Abstract
Laboratory diagnosis of tuberculosis is often difficult. Immunodetection of circulating Mycobacterium tuberculosis proteins shed during active infection would not depend on an intact host immune response and could take advantage of the speed and low costs afforded by antibody-based assays. We previously showed that patients with active tuberculosis had increased levels of circulating antigen 85 (Ag85) proteins independent of their tuberculin skin test status (S. I. Bentley-Hibbert, X. Quan, T. Newman, K. Huygen, and H. P. Godfrey, Infect. Immun. 67:581–588, 1999). To extend these observations to a Mycobacterium bovis BCG-vaccinated population and to another secreted mycobacterial protein, Ag85 and PstS-1 (protein antigen B, p38 antigen) were quantified in sera from 97 Chilean tuberculosis patients and healthy controls (many of whom had received BCG as children) using dot immunobinding, mouse monoclonal anti-BCG Ag85 complex antibody, and chicken antipeptide antibodies reactive with M. tuberculosis Ag85B and PstS-1. The latter antibodies had been raised to peptide-derived immunogens expressed on a novel proprietary protein carrier in Escherichia coli. Median serum Ag85 levels measured by using either anti-Ag85 antibody were significantly higher in patients with active tuberculosis than in healthy controls (P, <0.001 to 0.01); the median serum PstS-1 levels were similar in patients and controls. The sensitivity of significantly elevated circulating Ag85 levels in patients with pulmonary tuberculosis measured by anti-Ag85 complex or anti-Ag85B antibodies was 60 and 55%, respectively, but increased to 77% when results obtained with both anti-Ag85 antibodies were considered jointly (P < 0.02). The corresponding specificities for individual and joint consideration were 95, 85, and 80%, respectively. These results indicate that elevated Ag85 levels can be detected in patients with active tuberculosis even after BCG vaccination and suggest that combinatorial use of antibodies directed at different epitopes of this protein could provide a viable strategy for developing new host immune response-independent diagnostic tests for tuberculosis.
Tuberculosis is caused by organisms of the Mycobacterium tuberculosis complex (4). It is responsible for about 2 million deaths worldwide annually and is one of the most common worldwide causes of adult death from a single infectious agent. Its recent global resurgence has been linked to the human immunodeficiency virus (HIV) epidemic, although worsening socioeconomic parameters among certain population segments are also involved at least in part (15).
Diagnosis of tuberculosis is often difficult (29). Skin reactivity to purified protein derivative of tuberculin (PPD), particularly among people not immunized to mycobacterial antigens by vaccination with M. bovis BCG, serves as an important diagnostic tool (17). PPD skin reactivity is a major element in the diagnosis of tuberculosis and mycobacterial infection in the United States (5), but it requires an intact host immune system. Indeed, tuberculin anergy occurs in 15 to 25% of non-HIV-infected tuberculosis patients and is at least twice as high in populations infected with both M. tuberculosis and HIV (31). Thus, alternative diagnostic methods that do not depend on an intact host immune response are greatly needed.
Bacteriologic culture of M. tuberculosis is definitive but can take 2 to 3 weeks to yield results even under optimal conditions (34). Morphologic identification of acid-fast bacilli in sputum smears is more rapid but less sensitive than culture since it requires a much larger number of organisms (only roughly 50% of cases are positive overall) (3, 8, 10, 34) and is labor-intensive. Molecular methods for diagnosis of tuberculosis based on nucleic acid amplification are rapid, highly specific, and more sensitive than microscopic examination of smears but less sensitive than culture in smear-negative cases (3, 37). They are also expensive and technically complex and require a high degree of quality control for accurate performance. Although dependent on the host immune response and therefore of limited use in HIV-infected patients, detection of circulating antibodies to mycobacterial antigens is easy and cost-effective but has not provided a generally accepted diagnostic method for tuberculosis because of low sensitivity, poor specificity, or both (10, 17, 26).
Actively growing mycobacteria secrete many proteins. The three closely related proteins of the antigen 85 complex (Ag85A, Ag85B, and Ag85C) are major secretory proteins of M. tuberculosis (36). These 30- to 32-kDa mycolyl transferases are involved in cell wall synthesis (6, 36) and readily bind to plasma and cellular fibronectins (1, 18). They appear in culture fluids of exponentially growing M. tuberculosis by day 2 to 4 of culture (2, 35, 36) and can account for up to 30% of secreted proteins (36). PstS-1 (protein antigen B, p38 antigen, PhoS) is also secreted early in the growth phase (19, 35). This 38-kDa phosphate binding lipoprotein is the mycobacterial equivalent of the PstS protein component of the phosphate uptake system found in other bacteria (9, 19). It accounts for about 10% of mycobacterial culture filtrate proteins (19, 35).
Ag85 complex proteins can be detected immunologically in the sera of patients with active tuberculosis who are PPD negative and HIV positive (7). Because PstS-1 is also a secreted M. tuberculosis protein and anti-PstS-1 antibodies have high specificity for infection with M. tuberculosis (12), it seemed reasonable to determine if high levels of PstS-1 protein could be demonstrated in sera from patients with active tuberculosis. To extend these observations to a BCG-vaccinated population, mycobacterial secretory proteins were quantified by immunoassay in sera from 97 adult Chilean tuberculosis patients and healthy controls, many of whom had received BCG as children. A dot-immunobinding format was chosen so that the speed and low costs afforded by an antibody-based test could be available to laboratories with limited facilities (25). To complement available anti-mycobacterial secretory protein antibodies, IgY chicken antipeptide antibodies were raised against immunogens containing peptides derived from Ag85B and PstS-1 sequences linked to a proprietary carrier sequence (22). The resulting antipeptide antibodies specifically detected recombinant and native mycobacterial antigens by Western analysis and indirect enzyme-linked immunosorbent assay, possessed sufficient sensitivity to allow the detection of mycobacterial antigens by dot immunobinding in the sera of human patients with active tuberculosis, and improved the sensitivity of detection of circulating Ag85 in patients with active tuberculosis.
