Abstract
Background
Vaccine effectiveness against COVID-19 beyond 6 months remains incompletely understood. We aimed to investigate the effectiveness of COVID-19 vaccination against the risk of infection, hospitalisation, and death during the first 9 months after vaccination for the total population of Sweden.
Methods
This retrospective, total population cohort study was done using data from Swedish nationwide registers. The cohort comprised all individuals vaccinated with two doses of ChAdOx1 nCoV-19, mRNA-1273, or BNT162b2, and matched unvaccinated individuals, with data on vaccinations and infections updated until Oct 4, 2021. Two outcomes were evaluated. The first was SARS-CoV-2 infection of any severity from Jan 12 to Oct 4, 2021. The second was severe COVID-19, defined as hospitalisation for COVID-19 or all-cause 30-day mortality after confirmed infection, from March 15 to Sept 28, 2021.
Findings
Between Dec 28, 2020, and Oct 4, 2021, 842 974 individuals were fully vaccinated (two doses), and were matched (1:1) to an equal number of unvaccinated individuals (total study cohort n=1 685 948). For the outcome SARS-CoV-2 infection of any severity, the vaccine effectiveness of BNT162b2 waned progressively over time, from 92% (95% CI 92 to 93; p<0·001) at 15–30 days, to 47% (39 to 55; p<0·001) at 121–180 days, and to 23% (−2 to 41; p=0·07) from day 211 onwards. Waning was slightly slower for mRNA-1273, with a vaccine effectiveness of 96% (94 to 97; p<0·001) at 15–30 days and 59% (18 to 79; p=0·012) from day 181 onwards. Waning was also slightly slower for heterologous ChAdOx1 nCoV-19 plus an mRNA vaccine, for which vaccine effectiveness was 89% (79 to 94; p<0·001) at 15–30 days and 66% (41 to 80; p<0·001) from day 121 onwards. By contrast, vaccine effectiveness for homologous ChAdOx1 nCoV-19 vaccine was 68% (52 to 79; p<0·001) at 15–30 days, with no detectable effectiveness from day 121 onwards (−19% [–98 to 28]; p=0·49). For the outcome of severe COVID-19, vaccine effectiveness waned from 89% (82 to 93; p<0·001) at 15–30 days to 64% (44 to 77; p<0·001) from day 121 onwards. Overall, there was some evidence for lower vaccine effectiveness in men than in women and in older individuals than in younger individuals.
Interpretation
We found progressively waning vaccine effectiveness against SARS-CoV-2 infection of any severity across all subgroups, but the rate of waning differed according to vaccine type. With respect to severe COVID-19, vaccine effectiveness seemed to be better maintained, although some waning became evident after 4 months. The results strengthen the evidence-based rationale for administration of a third vaccine dose as a booster.
Funding
None.
Introduction
Randomised clinical trials have shown a high efficacy of the BNT162b2 (Pfizer-BioNTech),1 mRNA-1273 (Moderna),2 and ChAdOx1 nCoV-19 (Oxford-AstraZeneca) COVID-19 vaccines,3, 4 and observational studies have estimated a high real-world effectiveness.5, 6, 7, 8 However, reports on breakthrough infections9 and waning immunity10, 11, 12, 13, 14 have raised concerns regarding the duration of protection.
With respect to severe COVID-19 outcomes such as hospitalisation or death, follow-ups of clinical trials showed that after 4 months the efficacy of BNT162b2 was about 84%15 and the efficacy of mRNA-1273 was about 92%,16 with similar results reported by the US Centers for Disease Control and Prevention.17 Observational studies from the USA and Qatar also showed that the effectiveness of BNT162b2 against hospitalisation and death persisted up to 6 months,18, 19 whereas preliminary data from the UK indicate a slight waning, especially in older adults and of ChAdOx1 nCoV-19 compared with BNT162b2.20 In terms of ChAdOx1 nCoV-19, another observational study reported waning effectiveness against hospitalisation and death within 3 months in Brazil and Scotland.21 Altogether, although evidence suggests that vaccine effectiveness against severe COVID-19 is relatively well maintained, the data are inconsistent. Similarly, the duration of protection against SARS-CoV-2 infection of any severity is unclear. After 4–5 months of follow-up, the effectiveness of BNT162b2 has been estimated as greater than 80% in one study,15 around 50% in two other studies,19, 20 and as low as around 20% in a study from Qatar.18 For ChAdOx1 nCoV-19, preliminary data from the UK suggest about 50% remaining effectiveness after 5 months of follow-up,20 whereas a published study showed that the effectiveness was down to about 50% in Scotland and 60% in Brazil after about 4 months.21
Research in context.
Evidence before this study
We did not conduct a formal literature search; however, we searched standard databases such as PubMed for published studies and used Google to identify relevant preprint articles. Randomised clinical trials have shown high efficacy of COVID-19 vaccines against infection and severe illness. However, reports on breakthrough infections and waning immunity have raised concerns regarding the duration of vaccine protection, and whether additional doses are warranted. There is some evidence to suggest waning vaccine effectiveness against infection up to 6 months after vaccination, whereas protection against severe illness seems to be better maintained. However, the evidence is limited and inconsistent, in part due to evaluations of vaccines that might have different long-lasting effects, different age of study participants, and varying follow-up times.
Added value of this study
The findings from this study show there was a progressive waning vaccine effectiveness of BNT162b2 against SARS-CoV-2 infection of any severity, with no vaccine effectiveness detected from 7 months onwards. The vaccine effectiveness of mRNA-1273 and heterologous ChAdOx1 nCoV-19 plus an mRNA vaccination waned slightly more slowly, whereas vaccine effectiveness of homologous ChAdOx1 nCoV-19 vaccination waned faster. For the outcome of COVID-19 hospitalisation or death, vaccine effectiveness was better maintained, although waned from 4 months onwards. Generally, there was some evidence for lower vaccine effectiveness in men than in women and in older individuals than in younger individuals.
Implications of all the available evidence
Our results suggest that vaccine protection against SARS-CoV-2 infections of any severity wanes progressively over time across all subgroups, but the rate of waning seems to be influenced by the type of vaccine. The protection against COVID-19 hospitalisation or death seems to be better maintained, although with some waning more than 4 months after vaccination. The results strengthen the evidence-based rationale for administration of a third vaccine dose as a booster to specific high-risk populations.
