured in cardiac macrophages isolated from WT and β2ARKO BMT mice administered vehicle or isoproterenol using RT-qPCR. Levels were normalized to the housekeeping gene, TPT-1, and expressed relative to macrophages from vehicle-treated WT BMT mice. n = 6, two-way ANOVA, *P < 0.05. E: representative BrdU staining (green) from WT or β2ARKO BMDM grown in complete media or media supplemented with mCSF. DAPI (blue) was used to identify nuclei. The number of BrdU-positive nuclei were quantified (F). n = 6, two-way ANOVA, *P < 0.05. G: a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay was used to measure cell numbers from WT or β2ARKO BMDM grown in complete media or media supplemented with mCSF. Values are expressed relative to control cells from the same mouse. n = 6, two-way ANOVA, *P < 0.05. β2ARKO, β2-adrenergic receptor knockout; BMDM, bone marrow-derived macrophage; BMT, bone marrow transplant; BrdU, bromodeoxyuridine; Iso, isoproterenol; mCSF, macrophage colony stimulating factor; NS, not significant; RT-qPCR, reverse transcription quantitative PCR; TPT-1, tumor protein 1; Veh, vehicle; WT, wild-type.
To determine whether the local macrophage proliferation was responsible for the changes in macrophage numbers and phenotype observed between WT and β2ARKO BMT mice, the expression of the proliferation marker, Ki67, was quantified in cardiac macrophages isolated from WT and β2ARKO BMT mice treated with vehicle or isoproterenol. Isoproterenol infusion increased Ki67 levels in both WT and β2ARKO BMT macrophages (Fig. 4D); however, β2ARKO BMT macrophages have enhanced expression compared with WT BMT. To confirm that lack of β2AR does not diminish macrophage proliferation, BMDM were generated from WT and β2ARKO mice and grown in the presence or absence of mCSF. mCSF increased macrophage proliferation in both WT and β2ARKO BMDM as shown by BrdU incorporation (Fig. 4, E and F, and Supplemental Fig. S8) and cell number, which was measured using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Fig. 4G), indicating that β2ARKO macrophages have normal proliferative capacity.
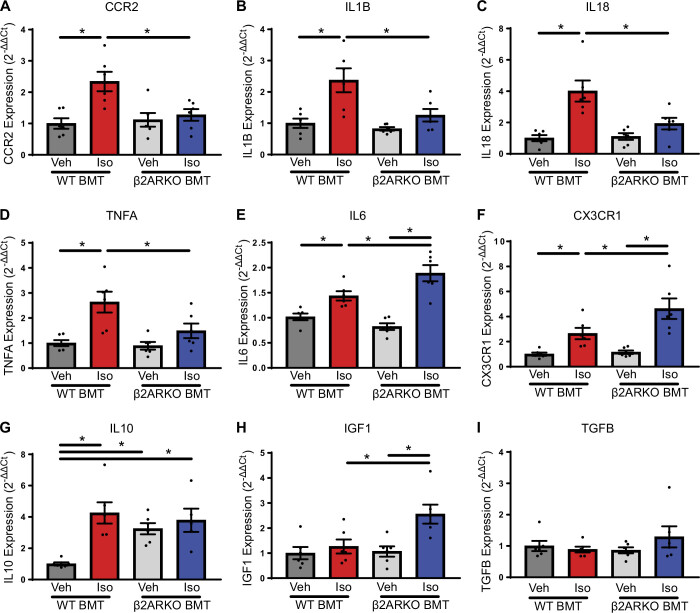
To further characterize the phenotype of cardiac macrophages, WT or β2ARKO BMT mice were administered vehicle or isoproterenol; macrophages were isolated and analyzed for transcript expression of a variety of inflammatory mediators known to represent various macrophage populations. In confirmation with flow cytometry experiments, C-C chemokine receptor type 2 (CCR2) expression, a infiltrating, proinflammatory macrophage marker, was elevated in WT BMT following isoproterenol treatment (Fig. 5A). This response was blunted in β2ARKO BMT animals (Fig. 5A). In accordance with these findings, a number of proinflammatory cytokines were increased in isoproterenol-treated WT BMT cardiac macrophages including IL1B, IL18, TNFA, and IL6 transcript expression (Fig. 5, B–E). With the exception of IL6, cardiac macrophages from isoproterenol-treated β2ARKO BMT mice were lower than WT BMT levels and not different than vehicle-treated β2ARKO BMT levels (Fig. 5, B–E). Interestingly, cardiac macrophages from β2ARKO BMT mice had augment IL6 expression compared with isoproterenol administered WT BMT animals (Fig. 5E).
Figure 5.
Cardiac macrophage inflammatory mediator profile with immune cell β2ARKO. CCR2 (A), IL1B (B), IL18 (C), TNFA (D), IL6 (E), CX3CR1 (F), IL10 (G), IGF1 (H), and TGFB (I) transcript expression from macrophages isolated from vehicle and isoproterenol-treated WT or β2ARKO BMT mice was quantified by RT-qPCR. Values were normalized to the housekeeping gene TPT-1 and expressed relative to macrophages from vehicle-treated WT BMT mice. n = 6, two-way ANOVA, *P < 0.05. β2ARKO, β2-adrenergic receptor knockout; BMT, bone marrow transplant; IGF1, insulin-like growth factor 1; Iso, isoproterenol; RT-qPCR, reverse transcription quantitative PCR; TPT-1, tumor protein 1; Veh, vehicle; WT, wild-type.
In accordance with the flow cytometry findings, C-X3-C motif chemokine receptor 1 (CXCR1) expression was elevated following isoproterenol infusion in cardiac macrophages from both WT and β2ARKO BMT mice, with a greater increase being observed in β2ARKO BMT mice (Fig. 5F). The anti-inflammatory cytokine IL10 was elevated in macrophages from vehicle-treated β2ARKO BMT mice compared with WT BMT mice (Fig. 5G). Isoproterenol infusion increased the IL10 levels in macrophages from WT BMT mice, but produced no further elevations in β2ARKO BMT animals (Fig. 5G). Insulin-like growth factor 1 (IGF1), a mediator produced by reparative macrophages, was unaltered in WT BMT macrophages; however, isoproterenol administration increased IGF1 expression in macrophages from β2ARKO BMT mice (Fig. 5H). TGFB, a growth factor that has been implicated in the transition from phagocytic to reparative macrophages in the heart, was unaltered in any condition (Fig. 5I).
