Abstract
J Clin Hypertens (Greenwich). © 2010 Wiley Periodicals, Inc.
The purpose of the present study was to determine and compare the effect of interval and continuous training programs in the management of hypertension. Three hundred fifty‐seven male patients with essential hypertension were age matched and grouped into interval, continuous, and control groups. The interval (n=140; 58.90±7.35 years) and continuous (n=112; 58.63±7.22 years) groups were involved in 8 weeks of interval (60%–79% maximum heart rate) and continuous (60%–79% maximum heart rate) programs of between 45 to 60 minutes, while the control group (n=105; 58.27±6.24 years) remained sedentary during this period. Findings of the study revealed significant effect of both training programs on maximum oxygen consumption, systolic blood pressure, diastolic blood pressure, heart rate, pulse pressure, and mean arterial pressure at P<.05. The maximum oxygen consumption significantly and negatively correlated with systolic blood pressure, diastolic blood pressure, rate–pressure product, pulse pressure, and mean arterial pressure at P<.01. It was concluded that both training programs are effective adjunct nonpharmacological management of hypertension. The recommendation of the paper was that both interval and continuous training programs should form part of the kit in the management of hypertension. J Clin Hypertens (Greenwich). 2010;12:841–849.
It is a well‐established fact that a sedentary lifestyle contributes to increased risk of cardiovascular disease, especially hypertension. Indeed hypertension is a major independent risk factor for cardiovascular and renal disease, increasing the risk of myocardial infarction, stroke, and heart failure. Hypertension and its complications are largely responsible for morbidity and mortality of all age groups. 1 , 2 Despite the well established benefit of blood pressure (BP) reduction in reducing the risk of these clinical events, the rate of BP control remains poor. 3 Drug therapy remains the mainstay of hypertension management. However, antihypertensive drugs are not without their side effects and long‐term complications. The difficulty of accepting lifelong drug therapy and the high cost of drugs has led to the development of interest in searching for adjunct nonpharmacological management. One of these approaches includes therapeutic exercise. 4 , 5 , 6 , 7 , 8 , 9 , 10
Many studies 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 have shown that exercise training lowers BP in hypertensive patients. Exercise scientists, physiotherapists, and other health‐related professionals have used various training methods to evaluate these physiological changes. Two of these methods of training are widely used and they are frequently labeled as continuous, which includes exercise preformed at low to moderate intensities without rest intervals, and interval exercise which consists of a repeated series of exercise bouts with intervening rest periods. 15 , 16 Although there appears to be a general agreement regarding the fact that both continuous and interval training programs can beneficially alter many physiological parameters, there is still controversy regarding the level and format of exercise, particularly intensities that can yield optimal beneficial effects, particularly in hypertension. 17 , 18 Also, only a few studies have systematically attempted to determine which exercise program optimally affects each of these variables in hypertensive patients, particularly in older individuals. Therefore the purpose of the present study was to determine and compare the therapeutic efficacy of both continuous and interval training programs on cardiovascular parameters among participants with hypertension.
Methodology
Research Design
In the present study, age‐matched, randomized, double‐blind, independent groups design was used to determine the influence of the interval and continuous training programs on cardiovascular parameters. Participants’ ages were arranged in ascending order (50–70 years) and then assigned to interval, continuous, and control groups in an alternating pattern (age matched). A one‐week washout period was established and pretest was administered to all participants on the last day of the washout period. Following washout and pretest, all participants were placed on methyldopa, the interval and continuous groups were involved in interval and continuous training programs for 8 weeks, while the control group remained sedentary during this period. At the end of the training and sedentary period, another 1‐week washout period was established, and a posttest was administered to all participants on the last day of the washout period.
Participants
The study population was male essential hypertensive participants attending the hypertensive clinic at Murtala Muhammed Specialist Hospital (MMSH), Kano, Nigeria. Participants were fully informed of the experimental procedures, protocol, and risk, after which they gave their informed consent.
Inclusion Criteria
Only those who volunteered to participate in the study were recruited. Participants between the age range of 50 and 70 years with chronic, mild‐to‐moderate, and stable (>1 year duration) hypertension (systolic blood pressure [SBP] between 140–179 mm Hg and diastolic blood pressure [DBP] between 90–109 mm Hg) were selected. Only participants with hypertension who had stopped taking antihypertensive drugs or were on a single antihypertensive medication were recruited. 11 They were sedentary and had no history of psychiatry or psychological disorders or abnormalities.
Exclusion Criteria
Obese or underweight (body mass index [BMI] between 20 and 30 kg/m2), smokers, alcoholic, diabetic, and other cardiac, renal, and respiratory disease patients were excluded. Those involved in vigorous physical activities and above average physically fit people (maximum oxygen consumption [VO2 max] >27 and >33 mL/kg/min for over 60 and 50 years old respectively) were also excluded.
A total of 485 chronic and stable, essential, mild‐to‐moderate, male patients with hypertension satisfied the necessary study criteria. Participants were aged‐matched and randomly grouped into interval (162), continuous (162), and control (161) groups. They were fully informed about the experimental procedures, risk, and protocol, after which they gave their informed consent in accordance with the American College of Sports Medicine (ACSM) guidelines, regarding the use of human subjects 19 as recommended by the human subject protocol. Ethical approval was granted by the Ethical Committee of Kano State Hospitals Management Board.