The different results in recent studies might relate to several factors, such as the evaluations of vaccines that might have different long-lasting effects,16, 18, 19, 20 different age of the participants,18 varying and relatively short follow-up times,15, 16, 22 different patterns of risk compensation in the populations, different severities and definitions of infections included as outcomes, and variations in infection pressure and variant exposure during follow-up. Collectively, vaccine effectiveness beyond 6 months remains incompletely understood. In this study, we investigated the effectiveness of COVID-19 vaccination against the risk of infection, hospitalisation, and death during the first 9 months after vaccination for the total population of Sweden.
Methods
Study design and participants
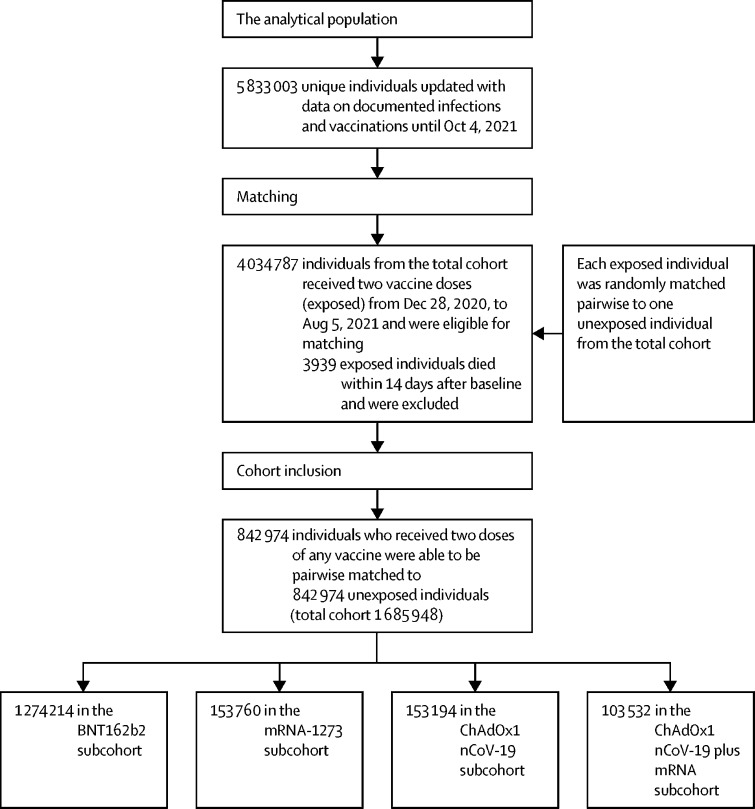
This retrospective, total population cohort study was done in Sweden. We included all individuals (n=3 640 421) vaccinated with at least one dose of any COVID-19 vaccine (ChAdOx1 nCoV-19, BNT162b2, or mRNA-1273) in Sweden until May 26, 2021, and all individuals with a documented SARS-CoV-2 infection until May 24, 2021 (n=1 331 989). Each individual was then matched (1:1) by Statistics Sweden, the national agency for statistics, to one randomly sampled individual from the total population of Sweden on birth year, sex, and municipality. In total, the cohort (vaccinated, those with documented infection, and matches) consisted of 5 833 003 individuals. This cohort was updated with respect to data on vaccinations and documented infections until Oct 4, 2021. From this cohort, each individual who was vaccinated with two doses, with no documented SARS-CoV-2 infection and alive within 14 days of vaccination, was matched (1:1) to one randomly sampled individual from the rest of the cohort on birth year and sex. Baseline for both individuals in each matched pair was set to the date of the second dose of vaccine in the vaccinated individual. Matched individuals were excluded if they received a first dose of vaccine, had a documented previous SARS-CoV-2 infection, or died within 14 days of baseline, whereby a new individual was searched from the remaining total cohort. This procedure was repeated five times. Data on individuals vaccinated against COVID-19 and data on documented SARS-CoV-2 infections were collected from the Swedish Vaccination Register and the SmiNet register, respectively, both of which are managed by the Public Health Agency of Sweden.23, 24 All health-care providers in Sweden are obliged to report to these registers according to Swedish law, with 100% coverage of the total population.
In the main cohort, cases of SARS-CoV-2 infections of any severity were recorded from Jan 12 to Oct 4, 2021, and cases of severe COVID-19 were recorded from March 15 to Sept 28, 2021. From the main cohort, we also formed four subcohorts according to specific vaccine types (BNT162b2, mRNA-1273, ChAdOx1 nCoV-19) and schedule (heterologous ChAdOx1 nCoV-19 plus an mRNA vaccine).
We also formed a second cohort to be used in a sensitivity analysis. This second cohort was formed using less strict matching criteria to increase the size of the cohort. In this dataset, each vaccinated individual was matched to the rest of the cohort on age only, with an allowance of a 5-year difference in age within each pair. This procedure was repeated ten times and one matched unvaccinated individual could be paired with several vaccinated individuals.
This study was approved by the Swedish Ethical Review Authority (number 495/2021), who waived the requirement of obtaining informed consent given the retrospective study design.
Exposures and outcomes
The exposure variable was vaccination status (vaccinated with two doses vs unvaccinated). Vaccination status was defined according to each specific vaccine schedule, as well as a composite variable (any vaccine). There were two outcomes of the study. The first was SARS-CoV-2 infection of any severity until Oct 4, 2021. In 94·4% of cases, infections were confirmed using PCR and in 4·8% by sequencing, according to the SmiNet register. The second outcome was a composite endpoint of severe COVID-19, defined as inpatient hospitalisation with COVID-19 as the main diagnosis, and all-cause mortality within 30 days after confirmed SARS-CoV-2 infection. This outcome was collected until Sept 28, 2021. Data on patients admitted to hospital were collected from the Swedish National Inpatient Register using the International Classification of Diseases version 10 (ICD-10), code U071, and Statistics Sweden provided data on mortality. All outcomes were collected from more than 14 days after baseline.