Immune Cell β2-Adrenergic Receptors Contribute to Maladaptive Cardiac Remodeling with Chronic Isoproterenol Administration
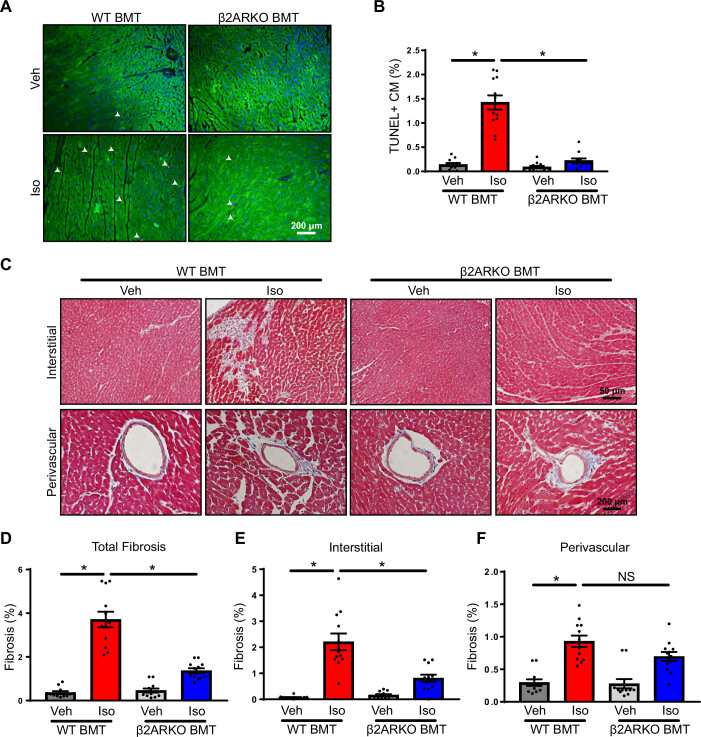
To determine the impact of immune cell β2AR on pathological remodeling, cardiomyocyte apoptosis was measured by TUNEL staining and demonstrated elevations in cardiomyocyte death following isoproterenol infusion in WT BMT animals (Fig. 6, A and B). In addition, Masson’s trichrome staining was used to evaluate fibrosis and showed that isoproterenol significantly increased fibrosis in WT BMT mice (Fig. 6, C–F). However, cardiomyocyte death was diminished in β2ARKO BMT mice administered isoproterenol (Fig. 6, A and B). Elevations of total fibrosis with isoproterenol infusion were largely prevented in β2ARKO mice (Fig. 6, C and D). This occurred primarily through decreases in interstitial fibrosis (Fig. 6, C and E), whereas perivascular fibrosis was equally increased in both WT and β2ARKO BMT mice (Fig. 6, C and F).
Figure 6.
Immune cell β2AR influences remodeling in heart failure. A: representative TUNEL staining (red) from hearts of WT and β2ARKO BMT mice infused with vehicle or isoproterenol. Sections were counterstained with troponin I (green) to identify cardiomyocytes and DAPI (blue) to label nuclei. The number of TUNEL-positive cardiomyocytes (CMs) were quantified and expressed as a percentage of total cardiomyocyte nuclei (B). n = 12, two-way ANOVA, *P < 0.05. C: representative Masson trichrome staining (fibrosis = blue, cytoplasm = red, nuclei = black) of vehicle or isoproterenol-treated hearts from WT and β2ARKO BMT mice. Total (D), interstitial (E), and perivascular (F) fibrosis was quantified from Masson trichrome-stained hearts and expressed as a percentage of the total LV area. n = 12, two-way ANOVA, *P < 0.05. β2AR, β2-adrenergic receptor; β2ARKO BMT, β2-adrenergic receptor knockout bone marrow transplant; Iso, isoproterenol; LV, left ventricular; NS, not significant; Veh, vehicle; WT, wild-type.
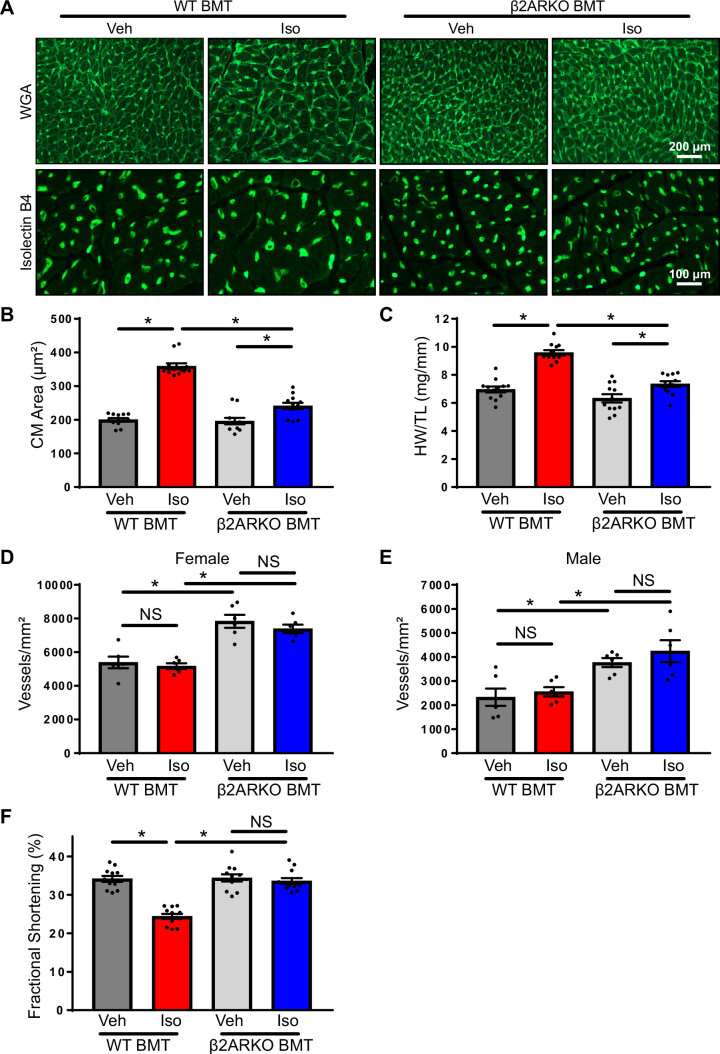
Cardiomyocyte hypertrophy was apparent after isoproterenol infusion at the cellular level in WT BMT and greatly reduced in β2ARKO BMT mice (Fig. 7, A and B). Left-ventricular hypertrophy at the organ level was also apparent after isoproterenol infusion in WT BMT mice as indicated by increases in the heart weight to tibia length (HW/TL) ratio (Fig. 7C) and wall thicknesses as measured by echocardiography (Table 1). These alterations were prevented in β2ARKO BMT animals (Fig. 7C and Table 1).