MATERIALS AND METHODS
Study population.
Serum was obtained from 97 patients and healthy controls aged 15 to 80 years (median age, 47 years) at tuberculosis clinics in Santiago, Chile. All patients were Hispanic, 65% were male, and 42% were under 40 years. There was no significant difference in the age distributions of male and female patients. Patients under 40 years were highly likely to have received one or more BCG vaccinations as neonates and/or during childhood as part of the increasingly effective Chilean national BCG vaccination program over the past 40 years (28). At present, over 98% of neonates in Chile receive BCG vaccination (27). Because of this widespread use of BCG, many healthy adults in Chile have positive tuberculin skin tests (28) and the use of PPD skin reactivity in the diagnosis of tuberculosis is limited. Diagnoses of pulmonary tuberculosis in the study population were made on the basis of clinical findings including cough and expectoration lasting longer than 2 weeks, sputum smear, culture, and, for patients older than 50 years, chest X ray, in accordance with guidelines of the Chilean Ministry of Health. Pulmonary tuberculosis was diagnosed in a smear-negative and culture-negative patient on the basis of clinical and radiographic findings and response to antituberculosis therapy. Diagnoses of extrapulmonary tuberculosis were made on the basis of clinical and radiographic findings, histopathologic examination and culture of biopsy material, and response to therapy in accordance with guidelines of the Chilean Ministry of Health. Diagnoses included smear-positive pulmonary tuberculosis (51 patients); smear-negative, culture-positive pulmonary tuberculosis (8 patients); smear-negative, culture-negative pulmonary tuberculosis (1 patient); extrapulmonary tuberculosis (4 patients; one case each of smear-positive renal tuberculosis, biopsy-positive lymph node tuberculosis, culture-positive pleural tuberculosis, and culture-negative miliary tuberculosis); inactive tuberculosis (documented successful completion of treatment for active tuberculosis 0.5 to 21 years previously) (13 patients); and no disease (healthy controls) (20 patients). All patients with active tuberculosis were tested for HIV infection; the single HIV-positive patient in the present study had smear-positive pulmonary tuberculosis. All patients with active tuberculosis were treated with regimens that included isoniazid (5 mg/kg daily or 15 mg/kg weekly; maximal weekly dose, 900 mg) in accordance with guidelines of the Chilean Ministry of Health. Serum was collected with informed consent from all members of the study population, coded, and stored frozen at −80°C until analyzed. Sera from patients with tuberculosis were stratified as to whether they were collected before or after 15 days of antimicrobial therapy. The code was not broken until all assay measurements had been completed.
Development and purification of oligoclonal chicken antipeptide antibodies.
Peptide sequences for M. tuberculosis Ag85B (fnpB, Rv1886c, GenBank accession no. P31952) and PstS-1 (phoS1, Rv0934, GenBank accession no. P15712) were analyzed for predicted antigenicity, surface probability, hydrophilicity, and hydrophobicity using algorithms in Protean (DNAStar). Peptide sequences with possibly suitable properties for use in raising antipeptide antibodies were subjected to standard protein-protein BLAST (BLASTP) and position-specific iterative BLAST (PSI-BLAST) searches (http://www.ncbi.nlm.nih.gov/blast/) to screen for homology to known protein sequences. Two peptide sequences were chosen from each protein. SSDPAWERNDPT was present only in Ag85B of M. tuberculosis and BCG. Homologous sequences in M. tuberculosis Ag85A and Ag85C and in Ag85 complex proteins from other mycobacterial species (identified by BLASTP) differed primarily by substitution of a glutaminyl or an alanyl residue for the glutamyl (E) residue at position 7. LNAMKGDLQSSL was present only in Ag85B of M. tuberculosis and BCG. Homologous sequences identified by BLASTP in other Ag85 complex proteins of M. tuberculosis and other mycobacterial species differed primarily by substitution of a prolyl or an alanyl for the glycyl (G) residue at position 6. GSKPPSGSPETGAG and LDQASQRGLGE were identified only in M. tuberculosis PstS-1 by BLASTP analysis. Very weakly homologous sequences to GSKPPSGSPETGAG identified by PSI-BLAST differing in multiple positions were present in hypothetical proteins of unknown function in M. tuberculosis and other bacteria. Very weakly homologous sequences to LDQASQRGLGE identified by PSI-BLAST were present in PstS subunits of ATP binding cassette transporters in M. intracellulare and in other bacteria but differed at multiple positions (positions 2 through 7) from the M. tuberculosis PstS-1 sequence. Antigens for immunization were generated by cloning oligonucleotides encoding SSDPAWERNDPT, LNAMKGDCQSSL, GSKPPSGSPETGAG, or LNAMKGDCQSSL into the proprietary SSNAP system (Promega Corp., Madison, Wis.) for expression of fusion proteins (22). Briefly, the SSNAP vector attaches the antigen sequence to a proprietary nonantigenic protein of limited solubility in aqueous solutions that allows for easy purification of the expressed fusion protein by centrifugation. Oligoclonal IgY antibodies were raised to purified fusion proteins in chickens (Rockland, Gilbertsville, Pa.) (22), and IgY was isolated from eggs of immunized chickens using the EGGstract purification system (Promega). In some cases, IgY antibodies were further purified by affinity purification against their immunogen peptides (Genosys, The Woodlands, Tex.) coupled to an activated agarose resin (Pierce Chemical Co., Rockford, Ill.).