Covariates
From Statistics Sweden, we obtained information on whether individuals were born in Sweden or not, birth year, birth month, and sex for all individuals. From Statistics Sweden, we also obtained individual-level data on highest education during 2019. Individual-level data regarding diagnoses, prescription medications, and homemaker services were obtained from national registers managed by the Swedish National Board of Health and Welfare. Homemaker services include domestic services provided to individuals (primarily older individuals) who live at home but need help with shopping, cleaning, meal preparation, and similar tasks. From the Swedish National Inpatient Register and National Outpatient Register for specialist care, diagnoses from 1998 and 2001 and later were obtained using ICD-10 codes. Prescription medications from 2018 and later were obtained from the Prescribed Drug Register using Anatomic Therapeutic Chemical classification system codes. These three registers are complete for all specialist care and medications prescribed in Sweden for the years selected. The diagnoses and medications selected as covariates for this study were selected a priori based on the results from a previous nationwide study.25 Definitions of comorbidities are shown in the appendix (p 2).
Statistical analysis
Hazards over time for the outcome SARS-CoV-2 infection of any severity, based on exposure status (vaccinated vs unvaccinated), are shown using proportional hazards models with 95% CIs and restricted cubic splines. To compare the risk of the outcomes based on exposure status (vaccinated vs unvaccinated), Cox regression was used to calculate hazard ratios (HRs). To adjust for the matched samples, 95% CIs were estimated using robust SEs by the variance-covariance matrix of the estimators procedure and robust option in Stata. To formally test whether the associations were time dependent, Schoenfeld's residuals were evaluated using estat phtest command (Stata software). Given that the test indicated that the proportional hazard assumption was violated (χ2 = 3184·25; p<0·001) in the main analyses, the associations were evaluated in time intervals. The first model was adjusted for age and baseline date (date of second dose of vaccine) to adjust for variations in infection pressure during follow-up. The second model included the additional covariates sex, homemaker service (yes or no), education (six categories), whether the individual was born in Sweden or not, and eight diagnoses at baseline (yes or no). The HR was used to calculate vaccine effectiveness using the following formula: vaccine effectiveness=(1 – HR) × 100%. To investigate whether vaccine effectiveness was influenced by the prespecified covariates, interaction analyses were done, using product terms created by multiplying the variable coding for vaccination status at baseline (vaccinated vs unvaccinated) by each respective covariate, which were added to the fully adjusted Cox model. Given that the interaction terms were highly significant (p<0·001) for age, sex, homemaker service, and all diagnoses at baseline except asthma, vaccine effectiveness was also estimated in subgroups according to these covariates. Follow-up time in days was counted until date of confirmed outcome, date of first vaccination after baseline among unvaccinated individuals, death, or end of possible follow-up time (described earlier), whichever occurred first. All analyses were done in SPSS (version 27.0 for Mac), and Stata (version 16.1 for Mac). A two-sided p value less than 0·05 or HR with 95% CIs not crossing one were considered significant.
Role of the funding source
There was no funding source for this study.
Results
Between Dec 28, 2020, and Oct 4, 2021, 842 974 individuals were fully vaccinated (two doses), and were matched (1:1) to an equal number of unvaccinated individuals. Thus, the total study cohort comprised 842 974 pairs (n=1 685 948; figure 1 ). The mean date for the second dose of vaccine in the vaccinated group according to each vaccine schedule is shown in table 1 , together with baseline characteristics. Compared with unvaccinated individuals, vaccinated individuals more often had homemaker service, were more often born in Sweden, had more comorbidities, and had a higher level of education at baseline (p<0·001 for all; table 1). Similar differences were evident between vaccinated and unvaccinated individuals in the different vaccine subcohorts. SARS-CoV-2 variants sequenced in Sweden during the study period are shown in the appendix (p 2).
Figure 1.
Selection of the cohort
Table 1.
Baseline characteristics of the cohort at second dose of vaccine, according to vaccine schedule and in total
|
Total study cohort (any vaccine) |
BNT162b2 subcohort |
mRNA-1273 subcohort |
ChAdOx1 nCoV-19 subcohort |