Figure 7.
Hypertrophy and cardiac function with β2ARKO BMT. A: representative WGA and isolectin B4 staining of hearts from WT and β2ARKO BMT receiving 1 wk vehicle or isoproterenol infusion. Quantification of cardiomyocyte area (B) and gravimetric analysis (C) of heart weight (HW) normalized to tibia length (TL) for WT and β2ARKO BMT mice treated for 1 wk with vehicle or isoproterenol. n = 12, two-way ANOVA, *P < 0.05. Quantification of isolectin B4 staining for capillary density for vehicle and isoproterenol-treated WT or β2ARKO BMT female (D) or male (E) mice. n = 6, two-way ANOVA, *P < 0.05. F: fractional shortening was determined by echocardiography in WT and β2ARKO BMT animals administered vehicle or isoproterenol. n = 12, two-way ANOVA, *P > 0.05. β2ARKO BMT, β2-adrenergic receptor knockout bone marrow transplant; Iso, isoproterenol; NS, not significant; Veh, vehicle; WGA, wheat germ agglutinin; WT, wild-type.
Table 1.
Echocardiographic measurements in wild-type or β2ARKO BMT mice treated with vehicle or Isoproterenol
| WT BMT Veh |
WT BMT Iso |
β2ARKO BMT Veh |
β2ARKO BMT Iso |
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1 wk | Baseline | 1 wk | Baseline | 1 wk | Baseline | 1 wk | |
| n | 12 | 12 | 12 | 12 | ||||
| LV volume, μL | ||||||||
| Systolic | 19.6 ± 1.6 | 20.7 ± 2.8 | 22.4 ± 2.2 | 37.1 ± 4.7* | 18.0 ± 2.6 | 18.2 ± 3.3 | 17.3 ± 2.3 | 21.0 ± 4.5† |
| Diastolic | 58.8 ± 4.0 | 58.2 ± 6.2 | 63.0 ± 3.6 | 78.3 ± 7.6 | 51.7 ± 4.9 | 52.4 ± 6.4 | 52.6 ± 5.5 | 60.4 ± 9.7 |
| LVID, mm | ||||||||
| Systolic | 1.7 ± 0.3 | 2.3 ± .01 | 1.6 ± 0.2 | 3.2 ± 0.2* | 1.6 ± 0.2 | 2.4 ± 0.1 | 1.7 ± 0.2 | 2.2 ± 0.1† |
| Diastolic | 3.5 ± 0.1 | 3.4 ± 0.1 | 3.7 ± 0.1 | 4.3 ± 0.2* | 3.4 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.1 | 3.6 ± 0.2† |
| Ejection fraction, % | 66.7 ± 1.1 | 64.9 ± 1.2 | 64.9 ± 1.6 | 53.5 ± 1.9* | 65.9 ± 2.1 | 66.4 ± 2.0 | 67.6 ± 1.0 | 65.8 ± 2.2† |
| Fractional shortening, % | 36.2 ± 0.9 | 34.9 ± 0.8 | 35.0 ± 1.2 | 27.3 ± 1.2* | 35.5 ± 1.5 | 35.9 ± 1.4 | 36.8 ± 0.7 | 35.6 ± 1.7† |
| Cardiac output, mL/min | 21.1 ± 1.5 | 19.8 ± 2.3 | 22.0 ± 0.7 | 22.1 ± 1.5 | 16.9 ± 1.4 | 16.9 ± 1.4 | 18.3 ± 1.4 | 20.8 ± 4.4 |
| Stroke volume, µL | 39.2 ± 2.6 | 37.5 ± 3.4 | 40.6 ± 1.7 | 40.0 ± 2.8 | 33.7 ± 2.5 | 34.2 ± 3.3 | 35.3 ± 3.2 | 39.8 ± 7.8 |
| Heart rate, beats/min | 538 ± 7 | 526 ± 22 | 544 ± 12.8 | 554 ± 19 | 501 ± 12 | 499 ± 15 | 521 ± 8 | 519 ± 17 |
| LVAW, mm | ||||||||
| Systolic | 1.35 ± 0.06 | 1.46 ± 0.09 | 1.42 ± 0.05 | 1.53 ± 0.13 | 1.25 ± 0.09 | 1.54 ± 0.03 | 1.44 ± 0.03 | 1.67 ± 0.09 |
| Diastolic | 1.03 ± 0.06 | 1.07 ± 0.06 | 1.01 ± 0.03 | 1.13 ± 0.06 | 0.87 ± 0.05 | 1.07 ± 0.03 | 1.04 ± 0.05 | 0.93 ± 0.10 |
| LVPW, mm | ||||||||
| Systolic | 1.21 ± 0.03 | 1.21 ± 0.09 | 1.23 ± 0.06 | 1.19 ± 0.09 | 1.12 ± 0.05 | 1.22 ± 0.08 | 1.11 ± 0.04 | 1.28 ± 0.08 |
| Diastolic | 0.76 ± 0.03 | 0.78 ± 0.03 | 0.82 ± 0.02 | 0.85 ± 0.06 | 0.79 ± 0.04 | 0.85 ± 0.05 | 0.74 ± 0.05 | 0.89 ± 0.65 |
| LV mass, mg | 96 ± 7 | 114 ± 9 | 105 ± 6 | 165 ± 25* | 91 ± 7 | 97 ± 9 | 92 ± 3 | 106 ± 9† |
Values are means ± SE; n, number of mice per group. β2ARKO, β2-adrenergic receptors knockout; LV, left ventricular; LVAW, LV anterior wall thickness; LVID, LV internal dimension; LVPW, LV posterior wall thickness. Two-way ANOVA. P < 0.05 vs. wild-type (WT) bone marrow transplant (BMT) *vehicle (Veh) or †isoproterenol (Iso) at same time point.