Pretest Procedure
Washout Period
All participants on antihypertensive drugs were asked to stop all forms of medication and in their place, were given placebo tablets (which consisted of mainly lactose and inert substance) in a single blind method. 20 , 21 All participants, including those not on any antihypertensive medications, were placed on placebo tablets for 1 week (7 days); this is known as “washout period.” The purpose of the washout period was to get rid of the effects of previously taken antihypertensive drugs/medications. During the washout period all participants were instructed to report to the hypertensive clinic for daily BP monitoring and general observation. The pretest procedure was conducted on the last day of the washout period in the Department of Physiotherapy at MMSH, Kano between 8 am and 10 am.
Physiological Measurement
Participants resting heart rate (HR), SBP, and DBP were monitored using the right arm as described by Walker and colleagues 22 and Musa and colleagues 23 using an automated digital electronic BP monitor (Omron digital BP monitor, Model 11 EM 403c; Tokyo, Japan). These measurements were taken between 8 am and 10 am each test day.
Anthropometric Measurement
Participants’ physical characteristics (weight [kg] and height[m]) and body composition (BMI [kg/m2]) assessment were measured in accordance with standardized anthropometric protocol. 24 , 25
Stress Test
The Young Men Christian Association (YMCA) submaximal cycle ergometry test protocol was used to assess participants’ aerobic power as described by the ACSM 26 and Golding and associates. 27 The YMCA protocol uses 2 to 4 3‐minute stages of continuous exercise; 2 HR‐power output data points were needed (steady state HR) of between 110 and 150 beats/min. The two steady state HR were plotted against the respective workload on the YMCA graph sheet. A straight line was drawn through the 2 points and extended to the participant’s predicted maximum HR (220 – age). The point at which the diagonal line intersects the horizontal predicted maximum HR (HR max) line represents the maximal working capacity for the participant (HR max). A perpendicular line was dropped from this point to the baseline where the maximal physical workload capacity was read in kg/min, which was used to predict the participants VO2 max. This procedure was done for both pre‐ and posttest stress test.
Test Procedure
The test procedure was conducted in the Department of Physiotherapy of MMSH, Kano between 8 am and 10 am.
Training Program
Following the stress test and prior to the exercise training, all participants in the control, interval, and continuous groups were reassessed by the physician and were prescribed with methyldopa as necessary. Participants maintain these prescriptions with regular medical consultation and observation throughout the period of training.
The Interval Group (Group 1)
Participants in the interval group exercised on a bicycle ergometer at a moderate intensity of between 60% and 79% of their HR max reserve that was estimated from 220 minus the age of a participant (220 – age) as recommended by the ACSM. 14 The duration of the exercise session was 45 minutes in the first 2 weeks of training. This duration was then gradually increased to 60 minutes in the remaining part of the training. 14 Exercise sessions 3 times per week were maintained throughout the 8‐week period of training for the interval group.
The Continuous Group (Group 2)
The starting workload was 100 kg/min (17 watts) which was increased at a pedal speed of 50 rpm to obtain the starting point of HR max 60%, which was increased in the first 2 weeks to 79% HR max and maintained throughout the remaining part of the training period. The duration of the exercise session was 45 minutes in the first 2 weeks of training; the duration was then gradually increased to 60 minutes in the remaining part of the training.
The Control Group (Group 3)
Participants in the control group were instructed not to undertake any vigorous physical activity during the 8‐week period of study.
During the training and sedentary period (8 weeks) all participants were placed on methyldopa according to their prerecruitment doses and responses of 250 and 500 mg daily. Methyldopa was preferred because it does not alter normal hemodynamic responses to exercise. 28 It is well tolerated and the highest prescribed antihypertensive drug in Nigeria, 29 particularly Northern Nigeria where the study was conducted, and it is also useful in the treatment of mild to moderately severe hypertension. 30
Posttest Procedure
Washout Period
At the end of the 8‐week training and sedentary period, all participants were asked to stop methyldopa and participants were prescribed placebo tablets in a single blinded method for 1 week in order to rid the effect of the methyldopa taken during the training period.
Post training physiological (SBP, DBP, HR, rate–pressure product (RPP), pulse pressure [PP], and mean arterial pressure [MAP]) assessment and stress test were conducted as earlier described in the pretest procedures using standardized protocols, techniques, and methods.
All pre‐ and posttest measurements were recorded on a data sheet. Three hundred fifty‐seven participants (140 from interval, 112 continuous, and 105 from control group) completed the 8‐week training program. One hundred twenty‐eight participants (22 from interval, 50 from continuous, and 56 from control group) dropped out because of noncompliance, unfavorable responses to methyldopa and exercise training, or had incomplete data; therefore, the data of 357 participants were used in the statistical analysis (Figure 1).
Figure 1.
