Although kidney-related complications are not a common side effect of coronavirus disease-19 (COVID-19) vaccination, complications such as abnormal urinalysis and worsening renal function have recently been reported in patients with underlying chronic glomerulonephritis [1]. Herein we report a case of gross hematuria and exacerbation of proteinuria after COVID-19 vaccination in a patient with known immunoglobulin A nephropathy (IgAN) (Fig. 1).
Figure 1. Clinical course of the patient.
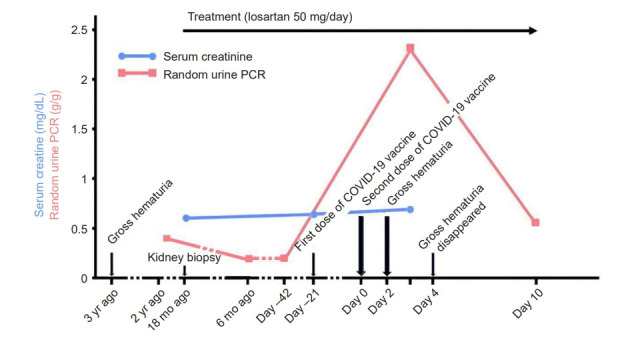
Immunoglobulin A nephropathy was diagnosed 18 months earlier. Gross hematuria appeared two days after the second messenger RNA coronavirus disease 2019 (COVID-19) vaccine dose with a peak increase in random urine protein-to-creatinine ratio (PCR). Although urine PCR improved quickly, a complete recovery to the previous state was not seen. Serum creatinine levels were stable before and after vaccination.
A 27-year-old female presented with brown-red hematuria 2 days after the second dose of messenger RNA (mRNA) COVID-19 vaccine (Pfizer-BioNTech). Hematuria did not appear after the first dose. Chills and myalgia were felt several hours after vaccination, however, improved quickly within 24 hours of injection. Laboratory findings were as follows: urine blood, 3+; urine red blood cell (RBC), >20/high power field (HPF); urine protein, 4+; random urine protein-to-creatinine ratio (PCR), 2.295 g/g; and serum creatinine, 0.69 mg/dL. On the 4th day post-vaccine, gross hematuria resolved with conservative treatment. On the tenth day, laboratory findings were as follows: urine blood, 3+; urine RBC, 11–20/HPF; and random urine PCR, 0.541 g/g. The initial IgAN diagnosis (Oxford MEST-C classification as M1-E0-S1-T0-C0) was made 1.5 years before this event during an evaluation for asymptomatic urinalysis with normal kidney function (urine RBC, 11–20/HPF; urine PCR, 0.543 g/g; and serum creatinine, 0.57 mg/dL). The patient was receiving treatment with losartan 50 mg per day and proteinuria and kidney function were stable (urine PCR, 0.17–0.18 g/g and serum creatinine, 0.65 mg/dL) until this event. Gross hematuria self-resolved without intervention and proteinuria level improved, but proteinuria did not return to baseline and remained higher than usual.
Table 1 summarizes cases that showed renal complications after COVID-19 vaccination in patients with IgAN. We observed some important findings. First, gross hematuria can be the first manifestation of newly diagnosed IgAN in previously healthy patients. Second, IgAN flares can relapse after just single injection; however, de novo IgAN only follows a second injection. Third, the latent period from injection to gross hematuria was short (even within a day) in all cases. Fourth, increased proteinuria and serum creatinine level can accompany gross hematuria. Finally, all cases of gross hematuria occurred in patients who received the mRNA COVID-19 vaccine, not other vaccine types.
Table 1.
Reported cases of gross hematuria following COVID-19 vaccination in IgAN patients
| IgAN | Age (yr)/sex | Vaccine | Dose | Latent period (day) | Systemic sign/symptom | Initial sign | Duration of gross hematuria | Residual sign | Reporta |
|---|---|---|---|---|---|---|---|---|---|
| Known | 22/male | MO | 1st | 2 | Arthralgia | H, P | ND | No | Perrin et al., 2021 |
| 2nd | 2 | ||||||||
| Known | 41/female | PB | 1st | 2 | Leukocytosis | C | ND | No | Perrin et al., 2021 |
| Known | 13/male | PB | 2nd | <1 | Vomiting | H, C | 2 days | No | Hanna et al., 2021 |
| Known | 38/female | MO | 2nd | 1 | Fever | H, P | 3 days | P | Negrea et al., 2021 |
| Known | 38/female | MO | 2nd | 1 | Fever | H | 3 days | No | Negrea et al., 2021 |
| Known | 52/female | PB | 2nd | 1 | Fever | H, P | 5 days | P | Rahim et al., 2021 |
| Known | ND/male | PB | 2nd | 1 | Fever | H, P | 3 days | No | Plasse et al., 2021 |
| Known | 22/female | MO | 2nd | <2 | ND | H, P | <1 month | P | Park et al., 2021 |
| Known | 39/female | MO | 2nd | <2 | ND | H, P | <1 month | No | Park et al., 2021 |
| Known | 27/female | PB | 2nd | 2 | Pancytopenia | H | ND | No | Perrin et al., 2021 |
| Known | ND/male | PB | 2nd | 6 | Myalgia | H, C, P | ND | C, P | Plasse et al., 2021 |
| Known | 78/female | MO | ND | 7 | Diarrhea | H | 15 days | No | Obeid et al., 2021 |
| Known | 27/female | PB | 2nd | 2 | Fever | H, P | 4 days | No | Presenting case |
| De novo | 39/male | MO | 2nd | Immediately | Fever | H, C, P | weeks | H | Anderegg et al., 2021 |
| De novo | 17/male | PB | 2nd | <1 | Hypertension | H, C, P | 5 days | C, P | Hanna et al., 2021 |
| De novo | 41/female | PB | 2nd | 1 | Myalgia | H, C, P | ND | ND | Tan et al., 2021 |
| De novo | 50/male | MO | 2nd | 1 | Rash | H, C, P | <1 month | C, P | Park et al., 2021 |
| De novo | 50/female | MO | 2nd | 2 | Fever | H, C, P | 5 days | ND | Kudose et al., 2021 |
| De novo | 19/male | MO | 2nd | 2 | ND | H, C | 2 days | ND | Kudose et al., 2021 |
| De novo | 30/male | MO | 2nd | 1 | Fever | H, P | 2 days | P | Abramson et al., 2021 |
C, increased creatinine; COVID-19, coronavirus disease-19; F, female; H, gross hematuria; IgAN, immunoglobulin A nephropathy; M, male; MO, Moderna; ND, no data; P, increased proteinuria; PB, Pfizer-BioNTech.