Capillary density was measured by isolectin B4 staining to determine the impact of immune cell β2ARKO deletion (Fig. 7, D and E). Significant differences were observed between male and female animals, so data are presented separately. Although isoproterenol infusion had no impact on capillary density in either WT or β2ARKO BMT animals, in both female (Fig. 7D) and male (Fig. 7E) mice, β2ARKO BMT mice had significantly increased levels of capillaries when compared with WT BMT animals.
Cardiac function was monitored using echocardiography (Fig. 7F and Table 1), and cardiac structure was assessed following infusion to assess the impact of immune cell β2AR. Contractile dysfunction was apparent in WT BMT treated with isoproterenol, with significant decreases in the fractional shortening (Fig. 7F) and ejection fraction (Table 1) in comparison with vehicle-treated animals. Isoproterenol-mediated changes in contractility were prevented in β2ARKO BMT mice (Fig. 7F and Table 1). These findings demonstrate the importance of immune cell β2AR expression in the detrimental cardiac effects observed with chronic catecholamine elevations.
DISCUSSION
Our findings demonstrate a major influence of immune cell-expressed β2AR in the heart’s adverse response to elevated catecholamines. WT mice infused with the endogenous catecholamine norepinephrine or β-adrenergic receptor agonists exhibit many of the hallmarks of HF including maladaptive remodeling and declines in function (26, 27, 32). Similarly, our study shows decreases in cardiomyocyte survival and contractility and increases in hypertrophy and fibrosis in WT BMT control animals administered isoproterenol. In the past, these alterations have largely attributed hemodynamic changes and β1AR expression on cardiomyocytes, which β-blocker downregulates in HF, impairing signaling and resulting in cardiomyocyte death (33, 34). β-Blocker therapy, a primary treatment clinically, is thought to normalize these responses and restore β1AR signaling to improve outcome in HF (35). β1ARs are thought to be the primary cardiac subtype responsible for these effects and clinical studies, including the Carvedilol or Metoprolol European Trial (COMET), show benefits of nonselective βAR inhibition over β1AR-selective antagonists (11, 36). Furthermore, effective doses of β1AR antagonists are often higher than would be selective for β1AR, suggesting an important role of β2AR inhibition in the protective effects of β-blocker therapy (37). β2ARs are widely expressed on immune cell subpopulations where they can influence a variety of functions in a cell type and context-dependent manner (6). Our previous findings demonstrated a critical role of immune cell-expressed β2AR in myeloid cell recruitment to the heart following myocardial infarction (8–10). Because of the importance of immune cells in many facets of cardiac biology, we hypothesized that immune cell β2AR expression plays an important role in the heart’s response to elevated catecholamines. Indeed, we found that β2ARKO BMT mice that lack β2AR specifically in cells of hematopoietic origin have decreased cardiomyocyte death, reduced fibrosis, blunted hypertrophy, and improvements in contractility following isoproterenol infusion compared with their WT BMT counterparts.
β2ARKO BMT animals were found to have decreased cardiomyocyte death with chronic isoproterenol infusion compared with their WT BMT counterparts, suggesting that a large portion of the cardiomyocyte death observed with isoproterenol treatment is a result of β2AR expression on immune cells. Although direct β1AR activation is known to induce cardiomyocyte death through PKA-dependent mechanisms (12, 18), our findings suggest these responses are potentiated by the β2AR-dependent regulation of immune cells. Cells of the immune system, particularly inflammatory leukocytes such as neutrophils and macrophages, have been associated with cytotoxic cardiomyocyte injury through release of inflammatory factors such as reactive oxygen species and cytokines; however, the extent of their contribution has been debated (38, 39). In myocardial infarction models, there is extensive leukocyte infiltration into the peri-infarcted region, which promotes cardiomyocyte injury in part, through TNFα secretion (40). Changes in proinflammatory cytokines are likely responsible for the differences in cardiomyocyte death between WT and β2ARKO BMT mice, as many proinflammatory cytokines that were elevated with isoproterenol in WT BMT mice including IL-1β, IL-18, and TNFα were not observed in β2ARKO BMT animals.
Fibrosis in the heart occurs in three forms: perivascular, reactive interstitial, and replacement fibrosis, all of which are observed with isoproterenol administration (41, 42). As anticipated, our study identified elevations of fibrosis in WT BMT mice. Moderate levels of fibrosis were observed in mice lacking immune cell β2AR, which may be partially due to the proliferative effects of β2AR activation on cardiac fibroblasts (5, 43). However, fibrosis levels were significantly diminished in β2ARKO BMT animals compared with WT BMT animals receiving isoproterenol treatment. This is likely due, in part, to the decreased cardiomyocyte death observed in these animals, as much of the isoproterenol-induced fibrosis is thought to occur as a result of necrotic cardiomyocyte loss (44, 45). However, β1/β2AR BMT mice have been reported to have decreased basal systolic and mean arterial blood pressure (46), which could influence interstitial and perivascular fibrosis. In addition, a number of immune cell populations, including neutrophils, macrophages, and T cells, which were shown to be reduced in β2ARKO BMT animals, have been shown to influence fibroblast activation through secreted factors such as cytokines, reactive oxygen species, growth factors, and proteases (47–49). Thus, the reduced infiltrating immune cells observed in isoproterenol-treated β2ARKO BMT mice is likely also contributing to the reduction in fibrosis.