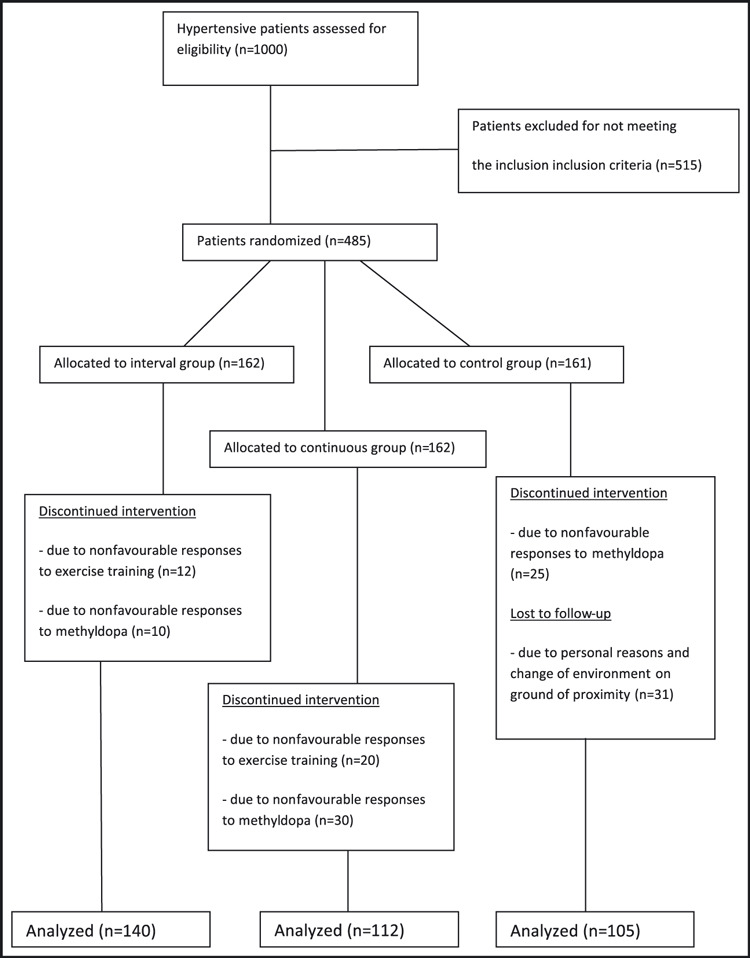
Study design flow chart.
Statistical Analysis
Following data collection, the measured and derived variables were statistically analyzed. The descriptive statistics (mean and standard deviation [SD]) of the participants’ physical characteristics, estimated VO2 max, and other cardiovascular parameters were determined. Analysis of variance (ANOVA), Turkey and Pearson product‐moment correlation tests were computed for the variables of interest. In the ANOVA and correlation tests, the difference between participants post‐training and pre‐training measurements (changed score) were used as dependent measures. All statistical analysis was performed on a Toshiba compatible microcomputer using the statistical package for the social science (SPSS), (Windows Version 16.0; Chicago, IL). The probability level for all of the above tests was set at .05 to indicate significance.
Results
The participants’ ages ranged between 50 and 70 years. Mean age, height, weight, and BMI ± SD for the interval group were 58.63±7.22 years, 173.16±6.97 cm, 67.49±10.16 kg, and 22.48± 2.89 kg/m2, respectively; 58.40±6.91 years, 167.78±7.81 cm, 70.18±11.37 kg, 24.96±3.88 kg/m2, respectively for the continuous group; and 58.27±6.24 years, 167.89±5.31 cm, 68.47±17.07 kg, 24.16±4.91 kg/m2, respectively for the control group. There was no significant age difference in groups (F=0.245, P=.783).
Participants’ mean and standard variation values for both pre‐ and posttest values are depicted in Table I. Table II shows ANOVA and changed score values. The results indicated a significant difference in groups SBP, DBP, HR, RPP, PP, MAP, and VO2 max at P<.05. Tukey’s Honestly Significantly Different (HSD) test indicated significant differences in all groups’ paired cardiovascular parameters.
Table I.
Groups’ Mean (X) and Standard Deviation (SD) (N=357)
| Variables | Interval Group X±SD | Continuous Group X±SD | Control Group X±SD | |||
|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | Pretest | Posttest | |
| SBP (mm Hg) | 166.05±14.10 | 150.35±16.67 | 168.81±12.73 | 154.38±12.63 | 160.87±12.23 | 163.47±14.88 |
| DBP (mm Hg) | 96.80±3.38 | 94.98±5.04 | 100.63±7.06 | 94.44±8.77 | 97.17±1.43 | 96.10±2.67 |
| HR (beats/min) | 85.05±10.26 | 76.83±8.51 | 82.25±10.23 | 70.78±9.82 | 82.60±22.62 | 80.50±21.99 |
| VO2 max (mL/kg/min) | 23.62±9.15 | 37.46±7.45 | 20.69±12.49 | 28.68±13.60 | 21.23±5.76 | 22.82±7.44 |
| RPP (mm Hg × beats /min) | 1.41a±2.28b | 1.15a±1.6b | 1.40a±1.9b | 1.13a±2.6b | 1.34a±4.46b | 1.32a±4.25b |
| PP (mm Hg) | 69.25±13.29 | 55.38±14.88 | 72.88±13.45 | 63.00±21.83 | 63.79±12.21 | 67.37±14.12 |
| MAP (mm Hg) | 119.65±5.85 | 113.25±7.73 | 121.61±8.83 | 115.61±10.87 | 118.19±5.12 | 118.33±5.79 |
Abbreviations: DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; PP, pulse pressure; RPP, rate–pressure product; SBP, systolic blood pressure; VO2 max, maximum oxygen consumption . a× 104, b× 103.