ChAdOx1 nCoV-19 and an mRNA vaccine*subcohort |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated (n=842 974) | Unvaccinated (n=842 974) | Vaccinated (n=637 107) | Unvaccinated (n=637 107) | Vaccinated (n=76 880) | Unvaccinated (n=76 880) | Vaccinated (n=76 597) | Unvaccinated (n=76 597) | Vaccinated (n=51 766) | Unvaccinated (n=51 766) | ||
| Mean baseline date | May 5, 2021 | May 5, 2021 | April 27, 2021 | April 27, 2021 | May 20, 2021 | May 20, 2021 | June 5, 2021 | June 5, 2021 | May 28, 2021 | May 28, 2021 | |
| Days between doses | 42 (28–45) | .. | 37 (24–42) | .. | 42 (29–42) | .. | 70 (65–82) | .. | 87 (82–97) | .. | |
| Age, years | 52·7 (37·0–67·5) | 52·7 (37·0–67·5) | 54·8 (39·2–68·5) | 54·8 (39·1–68·5) | 41·1 (34·7–61·7) | 48·0 (34·7–61·7) | 64·6 (36·5–71·0) | 64·6 (36·5–71·0) | 35·2 (28·3–43·0) | 35·2 (28·3–43·0) | |
| Male | 342 677 (40·7%) | 342 677 (40·7%) | 263 866 (41·4%) | 263 866 (41·4%) | 34 461 (44·8%) | 34 461 (44·8%) | 30 141 (39·4%) | 30 141 (39·4%) | 13 926 (26·9%) | 13 926 (26·9%) | |
| Female | 500 297 (59·3%) | 500 297 (59·3%) | 373 241 (58·6%) | 373 241 (58·6%) | 42 419 (55·2%) | 42 419 (55·2%) | 46 456 (60·6%) | 46 456 (60·6%) | 37 840 (73·1%) | 37 840 (73·1%) | |
| Homemaker service | 87 004 (10·3%) | 30 680 (3·6%) | 81 704 (12·8%) | 25 718 (4·0%) | 4297 (5·6%) | 1950 (2·5%) | 698 (0·9%) | 2823 (3·7%) | 262 (0·5%) | 174 (0·3%) | |
| Born in Sweden | 703 666 (83·5%) | 578 647 (68·6%) | 533 572 (83·8%) | 442 799 (69·5%) | 63 288 (82·3%) | 50 259 (65·4%) | 64 951 (84·8%) | 50 178 (65·5%) | 41 363 (79·9%) | 35 011 (67·6%) | |
| Education | |||||||||||
| Elementary school, <9 years | 61 022 (7·2%) | 79 375 (9·4%) | 51 598 (8·1%) | 63 360 (9·9%) | 4234 (5·5%) | 6381 (8·3%) | 4420 (5·8%) | 7608 (9·9%) | 737 (1·4%) | 1967 (3·8%) | |
| Elementary school, 9 years | 81 455 (9·7%) | 97 948 (11·6%) | 61 814 (9·7%) | 73 709 (11·6%) | 8309 (10·8%) | 9458 (12·3%) | 6929 (9·1%) | 9084 (11·9%) | 4344 (8·4%) | 5621 (10·9%) | |
| Secondary school, 2 years | 180 672 (21·4%) | 182 971 (21·7%) | 143 917 (22·6%) | 145 325 (22·8%) | 14 824 (19·3%) | 15 810 (20·6%) | 16 391 (21·4%) | 16 065 (21·0%) | 5424 (10·5%) | 5641 (10·9%) | |
| Secondary school, >2 years | 171 349 (20·3%) | 168 922 (20·0%) | 125 590 (19·7%) | 122 362 (19·2%) | 15 848 (20·6%) | 16 511 (21·5%) | 15 669 (20·5%) | 14 927 (19·5%) | 14 117 (27·3%) | 14 982 (28·9%) | |
| University education | 324 660 (38·5%) | 275 444 (32·7%) | 237 148 (37·2%) | 204 663 (32·1%) | 30 503 (39·7%) | 24 708 (32·1%) | 31 973 (41·7%) | 24 994 (32·6%) | 24 770 (47·9%) | 20 893 (40·4%) | |
| Unknown | 23 816 (2·8%) | 38 314 (4·6%) | 17 040 (2·7%) | 27 688 (4·4%) | 3162 (4·1%) | 4012 (5·2%) | 1215 (1·6%) | 3919 (5·1%) | 2374 (4·6%) | 2662 (5·1%) | |
| Comorbidities | |||||||||||
| Myocardial infarction | 21 885 (2·6%) | 18 530 (2·2%) | 18 167 (2·9%) | 15 190 (2·4%) | 1637 (2·1%) | 1335 (1·7%) | 1974 (2·6%) | 1910 (2·5%) | 99 (0·2%) | 86 (0·2%) | |
| Stroke | 29 493 (3·5%) | 16 808 (2·0%) | 26 037 (4·1%) | 13 727 (2·2%) | 1751 (2·3%) | 1185 (1·5%) | 1543 (2·0%) | 1785 (2·3%) | 143 (0·3%) | 101 (0·2%) | |
| Diabetes | 91 203 (10·8%) | 62 198 (7·4%) | 74 361 (11·7%) | 49 614 (7·8%) | 8136 (10·6%) | 4880 (6·4%) | 6944 (9·1%) | 6744 (8·8%) | 1698 (3·3%) | 922 (1·8%) | |
| Hypertension | 262 659 (31·2%) | 207 862 (24·7%) | 212 647 (33·4%) | 170 772 (26·8%) | 21 358 (27·8%) | 15 295 (19·9%) | 24 624 (32·2%) | 19 387 (25·3%) | 3857 (7·5%) | 2281 (4·4%) | |
| Kidney failure | 20 027 (2·4%) | 10 317 (1·2%) | 16 711 (2·6%) | 8481 (1·3%) | 2251 (2·9%) | 706 (0·9%) | 815 (1·1%) | 990 (1·3%) | 242 (0·5%) | 134 (0·3%) | |
| COPD | 17 257 (2·1%) | 13 353 (1·6%) | 14 709 (2·3%) | 10 768 (1·7%) | 1248 (1·6%) | 928 (1·2%) | 1189 (1·6%) | 1563 (2·0%) | 102 (0·2%) | 83 (0·2%) | |
| Asthma | 50 341 (6·0%) | 36 671 (4·4%) | 38 234 (6·0%) | 27 717 (4·4%) | 5118 (6·7%) | 3267 (4·3%) | 3710 (4·8%) | 3254 (4·3%) | 3242 (6·3%) | 2400 (4·6%) | |
| Cancer | 48 512 (5·8%) | 37 092 (4·4%) | 39 720 (6·2%) | 30 696 (4·8%) | 3908 (5·1%) | 2613 (3·4%) | 4225 (5·5%) | 3323 (4·3%) | 635 (1·2%) | 438 (0·9%) | |
| SARS-CoV-2 infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
Data are median (IQR) or n(%), unless otherwise specified. COPD=chronic obstructive pulmonary disease.
Either BNT162b2 or mRNA-1273.
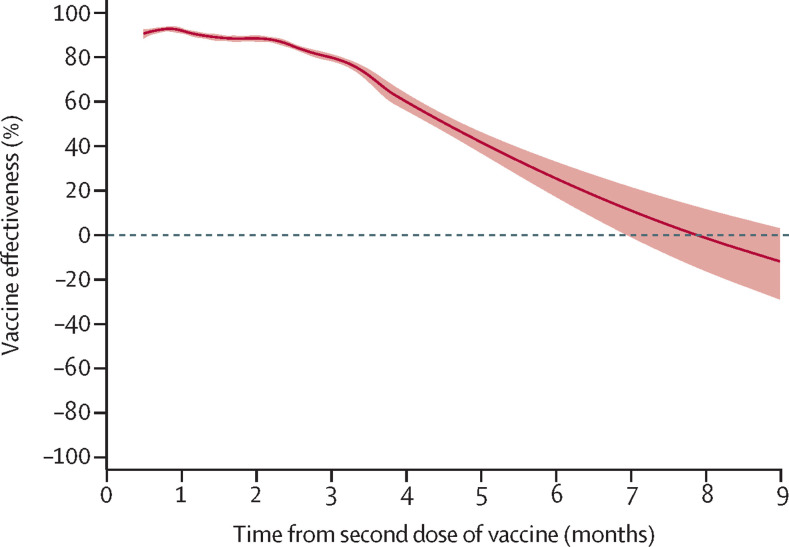
During a median follow-up of 108 days (IQR 69–145), a SARS-CoV-2 infection was confirmed in 27 918 individuals, of whom 6147 were vaccinated (4·9 infections per 100 000 person-days) and 21 771 were unvaccinated (31·6 infections per 100 000 person-days). The vaccine effectiveness associated with two doses of any vaccine peaked at 15–30 days (92% [95% CI 91 to 93]; p<0·001) and declined marginally at 31–60 days (89% [88 to 89]; p<0·001; table 2 , figure 2 ). From thereon, the waning became more pronounced, and from day 211 onwards there was no remaining detectable vaccine effectiveness (23% [–2 to 41]; p=0·07).