Proteins and monoclonal antibodies.
Concentrated culture filtrate proteins from BCG and M. avium ATCC 25291 were prepared as described previously (14) from zinc-supplemented mycobacterial cultures. Purified BCG Ag85 complex proteins contained 60% Ag85A, 30% Ag85B, and 10% Ag85C as judged by Western blotting against monoclonal anti-BCG Ag85A, Ag85B, and Ag85C. The initial lot of Ag85 complex proteins was designated as containing 1 mU of Ag85 complex immunoreactive units per mg (7). Recombinant M. tuberculosis Ag85B and PstS-1 (11) were used as standards in Western and dot blot analyses. Initial lots of recombinant antigens were defined as containing 1 mU of Ag85B and PstS-1 immunoreactive units, respectively, per mg. Immunoreactive unitage was not comparable between individual antigen standards but was constant between different aliquots within any single lot of standard. Unitage for each subsequent antigen preparation was determined by parallel-line analysis of dot blots of initial and subsequent materials (7). Mouse immunoglobulin G1 (IgG1) anti-BCG Ag85 complex, clone 240 (7), reacted strongly with purified M. tuberculosis and M. bovis BCG Ag85A and Ag85B and weakly with M. tuberculosis and M. bovis BCG Ag85C (14). This antibody reacted minimally with purified M. avium Ag85B, strongly with some or all purified Ag85 proteins from other mycobacterial species (M. kansasii, M. gordonae, M. fortuitum, M. phlei, and M. xenopi) and not at all with purified Ag85 proteins from M. smegmatis (14). The Ag85 epitope recognized by clone 240 is not known but is probably conformational (reference 21 and our unpublished observations).
Immunoblotting.
Recombinant and native mycobacterial antigens were separated on a 4 to 20% Novex polyacrylamide gel, transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, Calif.), and developed using 1 μg of primary antibody per ml, appropriate alkaline phosphatase-conjugated second antibodies (Promega), and Western blue AP substrate (Promega) (22).
Dot immunobinding.
Mycobacterial antigen levels in human sera were determined by dot immunobinding using mouse anti-BCG Ag85 complex (clone 240), chicken anti-Ag85B (85CPL4) or chicken anti-PstS-1 (38CPL2) antibodies, appropriate horseradish peroxidase-conjugated second antibodies, enhanced chemiluminescence technology (ECL or ECL-Plus; Amersham Pharmacia Biotech), and standard X-ray film (Kodak) (7). Developed X-ray films were evaluated visually by comparison to antigen standards included on each blot. Previous studies have indicated that visual and densitometric quantitation yield equivalent results (reference 32 and our unpublished observations). Serum samples were assayed in triplicate for their content of immunoreactive Ag85 complex, Ag85B, and PstS-1, using native BCG Ag85 complex, recombinant M. tuberculosis Ag85B, or recombinant M. tuberculosis PstS-1, respectively, as antigen standards. The results are reported as geometric means in immunoreactive units. For purposes of computation, samples not reactive at 4 μU/ml were assumed to contain 0.4 μU/ml. For analysis of serum antigen levels, significantly elevated levels were defined as values more than 6 standard errors of the mean (SEM) above mean values in healthy controls; these were values greater than 23 μU/ml for Ag85 complex, 170 μU/ml for Ag85B, and 64 μU/ml for PstS-1.
Statistical analysis.
The significance of differences in medians was determined by Kruskal-Wallis one-way analysis of variance by ranks and a Dunn multiple comparison posttest or by the Mann-Whitney test (13). The significance of differences in levels of mycobacterial immunoreactivity and sensitivity and specificity were determined by Fisher's exact test.
RESULTS
Characterization of antibodies.
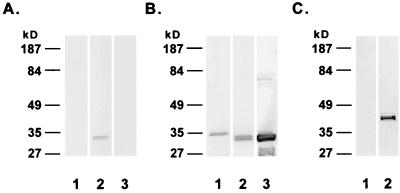
Mouse IgG1 anti-BCG Ag85 complex antibody reacted primarily if not exclusively with a 32-kDa band in 50 ng of purified native Ag85 complex proteins (Fig. 1A, lane 2). It did not react in immunoblotting analyses with 50 ng of M. tuberculosis rAg85B (lane 1) or with 200 ng of a concentrated M. avium culture filtrate (lane 3). This monoclonal antibody has been previously shown to react minimally if at all with high doses of purified M. avium Ag85 complex proteins (14) and not to react significantly with sera from patients with disseminated M. avium disease (7). These latter observations are consistent with its lack of reactivity with M. avium Ag85 in immunoblotting (lane 3).
FIG. 1.
Specificity of mouse monoclonal antibody (A) and chicken antipeptide antibodies (B and C) for secreted mycobacterial proteins. (A and B) Reactivity of mouse monoclonal IgG1 anti-BCG Ag85 complex (1 μg/ml) (A) and chicken IgY antipeptide antibody 85CPL4 (anti-Ag85B) (1 μg/ml) (B) with 50 ng of purified M. tuberculosis rAg85B (lanes 1), 50 ng of purified native BCG Ag85 complex proteins (lanes 2), and 200 ng of M. avium culture filtrate (lanes 3). (C) Reactivity of IgY antipeptide antibody 38CPL2 (anti-PstS-1) (1 μg/ml) with 50 ng of M. tuberculosis rAg85B (lane 1) and 50 ng of M. tuberculosis rPstS-1 (lane 2).