Table II.
ANOVA and Groups’ Changed Score Values
| Variables | Interval | Continuous | Control | F Value | P Value |
|---|---|---|---|---|---|
| SBP (mm Hg) | −16.40±13.16 | −13.94±12.60 | 2.61±7.85 | 119.345 | .000a |
| DBP (mm Hg) | −4.01±4.34 | −7.41±6.27 | −1.07±1.76 | 53.171 | .000a |
| HR (beats/min) | −8.23±8.85 | −11.47±12.36 | −2.10±9.46 | 23.325 | .000a |
| VO2 max (mL/kg/min) | 13.85±9.94 | 7.99±6.62 | 1.59±5.54 | 285.429 | .000a |
| RPP (mm Hg × beats/min ) | −2.60b±70c | −2.72b±.04c | −1.8b±1.4c | 49.266 | .000a |
| PP (mm Hg) | −13.88±13.81 | −9.89±25.03 | 3.67±8.60 | 33.168 | .000a |
| MAP (mm Hg) | −6.40±5.77 | −6.00±13.11 | 0.15±2.46 | 33.429 | .000a |
Abbreviations: ANOVA, analysis of variance; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; PP, pulse pressure; RPP, rate–pressure product; SBP, systolic blood pressure; VO2 max, maximum oxygen consumption. aSignificant, P<.05; b× 104; c× 103.
Pearson correlation results indicated negative significant correlation between changes in VO2 max and changes in other parameters; SBP (r=−0.199), DBP (r=−0.232), HR (r=−0.326), RPP (r=−0.395), PP (r=−0.140), and MAP (r=−282), for the interval group at P<.01 (Figure 2).
Figure 2.
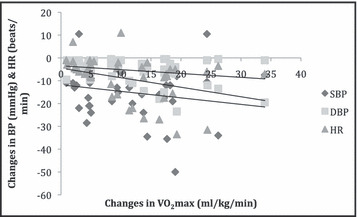
Correlation between training changes in maximum oxygen consumption (VO2 max) and blood pressure (BP) and heart rate (HR) (N=252). DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
Discussion
VO2 max
The present study revealed considerable changes in estimated VO2 max in experimental groups over control group. In the experimental groups, the interval group indicated a significant increase in VO2 max above the continuous group. Result of the present study is consistent with the study of Smith and colleagues 31 ; they investigated the effect of aerobic exercise and weight reduction intervention on 133 sedentary hypertensive men and women. Participants were grouped into an aerobic group, an aerobic group with weight reduction, and a control group. Participants engaged in a 6‐month treatment period. They reported a significant improvement in aerobic capacity in the treatment groups compared to the control group.
A recent study conducted by Jones and colleagues 32 that agrees with the present study exercised 35 participants (22 Caucasians and 13 African Americans) for 6 months. Their report indicated that aerobic exercise significantly increased participants maximal aerobic consumption (from 24±08 to 28±1 mL/kg/min) at P<.001. The present finding is also consistent with the report of Mughal and colleagues, 33 who studied the effect of aerobic exercise in patients with essential hypertension. Twenty stage 1 and 2 hypertensives participated in a 12‐week endurance ergometer program at 50% VO2 max, 3 to 5 times/week for 30 minutes. They reported significant increase in VO2 max at P<.05. Dengel and associates 34 conducted a study on the effect of physical training on maximum aerobic capacity. Thirty‐one (63±1 years) hypertensive (153±2/88±1 mm Hg) individuals participated in a 6‐month aerobic exercise training program at 75% VO2 max, 3 times weekly for 40 minutes. They reported a significant increase in maximal aerobic capacity (VO2 max: 18.3±3.8 vs output of 20.7±4.2 mL/kg/min, at P<.017). Despite these outstanding results, the limitation of the 2 studies, however, was lack of control groups.
Another related study was conducted by Ferrier and colleagues, 35 investigating the effect of aerobic exercise on VO2 max and maximum workload. Twenty (10 men and 10 women) participants with isolated systolic hypertension (ISH) aged (64±7) and 20 age‐ (64±7) and sex‐matched normal (control) control participants were recruited. Participants were involved in a randomized crossover study of moderate‐intensity exercise (60% HR max) for 40 minutes, 3 times/week for 8 weeks followed by another 8 weeks of sedentary activity. They reported a significant increase in VO2 max by 13%±5% (P=.04) and workload increase by 8±4 (P=.05) ISH patients in the ISH group. The probable reasons for the significant effect of interval training in the continuous and control groups might be due to the ability of intermittent exercise to intermittently overload the heart for a brief period beyond that which could be achieved during a single continuous bout at the same intensity. Also, the alternate work and rest periods in the interval training are proposed to allow for more cardiovascular work to be accomplished in the training period. Furthermore, the improved anaerobic threshold and work economy increase the participant’s ability to cope with the physical demand of daily activity. Finally, the motivating factor of exercise and rest period (varied procedure followed during each training) could not be ruled out. 33 , 34 , 35 , 36 , 37 , 38 , 39
Blood Pressure
Findings from the present study revealed a significant decrease in SBP and DBP in the experimental groups over placebo group. The favorable changes resulting from aerobic training on both SBP and DBP demonstrated in the present study are consistent with the study of Smith and colleagues 28 that investigated the effect of aerobic exercise and weight reduction intervention on 133 sedentary hypertensive (SBP: 130–180 mm Hg; DBP: 85–110 mm Hg) men and women. Participants were grouped into aerobic group, aerobic with weight reduction group, and control group. Participants engaged in a 6‐month treatment period. They reported a significant reduction in both SBP and DBP in the treatment groups compared to the placebo group. In their study, Westhoff and colleagues 40 investigated the effects of aerobic exercise on elderly (≥60 years) hypertensive patients (SBP ≥140 mm Hg and DBP ≥90 mm Hg). Fifty‐four patients were randomly assigned to exercise and control groups. The exercise group engaged in a 12‐week treadmill exercise program, while the control group did not. They reported a significant decrease in SBP and DBP by 8.5±8.2 mm Hg and 5.1±3.7 mm Hg, respectively, at P<.001.Their results concur with the findings of the present study.