Table 2.
Vaccine effectiveness against SARS-CoV-2 infection of any severity up to 9 months after full vaccination (>14 days after the second dose) by number of days after the second dose
| Number of individuals |
Vaccinated |
Unvaccinated |
Vaccine effectiveness (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| Number of events | Incidence per 100 000 person-days | Number of events | Incidence per 100 000 person-days | Adjusted for age and baseline date | Fully adjusted* | |||
| Total study cohort (any vaccine) | 1 685 948 | 6147 | 4·9 | 21 771 | 31·6 | 84% (83 to 84) | 84% (83 to 84) | |
| 15–30 days | 1 685 948 | 397 | 1·6 | 4719 | 19·5 | 92% (91 to 93) | 92% (91 to 93) | |
| 31–60 days | 1 544 326 | 1254 | 2·5 | 8908 | 22·5 | 89% (88 to 90) | 89% (88 to 89) | |
| 61–120 days | 1 363 616 | 2436 | 2·6 | 7522 | 14·4 | 83% (82 to 83) | 82% (81 to 83) | |
| 121–180 days | 635 402 | 820 | 1·0 | 399 | 1·8 | 52% (46 to 58) | 48% (41 to 54) | |
| 181–210 days | 327 257 | 718 | 1·2 | 161 | 2·1 | 42% (31 to 51) | 32% (19 to 43) | |
| >210 days | 239 822 | 522 | 1·0 | 62 | 1·2 | 23% (0 to 41) | 23% (−2 to 41) | |
| BNT162b2 subcohort | 1 274 214 | 5062 | 5·1 | 19 121 | 36·4 | 84% (84 to 85) | 85% (84 to 85) | |
| 15–30 days | 1 274 214 | 333 | 1·7 | 4039 | 22·1 | 92% (91 to 93) | 92% (92 to 93) | |
| 31–60 days | 1 166 247 | 1095 | 2·9 | 7982 | 26·7 | 89% (88 to 90) | 89% (88 to 90) | |
| 61–120 days | 1 032 971 | 1796 | 2·6 | 6601 | 16·6 | 85% (84 to 85) | 84% (84 to 85) | |
| 121–180 days | 480 153 | 631 | 1·0 | 292 | 1·7 | 52% (45 to 58) | 47% (39 to 55) | |
| 181–210 days | 304 298 | 688 | 1·2 | 145 | 2·1 | 39% (26 to 49) | 29% (15 to 41) | |
| >210 days | 231 006 | 519 | 1·1 | 62 | 1·3 | 23% (1 to 41) | 23% (−2 to 41) | |
| mRNA-1273 subcohort | 153 760 | 300 | 2·9 | 1722 | 28·2 | 89% (88 to 91) | 89% (88 to 90) | |
| 15–30 days | 153 760 | 20 | 0·9 | 493 | 22·5 | 96% (94 to 98) | 96% (94 to 97) | |
| 31–60 days | 139 532 | 67 | 1·5 | 743 | 21·1 | 93% (91 to 95) | 93% (90 to 94) | |
| 61–120 days | 123 610 | 116 | 1·4 | 418 | 9·0 | 86% (82 to 88) | 85% (82 to 88) | |
| 121–180 days | 52 254 | 65 | 1·0 | 53 | 2·6 | 72% (59 to 80) | 71% (56 to 80) | |
| >180 days | 22 755 | 32 | 0·8 | 15 | 2·4 | 69% (44 to 83) | 59% (18 to 79) | |
| ChAdOx1 nCoV-19 subcohort | 153 194 | 465 | 5·0 | 469 | 7·2 | 49% (42 to 55) | 44% (36 to 52) | |
| 15–30 days | 153 194 | 33 | 1·4 | 93 | 4·2 | 66% (50 to 77) | 68% (52 to 79) | |
| 31–60 days | 144 772 | 53 | 1·2 | 88 | 2·3 | 55% (36 to 68) | 49% (28 to 64) | |
| 61–120 days | 129 103 | 293 | 3·5 | 262 | 4·9 | 48% (39 to 56) | 41% (29 to 51) | |
| >120 days | 53 060 | 86 | 1·6 | 26 | 1·4 | 0% (−55 to 36) | −19% (−98 to 28) | |
| ChAdOx1 nCoV-19 and an mRNA vaccine† subcohort | 103 532 | 316 | 4·8 | 442 | 11·8 | 68% (63 to 72) | 65% (58 to 70) | |
| 15–30 days | 103 532 | 11 | 0·7 | 92 | 6·2 | 89% (79 to 94) | 89% (79 to 94) | |
| 31–60 days | 92 623 | 37 | 1·2 | 88 | 4·0 | 74% (62 to 82) | 72% (59 to 82) | |
| 61–120 days | 76 924 | 230 | 3·8 | 234 | 8·8 | 63% (55 to 69) | 55% (45 to 64) | |
| >120 days | 49 664 | 38 | 0·8 | 28 | 1·8 | 61% (36 to 76) | 66% (41 to 80) | |
Adjusted for age, baseline date, sex, homemaker service, place of birth, education, and comorbidities at baseline.
The mRNA vaccine was either BNT162b2 or mRNA-1273.
Figure 2.
Vaccine effectiveness (any vaccine) against SARS-CoV-2 infection of any severity in 842 974 vaccinated individuals matched to an equal number of unvaccinated individuals for up to 9 months of follow-up
The association is shown using proportional hazards models with 95% CIs (shaded areas) and restricted cubic splines. The model was adjusted for age, baseline date, sex, homemaker service, place of birth, education, and comorbidities at baseline.