Alterations in the recruitment of immune cells with β2ARKO is likely a major contributing factor to the improvements in cardiac remodeling and functional parameters observed in β2ARKO BMT mice. Damage or death of cardiomyocytes causes the recruitment of proinflammatory populations including neutrophils and macrophages to clear the tissue and initiate reparative mechanisms, whereas alternative populations including lymphocytes and reparative macrophages facilitate tissue remodeling and repair (44, 50–54). β2ARs are expressed on most immune cell subpopulations where they regulate a variety of cellular functions (6). In the heart, isoproterenol administration increased immune cell infiltration to the heart in WT BMT animals. The majority of immune cells present in the naïve heart expressed markers of the monocyte/macrophage lineage, which is in line with the large number of resident macrophages known to exist in the adult heart (53, 55). Alterations with isoproterenol occurred primarily through increases in monocyte/macrophage populations including a large increase in M1-like macrophages and a more modest increase in M2-like macrophages. For the purpose of this study, we considered M1-like macrophages to have high expression of CCR2, which are known to infiltrate to the heart after injury and be responsible for many of the proinflammatory macrophage effects. Our study confirms that the macrophages isolated from WT BMT hearts after isoproterenol infusion express high levels of proinflammatory cytokines including IL-1β, IL-18, and TNFα. Here, we consider CX3CR1+ macrophages as M2-like because of their reparative properties. This population likely represents the resident macrophage populations in the heart (53, 56–58). In addition to difference in macrophages, lymphocyte levels were also elevated compared with vehicle-treated WT BMT mice, although to a lesser magnitude than myeloid populations. β2AR BMT mice had an overall decrease in CD45+ cells, demonstrating a reduced infiltration of immune cells. This was particularly evident in the proinflammatory macrophage population, whereas increases in reparative macrophages were not different between WT and β2AR BMT groups. Interestingly, β2AR expression is higher on lymphocyte populations than macrophages, and the greater impact of β2AR deletion on macrophages may be due to the type or timing of injury (6). Changes in cardiac immune cell populations could occur through local proliferation of resident populations or recruitment (59). For CX3CR1+ macrophages, this is likely the case since increases in levels were observed with isoproterenol treatment in both WT and β2ARKO BMT animals. Furthermore, macrophages isolated from the hearts of WT and β2ARKO BMT animals had increased expression of the proliferation marker, Ki67, and WT and β2ARKO BMDM had the same capacity to proliferate in response to mCSF.
Infiltration of immune cells to a tissue is dependent on a number of events including egress from primary and secondary lymphoid organs, homing and extravasation into the tissue. A number of studies have implicated β2AR in these functions. Global β2ARKO mice have trends toward increased spleen, lymph node, and thymus size; however, these changes were not significant, possibly due to low sample sizes (60). β2ARKO BMT mice have been shown to have increased spleen size compared with their WT BMT counterparts (9), which also occurs with β2AR antagonist administration (8). Of note, murine studies do not always recapitulate human pathophysiology and species differences may occur; however, β2AR agonists have been shown in multiple species including human to reduce spleen size through the vascular-independent release of immune cells (61–65). β2AR has also been shown to influence lymphocyte egress from lymph nodes (66). On chronic isoproterenol administration, WT BMT animals had a robust recruitment of macrophages to the heart, which was diminished in β2ARKO BMT mice. Although this increase occurred both in macrophage populations expressing CCR2 and CX3CR1, only CCR2high-expressing macrophages were decreased in β2ARKO BMT animals. This would suggest the involvement of β2AR in mounting a proinflammatory macrophage response, while reparative and maintenance functions were unaltered. However, we cannot discount the possibility that β2ARKO macrophages have enhanced reparative properties. Increased capillary density was observed in both vehicle and isoproterenol-treated β2ARKO and several anti-inflammatory cytokines and protective growth factors such as IL-10 and insulin-like growth factor 1 (IGF1) were increased in isolated cardiac macrophages. There have been many in vitro studies examining the role of β2AR activation in dampening proinflammatory cytokine release from macrophages (67–69). However, our findings suggest a role for β2AR in macrophage polarization or recruitment. Studies examining the effects of β2AR on macrophage polarization suggest that β2AR promotes a unique phenotype that is on the M2-spectrum; however, this study was in the absence of other stimuli and the role of β2AR in macrophage polarization in a pathological setting is unknown (70). In the setting of endotoxemia, β2AR was identified as being a positive regulator of M2-macrophage polarization (71). These studies would imply that KO of β2AR would negatively impact M2-macrophage levels, which is in contrast to what is observed in the present study. This could be due to differences in the macrophage populations examined. Resident macrophage populations are thought to be derived from yolk-sac erythro-myeloid progenitors (72). There populations are expanded via local proliferation and are thought to serve a tissue maintenance and reparative function (58). In contrast, a unique subset of macrophages is recruited to the heart during pathological states (58). This population is monocyte derived, originates from hematopoietic stem cell precursors, and is responsible for the majority of the proinflammatory macrophage effects in the heart (73). Similar to what is observed with M1-like macrophages, their precursors, Ly-6Chigh monocytes (74), were reduced with β2AR deficiency. This might suggest that in contrast to altering differentiation and maturation of the macrophage lineage, β2AR might be influencing migration or transmigration. Although very little research has investigated the involvement of β2AR in regulating these processes, previous work has demonstrated increased vascular cell adhesion protein 1 (VCAM-1) in β2ARKO macrophages, a protein involved in immune cell adhesion to the vascular endothelium and transmigration (74), which negatively impacts egress of cells (9). Furthermore, β2ARKO BM and circulating immune cells have decreased levels of CCR2, a receptor involved in monocyte chemotaxis (8, 10).
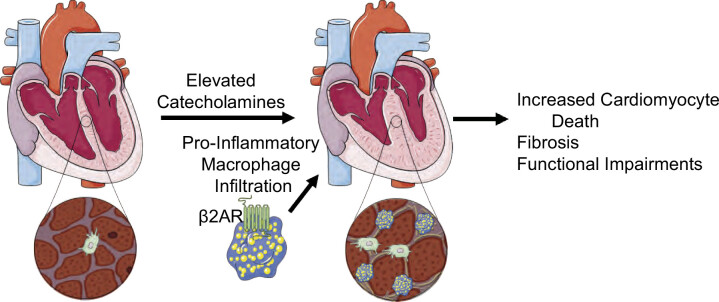
Clinically, inflammation has been implicated in the progression of heart failure, suggesting that anti-inflammatory strategies may be beneficial (75, 76). However, to date, anti-inflammatory strategies have been disappointing demonstrating the complexity of immune responses in the heart and a need for better understanding of how the immune system is regulating heart failure advancement (77). Our findings demonstrate that immune cell-specific deletion of β2AR protects the heart in a chronic isoproterenol model of heart failure through altering the infiltration of immune cells to the heart, particularly proinflammatory macrophages (Fig. 8). These results suggest that β-blockers, a common heart failure therapy, might benefit the heart through the reduction of inflammation rather than through alterations in hemodynamics and direct actions on cardiomyocytes. However, whether these processes are relevant to other types of heart failure including ischemic or pressure overload is not yet known. Although the mechanisms of cardiac immune cell changes were not carefully examined in this study, these findings demonstrate importance of the immunomodulatory role of β2AR in heart failure and identify a novel therapeutic strategy for the treatment of heart failure.
