Abstract
J Clin Hypertens(Greenwich). 2010;12:597–602. © 2010 Wiley Periodicals, Inc.
Microalbuminuria is a prognostic marker of cardiovascular disease and is related to metabolic syndrome (MetS). For this purpose, the authors examined the relationship of low grade albuminuria to MetS, using 4 current definitions and a MetS score. They studied 6650 consecutive, nondiabetic, hypertensive patients with normal microalbumin excretion. MetS was defined by Adult Treatment Panel III, American Heart Association, World Heart Organization, International Diabetes Federation criteria, and MetS Gruppo Italiano per lo Studio della Streptochinasi nell’Infarcto Miocardico (GISSI) score. Urine microalbumin concentration was measured after a 24‐hour urine collection by immunonephelometry. By all definitions, hypertensive patients with MetS had higher microalbumin levels. Significantly higher microalbumin levels were observed as the number of metabolic components rose. After adjustment for systolic blood pressure, the strength of this association was reduced to a nonsignificant level. Microalbumin levels, within normal range, are increased in patients with MetS, irrespective of the definition criteria.
The significance of microalbuminuria, as a prognostic marker of cardiovascular disease, has been established during the past few years. 1 , 2 , 3 , 4 , 5 In essential hypertensives, increased albumin excretion has been associated with blood pressure (BP) values and hyperinsulinemia, as an expression of insulin resistance. 6 , 7 , 8 , 9 Obesity, smoking, and genetics have also been implicated as determinants of microalbuminuria in some studies. 10 Although the association of higher levels of albumin excretion with future cardiovascular events is well described, only recently the importance of low‐grade albuminuria has been investigated. 2 , 5 , 11 , 12 Increases of albuminuria, even within the accepted normal range, are associated with higher cardiovascular risk. 13 , 14
In the general population, metabolic syndrome (MetS) is associated with increased cardiovascular mordibity 15 and mortality. 16 Hypertensive patients with MetS exert 2‐fold greater cardiovascular risk compared to those without MetS. Despite the debate on the distinct definitions of MetS, focusing either on central obesity or on insulin resistance, microalbuminuria is significantly associated with the prevalence of MetS. 17 Moreover, microalbuminuria is included in the determinant criteria of MetS according to the World Health Organization (WHO), but the association of low‐grade albuminuria with MetS has not been fully explored.
The aim of the present study was to investigate whether a continuum of increasing levels of albumin excretion, within the normal range, reflects increasing prevalence of MetS, according to the definitions of WHO, of the National Cholesterol Education Program Adult Treatment Panel III (NCEP‐ATP III), of the American Heart Association (AHA) and the International Diabetes Federation (IDF), and MetS Gruppo Italiano per lo Studio della Streptochinasi nell’Infarcto Miocardico (GISSI) score.
Materials and Methods
Study Population
The study included 7500 patients who attended our outpatient clinics for the first time. Patients with acute and chronic inflammatory disease, endocrine disorders, chronic obstructive pulmonary disease, malignancy, renal insufficiency (serum creatinine >1.5 mg/dL for men and >1.3 mg/dL for women), heart failure, recent (<6 months) cerebrovascular event, coronary artery disease (history of stable, unstable angina or past myocardial infraction), ventricular arrhythmia, atrial fibrillation, sinus bradycardia (<55 beats per minute), sinus tachycardia (>100 beats per minute) or atrioventricular conduction disturbance, and any condition preventing technically adequate ambulatory BP monitoring, were excluded from the study. Patients with microalbuminuria, defined as albumin excretion rate >20 μg/min were also excluded from the study.
The study protocol was approved by the Ethics Committee of Hippokration Hospital. All patients included in the study were subjected to the following procedures: physical examination, medical history, repeated clinical BP measurement, blood sampling for routine laboratory examinations, and 24‐hour ambulatory BP monitoring.
Thus the final cohort comprised 6650 consecutive patients (mean age 53.8 years, 3381 men and 3269 women). Essential hypertension was defined as systolic BP (SBP) >140 mm Hg and/or diastolic BP >90 mm Hg in the sitting position. The diagnosis of hypertension was based on office BP measurements which were taken in 3 consecutive visits 1 week apart. At each visit, BP was measured 3 times with 1‐minute intervals and with the patient resting comfortably, back supported in the sitting position after a 10–15 minute relax period. A mercury sphygmomanometer was used for all measurements with a medium or a large size cuff, according to the patients arm circumference. All patients underwent a 24‐hour ambulatory BP monitoring (Spacelabs 90207; Redmond, WA).