Laterza and colleagues 41 conducted a study investigating the effect of aerobic exercise on patients with hypertension. Twenty sedentary hypertensives were randomly assigned to 2 groups; exercise group (n=11; age=46±2 years), nonexercise group (n=12; age=42±2 years), and age‐matched normotensive control (n=12; age 42±2). The exercise training consisted of one 60‐minute exercise session per week for 4 months. They reported a significant decrease in BP of the hypertensives following exercise at P<.01 compared to the nonexercise hypertensive group.
Jones and colleagues 32 investigated the effect of a 6‐month aerobic exercise program on the BP of 35 sedentary prehypertensive and hypertensive participants (22 Caucasians; 13 African Americans). They reported nonsignificant changes in the BP of both the Caucasians and African‐American participants. Sohn and colleagues 42 investigated the effect of walking an extra 30 minutes a day on BP. Nineteen newly diagnosed hypertensive African‐American adults were randomly assigned to inter‐vention and control groups. The intervention group was advised to walk an extra 30 minutes per day. The control group was not given this advice. All participants used pedometers to record the numbers of daily steps. Also all participants were controlled for age and BMI. They reported a significant reduction in adjusted mean SBP by 9% for those in the intervention group and 2.33% for those in the control group. Similarly, adjusted mean DBP dropped by 7.42% for the intervention group and remained essentially unchanged for the control group at P=.08.
Heart Rate
The present study revealed significant reduction in both interval and continuous programs over control group in resting HR. The result of the present study is in agreement with the study of Laterza and colleagues 41 that investigated the effect of aerobic exercise training on HR in patients with hypertension. Twenty sedentary hypertensive patients were randomly divided into 2 groups; exercise: exercise trained (n=11; age 46±2 years) and untrained (n=9; age 42±2 years). An age‐matched normotensive exercise trained group (n=12; age 42±2 years) was also studied. Participants were involved in an aerobic exercise training program of 3 60‐minute exercise sessions per week for 4 months. They reported a significant decrease in HR following exercise in the hypertensive group. Ferrier and colleagues 35 investigated the effect of aerobic exercise on resting HR. Twenty (10 men and 10 women) ISH participants and 20 age‐ and gender‐matched normal control participants were recruited. Participants were involved in a randomized crossover study of moderate‐intensity excercise (60% HR max) for 40 minutes for 8 weeks followed by another 8 weeks of sedentary activity. They reported no significant effect of exercise on HR in ISH patients.
Himeno and colleagues 43 studied the effect of aerobic exercise on HR in mild‐to‐moderately hypertensive, obese participants. Fourteen mildly hypertensive, obese participants (SBP, 140–160 mm Hg or DBP, 90–100 mm Hg) and 22 normotensive, obese participants, age range from 22 to 51 years (mean ± SD, 35±9 years) (all participants had a BMI of >26 kg/m2. Participants were placed on a hypocaloric diet and exercise program; 33 participants exercised on a cycle ergometer and 3 participants on a treadmill at an HR corresponding to the anaerobic threshold (AT) for 60 minutes, 3 times/week for a total period of 12 weeks. The exercise was maintained for a 1‐year follow‐up. They reported a nonsignificant effect of exercise on HR in both the hypertensive and normotensive groups following the 12‐week training and after 1 year post‐training follow‐up at P<.05.
RPP, PP and MAP
Results of the present study revealed a significant reduction in both interval and continuous groups’ RPP, PP, and MAP over control. This finding is in agreement with the report of Silver and colleagues, 44 who studied the effects of acute and chronic exercise on the arterial baroreflex and chemosensitive cardiopulmonary baroreflex in spontaneously hypertensive rats. Arterial baroreflex and cardiopulmonary baroreflex were evaluated in normotensive rats (n=11) and spontaneously hypertensive rats (SHRs) (n=5) at rest and after 30 minutes of an acute bout of exercise (45 minutes at 50% of VO2 max). In addition, these baroreflexes were evaluated in sedentary (n=5) and exercise‐trained (n=9) SHRs. Exercise training was performed on a motor treadmill, 5 days/week, for 60 minutes, at 50% of VO2 max. They reported that exercise training markedly improved baroreflex bradycardia and tachycardia in SHRs (1.9±0.1 vs 0.7±0.1 and 2.9±0.1 vs 1.8±0.2 beats/min/mm Hg, respectively. They also reported that during the recovery period, SHRs showed a significant fall in MAP compared with their respective baseline levels (25 minutes, −11±4 mm Hg; 35 minutes, −11±5 mm Hg; and 45 minutes, −10±5 mm Hg). In normotensive rats, MAP was not changed during the recovery period.