Immunogens for all four Ag85B and PstS-1 peptide sequences were cloned, expressed, and purified in parallel within a week. They were used to raise antipeptide antibodies which were then screened for reactivity against the parent recombinant protein antigens by indirect enzyme-linked immunosorbent assay. Two chicken IgY antipeptide antibodies, 85BCPL4 against the sequence SSDPAWERNDPT (anti-Ag85B) and 38CPL2 against the sequence GSKPPSGSPETGAG (anti-PstS-1), showed the highest level of immunoreactivity against the purified parent antigen proteins and were further characterized.
IgY fractions of chicken anti-Ag85B easily detected 25 ng of purified M. tuberculosis rAg85B (Fig. 1B, lane 1) on immunoblots. After affinity purification, anti-Ag85B detected as little as 1.5 ng of rAg85B (data not shown). Anti-Ag85B detected two bands in the purified BCG Ag85 complex (lane 2), one at 32 kDa containing Ag85A and Ag85C and the other at 30 kDa containing Ag85B (14, 20). The 30-kDa band was consistently darker than the 32-kDa band. The molecular mass of rAg85B in the immunoblots was larger than that of native Ag85B (the lower band in the doublet in lane 2) because of the presence of 6His in the recombinant material (11). Anti-M. tuberculosis Ag85B was cross-reactive with M. avium Ag85 and detected a doublet of 30 and 32 kDa in immunoblots of a concentrated culture filtrate of M. avium (lane 3). The results in Fig. 1A and B indicate that mouse and chicken anti-Ag85 antibodies recognize distinct epitopes.
IgY fractions of chicken anti-PstS-1 reacted with 25 ng of 38-kDa rPstS-1 (Fig. 1C, lane 2) but not with 25 ng of rAg85B (lane 1). This antibody also reacted weakly with a 38-kDa protein in M. avium culture filtrates (data not shown). After affinity purification, the sensitivity of anti-PstS-1 for immunoblotted rPstS-1 increased from 25 to 6.25 ng (data not shown).
Circulating mycobacterial antigens in patients with active tuberculosis.
Dot immunobinding using monoclonal anti-Ag85 complex, anti-Ag85B, and anti-PstS-1 antibodies provided a simple and reproducible means of measuring circulating mycobacterial proteins in patient sera. The mean coefficient of variation within runs was 14% (15% for low-positive specimens), and the interrun coefficient of variation was 32% (32% for low-positive specimens).
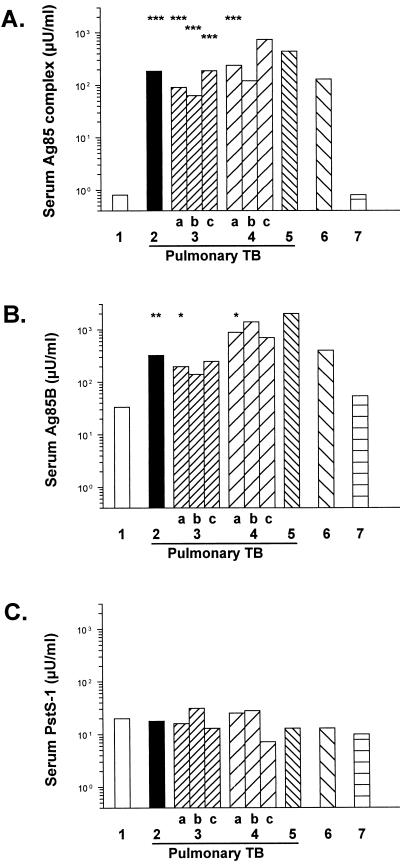
The median levels of circulating Ag85 complex proteins measured with monoclonal anti-Ag85 complex antibody were 60 to 900 times higher in patients with active smear-positive or culture-positive pulmonary tuberculosis than in healthy controls with no active disease and were also significantly higher than those in patients with inactive tuberculosis (P < 0.001 to P < 0.05; Kruskal-Wallis one-way analysis of variance with a Dunn multiple comparison posttest) (Fig. 2). There were no significant differences in serum Ag85 levels measured with monoclonal antibody between smear- and culture-positive patients with pulmonary tuberculosis (Fig. 2), between patients with pulmonary tuberculosis under and over 40 years of age (data not shown), or between patients with inactive tuberculosis and healthy controls (Fig. 2). Median levels of circulating Ag85 detected by monoclonal anti-Ag85 antibody were 3 to 15 times higher in patients with smear- or culture-positive pulmonary tuberculosis who had been treated for more than 15 days than in those who had been treated for less than 15 days, but this difference was not statistically significant (Fig. 2).
FIG. 2.
Circulating median levels (in microunits per milliliter) of Ag85 complex (A), Ag85B (B), and PstS-1 (C) measured by dot immunobinding in Chilean patients with tuberculosis (TB) and controls. Key (numbers of subjects): 1, healthy controls (20 patients); 2, total pulmonary tuberculosis (60 patients); 3, smear-positive tuberculosis (a, total [51 patients]; b, treated less than 15 days before the serum sample was obtained [33 patients]; c, treated more than 15 days before the serum sample was obtained [18 patients]); 4, culture-positive tuberculosis (a, total [8 patients]; b, treated less than 15 days before the serum sample was obtained [6 patients]; c, treated more than 15 days before the serum sample was obtained [2 patients]); 5, smear- and culture-negative tuberculosis (1 patient); 6, extrapulmonary tuberculosis (4 patients); 7, inactive tuberculosis (13 patients). See Materials and Methods for details of the patient populations and assay. Unitages for individual mycobacterial antigens cannot be compared to each other. Median serum Ag85 complex and Ag85B levels were significantly higher in patients with active pulmonary tuberculosis than in healthy controls (∗∗∗, P < 0.001 [Kruskal-Wallis test with Dunn posttest]; ∗∗, P < 0.01, ∗, P < 0.025 [Mann-Whitney test]).