Anthropometric and Biochemical Measurements
In each subject weight and height were measured, while body mass index (BMI) and waist to height were also calculated. Waist circumference was measured at the midpoint between the bottom of the rib cage and above the top of the iliac crest from patients at minimal respiration to the nearest 0.1 cm.
All subjects underwent full laboratory evaluation (blood test, lipids, liver, and kidney function indices). The blood samples were collected from the antecubital vein between 8:00 am and 10:00 am, in a sitting position, after a 12‐hour fast and alcohol absence. The biochemical evaluation was carried out in the same laboratory that followed the criteria of the WHO Lipid Reference Laboratories.
Patients with fasting blood glucose >100 mg/dL were subject to a 2‐hour oral glucose tolerance test with 75 g glucose. Urine microalbumin concentration was measured after a 24‐hour urine collection by immunonephelometry (Dade Behring, Marburg, Germany).
Determination of MetS
The definition of MetS was based on the following criteria:
-
•
ATP III criteria (3 or more of the following including hypertension): waist circumference >102 cm for men or >88 cm for women, serum triglycerides >150 mg/dL, high‐density lipoprotein [HDL] cholesterol <40 mg/dL for men or <50 mg/dL for women, and fasting glucose levels >110 mg/dL.
-
•
AHA (updated ATP III criteria): similar to ATP III, but with lower glucose threshold (glucose >100 mg/dL)
-
•
WHO criteria: impaired glucose tolerance or impaired fasting glucose or insulin resistance plus 2 of the following plus hypertension: waist to hip ratio >0.90 for men or >0.85 for women or BMI >30 kg/m2, serum triglycerides >150 mg/dL, HDL cholesterol <35 mg/dL for men or <39 mg/dL for women, and microalbuminuria defined as albumin excretion rate of 20 mg/L or urinary albumin to creatinine ratio >30 mg/g. Diabetes mellitus was excluded from the WHO criteria.
-
•
IDF criteria: central obesity defined as waist circumference >94 cm for men or 80 cm for women plus any 2 of the following including hypertension: serum triglycerides >150 mg/dL, HDL cholesterol <40 mg/dL for men or <50 mg/dL for women and fasting glucose >100 mg/dL
-
•
GISSI score: According to the GISSI study, points were assigned for each risk factor including male gender, age, hypertension, BMI, serum triglycerides, HDL cholesterol, and fasting glucose. The score was obtained by summing all the individual points. The cutpoint of the total score for the diagnosis of MetS was 28.
Statistical Analysis
Statistical analysis was performed using SPSS package for Windows version 13.0 (SPSS, Chicago, IL.). Values were expressed as means ± standard deviation or as percentages. Means were compared using independent samples Student t‐test or after analysis of variance when appropriate. Analysis of categorical data was carried out with the chi‐square test. The limit of statistical significance was set at P<.05.
Results
Overall, the study population was divided into 4 groups according to urine microalbumin quartiles. Baseline characteristics of the study cohort are presented in Table I. Patients in the fourth group of microalbumin were significantly older, had higher waist‐to‐height values and were mostly men (P<.001). The distribution of patients according to different MetS definition criteria (AHA, ATP III, IDF, WHO, and GISSI score) is also presented in Table I.
Table I.