A similar result was reported by Mughal and colleagues, 33 who investigated the effects of aerobic exercise in patients with essential hypertension. A 12‐week aerobic exercise intervention trial was conducted to examine the influence of brisk walking on resting PP and MAP in patients with essential hypertension. Twenty‐seven men with stage 1 or 2 essential hypertension (not on antihypertensive medication) participated in the study. The aerobic exercise training protocol consisted of 30 minutes of brisk walking 3 to 5 times/week, at 50% of VO2 max on an ergometer cycle. They reported a statistically significant decrease in PP, from the baseline value of −3.7 mm Hg (P<.01), and in MAP, of −3.4 mm Hg (P<.01) was noted. They concluded that aerobic exercise caused small reductions in MAP and PP in men with stage 1 or 2 essential hypertension.
Randon and colleagues 45 studied 24 elderly hypertensive patients (age 68.9±1.5 years) and 18 age‐matched normotensive control participants (age 68.1±1.2 years). Cardiac output (carbon dioxide rebreathing) and BP (auscultatory) were measured at rest and after a 45‐minute period of low‐intensity bicycle exercise (50% maximal oxygen uptake) and at 15, 30, 60, and 90 minutes after exercise. Left ventricular function (by Doppler echocardiography) was also evaluated. Ambulatory BP monitoring was evaluated after 45 minutes of exercise or 45 minutes of rest, in a randomized order. They reported that in the hypertensive patients, exercise provoked a significant reduction in mean BP during a 22‐hour period, at daytime and nighttime. They concluded that the short‐term positive effect noted after exercise in elderly hypertensive patients is associated with a decrease in stroke volume and left ventricular end‐diastolic volume.
Generally, the factors responsible for the potential antihypertensive effect of long‐term aerobic exercises are incompletely understood and unclear. 46 However, it is theorized that the biochemical, neural, and hormonal changes in the blood vessel walls induce an acute and long‐term blood vessel relaxation. When a person engages in aerobic exercise, the SBP and HR will increase, while the DBP changes little. Immediately following the exercise bout, the SBP of hypertensive individuals will fall below pre‐exercise values by 20 to 30 mm Hg and that of normotensive individuals by 8 to 12 mm Hg, an effect that lasts for 12 hours in healthy individuals and beyond 12 hours in hypertensive patients. 47 , 48 This decrease below the baseline following aerobic exercise is called “post‐exercise hypotension.” 49
There are many potential mechanisms responsible for the “post‐exercise hypotensive effects,” including relaxation and vasodilatation of blood vessels in the legs and visceral organ areas. 48 The blood vessels may relax after each exercise session because of body warming effects, local production of certain chemicals (such as lactic acid and nitric oxide), decreases in nerve activity, and changes in certain hormones and their receptors. 47 , 48 Over time, as the exercise is repeated, there is growing evidence that a long lasting reduction in resting BP can be measured, which may partly be due to the acute drop in BP that occurs after each bout. 47
Another mechanism is that the post‐exercise hypotension is accompanied by a decrease in serum catecholamines, norepinephrine, dopamine, cortisol, and sympathetic nervous system and plasma rennin activity. 49 , 50 , 51 , 52 In addition to a reduction in sympathetic activities, a fall in plasma volume and cardiac index may also be important in the antihypertensive effect of aerobic exercise. 46 Exercise has been reported to improve abnormal baroreflex function in patients with hypertension. 53 , 54
Conclusions
Based on the results of the present study, it is concluded that continuous and interval training programs are effective adjunct, nonpharmacological management of chronic essential hypertension. However, there seems to be no clear‐cut supremacy of one training program over the other.
Recommendation
The recommendation of the present paper is that both training programs should form part of the kit in the management of hypertension.