See the Supplementary material (available online) for the references below.
The pathogenesis of gross hematuria in IgAN is not fully understood. The present consensus ‘multi-hit hypothesis’ theoretically explains the pathogenesis as a four-step model: elevation of circulating galactose-deficient IgA1 (Gd-IgA1) level; production of anti-glycan antibody against Gd-IgA1; formation of an immune complex consisting of Gd-IgA1 and anti-glycan antibody; and, finally, deposition of an immune complex in the glomerular mesangium [2]. However, the role of mRNA COVID-19 vaccines in the pathogenesis of IgAN remains unclear. Considering the short latent period from injection to gross hematuria and the finding that neutralizing antibody does not increase during the 7 days after the first injection in the serum of vaccinated patients [3], it is reasonable to postulate that gross hematuria is not associated with neutralizing antibody, but with more immediate immunologic factors such as cytokines or IgA1 anti-glycan immune responses [4].
There have been reports of other glomerulonephritis (including Henoch-Schönlein purpura) relapse or new diagnosis after viral vector-based COVID-19 vaccines (manufactured by Oxford-AstraZeneca) [1,5–7]; however, all gross hematuria in patients with IgAN were reported only after an mRNA COVID-19 vaccine. The reason for this remains to be elucidated. In viral vector-based COVID-19 vaccines, the gene encoding the spike protein of severe acute respiratory syndrome coronavirus-2 is delivered to host cells by an adenovirus vector [8]. For the preexisting anti-adenovirus antibody that circulates in majority of population and can be cross-reactive with various serotypes of adenovirus, vaccine immunogenicity can be attenuated by preexisting antibody [8]. However, mRNA COVID-19 vaccines use a lipid nanoparticle as delivery vehicle (not adenovirus); therefore, interference from a preexisting antibody is not relevant to immunogenicity [8]. We assume that such differences in vaccine mechanism and immunogenicity may be one potential answer to question of why gross hematuria is reported after only a specific type of vaccine in IgAN patients.
The present case described gross hematuria and exacerbation of proteinuria without acute kidney injury following mRNA COVID-19 vaccine in an IgAN patient. To the best of our knowledge, this is the first such case report in Korea. Our findings suggest the following important points: First, gross hematuria can occur as the first manifestation of de novo IgAN even within a day after mRNA COVID-19 vaccine injection. Second, patients should be warned about the possibility of relapse or de novo IgAN before their first or second dose of mRNA COVID-19 vaccine, respectively. Third, serial assessment of urine protein and PCR for increased proteinuria and serum creatinine should be performed in at-risk patients. Finally, kidney biopsy, if indicated, should be performed to diagnose de novo IgAN. Further study is required to clarify the role of mRNA COVID-19 vaccines in the pathogenesis of IgAN.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This work was supported by a National Research Foundation (NRF) of Korea grant funded by the Korean government (Ministry of Science and ICT) (2020R1A2C2003438) and Soonchunhyang University Research Fund.
Authors’ contributions
Conceptualization: JSP, EYL
Data curation: JSP
Funding acquisition: EYL
Investigation: EYL
Writing–original draft: JSP
Writing–review & editing: JSP, EYL
All authors read and approved the final manuscript.
Supplementary Materials
References
- 1.Bomback AS, Kudose S, D’Agati VD. De novo and relapsing glomerular diseases after COVID-19 vaccination: what do we know so far? Am J Kidney Dis. 2021;78:477–480. doi: 10.1053/j.ajkd.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pattrapornpisut P, Avila-Casado C, Reich HN. IgA nephropathy: core curriculum 2021. Am J Kidney Dis. 2021;78:429–441. doi: 10.1053/j.ajkd.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 3.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 4.Kudose S, Friedmann P, Albajrami O, D’Agati VD. Histologic correlates of gross hematuria following Moderna COVID-19 vaccine in patients with IgA nephropathy. Kidney Int. 2021;100:468–469. doi: 10.1016/j.kint.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villa M, Díaz-Crespo F, Pérez de José A, et al. A case of ANCA-associated vasculitis after AZD1222 (Oxford-AstraZeneca) SARS-CoV-2 vaccination: casualty or causality? Kidney Int. 2021;100:937–938. doi: 10.1016/j.kint.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillion V, Jadoul M, Demoulin N, Aydin S, Devresse A. Granulomatous vasculitis after the AstraZeneca anti-SARS-CoV-2 vaccine. Kidney Int. 2021;100:706–707. doi: 10.1016/j.kint.2021.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sirufo MM, Raggiunti M, Magnanimi LM, Ginaldi L, De Martinis M. Henoch-Schönlein purpura following the first dose of COVID-19 viral vector vaccine: a case report. Vaccines (Basel) 2021;9:1078. doi: 10.3390/vaccines9101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendonça SA, Lorincz R, Boucher P, Curiel DT. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines. 2021;6:97. doi: 10.1038/s41541-021-00356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