The estimated vaccine effectiveness was influenced significantly by vaccine type, age, sex, homemaker service, and all diagnoses at baseline (pinteraction<0·001 for all), except asthma (pinteraction=0·86). At 61–120 days, vaccine effectiveness declined to 50% (95% CI 30 to 64; p<0·001) in individuals aged 80 years or older, and to 61% (47–72; p<0·001) in individuals with homemaker service (table 3 ). With respect to sex, there was no detectable vaccine effectiveness in men (17% [95% CI –13 to 40]; p=0·23) from day 181 onwards, whereas it remained in women (34% [22 to 45]; p<0·001). With respect to vaccine type, vaccine effectiveness waned progressively for all vaccines during follow-up, but at different speeds (table 2). The vaccine effectiveness of BNT162b2 was 92% (95% CI 92 to 93; p<0·001) at 15–30 days, 47% (39 to 55; p<0·001) at 121–180 days, and 23% (−2 to 41; p=0·07) from day 211 onwards. Waning was slightly slower for mRNA-1273, with a vaccine effectiveness of 96% (94 to 97; p<0·001) at 15–30 days and 59% (18 to 79; p=0·012) from day 181 onwards. Waning was also slightly slower for heterologous ChAdOx1 nCoV-19 plus mRNA vaccine schedules, with a vaccine effectiveness of 89% (79 to 94; p<0·001) at 15–30 days and 66% (41 to 80; p<0·001) from day 121 onwards. By contrast, vaccine effectiveness for homologous ChAdOx1 nCoV-19 was 68% (52 to 79; p<0·001) at 15–30 days, with no detectable effectiveness from day 121 onwards (−19% [95% CI –98 to 28]; p=0·49).
Table 3.
Vaccine effectiveness against SARS-CoV-2 infection of any severity up to 9 months after full vaccination with any vaccine (>14 days after the second dose) by number of days after the second dose, according to sex, age, homemaker service, and any comorbidity at baseline
| Number of individuals |
Vaccinated |
Unvaccinated |
Vaccine effectiveness (95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Number of events | Incidence per 100 000 person-days | Number of events | Incidence per 100 000 person-days | Adjusted for age and baseline date | Fully adjusted* | |||
| 15–30 days | 1 685 948 | .. | .. | .. | .. | .. | .. | |
| Men | 685 354 | 133 | 1·3 | 1687 | 17·1 | 93% (91 to 94) | 93% (91 to 94) | |
| Women | 1 000 594 | 264 | 1·8 | 3032 | 21·1 | 92% (91 to 93) | 92% (91 to 93) | |
| Age <50 years | 769 391 | 191 | 1·7 | 3494 | 31·6 | 95% (95 to 96) | 95% (94 to 95) | |
| Age 50–64 years | 431 159 | 106 | 1·6 | 876 | 13·9 | 88% (86 to 90) | 88% (86 to 90) | |
| Age 65–79 years | 327 850 | 47 | 1·0 | 213 | 4·5 | 80% (72 to 85) | 82% (75 to 88) | |
| Age ≥80 years | 157 548 | 53 | 2·3 | 136 | 6·3 | 67% (55 to 76) | 74% (63 to 82) | |
| Any comorbidity | 619 248 | 184 | 1·8 | 897 | 11·7 | 85% (83 to 87) | 86% (84 to 88) | |
| Homemaker service | 117 684 | 72 | 2·8 | 68 | 7·9 | 76% (65 to 84) | 76% (65 to 84) | |
| 31–60 days | 1 544 326 | .. | .. | .. | .. | .. | .. | |
| Men | 629 873 | 361 | 1·8 | 2900 | 17·9 | 90% (89 to 91) | 90% (89 to 91) | |
| Women | 914 453 | 893 | 3·0 | 6008 | 25·8 | 88% (87 to 89) | 88% (87 to 89) | |
| Age <50 years | 704 877 | 706 | 3·1 | 6683 | 37·2 | 91% (91 to 92) | 91% (90 to 92) | |
| Age 50–64 years | 410 305 | 303 | 2·3 | 1776 | 15·7 | 85% (83 to 87) | 85% (83 to 87) | |
| Age 65–79 years | 298 770 | 145 | 1·5 | 315 | 4·2 | 69% (62 to 74) | 71% (64 to 76) | |
| Age ≥80 years | 130 374 | 100 | 2·1 | 134 | 5·0 | 69% (60 to 76) | 73% (65 to 79) | |
| Any comorbidity | 563 605 | 439 | 2·1 | 1571 | 13·2 | 84% (83 to 86) | 85% (83 to 86) | |
| Homemaker service | 108 919 | 149 | 2·9 | 64 | 5·1 | 71% (59 to 79) | 70% (59 to 79) | |
| 61–120 days | 1 363 616 | .. | .. | .. | .. | .. | .. | |
| Men | 558 636 | 721 | 2·0 | 2360 | 10·9 | 84% (82 to 85) | 83% (82 to 85) | |
| Women | 804 980 | 1715 | 3·1 | 5162 | 16·8 | 82% (81 to 83) | 82% (81 to 83) | |
| Age <50 years | 618 008 | 1531 | 3·7 | 5697 | 24·3 | 84% (83 to 84) | 83% (82 to 84) | |
| Age 50–64 years | 380 804 | 492 | 2·1 | 1510 | 9·5 | 81% (79 to 83) | 81% (79 to 83) | |
| Age 65–79 years | 260 405 | 227 | 1·2 | 255 | 2·6 | 66% (59 to 72) | 65% (56 to 72) | |
| Age ≥80 years | 104 399 | 186 | 2·0 | 60 | 2·0 | 48% (30 to 61) | 50% (30 to 64) | |
| Any comorbidity | 497 270 | 852 | 2·2 | 1252 | 8·3 | 79% (77 to 81) | 79% (77 to 80) | |
| Homemaker service | 101 580 | 247 | 2·5 | 64 | 3·5 | 64% (51 to 73) | 61% (47 to 72) | |
| 121–180 days | 635 402 | .. | .. | .. | .. | .. | .. | |
| Men | 220 596 | 273 | 1·0 | 97 | 1·2 | 33% (15 to 47) | 29% (9 to 45) | |
| Women | 414 806 | 547 | 1·1 | 302 | 2·1 | 58% (52 to 64) | 54% (46 to 61) | |
| Age <50 years | 269 241 | 503 | 1·6 | 293 | 2·7 | 55% (48 to 61) | 51% (43 to 58) | |
| Age 50–64 years | 115 938 | 161 | 1·0 | 36 | 1·1 | 40% (14 to 58) | 29% (−5 to 52) | |
| Age 65–79 years | 156 187 | 92 | 0·5 | 27 | 0·5 | 40% (3 to 63) | 30% (−16 to 58) | |
| Age ≥80 years | 94 036 | 64 | 0·5 | 43 | 1·3 | 53% (31 to 68) | 46% (15 to 66) | |
| Any comorbidity | 269 919 | 273 | 0·7 | 97 | 1·4 | 58% (47 to 67) | 55% (42 to 65) | |
| Home maker service | 90 347 | 81 | 0·6 | 24 | 1·5 | 35% (−14 to 63) | 29% (−24 to 59) | |
| >180 days | 327 257 | .. | .. | .. | .. | .. | .. | |
| Men | 104 220 | 351 | 1·7 | 51 | 2·1 | 25% (0 to 45) | 17% (−13 to 40) | |
| Women | 223 037 | 889 | 2·0 | 172 | 3·1 | 41% (30 to 50) | 34% (22 to 45) | |
| Age <80 years | 260 172 | 1005 | 1·9 | 204 | 3·3 | 40% (30 to 48) | 33% (21 to 43) | |
| Age ≥80 years | 67 085 | 235 | 1·8 | 19 | 1·0 | 4% (−50 to 39) | 5% (−53 to 41) | |
| Any comorbidity | 160 790 | 536 | 1·6 | 41 | 1·6 | 22% (−8 to 43) | 15% (−17 to 38) | |
Adjusted for age, baseline date, sex, homemaker service, place of birth, education, and comorbidities at baseline.