References
- 1. Jean‐Michel M, Bernard C, Roland A, et al. Twenty four hour ambulatory blood pressure monitoring efficacy of peridopril/Indapamide first line combination in hypertensive patients. Am J Hypertens. 2004;17:245–251. [DOI] [PubMed] [Google Scholar]
- 2. Benegas JR. Epidemiologia de la hypertension arterial en Espana. Prevalencia, conocimneto Y control. Hypertension. 1999;16:315–322. [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on prevention, Detection Evaluation and Treatment of high blood pressure. The JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 4. Guidelines Committee European Society of Hypertension European Society of Cardiology . Guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. [DOI] [PubMed] [Google Scholar]
- 5. Grossman E, Grossman A, Schein MH, et al. Breathing control lowers blood pressure. J Human Hypertens. 2001;15:263–269. [DOI] [PubMed] [Google Scholar]
- 6. Shein MH, Gavish B, Henz M, et al. Treating hypertension with device that slow and regularizes breathing: a randomized double blind control study. J Hum Hypertens. 2001;15:271–278. [DOI] [PubMed] [Google Scholar]
- 7. Joint National Committee on Prevention, Detection, Evaluation and Treatment (JNC) of High Blood Pressure . The six report of the JNC. Arch Intern Med. 1997; 157, 2413–2446. [DOI] [PubMed] [Google Scholar]
- 8. Cappuccio FP, MacGregor GA. Does potassium suppliemntation lower blood pressure? A meta‐analysis of published trials. J Hypertens. 1991;9:465–473. [DOI] [PubMed] [Google Scholar]
- 9. Elliott P. Observation studies of salt and BP. Hypertens. 1991;17(Suppl 1):1–8. [Google Scholar]
- 10. Patel C, Margot MG, Terry DJ, et al. Trial of relaxation in reducing coronary risk. Four year follow up. Br Med J. 1985;290:1103–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart KJ, Bacher AC, Turner KL, et al. Effects of exercise on blood pressure in older person. Arch Intern Med. 2005;165:756–762. [DOI] [PubMed] [Google Scholar]
- 12. Ishikawa‐Takata K, Ohta T, Tanaka H. How much exercise is required to reduce blood pressure in essential hypertension? A dose response study. Am J Hypertens. 2003;16:629–633. [DOI] [PubMed] [Google Scholar]
- 13. Engstrom G, Hedblad B, Janzon L. Hypertensive men who exercise regularly have lower rate of cardiovascular mortality. J Hypertens. 1999;17:737–742. [DOI] [PubMed] [Google Scholar]
- 14. American College of Sport Medicine . Physical activity, physical fitness and hypertension. Med Sci Sports Exerc. 1993;25:i–x. [PubMed] [Google Scholar]
- 15. Heyward VH. Advanced Fitness Assessment and Exercise Prescription, 2nd ed, Champaign, IN: Human Kinetic Books; 1991. [Google Scholar]
- 16. Gregory LW. The development of aerobic capacity. A comparison of continuous and interval training. Res Q Exerc Sport. 1979;50:199–206. [PubMed] [Google Scholar]
- 17. Fletcher GF, Balady GJ, Amsterdam EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. [DOI] [PubMed] [Google Scholar]
- 18. Shephard RJ, Balady GJ. Exercise as cardiovascular therapy. Circulation. 1999;99:963–972. [DOI] [PubMed] [Google Scholar]
- 19. American College of Sport Medicine . Guide Lines for Exercise Testing and Prescription, 4th ed. Philadelphia, PA: Lea & Febiger; 1991. [Google Scholar]
- 20. Townsend RR, Mcfadden TC, Ford V, et al. A randomized double blind, placebo‐controlled trial of casein protein hydrolysnte(C12 peptide) in human essential hypertension. Am J Hypertens. 2004;17:1056–1058. [DOI] [PubMed] [Google Scholar]
- 21. Waeber B, Nussberger J, Brunner HR. The rennin angiotension system: role in experimental and human hypertension. In: Zanchetti A, Tarazi RC, eds. Pathophysiology of Hypertension: Regulatory Mechanisms. Amsterdam, the Netherlands: Elsevier; 1986: 489–519. [Google Scholar]
- 22. Walker AJ, Bassett DR, Duey WJ, et al. Cardiovascular and plasma cathecolamae responses to exercise in blacks and whites. Hypertension. 1992;20:542–548. [DOI] [PubMed] [Google Scholar]
- 23. Musa DI, Ibrahim DM, Toriola AL. Cardiorespiratory fitness and risk factors of CHD in pre‐adolescent Nigerian girls. J Hum Mov Studies. 2002;42:455–460. [Google Scholar]
- 24. International Society for the Advancement of Kinanthropometry (ISAK) . International Standards for Anthropometric Assessment. Patche Fstroom, South Africa: ISAK; 2001. [Google Scholar]
- 25. Ross WD, Marfell‐Jones MJ. Physiological testing of the high performance athletes. In: MacDugall JD, Wenger A, Green HJ, eds. Kinanthropometry. Champaign, IL: Human Kinetics Books; 1991: 223–308. [Google Scholar]
- 26. American College of Sports Medicine . ASCM’s Guidelines for Exercise Testing and Prescription, 5th ed. Baltimore, MD: Williams & Wilkins; 1995. [Google Scholar]
- 27. Golding LA, Meyers CR, Sinniny WE. Way to Physical Fitness. The Complete Carnote to Fitness Testing and Instruction, 3rd ed. Champaign, IL: Human Kinetics Publishers; 1989. [Google Scholar]
- 28. Katzung BG. Basic and Clinical Pharmacology, 7th ed. New York, NY: Lange Medical Books/McGraw Hill; 1998. [Google Scholar]
- 29. Mancia G, Ferari L, Gregorini L, et al. Effects of treatment with methyldopia on basal haemodynamic and on rural control. In: Robertson JS, Pickering GW, Goldwell ADS, eds. The Therapeutics of Hypertension. London, UK: Royal Society of Medicine and Academic Press Inc. Ltd; 1980: 70–78. [Google Scholar]