Patient Baseline Characteristics According to Microalbumin Group
| Group 1 | Group 2 | Group 3 | Group 4 | P Value | |
|---|---|---|---|---|---|
| N (M) | 1659 (767) | 1675 (849) | 1656 (869) | 1660 (896) | NS |
| Age, y | 53.5±13.0 | 53.1±12.2 | 53.3±12.8 | 54.9±12.8 | <.001 |
| WtoHeight | 5.30±6.41 | 5.36±6.41 | 5.39±6.24 | 5.53±6.41 | <.001 |
| BMI, kg/m2 | 26.8±4.2 | 27.4±3.9 | 27.4±4.0 | 28.0±4.2 | <.001 |
| Waist, %a | 27.7 | 27.7 | 29.6 | 35.0 | <.001 |
| MLK, mg/L | 5.8±2.03 | 9.61±7.12 | 12.84±1.28 | 17.67±1.38 | <.001 |
| Male sex, % | 46.2 | 50.7 | 52.5 | 54 | <.001 |
| MetS‐WHO, % | 16 | 17.8 | 22.2 | 26.7 | <.001 |
| MetS‐NCEP, % | 22.1 | 30.0 | 31.6 | 39 | <.001 |
| MetS‐GISSI, % | 22.3 | 26.3 | 32.1 | 37.7 | <.001 |
| ATP‐MetS, % | 16 | 23.7 | 24.7 | 29.9 | <.001 |
| MetS‐IDF, % | 31.3 | 39 | 41.8 | 46.1 | <.001 |
| IGT, % | 8.6 | 11.3 | 13.8 | 14.2 | <.001 |
| IFG, % | 16 | 16.3 | 17.8 | 22.7 | <.001 |
Abbreviations: ATP‐MetS, metabolic syndrome definition according to Adult Treatment Panel III; BMI, body mass index; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; M, male; MLK, microalbumin; MetS‐IDF, metabolic syndrome definition according to the International Diabetes Federation; MetS‐GISSI, metabolic syndrome Gruppo Italiano per lo Studio della Streptochinasi nell’Infarcto Miocardico score system; MetS‐NCEP, metabolic syndrome definition according to the National Cholesterol Education Program; MetS‐WHO, metabolic syndrome definition according to the World Health Organization; NS, not significant; WtoHeight, waist to height. aWaist, % is >102 cm for men and >88 cm for women.
Further, we analyzed the presence of MetS according to each different MetS definition criteria separately for each one of the 4 microalbumin quartiles. In group 1 (lowest microalbumin levels) there were significantly higher microalbumin levels in patients with MetS according to GISSI score (P=.01), while in group 4 (highest microalbumin levels) there were significantly higher microalbumin levels in patients with MetS according to AHA (P=.002), GISSI score (P<.001), and ATP III (P=.0034) (Table II).
Table II.
Distribution of Microalbumin Quartiles According to Each of the 5 Definition Criteria Used
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | P Value | 0 | 1 | P Value | |
| MetS‐WHO | 5.77±2.06 | 5.86±1.96 | NS | 9.62±0.70 | 9.59±0.72 | NS |
| MetS‐NCEP | 5.76±2.03 | 5.93±2.02 | NS | 9.61±0.70 | 9.61±0.73 | NS |
| MetS‐GISSI | 5.73±2.05 | 6.03±1.92 | .01 | 9.60±0.70 | 9.61±0.73 | NS |
| ATP‐MetS | 5.76±2.03 | 5.97±2.01 | NS | 9.60±0.70 | 9.60±0.74 | NS |
| MetS‐IDF | 5.77±2.05 | 5.86±1.96 | NS | 9.62±0.70 | 9.59±0.72 | NS |
| Group 3 | Group 4 | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | P | 0 | 1 | P | |
| MetS‐WHO | 12.83±1.26 | 12.87±1.31 | NS | 17.63±1.39 | 17.72±1.37 | NS |
| MetS‐NCEP | 12.83±1.26 | 12.87±1.40 | NS | 17.59±1.39 | 17.81±1.37 | .002 |
| MetS‐GISSI | 12.84±1.26 | 12.86±1.32 | NS | 17.55±1.38 | 17.87±1.37 | <.001 |
| ATP‐MetS | 12.85±1.27 | 12.83±1.33 | NS | 17.63±1.38 | 17.78±1.38 | .034 |
| MetS‐IDF | 12.83±1.26 | 12.87±1.31 | NS | 17.63±1.39 | 17.72±1.37 | NS |
Abbreviations: ATP‐MetS, metabolic syndrome definition according to Adult Treatment Panel III; MetS‐IDF, metabolic syndrome definition according to the International Diabetes Federation; MetS‐GISSI, metabolic syndrome Gruppo Italiano per lo Studio della Streptochinasi nell’Infarcto Miocardico score system; MetS‐NCEP, metabolic syndrome definition according to the National Cholesterol Education Program; MetS‐WHO, metabolic syndrome definition according to the World Health Organization.