Figure 8.
Summary schematic. The normal heart contains a supply of resident macrophages that helps maintain homeostasis. With an excess of catecholamines, there is proliferation of resident macrophage populations that occurs independent of the β2AR and recruitment of proinflammatory macrophage populations that is enhanced by β2AR, which potentiates cardiomyocyte death through the secretion of proinflammatory cytokines, leading to adverse remodeling and disease progression. β2AR, β2-adrenergic receptor. Created using Servier Medical Art templates, licensed under Creative Commons Attribution 3.0 Uniported License (https://smart.servier.com).
SUPPLEMENTAL DATA
Supplemental Table S1 and Figs. S1–S8: https://doi.org/10.6084/m9.figshare.15167790.
GRANTS
This work was supported by American Heart Association Scientific Development Grant 17SDG33400114 (to L.A.G.) and National Heart, Lung, and Blood Institute Grant R01HL14808-01A1 (to L.A.G.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.A.G. conceived and designed research; M.A.T., C.A.M., and L.A.G. performed experiments; M.A.T. and L.A.G. analyzed data; L.A.G. interpreted results of experiments; L.A.G. prepared figures; L.A.G. drafted manuscript; L.A.G. edited and revised manuscript; M.A.T., C.A.M., and L.A.G. approved final version of manuscript.
REFERENCES
- 1.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation 101: 1634–1637, 2000. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Bauersachs J, Langer HF. Immune mechanisms in heart failure. Eur J Heart Fail 19: 1379–1389, 2017. doi: 10.1002/ejhf.942. [DOI] [PubMed] [Google Scholar]
- 3.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of β1- and β2-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation 100: 2210–2212, 1999. doi: 10.1161/01.CIR.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 4.Metrich M, Lucas A, Gastineau M, Samuel J-L, Heymes C, Morel E, Lezoualc’h F. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res 102: 959–965, 2008. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- 5.Turner NA, Porter KE, Smith WHT, White HL, Ball SG, Balmforth AJ. Chronic β2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc Res 57: 784–792, 2003. doi: 10.1016/S0008-6363(02)00729-0. [DOI] [PubMed] [Google Scholar]
- 6.Padro CJ, Sanders VM. Neuroendocrine regulation of inflammation. Semin Immunol 26: 357–368, 2014. doi: 10.1016/j.smim.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scanzano A, Cosentino M. Adrenergic regulation of innate immunity: a review. Front Pharmacol 6: 171, 2015. doi: 10.3389/fphar.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grisanti LA, de Lucia C, Thomas TP, Stark A, Strony JT, Myers VD, Beretta R, Yu D, Sardu C, Marfella R, Gao E, Houser SR, Koch WJ, Hamad EA, Tilley DG. Prior β-blocker treatment decreases leukocyte responsiveness to injury. JCI Insight 5: e99485, 2019. doi: 10.1172/jci.insight.99485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grisanti LA, Gumpert AM, Traynham CJ, Gorsky JE, Repas AA, Gao E, Carter RL, Yu D, Calvert JW, Garcia AP, Ibanez B, Rabinowitz JE, Koch WJ, Tilley DG. Leukocyte-expressed β2-adrenergic receptors are essential for survival after acute myocardial injury. Circulation 134: 153–167, 2016. doi: 10.1161/CIRCULATIONAHA.116.022304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grisanti LA, Traynham CJ, Repas AA, Gao E, Koch WJ, Tilley DG. β2-adrenergic receptor-dependent chemokine receptor 2 expression regulates leukocyte recruitment to the heart following acute injury. Proc Natl Acad Sci USA 113: 15126–15131, 2016. doi: 10.1073/pnas.1611023114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myagmar B-E, Flynn JM, Cowley PM, Swigart PM, Montgomery MD, Thai K, Nair D, Gupta R, Deng DX, Hosoda C, Melov S, Baker AJ, Simpson PC. Adrenergic receptors in individual ventricular myocytes: the beta-1 and alpha-1B are in all cells, the alpha-1A is in a subpopulation, and the beta-2 and beta-3 are mostly absent. Circ Res 120: 1103–1115, 2017. doi: 10.1161/CIRCRESAHA.117.310520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo BSu, Lemaire A, Mangmool S, Wolf MJ, Curcio A, Mao L, Rockman HA. β1-adrenergic receptors stimulate cardiac contractility and CaMKII activation in vivo and enhance cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol 297: H1377–H1386, 2009. doi: 10.1152/ajpheart.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lucia C, Eguchi A, Koch WJ. New insights in cardiac beta-adrenergic signaling during heart failure and aging. Front Pharmacol 9: 904, 2018. doi: 10.3389/fphar.2018.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, Simon AB, Rector T. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med 311: 819–823, 1984. doi: 10.1056/NEJM198409273111303. [DOI] [PubMed] [Google Scholar]
- 15.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med 307: 205–211, 1982. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 16.Jneid H, Addison D, Bhatt DL, Fonarow GC, Gokak S, Grady KL, Green LA, Heidenreich PA, Ho PM, Jurgens CY, King ML, Kumbhani DJ, Pancholy S. 2017 AHA/ACC Clinical performance and quality measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. J Am Coll Cardiol 70: 2048–2090, 2017. doi: 10.1016/j.jacc.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Bristow MR. Treatment of chronic heart failure with β-adrenergic receptor antagonists: a convergence of receptor pharmacology and clinical cardiology. Circ Res 109: 1176–1194, 2011. doi: 10.1161/CIRCRESAHA.111.245092. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Szeto C, Gao E, Tang M, Jin J, Fu Q, Makarewich C, Ai X, Li Y, Tang A, Wang J, Gao H, Wang F, Ge XJ, Kunapuli SP, Zhou L, Zeng C, Xiang KY, Chen X. Cardiotoxic and cardioprotective features of chronic β-adrenergic signaling. Circ Res 112: 498–509, 2013. doi: 10.1161/CIRCRESAHA.112.273896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52: 595–638, 2000. [PubMed] [Google Scholar]