During a median follow-up of 124 days (IQR 98–208), there were 277 cases of COVID-19 hospitalisation or death among vaccinated individuals (0·23 hospitalisations or deaths per 100 000 person-days) and 825 cases among unvaccinated individuals (1·20 hospitalisations or deaths per 100 000 person-days; appendix pp 3, 7). The vaccine effectiveness associated with two doses of any vaccine was 89% (95% CI 83 to 93; p<0·001) at 15–30 days, which declined to 64% (44 to 77; p<0·001) from day 121 onwards (appendix p 3).
In a sensitivity analysis using less strict matching criteria, a second matched cohort (1 983 315 matched pairs; n=3 996 630) more than twice the size of the original cohort was created. Mean age of vaccinated individuals was 5 years higher in the second cohort than in the main cohort, whereas all other characteristics were similar between the cohorts (appendix p 3). In this larger cohort, the waning vaccine effectiveness was confirmed with respect to a SARS-CoV-2 infection of any severity (appendix p 4), including the different rate of waning for different vaccine schedules (appendix p 5). In addition, it was confirmed that vaccine effectiveness was better maintained against the outcome of severe COVID-19 (appendix p 6), than against SARS-CoV-2 infection of any severity (appendix p 4).
Discussion
This study showed a progressive waning vaccine effectiveness against SARS-CoV-2 infection of any severity during up to 9 months of follow-up. In the main cohort, the estimated vaccine effectiveness was more than 90% in the first month, with a progressive waning starting soon thereafter, ultimately resulting in a non-detectable vaccine effectiveness after 7 months. Vaccine effectiveness waned across all subgroups, although differently according to vaccine schedule and type. Vaccine effectiveness with respect to the risk of COVID-19 hospitalisation or death seemed to be better maintained than effectiveness against infection, although some waning became evident after 4 months. Overall, there was also some evidence suggesting lower vaccine effectiveness in men than in women and in older individuals than in younger individuals.
Waning vaccine effectiveness against SARS-CoV-2 infection has previously been reported in preliminary observational studies from the UK and in published observational studies from the USA and Qatar,18, 19, 20 whereas follow-up studies of clinical trials show high remaining efficacy of both BNT162b2 after 4 months,15 and mRNA-1273 after more than 4 months.16 Our data add to these previous studies with a follow-up time of up to 9 months, about 28 000 confirmed SARS-CoV-2 infections in the main cohort, and the evaluation of four different vaccine schedules in a real-world setting. Overall, our results showed notable waning vaccine effectiveness against SARS-CoV-2 infection of any severity across all subgroups, although with higher remaining vaccine effectiveness for mRNA-1273 and for heterologous vaccine schedules. The latter finding is of particular interest, and is supported by clinical trials showing superior vaccine-elicited immunogenicity from heterologous vaccine schedules.26, 27 Our finding is in addition to previous observational studies estimating high vaccine effectiveness of heterologous schedules in the short-term.28, 29 By contrast, we were not able to detect any remaining vaccine effectiveness against SARS-CoV-2 infection of any severity from homologous ChAdOx1 nCoV-19 vaccination after more than 4 months. This finding contradicts preliminary evidence from the UK,20 but is in line with a recent study reporting waning vaccine effectiveness for this vaccine against both SARS-CoV-2 infection and severe COVID-19 in Brazil and Scotland within 3 months of the second dose.21 The different estimates of vaccine effectiveness in all of these studies could be influenced by several factors—eg, different patterns of risk compensation, undiagnosed previous infections in individuals used as controls, varying follow-up times, the prevalence of risk factors that reduce the immune response to vaccination, the severity and definition of infections included as outcomes, variations in infection pressure and SARS-CoV-2 variants during follow-up, and different age of the studied populations.
In the present study, vaccine effectiveness against severe COVID-19 was better maintained than against SARS-CoV-2 infections of any severity, although some waning was evident after more than 4 months. These results were confirmed in a sensitivity analysis done in a second, even larger cohort, and have some support from preliminary data originating from the UK.20 In the same report, waning seemed greater in individuals belonging to a clinically vulnerable group and in older adults,20 as indicated also from the sensitivity analysis in the present study. A reasonable explanation of waning effectiveness predominantly in older adults, would be that the adaptive immune response mediated by B cells that produce antibodies, as well as T cells is impaired with older age.30 In support, one of the risk factors associated with lower vaccine effectiveness in the present study was older age. Among other risk factors for lower vaccine effectiveness were male sex. Although there has been no previous study reporting waning vaccine effectiveness according to sex, these findings are supported by studies showing a lower vaccine-elicited immunogenicity along with a more rapid decline in neutralising antibody titres in men compared with in women.13, 31
The results of our study have important clinical implications, as they strengthen the evidence-based rationale for administration of a third vaccine dose as a booster, especially to specific high-risk populations. Recent preliminary phase 3 data from Pfizer-BioNTech show that a third dose of BNT162b2, administered a median of 11 months after the second dose, had 95·6% efficacy (95% CI 89·3–98·6) against symptomatic COVID-19 compared with those who had only received two primary doses, with consistent results irrespective of age, sex, and comorbidities.32 In addition, data from an Israeli observational study showed that individuals who received a third dose of BNT162b2 had a reduced rate of infections and hospitalisations compared with individuals given two doses.33 Currently, many countries are recommending a third vaccine dose as a booster to select populations at increased risk of severe COVID-19. The implication of the results from the present study and previous studies is that older individuals and individuals with known suboptimal or waning vaccine-elicited immunogenicity should be prioritised for booster doses, because these individuals also are at highest risk for severe COVID-19 manifestations if infected.