Moreover, in order to explore the burden posed to microalbumin levels as the number of components of MetS rose, the study population was divided into 5 groups: group 1 (hypertension), group 2 (hypertension + any 1 other component), group 3 (hypertension + any 2 other components), group 4 (hypertension + any 3 other components), and group 5 (all 5 components), and the distribution in the 4 microalbumin quartiles was investigated (Table III).
Table III.
Patients’ Distribution According to the Number of MetS Components
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | P Value | |
|---|---|---|---|---|---|---|
| N (M) | 2907 | 2147 | 1086 | 417 | 66 | NS |
| Age | 53.6±13.3 | 53.9±12.7 | 53.7±11.9 | 53.6±11.4 | 55.5±10.3 | NS |
| WtoHeight | 0.51±0.05 | 0.55±0.06 | 0.58±0.06 | 0.61±0.04 | 0.62±0.04 | <.001 |
| Male (%) | 50.1 | 50.3 | 52.8 | 53.7 | 51.5 | NS |
| MLK (mg/L) | 10.93±4.54 | 11.53±4.60 | 12.24±4.47 | 12.77±4.56 | 13.19±4.09 | <.001 |
| SBP, mm Hg | 160.40±11.72 | 161.93±11.65 | 163.85±11.66 | 164.67±10.62 | 165.30±9.37 | <.001 |
| DBP, mm Hg | 100.86±7.08 | 100.94±7.05 | 101.43±7.02 | 101.64±7.5 | 100.97±7.66 | NS |
| Glucose, mg/dL | 90.47±9.17 | 94.81±11.50 | 98.50±12.66 | 103.41±14.05 | 118.03±7.11 | <.001 |
Abbreviations: DBP, diastolic blood pressure; M, male; MLK, microalbumin; NS, not significant; WtoHeight, waist to height; SBP, Systolic blood pressure.
When comparing the 5 groups of MetS components, significantly higher microalbumin levels were observed as the number of components rose (Figure; P<.001), while the same significant trend was also observed in SBP levels (P<.001), fasting glucose levels (P<.001) and finally in waist‐to‐height levels (P<.001). After adjustment for SBP, glucose, and waist‐to‐height a nonsignificant relation between microalbumin levels and number of components was revealed (P = not significant).
Figure.
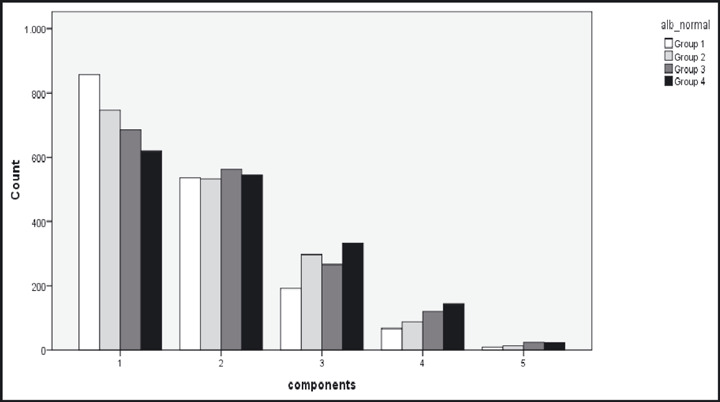
Different distributions of albumin quartiles according to the number of metabolic syndrome components.
Discussion
Microalbuminuria is an established marker of cardiovascular and renal disease and is included in the definition criteria of MetS, according to WHO. In the hypertensive patients, microalbuminuria has been proposed as an important risk factor, although it has not penetrated all guidelines. The groups of Bigazzi and Campese and coworkers 4 reviewed the importance of microalbuminuria as a cardiovascular risk predictor. Larger cohort studies confirmed this to be independent from other risk markers in the general hypertensive population (Multinational Monitoring of Trends and Determinants in Cardiovascular Disease [MONICA]), 3 a hypertensive cohort with left ventricular hypertrophy (Losartan Intervention for Endpoint Reduction in Hypertension [LIFE]), 18 and in individuals with already increased cardiovascular risk (Heart Outcomes Prevention Evaluation [HOPE]). 2
The main finding of the present study was that in hypertensive patients low grade albuminuria was significantly associated to MetS prevalence, irrespectively of the considered definition. For the first time we showed that albumin excretion, even within the currently defined normal levels, was related to MetS, using 4 variations of the definition and a score, interpreting the common pathophysiology pathways of the two. It has been reported that clinical and prognostic consequences of MetS may relate to the number of its individual components. 17 , 19 In the present study we observed that microalbumin levels were higher as more metabolic components rose, even among patients with MetS.