- 20.Mann DL. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 116: 1254–1268, 2015. doi: 10.1161/CIRCRESAHA.116.302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrillo-Salinas FJ, Ngwenyama N, Anastasiou M, Kaur K, Alcaide P. Heart inflammation: immune cell roles and roads to the heart. Am J Pathol 189: 1482–1494, 2019. doi: 10.1016/j.ajpath.2019.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 15: 117–129, 2015. doi: 10.1038/nri3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leimbach WN Jr, Wallin BG, Victor RG, Aylward PE, Sundlöf G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation 73: 913–919, 1986. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson DW, Berg WJ, Sanders JS. Clinical and hemodynamic correlates of sympathetic nerve activity in normal humans and patients with heart failure: evidence from direct microneurographic recordings. J Am Coll Cardiol 16: 1125–1134, 1990. doi: 10.1016/0735-1097(90)90544-Y. [DOI] [PubMed] [Google Scholar]
- 25.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res 93: 896–906, 2003. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 26.Rona G, Chappel CI, Balazs T, Gaudry R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol 67: 443–455, 1959. [PubMed] [Google Scholar]
- 27.Boluyt MO, Long X, Eschenhagen T, Mende U, Schmitz W, Crow MT, Lakatta EG. Isoproterenol infusion induces alterations in expression of hypertrophy-associated genes in rat heart. Am J Physiol Heart Circ Physiol 269: H638–H647, 1995. doi: 10.1152/ajpheart.1995.269.2.H638. [DOI] [PubMed] [Google Scholar]
- 28.Grisanti LA, Thomas TP, Carter RL, de Lucia C, Gao E, Koch WJ, Benovic JL, Tilley DG. Pepducin-mediated cardioprotection via β-arrestin-biased β2-adrenergic receptor-specific signaling. Theranostics 8: 4664–4678, 2018. doi: 10.7150/thno.26619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanner MA, Thomas TP, Maitz CA, Grisanti LA. β2-Adrenergic receptors increase cardiac fibroblast proliferation through the Gαs/ERK1/2-dependent secretion of interleukin-6. Int J Mol Sci 21: 8507, 2020. doi: 10.3390/ijms21228507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanner MA, Thomas TP, Grisanti LA. Death receptor 5 contributes to cardiomyocyte hypertrophy through epidermal growth factor receptor transactivation. J Mol Cell Cardiol 136: 1–14, 2019. doi: 10.1016/j.yjmcc.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, Wang G, Zou W. Bone marrow and the control of immunity. Cell Mol Immunol 9: 11–19, 2012. doi: 10.1038/cmi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grisanti LA, Repas AA, Talarico JA, Gold JI, Carter RL, Koch WJ, Tilley DG. Temporal and gefitinib-sensitive regulation of cardiac cytokine expression via chronic β-adrenergic receptor stimulation. Am J Physiol Heart Circ Physiol 308: H316–H330, 2015. doi: 10.1152/ajpheart.00635.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res 59: 297–309, 1986. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 34.Ungerer M, Bohm M, Elce JS, Erdmann E, Lohse MJ. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation 87: 454–463, 1993. doi: 10.1161/01.cir.87.2.454. [DOI] [PubMed] [Google Scholar]
- 35.Lymperopoulos A, Rengo G, Koch WJ. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ Res 113: 739–753, 2013. [Erratum in Circ Res 119: e38, 2016]. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole-Wilson PA, Swedberg K, Cleland JGF, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Di Remme WJ, Torp-Pedersen C, Scherhag A, Skene A; Carvedilol Or Metoprolol European Trial Investigators. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet 362: 7–13, 2003. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 37.Zebrack JS, Munger M, Macgregor J, Lombardi WL, Stoddard GP, Gilbert EM. Beta-receptor selectivity of carvedilol and metoprolol succinate in patients with heart failure (SELECT trial): a randomized dose-ranging trial. Pharmacotherapy 29: 883–890, 2009. doi: 10.1592/phco.29.8.883. [DOI] [PubMed] [Google Scholar]
- 38.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res 61: 481–497, 2004. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol 63: 185–195, 2014. doi: 10.1097/FJC.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, Spengler RN, Smith CW, Entman ML. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation 98: 699–710, 1998. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 41.Anderson KR, Sutton MG, Lie JT. Histopathological types of cardiac fibrosis in myocardial disease. J Pathol 128: 79–85, 1979. doi: 10.1002/path.1711280205. [DOI] [PubMed] [Google Scholar]
- 42.Park S, Ranjbarvaziri S, Lay FD, Zhao P, Miller MJ, Dhaliwal JS, Huertas-Vazquez A, Wu X, Qiao R, Soffer JM, Rau C, Wang Y, Mikkola HKA, Lusis AJ, Ardehali R. Genetic regulation of fibroblast activation and proliferation in cardiac fibrosis. Circulation 138: 1224–1235, 2018. doi: 10.1161/CIRCULATIONAHA.118.035420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pullar CE, Isseroff RR. The beta 2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J Cell Sci 119: 592–602, 2006. doi: 10.1242/jcs.02772. [DOI] [PubMed] [Google Scholar]
- 44.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K, Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol 62: 24–35, 2013. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 45.Liu T, Song D, Dong J, Zhu P, Liu J, Liu W, Ma X, Zhao L, Ling S. Current understanding of the pathophysiology of myocardial fibrosis and its quantitative assessment in heart failure. Front Physiol 8: 238, 2017. doi: 10.3389/fphys.2017.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmari N, Schmidt JT, Krane GA, Malphurs W, Cunningham BE, Owen JL, Martyniuk CJ, Zubcevic J. Loss of bone marrow adrenergic beta 1 and 2 receptors modifies transcriptional networks, reduces circulating inflammatory factors, and regulates blood pressure. Physiol Genomics 48: 526–536, 2016. doi: 10.1152/physiolgenomics.00039.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44: 450–462, 2016. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puhl SL, Steffens S. Neutrophils in post-myocardial infarction inflammation: damage vs. resolution? Front Cardiovasc Med 6: 25, 2019. doi: 10.3389/fcvm.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nevers T, Salvador AM, Velazquez F, Ngwenyama N, Carrillo-Salinas FJ, Aronovitz M, Blanton RM, Alcaide P. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J Exp Med 214: 3311–3329, 2017. doi: 10.1084/jem.20161791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo J-L, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo J-L, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325: 612–616, 2009. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 96: 881–889, 2005. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 53.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, Shankar TS, Selzman CH, Drakos SG, Lavine KJ. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 24: 1234–1245, 2018. doi: 10.1038/s41591-018-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail 10: e003688, 2017. doi: 10.1161/CIRCHEARTFAILURE.116.003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martini E, Kunderfranco P, Peano C, Carullo P, Cremonesi M, Schorn T, Carriero R, Termanini A, Colombo FS, Jachetti E, Panico C, Faggian G, Fumero A, Torracca L, Molgora M, Cibella J, Pagiatakis C, Brummelman J, Alvisi G, Mazza EMC, Colombo MP, Lugli E, Condorelli G, Kallikourdis M. Single-cell sequencing of mouse heart immune infiltrate in pressure overload-driven heart failure reveals extent of immune activation. Circulation 140: 2089–2107, 2019. doi: 10.1161/CIRCULATIONAHA.119.041694. [DOI] [PubMed] [Google Scholar]
- 56.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol 14: 986–995, 2013. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA 111: 16029–16034, 2014. [Erratum in Proc Natl Acad Sci USA 113: E1414, 2016]. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40: 91–104, 2014. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu L, Tai Y, Hu S, Zhang M, Wang R, Weijie Z, Tao J, Han Y, Wang Q, Wei W. Bidirectional role of β2-adrenergic receptor in autoimmune diseases. Front Pharmacol 9: 1313, 2018. doi: 10.3389/fphar.2018.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leposavic G, Arsenovic-Ranin N, Radojevic K, Kosec D, Pesic V, Vidic-Dankovic B, Plecas-Solarovic B, Pilipovic I. Characterization of thymocyte phenotypic alterations induced by long-lasting beta-adrenoceptor blockade in vivo and its effects on thymocyte proliferation and apoptosis. Mol Cell Biochem 285: 87–99, 2006. doi: 10.1007/s11010-005-9059-5. [DOI] [PubMed] [Google Scholar]
- 61.Ernström U, Sandberg G. Effects of adrenergic alpha- and beta-receptor stimulation on the release of lymphocytes and granulocytes from the spleen. Scand J Haematol 11: 275–286, 1973. doi: 10.1111/j.1600-0609.1973.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 62.Modin A, Pernow J, Lundberg JM. Comparison of the acute influence of neuropeptide Y and sympathetic stimulation on the composition of blood cells in the splenic vein in vivo. Regul Pept 47: 159–169, 1993. doi: 10.1016/0167-0115(93)90420-D. [DOI] [PubMed] [Google Scholar]
- 63.Moerman EJ, Scapagnini U, De Schaepdryver AF. Adrenergic receptors in the isolated perfused dog spleen. Eur J Pharmacol 5: 279–285, 1969. doi: 10.1016/0014-2999(69)90149-6. [DOI] [PubMed] [Google Scholar]
- 64.Harris TJ, Waltman TJ, Carter SM, Maisel AS. Effect of prolonged catecholamine infusion on immunoregulatory function: implications in congestive heart failure. J Am Coll Cardiol 26: 102–109, 1995. doi: 10.1016/0735-1097(95)00123-H. [DOI] [PubMed] [Google Scholar]
- 65.Bakovic D, Pivac N, Zubin Maslov P, Breskovic T, Damonja G, Dujic Z. Spleen volume changes during adrenergic stimulation with low doses of epinephrine. J Physiol Pharmacol 64: 649–655, 2013. [PubMed] [Google Scholar]
- 66.Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through beta2-adrenergic receptors. J Exp Med 211: 2583–2598, 2014. doi: 10.1084/jem.20141132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Izeboud CA, Mocking JA, Monshouwer M, van Miert AS, Witkamp RF. Participation of beta-adrenergic receptors on macrophages in modulation of LPS-induced cytokine release. J Recept Signal Transduct Res 19: 191–202, 1999. doi: 10.3109/10799899909036645. [DOI] [PubMed] [Google Scholar]
- 68.Deng J, Muthu K, Gamelli R, Shankar R, Jones SB. Adrenergic modulation of splenic macrophage cytokine release in polymicrobial sepsis. Am J Physiol Cell Physiol 287: C730–C736, 2004. doi: 10.1152/ajpcell.00562.2003. [DOI] [PubMed] [Google Scholar]
- 69.Keranen T, Hommo T, Moilanen E, Korhonen R. β2-receptor agonists salbutamol and terbutaline attenuated cytokine production by suppressing ERK pathway through cAMP in macrophages. Cytokine 94: 1–7, 2017. doi: 10.1016/j.cyto.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 70.Lamkin DM, Ho H-Y, Ong TH, Kawanishi CK, Stoffers VL, Ahlawat N, Ma JCY, Arevalo JMG, Steve WC, Sloan EK. β-Adrenergic-stimulated macrophages: comprehensive localization in the M1-M2 spectrum. Brain Behav Immun 57: 338–346, 2016. doi: 10.1016/j.bbi.2016.07.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grailer JJ, Haggadone MD, Sarma JV, Zetoune FS, Ward PA. Induction of M2 regulatory macrophages through the β2-adrenergic receptor with protection during endotoxemia and acute lung injury. J Innate Immun 6: 607–618, 2014. doi: 10.1159/000358524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald H-R. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518: 547–551, 2015. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 119: 853–864, 2016. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, Swirski FK, Weissleder R, Nahrendorf M. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 115: 284–295, 2014. doi: 10.1161/CIRCRESAHA.115.303567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 103: 2055–2059, 2001. doi: 10.1161/01.CIR.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 76.Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 102: 3060–3067, 2000. doi: 10.1161/01.CIR.102.25.3060. [DOI] [PubMed] [Google Scholar]
- 77.Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand J-L, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker-Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss H-P, Tschope C, Bilsen MV, Zannad F, McMurray J, Shah AM. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 11: 119–129, 2009. doi: 10.1093/eurjhf/hfn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 and Figs. S1–S8: https://doi.org/10.6084/m9.figshare.15167790.