Median levels of Ag85B in patients with active smear- or culture-positive pulmonary tuberculosis measured using IgY fractions of anti-Ag85B were 6 to 27 times higher than those in healthy controls (P = 0.011 to P = 0.026; Mann-Whitney test) (Fig. 2). In contrast, median levels of PstS-1 measured using IgY fractions of anti-PstS-1 antibody were similar in pulmonary tuberculosis patients and controls (Fig. 2). For both anti-peptide antibodies, the results obtained using affinity-purified antibodies were similar to those obtained using IgY fractions of these antibodies (data not shown).
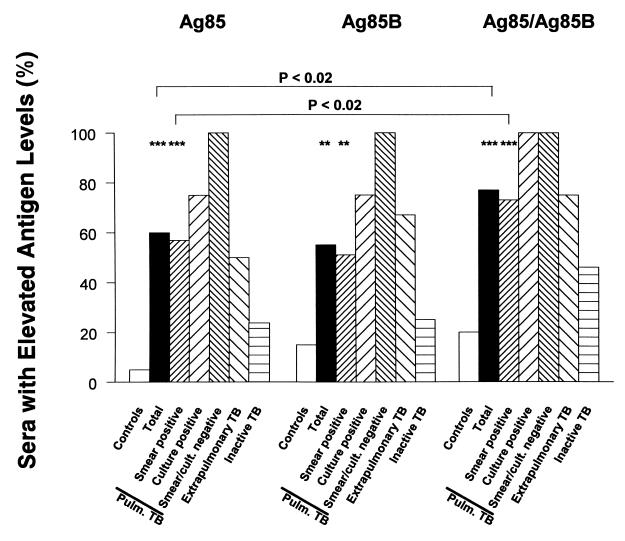
Sera from patients with active pulmonary tuberculosis were significantly more likely to contain significantly elevated levels (more than 6 SEM above mean values in healthy controls) of circulating immunoreactive Ag85 complex or Ag85B than were sera from healthy controls (P < 0.001 and P < 0.002, respectively; Fisher's exact test) (Fig. 3). The presence of significantly elevated levels of PstS-1 was not significantly different in patients with pulmonary tuberculosis and in controls (data not shown).
FIG. 3.
Percentage of sera showing significant elevations (values greater than 6 SEM above the means in healthy controls) of circulating mycobacterial antigens in Chilean patients with tuberculosis and in controls. See the legend to Fig. 2 and Materials and Methods for details. The percentage of sera showing significant elevation in Ag85 complex or Ag85B levels was significantly higher in patients with pulmonary tuberculosis than in healthy controls (∗∗∗, P < 0.001; ∗∗, P < 0.002 [Fisher exact test]). Sensitivity was significantly increased by combinatorial consideration of reagent results (P < 0.02, Fisher exact test).
The sensitivity and specificity of significantly elevated circulating Ag85 levels detected by using anti-Ag85 complex were 60 and 95%, respectively, while the sensitivity and specificity of detection of significantly elevated circulating Ag85 levels detected by using anti-Ag85B antibody were 55 and 85%, respectively. When the results obtained with both anti-Ag85 antibodies were considered jointly, the sensitivity and specificity of significantly elevated serum Ag85 levels were 77 and 80%, respectively (P < 0.001; Fisher's exact test). The increase in sensitivity when the results obtained with both Ag85 antibody reagents were considered jointly (Fig. 3) was significant (P < 0.02; Fisher's exact test). Of the four patients with extrapulmonary tuberculosis (Fig. 3), those with renal, lymph node, and pleural tuberculosis had marked elevations in circulating Ag85 complex protein and Ag85B levels while the patient with miliary tuberculosis showed a marked elevation only in circulating PstS-1 levels (data not shown). Joint consideration of results derived from experiments with all three antibodies (anti-Ag85 complex, anti-Ag85B, and anti-PstS-1) did not increase the sensitivity and specificity of the test (data not shown).
DISCUSSION
Circulating levels of Ag85 measured by using mouse monoclonal anti-Ag85 complex antibody have been previously shown to be significantly higher in patients with culture-positive tuberculosis from New York City than in controls with other diseases or no disease (7). The present study has confirmed this observation in patients from Santiago, Chile, and has extended it to a larger cohort of smear-positive patients with pulmonary tuberculosis. This study also examined whether anti-peptide antibodies reactive with Ag85B and PstS-1 could be used to increase the diagnostic sensitivity of this test. While significantly elevated levels of circulating Ag85B were detected in patients with active tuberculosis compared to healthy controls, there was no significant difference in circulating PstS-1 levels between tuberculosis patients and healthy controls.