The estimates of MetS prevalence are controversial due to different criteria used for its definition. 20 The level of agreement among the current definitions of MetS is not encouraging. 21 In our study we found that, even among the same microalbumin quartile, patients with MetS according to GISSI score had significantly higher microalbumin levels. GISSI score has the advantage, compared to all other diagnostic tools of MetS, of the weighted contribution of each individual metabolic component. 22 Moreover, male gender and age are also taken into account. 23 , 24 Despite the conflicting results depending on each definition of MetS, the relationship of low‐grade albuminuria to MetS is highlighted in patients with higher albumin excretion, within the normal range. These findings are in line with the proposed hypothesis that albuminuria should be interpreted as a continuum rather than as threshold cutoff values. 25
Whether MetS per se plays a regulator role in the process of albumin excretion or its contribution is mainly through its components such as SBP or hydrocarbonate metabolism abnormality is yet unknown. Adjusting for possible confounders such as SBP we found that the association of MetS to microalbumin levels was not independent. These results are in keeping with the findings of the recent studies showing that BP is the main determinant of developing albuminuria. 26 , 27
The limitation of the present study is the definition of microalbuminuria, which is still far from being standardized, with all of the confusing consequences. Although one should recognize the necessity for a clear definition of the term microalbuminuria, one should interpret the term with caution, because albuminuria is a continuous variable. In the present study, cutoff limits for high normal microalbumin levels were 20 mg/L in 24‐hour urinary collection.
In conclusion, the present study confirms the clinical importance of low‐grade albuminuria in essential hypertensive patients. A continuum of increasing levels of albumin excretion reflects increasing prevalence of MetS, and as shown in recent studies, increasing cardiovascular risk. More studies need to be assessed in order to explore the biologic complexity of low‐grade albuminuria and to challenge the existing definition of normal.
References
- 1. Redon J, Williams B. Microalbuminuria in essential hypertension: redefining the thresholds. J Hypertens. 2002;20:353–355. [DOI] [PubMed] [Google Scholar]
- 2. Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. [DOI] [PubMed] [Google Scholar]
- 3. Jencen JS, Feldt‐Rasmusen B, Strandgaard S, et al. Arterial hypertension, microalbuminuria and risk of ischemic heart disease. Hypertension. 2000;35:898–903. [DOI] [PubMed] [Google Scholar]
- 4. Bigazzi R, Bianchi S, Baldor D, et al. Microalbuminuria predicts cardiovascular events and renal insufficiency in patients with essential hypertension. J Hypertens. 1998;16:1325–1333. [DOI] [PubMed] [Google Scholar]
- 5. Vyssoulis P, Karpanou E, Michaelidis A, et al. Microalbuminuria and global myocardial function in patients with essential hypertensive. Int J Cardiol. 2008;126:268–272. [DOI] [PubMed] [Google Scholar]
- 6. Pedrineli R, Dell’Omo G, Di Bello V, et al. Microalbuminuria, an integrated marker of cardiovascular risk in essential hypertension. J Hum Hypertens. 2002;16:79–89. [DOI] [PubMed] [Google Scholar]
- 7. Podremoli R, Cheli V, Sofia A, et al. Prevalence of micro‐ and macro‐albuminuria and their relationship with other cardiovascular risk factors in essential hypertension. Nephrol Dial Transplant. 1995;16:6–9. [DOI] [PubMed] [Google Scholar]
- 8. Winocour PH, Harland JO, Millar JP, et al. Microalbuminuria and associated risk factors in the community. Atherosclerosis. 1992;93:71–81. [DOI] [PubMed] [Google Scholar]
- 9. Mykkanen L, Zaccaro DJ, Wegenknecht LE, et al. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47:793–800. [DOI] [PubMed] [Google Scholar]
