Abstract
J Clin Hypertens(Greenwich). 2010;12:613–620. © 2010 Wiley Periodicals, Inc.
The authors evaluated the risk for pregnancy‐related hypertension among previously healthy women who conceived within 5 years of exposure to drinking water contaminated with Escherichia coli O157.H7 in Walkerton, Canada (2000). Chronic hypertension was defined as systolic/diastolic blood pressure ≥140/90 mm Hg before 20 weeks gestation; gestational hypertension was defined as new onset systolic/diastolic blood pressure ≥140/90 mm Hg ≥20 weeks gestation. The incidence of hypertension was compared between women who were asymptomatic during the outbreak to those who experienced acute gastroenteritis. Blood pressure data were available for 135 of 148 eligible pregnancies. The adjusted relative risks for chronic and gestational hypertension were 1.5 (95% confidence interval [CI]: 0.3–7.7) and 1.0 (95% CI: 0.4–2.5), respectively. Mean arterial pressure before 20 weeks gestation was 2.7 mm Hg higher in women who had acute gastroenteritis (95% CI: 0.05–5.4). A trend toward higher chronic hypertension and mean arterial pressure in early pregnancy was observed among women who experienced gastroenteritis after exposure to bacterially‐contaminated drinking water.
In May of 2000, the municipal water supply in Walkerton, a small rural town in Ontario, Canada, became contaminated with bacteria, predominantly Escherichia coli O157:H7 and Campylobacter species. An unusually heavy rainfall and inadequate chlorination both contributed to the bacterial contamination of drinking water supplied by a shallow well. At the time of the outbreak, 5000 people lived in the town of Walkerton, of which 1000 (20%) were women aged 15 to 44 years. More than 2300 people became ill with symptoms of acute gastroenteritis; 27 cases of hemolytic uremic syndrome (HUS) were identified, and there were 7 deaths. 1 The magnitude of this event attracted worldwide media attention and sparked public concern about the safety of public drinking water. 2 , 3 Following the outbreak, the Walkerton Health Study (WHS) was initiated to monitor the potential long‐term health sequelae of the water contamination.
The Centers for Disease Control and Prevention estimate that E. coli O157:H7 infections cause between 50,000 and 120,000 gastroenteric illnesses annually in the United States, resulting in more than 2000 hospitalizations and 60 deaths. 4 , 5 , 6 However, apart from investigations into the long‐term sequelae of diarrhea‐associated HUS, 7 few studies have evaluated the long‐term health effects of exposure to this pathogen. In the developed world, it is rare for outbreaks of this magnitude to occur within a single community, 8 and this environmental catastrophe has allowed researchers to track long‐term health effects of E. coli O157:H7 exposure within a single cohort.
Receptors for E. coli O157:H7 are found in the kidney, and exposure to this pathogen may result in substantial nephron loss and subsequent hyperfiltration without presenting as HUS. 9 , 10 , 11 An earlier analysis of the WHS reported an increased risk for hypertension and reduced kidney function 4 years after infection among adults who were ill with acute gastroenteritis, but not HUS. 1
Hypertensive disorders during pregnancy complicate between 3% and 22% of pregnancies, and remain the leading cause of maternal and perinatal morbidity and mortality. 12 , 13 A subclinical renal injury from E. coli verotoxin may be unmasked by the physiologic demands of pregnancy and manifest as hypertension early in pregnancy. The present study of WHS participants evaluated the risk for pregnancy‐related hypertension (PRH) among previously healthy females who conceived within 5 years of drinking water contaminated with E. coli O157:H7. We compared the incidence of PRH, chronic, and gestational hypertension among participants who reported moderate to severe symptoms of gastroenteritis to participants who were asymptomatic at the time of the outbreak. Differences in mean arterial pressure (MAP) were also evaluated.
Methods
The Walkerton Health Study
WHS, a prospective cohort study, began in 2002 at which time residents of the Walkerton area, or anyone who had consumed municipal water at the time of the outbreak, were invited to attend a newly opened clinic and participate in the long term follow up. Written consent was obtained from all participants. Ethics approval for the study was obtained from the University of Western Ontario’s Research Ethics Board for Health Sciences. An analysis of the demographic characteristics found that the study sample was similar to the actual affected population and that potential response or selection biases would not lead to overestimates of risk between acute bacterial gastroenteritis and long term health sequelae. 14
All participants completed a 20–45 minute face‐to‐face, computer‐assisted interview that collected information on demographics, exposure to the water, illness at the time of the outbreak, medication history, and health conditions predating and since the outbreak, and family history. A complete pregnancy history was obtained, including date and location of birth and self‐reported hypertension during previous pregnancies. The presence of pre‐outbreak medical conditions was corroborated by medical chart review for 90% of participants.