- 30. Salako LA. Treatment of Hypertension: Cardiovascular Disease in Africa. Ibadan: Ciba Geigy Ltd; 1976. [Google Scholar]
- 31. Smith PJ, Blumenthal JA, Babyk MA, et al. Effects of exercise and weight loss on depressive symptoms among men and women with hypertensive. J Psychosom Res. 2007;63:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mughal M, Alvi I, Akhund I, et al. The effects of aerobic exercise training on resting blood pressure in hypertensive patients. J Pak Med Assoc. 2001;51:222–226. [PubMed] [Google Scholar]
- 33. Dengel DR, Brown MD, Rynoid TH, et al. Effect of aerobic training on blood pressure sensitivity to dietary sodium in older hypertensives. J Hum Hypertens. 2006;20:372–378. [DOI] [PubMed] [Google Scholar]
- 34. Ferrier KE, Wadded TK, Gaatzka D, et al. Aerobic exercise training does not modify large artery compliance in isolated systolic hypertension. Hypertension. 2001;38:222–226. [DOI] [PubMed] [Google Scholar]
- 35. Ulrik W, Asbjorn LP, Morten B, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients. Circulation. 2007;115:3086–3094. [DOI] [PubMed] [Google Scholar]
- 36. Gielen S, Adams V, Linke A, et al. Exercise training in chronic heart failure: correlation between reduced local inflammation and improved oxidative capacity in the skeletal muscle. Eur J Cardiovasc Prev Rehabil. 2005;12:393–400. [DOI] [PubMed] [Google Scholar]
- 37. Dubach P, Myers J, Dziekan G, et al. Effect of high intensity exercise training on central hemodynamic responses to exercise in men with reduced left ventricular function. J Am Coll Cardiol. 1997;29:1591–1598. [DOI] [PubMed] [Google Scholar]
- 38. Wielenga RP, Coats AJ, Mosterd WL, et al. The role of exercise training in chronic heart failure. Heart. 1997;74:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Westhoff TH, Franke N, Schmidt S, et al. Too old to benefit from sports? The cardiovascular effects of exercise training in elding subjects treated for isolated systolic hypertension. Kidney Blood Press Res. 2007;30:240–247. [DOI] [PubMed] [Google Scholar]
- 40. Laterza MC, Demator LD, Trombetta IC, et al. Exercise training restores baroreflex sensitivity in never trained hypertensive patients. Hypertension. 2007;49:1298–1306. [DOI] [PubMed] [Google Scholar]
- 41. Jones JM, Dowling TC, Park JJ, et al. Differential aerobic exercise‐induced changes in plasma aldosterol between Africa Americans and Caucasians. Exp Physiol. 2007;92:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sohn AJ, Hasnain M, Sinacore JM. Impact of exercise (walking) on blood pressure levels in African American adults with newly diagnosed hypertension. Ethn Dis. 2007;17:503–507. [PubMed] [Google Scholar]
- 43. Himeno E, Nishimo K, Okzaki T, et al. A weight reduction and weight maintenance program with long‐lasting improvement in left ventricular mass and blood pressure. AJH. 1999;12:682–690. [DOI] [PubMed] [Google Scholar]
- 44. Silva GJ, Brum PC, Negrão CE, et al. Acute and chronic effects of exercise on baroreflexes in spontaneously hypertensive rats. Hypertension. 1997;30:714–719. [DOI] [PubMed] [Google Scholar]
- 45. Rondon PB, Alves NN, Braga FW, et al. Postexercise blood pressure reduction in elderly hypertensive patients. J Am Coll Cardiol. 2002;39:676–682. [DOI] [PubMed] [Google Scholar]
- 46. Arakawa K. Antyhypertensive mechanism of exercise. J Hypertens. 1993;11:223. [DOI] [PubMed] [Google Scholar]
- 47. MacDonald MB, Laing GP, Wilson MP, et al. Prevalence and predictors of white coat response in patients with treated hypertension. MAJ. 1999;161:265–269. [PMC free article] [PubMed] [Google Scholar]
- 48. Halliwill JR. Mechanisms and clinical implications of post exercise hypertension in human exercise and sports. Sci Rev. 2001;29:65. [DOI] [PubMed] [Google Scholar]
- 49. Brooks GA, Fahey TD, White TP. Exercise Physiology, Human Bioenergetics and its Application, 2nd ed. Mountain View, NJ: May Field Publishing Company; 1996. [Google Scholar]
- 50. Hagberg JM. Physical activity, fitness, health and aging. In: Bouchard C, Shepard R, Stephens T, eds. Physical Activity, Fitness and Health International Proceedings and Consensus Statement. Champaign, IL: Human Kinetics Publishers; 1994: 998–1005. [Google Scholar]
- 51. Duncan JT, Farr JE, Upton SJ. The effects of aerobic exercise on plasma cathecolamines and blood pressure in patients with essential hypertension. JAMA. 1985;254:206. [PubMed] [Google Scholar]
- 52. Nelson L, Jannings GL, Esler MD, et al. Effect of changing levels of physical exercise on blood pressure and haemodynamics in essential hypertension. Lancet. 1986;2:437. [DOI] [PubMed] [Google Scholar]
- 53. Minami N, Yoshikawa T, Kataoka H, et al. Effect of exercise and β‐Blocker on blood pressure and baroreflexes in spontaneously hypertensive rats. AJA. 2003;16:966–972. [DOI] [PubMed] [Google Scholar]
- 54. Somers VK, Conway J, Johnston J, et al. Effects of endurance training on baroreflex sensitivity and blood pressure in borderline hypertension. Lancet. 1991;337:1363–1386. [DOI] [PubMed] [Google Scholar]