- 10. Redon J, Pascual JM. Development of microalbuminuria in essential hypertension. Curr Hypertens Rep. 2006;8:171–177. [DOI] [PubMed] [Google Scholar]
- 11. Hillege HL, Fidler V, Dierks GF, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002; 106: 1777–1782. [DOI] [PubMed] [Google Scholar]
- 12. Tsakiris A, Doumas M, Lagatouras D, et al. Microalbuminuria is determined by systolic and pulse pressure over a 12‐year period and related to peripheral artery disease in normotensive and hypertensive subjects: the Three Areas Study in Greece (TAS‐GR). Angiology. 2006;57:313–320. [DOI] [PubMed] [Google Scholar]
- 13. Klausen K, Borch Johnsen K, Feldt Rasmusen B, et al. Very low levels of microalbuminuria are associated with increased risk of coronary heart disease and death independently of renal function, hypertension and diabetes. Circulation. 2004;110:32–35. [DOI] [PubMed] [Google Scholar]
- 14. Arnlof J, Evans JC, Meigs JB, et al. Low grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. [DOI] [PubMed] [Google Scholar]
- 15. Mc Neil AM, Rosamond W, Girman C, et al. The metabolic syndrome and 11‐year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Ruotsalainen S, Moilanen L, et al. The metabolic syndrome predicts cardiovascular mortality: a 13‐year follow up study in elderly nondiabetic Finns. Eur Heart J. 2007;28:857–864. [DOI] [PubMed] [Google Scholar]
- 17. Schillaci G, Pirro M, Vaudo G, et al. Prognostic value of the metabolic syndrome in essential hypertension. J Am Coll Cardiol. 2004;43:1817–1822. [DOI] [PubMed] [Google Scholar]
- 18. Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients with left ventricular hypertrophy: The LIFE study. Ann Intern Med. 2003;139:901–906. [DOI] [PubMed] [Google Scholar]
- 19. Verdecchia P, Schillaci G, Guerrieri M, et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–536. [DOI] [PubMed] [Google Scholar]
- 20. Vyssoulis G, Pietri P, Karpanou E, et al. Differential impact of metabolic syndrome on arterial stiffness and wave reflections: focus on distinct definitions. Int J Cardiol. 2010;138:119–125.Epub 2008 Sep 19. [DOI] [PubMed] [Google Scholar]
- 21. Can AS, Bersot TP. Analysis of agreement among definitions of metabolic syndrome in nondiabetic Turkish adults: a methodological study. BMC Public Health. 2007;7:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Macchia A, Levantesi G, Borrelli G, et al; Associazione Nazionale Medici Cardiologi Ospedalieri; Istituto di Ricerche Farmacologiche Mario Negri‐Consorzio Mario Negri Sud, Santa Maria Imbaro; Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI)‐Prevenzione Investigators. A clinically practicable diagnostic score for metabolic syndrome improves its predictivity of diabetes mellitus: the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico (GISSI)‐Prevenzione scoring. Am Heart J. 2006;151:754. [DOI] [PubMed] [Google Scholar]
- 23. Mattix HJ, Hsu CY, Shaykevich S, et al. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;17:81–85. [DOI] [PubMed] [Google Scholar]
- 24. Jacobs DR Jr, Murtaugh MA, Steffes M, et al. Gender‐ and race‐specific determination of albumin excretion rate using albumin‐to‐creatinine ratio in single, untimed urine specimens: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155:1114–1119. [DOI] [PubMed] [Google Scholar]
- 25. Danziger J. Importance of low‐grade albuminuria. Mayo Clin Proc. 2008;83:806–812. [DOI] [PubMed] [Google Scholar]
- 26. Pascual JM, Rodilla E, Gonzalez C, et al. Long‐term impact of systolic blood pressure and glycemia on the development of microalbuminuria in essential hypertension. Hypertension. 2005;45:1125–1130. [DOI] [PubMed] [Google Scholar]
- 27. Schrier RW, Estacio RO, Esler A, et al. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes. Kidney Int. 2002;61:1086–1097. [DOI] [PubMed] [Google Scholar]