Other than the observational design, the present study has some limitations to consider. Although we adjusted our analyses for several potential confounders, the possibility of residual and unmeasured confounding remains, including a higher risk of selection bias in unvaccinated individuals with longer follow-up time. Moreover, although we excluded all individuals with a documented previous infection, some individuals with a previous asymptomatic infection are likely to have been included in the analyses. Furthermore, the SARS-CoV-2 infections registered in the SmiNet register included infections of any severity, and the definition of severe COVID-19 included death from any cause within 30 days after a confirmed infection. More strict definitions might have increased the estimates of vaccine effectiveness for both outcomes. However, it should be noted that vaccine effectiveness was greater than 90% early after vaccination. Finally, the follow-up in the present study was completed before the emergence of the recent omicron (B.1.1.529) variant of SARS-CoV-2. This study also has several important strengths. First, the results were confirmed in sensitivity analyses based on a second cohort where less strict matching criteria were used. Second, vaccinated individuals had received different types and combinations of vaccines, allowing us to investigate how this differentially affected vaccine effectiveness and duration of vaccine protection in a real-world setting. Third, all the registers used to obtain data on COVID-19 cases, vaccinations, hospitalisations, and deaths have a nationwide coverage and zero loss to follow-up. This reduces the risk of misclassification of unvaccinated individuals included in the analyses. Using these registers, we were also able to obtain covariates that have previously been identified as risk factors for COVID-19 in the Swedish population.25 Finally, the study cohort was based on the total population of Sweden, increasing the external validity of the findings to other countries with similar population structure.
In summary, our results suggest a substantial waning of vaccine protection against SARS-CoV-2 infection of any severity across all subgroups, but with variations related to vaccine types and schedules. By contrast, protection against severe COVID-19 was better maintained for up to 9 months of follow-up, although some waning became evident after more than 4 months. These findings might have implications for vaccination strategies and public health by strengthening the evidence-based rationale for administration of a third vaccine dose as a booster, where the priority should be specific populations who are at higher risk of severe consequences of COVID-19 due to weaker and more rapidly waning vaccine-elicited immunogenicity.
Data sharing
The data files used for the present study are publicly unavailable according to regulations under Swedish law. However, all data used for the present study can be applied for from the National Board of Health and Welfare, Statistics Sweden, and the Public Health Agency of Sweden.
Declaration of interests
We declare no competing interests.
Contributors
All authors conceived and designed the study. PN acquired the data. PN did the statistical analyses. PN and MB accessed and verified the underlying data. All authors interpreted the data. PN and MB drafted the manuscript. All authors critically revised the manuscript for intellectual content. PN and AN supervised the work. All authors gave final approval of the version to be published. All authors had full access to all the data and had final responsibility for the decision to submit for publication
Supplementary Material
References
- 1.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Costa Clemens SA, Madhi SA, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27:1614–1621. doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 6.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung H, He S, Nasreen S, et al. Effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe COVID-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374 doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keehner J, Horton LE, Binkin NJ, et al. Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. N Engl J Med. 2021;385:1330–1332. doi: 10.1056/NEJMc2112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/S0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naaber P, Tserel L, Kangro K, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iacobucci G. COVID-19: protection from two doses of vaccine wanes within six months, data suggest. BMJ. 2021;374 doi: 10.1136/bmj.n2113. [DOI] [PubMed] [Google Scholar]
- 13.Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas SJ, Moreira ED, Jr, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Self WH, Tenforde MW, Rhoads JP, et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews A, Tessier E, Stowe J, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK (preprint) https://khub.net/documents/135939561/338928724/Vaccine+effectiveness+and+duration+of+protection+of+covid+vaccines+against+mild+and+severe+COVID-19+in+the+UK.pdf/10dcd99c-0441-0403-dfd8-11ba2c6f5801
- 21.Katikireddi SV, Cerqueira-Silva T, Vasileiou E, et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet. 2022;399:25–35. doi: 10.1016/S0140-6736(21)02754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsey AR, Sobieszczyk ME, Hirsch I, et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Public Health Agency of Sweden The national vaccination register. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/vaccinationer/nationella-vaccinationsregistret/
- 24.Public Health Agency of Sweden SmiNet. https://www.folkhalsomyndigheten.se/smittskydd-beredskap/overvakning-och-rapportering/sminet/
- 25.Bergman J, Ballin M, Nordström A, Nordström P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol. 2021;36:287–298. doi: 10.1007/s10654-021-00732-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Normark J, Vikström L, Gwon Y-D, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N Engl J Med. 2021;385:1049–1051. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu X, Shaw RH, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–869. doi: 10.1016/S0140-6736(21)01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health Eur. 2021;11 doi: 10.1016/j.lanepe.2021.100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gram MA, Nielsen J, Schelde AB, et al. Vaccine effectiveness against SARS-CoV-2 infection, hospitalization, and death when combining a first dose ChAdOx1 vaccine with a subsequent mRNA vaccine in Denmark: a nationwide population-based cohort study. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lustig Y, Sapir E, Regev-Yochay G, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9:999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.BioNTech Pfizer and BioNTech announce phase 3 trial data showing high efficacy of a booster dose of their COVID-19 vaccine. Oct 21, 2021. https://investors.biontech.de/news-releases/news-release-details/pfizer-and-biontech-announce-phase-3-trial-data-showing-high
- 33.Bar-On YM, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against COVID-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data files used for the present study are publicly unavailable according to regulations under Swedish law. However, all data used for the present study can be applied for from the National Board of Health and Welfare, Statistics Sweden, and the Public Health Agency of Sweden.