The immunogens used to raise antipeptide antibodies were based on cloning and expression of peptide-carrier fusion proteins of limited aqueous solubility containing peptide sequences derived from secreted mycobacterial proteins (22). These rapidly and inexpensively produced immunogens generate antipeptide antibodies recognizing the parent proteins more readily than do peptide conjugates made with more immunogenic and more soluble proteins (22). Although anti-peptide antibodies raised against M. tuberculosis Ag85B and PstS-1 specifically recognized the parent proteins, they were cross-reactive with related mycobacterial proteins. The cross-reactivity of anti-PstS-1 peptide antibodies is consistent with the occurrence of closely related sequences in several entries in the unfinished M. avium genome. The high cross-reactivity of antibodies raised to the M. tuberculosis Ag85B SSDPAWERNDPT sequence with M. tuberculosis Ag85A/Ag85C and with Ag85 proteins in M. avium suggests that the change of the central amino acid residue from glutamic acid (E) to glutamine in these other Ag85 complex proteins is not associated with a major change in epitope specificity.
The broad antigenic specificity of anti-Ag85B was in sharp contrast with the narrower specificity of the monoclonal anti-Ag85 complex used in these studies. The latter antibody reacted strongly only with purified BCG Ag85 complex proteins, and its lack of reactivity with M. avium culture filtrate proteins was consistent with the lack of immunoreactivity in sera from HIV-positive patients with disseminated M. avium disease (7). The cross-reactivity of anti-Ag85B might in fact interfere with interpretation of Ag85 assay positivity in HIV-positive patients with M. avium disease in places where such disease is common (7). M. avium disease is, however, extremely rare in Chile even in HIV-positive persons (R. Sepulveda, unpublished observations), and the only HIV-positive person in our study had smear-positive tuberculosis and significant elevation of Ag85 levels detected by both anti-Ag85 antibodies. The cross-reactivity of anti-M. tuberculosis Ag85B with M. avium Ag85 in this patient was therefore unlikely to interfere with interpretation of the Ag85 antigen test. Pulmonary disease due to other nontuberculous mycobacteria is also very uncommon in Chile (Sepulveda, unpublished), so that the reactivities of monoclonal anti-BCG Ag85 complex with the Ag85 proteins of other mycobacterial species would also be unlikely to influence the interpretation of Ag85 tests.
High levels of circulating Ag85 complex proteins were readily demonstrable by both anti-Ag85 antibodies in patients with active tuberculosis, while healthy controls had low levels. The independence of these high Ag85 levels from the time of antimicrobial treatment was surprising, since all treatment regimens included isoniazid and since short-term isoniazid treatment has been associated with increased Ag85 secretion by M. tuberculosis in vitro (16) and with increased levels of Ag85 in sputum of patients (33). The results of Wallis et al. (33) in particular suggest that a 2-week period of treatment would be sufficient to observe an increase in secreted Ag85 levels.
Significant elevations of Ag85 immunoreactivity were more frequent in occasional sera from healthy patients with treated inactive tuberculosis than in healthy controls, a pattern previously observed in patients from New York City (7). The differences in the frequency of elevated Ag85 levels between patients with inactive tuberculosis and healthy controls were not significant (Fig. 2), and all patients with inactive tuberculosis with markedly elevated Ag85 levels have remained well. The cause of these elevated levels in patients with inactive tuberculosis remains unknown, but they would make serum Ag85 measurement a poor choice for a diagnostic assay for active disease in this patient group.
With regard to the healthy control population, the low Ag85 levels measured by using monoclonal anti-Ag85 in healthy controls from Santiago were similar to those previously found in healthy controls from New York City (7). Because Chile has had an extensive national BCG vaccination program for more than 40 years (27), these results indicate that elevated levels of circulating Ag85 can be detected in adult patients with active tuberculosis who were vaccinated with BCG as children.
The overall sensitivity (60 to 77%) and specificity (80 to 93%) of the host immune response-independent Ag85 immunoassay in detecting circulating mycobacterial proteins in patients with active tuberculosis was comparable to the sensitivities and specificities of host immune response-dependent serological tests to detect circulating antibodies to well-defined mycobacterial proteins. They were lower than the sensitivities and specificities of nonculture nucleic amplification methods. The latter methods have sensitivities and specificities of 66 to 97% and 85 to 99%, respectively (reviewed in reference 34 and 37). The sensitivities and specificities of tests for individual anti-mycobacterial antibodies range from 7 to 83% and from 80 to 97%, respectively, with the lowest sensitivities being found in sputum smear-negative and HIV-positive tuberculosis patients (8, 10, 25). Combinatorial evaluation of results of tests for host anti-mycobacterial antibody production increased the specificity (24, 26) but sometimes decreased the sensitivity (26).
Although anti-PstS-1 peptide antibody detected rPstS-1 as well as elevated levels of immunoreactive material in some patient sera, PstS-1 levels in the entire group of patient sera were not significantly different from those in healthy controls, and detection of significant elevations of circulating PstS-1 levels did not increase the number of tuberculosis patients showing elevated levels of circulating mycobacterial proteins. Patients with active tuberculosis are clearly exposed to secreted PstS-1 and make specific antibodies whose titer is maintained during antimicrobial treatment (12, 30). The reasons for the lack of detectably increased circulating levels of PstS-1 in patients with active tuberculosis are unclear. Whatever the exact mechanisms, our results suggest that it may not be possible to demonstrate increases in the levels of mycobacterial proteins other than Ag85 in sera of patients with active tuberculosis.
The results of the present study and a previous study (7) suggest that immunodetection of elevated levels of Ag85 in serum by dot immunobinding could provide a simple, rapid, and inexpensive diagnostic test for active tuberculosis. This test was not affected by host HIV infection (7), could distinguish between active infection with M. tuberculosis and prior BCG vaccination (this study) or prior infection with M. tuberculosis (7), could distinguish between active infection with M. tuberculosis and active infection with M. avium (7), and gave similar results in smear-positive and smear-negative tuberculosis patients (this study). Although the sensitivity of the Ag85 assay using monoclonal anti-Ag85 complex proteins was moderate, it could be increased by combining results obtained using a second anti-Ag85 antibody recognizing a different epitope. Unfortunately, this increase in sensitivity was accompanied by a loss of specificity. While the results of this study are preliminary due to the small population studied, they suggest that an optimal antibody cocktail could prove as useful for the development of a simple host immune system-independent diagnostic assay for active tuberculosis as an antigen cocktail appears to be in developing tuberculosis diagnostic assays dependent on the host anti-mycobacterial immune response (17, 23, 24).
ACKNOWLEDGMENTS
We thank Edwig C. Rodriguez for assistance in sample collection and Felipe C. Cabello for critical reading of the manuscript and many helpful discussions.
This work was supported by U.S. Public Health Service grants AI42452 (M.H.-F.), AI45925 (H.P.G), and AI36989 (M.L.G.) and by grant G-0355.95 from the Fonds voor Wetenschappelijk Onderzoek, Vlaanderen (K.H.).
REFERENCES
- 1.Abou-Zeid C, Ratliff T L, Wiker H G, Harboe M, Bennedsen J, Rook G A W. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infect Immun. 1988;56:3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen P, Askgaard D, Ljungqvist L, Bennedsen J, Heron I. Proteins released from Mycobacterium tuberculosis during growth. Infect Immun. 1991;59:1905–1910. doi: 10.1128/iai.59.6.1905-1910.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen P, Munk M E, Pollock J M, Doherty T M. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–1104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. The World Health Report 1999. Making a difference. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 5.Bass J B., Jr Tuberculin test, preventive therapy, and elimination of tuberculosis. Am Rev Respir Dis. 1990;141:812–813. doi: 10.1164/ajrccm/141.4_Pt_1.812. [DOI] [PubMed] [Google Scholar]
- 6.Belisle J T, Vissa V D, Sievert T, Takayama K, Brennan P J, Basra G S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biosynthesis. Science. 1997;276:1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 7.Bentley-Hibbert S I, Quan X, Newman T, Huygen K, Godfrey H P. Pathophysiology of antigen 85 in patients with active tuberculosis: antigen 85 circulates as complexes with fibronectin and immunoglobulin G. Infect Immun. 1999;67:581–588. doi: 10.1128/iai.67.2.581-588.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bothamley G H. Serological diagnosis of tuberculosis. Eur Respir J. 1995;8(Suppl. 20):676S–688S. [PubMed] [Google Scholar]
- 9.Braibant M, Lefèvre P, de Wit L, Peirs P, Ooms J, Huygen K, Andersen Å. A Mycobacterium tuberculosis gene cluster encoding proteins of a phosphate transporter homologous to the Escherichia coli Pst system. Gene. 1996;176:171–176. doi: 10.1016/0378-1119(96)00242-9. [DOI] [PubMed] [Google Scholar]
- 10.Chan E D, Heifets L, Iseman M D. Immunologic diagnosis of tuberculosis: a review. Tuberc Lung Dis. 2000;80:131–140. doi: 10.1054/tuld.2000.0243. [DOI] [PubMed] [Google Scholar]
- 11.Colangeli R, Jeijbel A, Williams A M, Manca C, Chan J, Lyashchenko K, Gennaro M L. Three-step purification of lipopolysaccharide-free, polyhistidine-tagged recombinant antigens of Mycobacterium tuberculosis. J Chromatogr. 1998;714:223–235. doi: 10.1016/s0378-4347(98)00094-2. [DOI] [PubMed] [Google Scholar]
- 12.Cole R A, Lu H M, Shi Y Z, Wang J, De-Hua T, Zhou A T. Clinical evaluation of a rapid immunochromatographic assay based on the 38 kDa antigen of Mycobacterium tuberculosis on patients with pulmonary tuberculosis in China. Tuberc Lung Dis. 1996;77:363–368. doi: 10.1016/s0962-8479(96)90103-3. [DOI] [PubMed] [Google Scholar]
- 13.Daniel W W. Applied non-parametric statistics. Boston, Mass: PWS-Kent Publishers; 1990. [Google Scholar]
- 14.Drowart A, De Bruyn J, Huygen K, Damiani G, Godfrey H P, Stelandre M, Yernault J-C, Van Vooren J-P. Isoelectric characterization of protein antigens present in mycobacterial culture filtrates and recognized by monoclonal antibodies directed against the Mycobacterium bovis BCG antigen 85 complex. Scand J Immunol. 1992;36:697–702. doi: 10.1111/j.1365-3083.1992.tb03130.x. [DOI] [PubMed] [Google Scholar]
- 15.Enarson D A, Murray J F. Global epidemiology of tuberculosis. In: Rom W N, Garay S M, editors. Tuberculosis. New York, N.Y: Little, Brown; 1996. pp. 57–75. [Google Scholar]
- 16.Garbe T R, Hibler N S, Deretic V. Insoniazid induces expression of the antigen 85 complex in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:1754–1756. doi: 10.1128/aac.40.7.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gennaro M L. Immunologic diagnosis of tuberculosis. Clin Infect Dis. 2000;30(Suppl. 3):S243–S246. doi: 10.1086/313868. [DOI] [PubMed] [Google Scholar]
- 18.Godfrey H P, Feng Z-H, Mandy S, Mandy K, Huygen K, De Bruyn J, Abou-Zeid C, Wiker H G, Nagai S, Tasaka H. Modulation of expression of delayed hypersensitivity by mycobacterial antigen 85 fibronectin-binding proteins. Infect Immun. 1992;60:2522–2528. doi: 10.1128/iai.60.6.2522-2528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harboe M, Wiker H G. The 38-kDa protein of Mycobacterium tuberculosis: a review. J Infect Dis. 1992;166:874–884. doi: 10.1093/infdis/166.4.874. [DOI] [PubMed] [Google Scholar]
- 20.Harth G, Lee B-Y, Wang J, Clemens D L, Horwitz M A. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect Immun. 1996;64:3036–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huygen K, Lozes E, Gilles B, Drowart A, Palfliet K, Jurion F, Roland I, Art M, Dufaux M, Nyabenda J, De Bruyn J, Van Vooren J-P, DeLeys R. Mapping of TH1 helper T-cell epitopes on major secreted mycobacterial antigen 85A in mice infected with live Mycobacterium bovis BCG. Infect Immun. 1994;62:363–370. doi: 10.1128/iai.62.2.363-370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knuth M W, Okragly A J, Lesley S A, Haak-Frendscho M. Facile generation and use of immunogenic polypeptide fusions to a sparingly soluble non-antigenic carrier. J Immunol Methods. 2000;236:53–69. doi: 10.1016/s0022-1759(99)00230-6. [DOI] [PubMed] [Google Scholar]
- 23.Lyashchenko K, Colangeli R, Houde M, Jahdali H A, Menzies D, Gennaro M L. Heterogeneous antibody responses in tuberculosis. Infect Immun. 1998;66:3936–3940. doi: 10.1128/iai.66.8.3936-3940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lyashchenko K P, Singh M, Colangeli R, Gennaro M L. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J Immunol Methods. 2000;242:91–100. doi: 10.1016/s0022-1759(00)00241-6. [DOI] [PubMed] [Google Scholar]
- 25.McDonough J A, Sada E D, Sippola A A, Ferguson L E, Daniel T M. Microplate and dot immunoassays for the serodiagnosis of tuberculosis. J Lab Clin Med. 1992;120:318–322. [PubMed] [Google Scholar]
- 26.Pottumarthy S, Wells V C, Morris A J. Comparison of seven tests for serological diagnosis of tuberculosis. J Clin Microbiol. 2000;38:2227–2231. doi: 10.1128/jcm.38.6.2227-2231.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sepulveda R L, Arredondo S, Rodriguez E, Leiva L E, Sorensen R U. Effect of human newborn BCG immunization on monocyte viability and function at 3 months of age. Int J Tuberc Lung Dis. 1997;1:122–127. [PubMed] [Google Scholar]
- 28.Sepulveda R L, Gonzalez B, Gerszencveig R, Ferrer X, Martinez B, Sorensen R U. The influence of BCG immunization on tuberculin reactivity in healthy Chilean women in the third trimester of pregnancy. Tuberc Lung Dis. 1995;76:28–34. doi: 10.1016/0962-8479(95)90576-6. [DOI] [PubMed] [Google Scholar]
- 29.Shinnick T M. Diagnostic test needs for evaluating antituberculosis vaccines. Clin Infect Dis. 2000;30(Suppl. 3):S276–S278. doi: 10.1086/313873. [DOI] [PubMed] [Google Scholar]
- 30.Sousa A O, Wargnier H, Poinsignon Y, Simonney N, Gerger F, Lavigne F, Herrmann J L, Lagrange P H. Kinetics of circulating antibodies, immune complex and specific antibody-secreting cells in tuberculosis patients during 6 months of antimicrobial therapy. Tuberc Lung Dis. 2000;80:27–33. doi: 10.1054/tuld.1999.0230. [DOI] [PubMed] [Google Scholar]
- 31.Toossi Z, Ellner J J. Mechanisms of anergy in tuberculosis. Curr Top Microbiol Immunol. 1996;215:221–238. doi: 10.1007/978-3-642-80166-2_10. [DOI] [PubMed] [Google Scholar]
- 32.Van Vooren J P, Turneer M, Yernault J C, De Bruyn J, Burton E, Legros F, Farber C M. A multidot immunobinding assay for the serodiagnosis of tuberculosis. Comparison with an enzyme-linked immunosorbent assay. J Immunol Methods. 1988;113:45–49. doi: 10.1016/0022-1759(88)90380-8. [DOI] [PubMed] [Google Scholar]
- 33.Wallis R S, Perkins M, Phillips M, Joloba M, Demchuk B, Namale A, Johnson J L, Williams D, Wolski K, Teixeira L, Dietze R, Mugerwa R D, Eisenach K, Ellner J J. Induction of the antigen 85 complex of Mycobacterium tuberculosis in sputum: a determinant of outcome in pulmonary tuberculosis treatment. J Infect Dis. 1998;178:1115–1121. doi: 10.1086/515701. [DOI] [PubMed] [Google Scholar]
- 34.Watterson S A, Drobniewski F A. Modern laboratory diagnosis of mycobacterial infections. J Clin Pathol. 2000;53:727–732. doi: 10.1136/jcp.53.10.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wiker H G, Harboe M, Nagai S. A localization index for distinction between extracellular and intracellular antigens of Mycobacterium tuberculosis. J Gen Microbiol. 1991;137:875–884. doi: 10.1099/00221287-137-4-875. [DOI] [PubMed] [Google Scholar]
- 36.Wiker H G, Harboe M. The antigen 85 complex: a major secretion product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woods G L. Molecular techniques in mycobacterial detection. Arch Pathol Lab Med. 2001;125:122–126. doi: 10.5858/2001-125-0122-MTIMD. [DOI] [PubMed] [Google Scholar]