Prepregnancy Body Mass Index, Blood Pressure and Renal Function. Information on prepregnancy height, weight, blood pressure (BP), and renal function was measured annually as part of WHS. Trained study personnel measured each participant’s height, weight, and BP using standardized protocols at each annual visit. Two automated BP readings and one manual BP reading, separated by 1 minute, were taken of seated participants with arm resting, palm facing upward, using a cuff size appropriate for measured arm circumference. 15 The automated readings were taken using a calibrated oscillometric device (Dinamap Pro 100 or 1846SX units, GE Medical Systems Information Technologies, Tampa, FL). Participants were asked to refrain from smoking 30 minutes prior to the measurements. Estimated glomerular filtration rate (eGFR) in mL/min/1.73 m2 was calculated using the Modification of Diet in Renal Disease equation (186 × serum creatinine−1.154 × age−0.203 × 0.742 [if female] × 1.21 [if black]). Serum creatinine was measured by the modified kinetic method of Jaffe using a Vitros 950 autoanalyzer with an interassay coefficient of variation <4% (Johnson and Johnson, Skillman, NJ). Albumin:creatinine ratio was measured on random spot urine samples using the IMAGE Beckman Coulter immunoassay for albumin (Beckman Coulter, Fullerton, CA).
Antenatal Chart Audit
A list of all pregnancies conceived after the outbreak was generated and the antenatal records were requested from the respective hospitals. Women with a history of hypertension, diabetes, or kidney disease prior to the outbreak were excluded from the study. Trained auditors conducted a detailed review of The Ontario Ministry of Health Antenatal 1 and Antenatal 2 forms and Record of Birth. Information was collected on BP during pregnancy, preexisting risk factors, and medical and obstetrical history.
Definition of Outcome. PRH was defined as any elevated systolic or diastolic BP observed during pregnancy: systolic/diastolic BP ≥140/90 mm Hg. Chronic hypertension was defined as elevated BP any time prior to 20 weeks gestation, and gestational hypertension was elevated BP for the first time ≥20 weeks gestation, with or without proteinuria. 16 Also, differences in MAP were measured because MAP may be a better predictor of PRH as compared to systolic BP and diastolic BP among low‐risk women. 17 , 18 MAP represents the steady component of BP and denotes a function of left ventricular contractility, heart rate, vascular resistance and elasticity averaged over time. 19 , 20 MAP was defined as twice the diastolic pressure, added to the systolic pressure, and divided by 3.
Definition of Exposure. Because of the demand on medical services at the time of the outbreak, adults were discouraged from submitting stool samples and advised not to seek medical attention unless absolutely necessary. As a result, severity of gastroenteritis symptoms at the time of the outbreak was largely determined by self‐report. In an effort to prevent nondifferential misclassification, which would bias the results toward a null association between acute gastroenteritis and long‐term health sequelae, self‐reported information about each participant’s symptoms at the time of the outbreak was confirmed for 90% of participants using physician and hospital charts using a standardized data abstraction form. Electronic records were also obtained from the regional health unit, which investigated illness during the outbreak.
Participants were divided into 2 groups according to the severity of illness at the time of the outbreak 21 : the asymptomatic group included participants with no symptoms of gastroenteritis at the time of the outbreak; the symptomatic group included participants with moderate to severe symptoms of acute gastroenteritis, specifically including any one or more of the following: diarrhea with more than 3 loose stools per day, or lasting more than 3 days, or any amount of bloody diarrhea. Participants with severe symptoms were more likely than those with moderate symptoms to report bloody diarrhea (39% vs 21%) and diarrhea lasting more than 3 days (54% vs 27%).
Analysis
To maximize statistical power, the outcomes of all pregnancies for a given woman were included; thus, the unit of analysis was a pregnancy. However, because a woman can have more than one pregnancy, standard errors were adjusted for the within‐woman correlation in all statistical models using a sandwich error estimation procedure. 22 The relative risk (RR) of PRH among symptomatic women compared to asymptomatic women was calculated using a modified Poisson regression model with 95% confidence intervals (CI). 22 MAP was regressed on exposure status after controlling for other known risk factors. The adjusted difference in MAP between the 2 groups was estimated directly from the coefficients in the linear model. We adjusted for the following known risk factors for PRH, selected a priori, maternal age at first obstetrical visit (in 1‐year increments), prepregnancy body mass index ≥30 kg/m2 (obese), gravida, family history of hypertension, diabetes mellitus (gestational or overt), previous history of premature birth, and smoking status (smoker before or during pregnancy: yes/no). Models were reduced using backward regression at alpha 0.15. Sensitivity analyses were performed to evaluate a dose‐response relationship between hypertension outcomes and exposure divided into 3 categories (none; moderate; severe).
Results
A total of 2553 women participated in the WHS between 2002 and 2006, and 148 were pregnant between January 2001 and May 2006. The sample flow is shown in Figure 1. After exclusions for miscarriages and abortion (n=19), preexisting hypertension prior to the outbreak (n=9) and past history of gestational hypertension (n=1), diabetes mellitus (n=1), kidney disease (n=2), and multiple pregnancies (n=3) (not mutually exclusive), 111 participants and 148 pregnancies were eligible for inclusion in the analysis. Chart audits were completed for 138 of the 148 pregnancies (93%) and BP data were available for 135 (91%) pregnancies.
Figure 1.
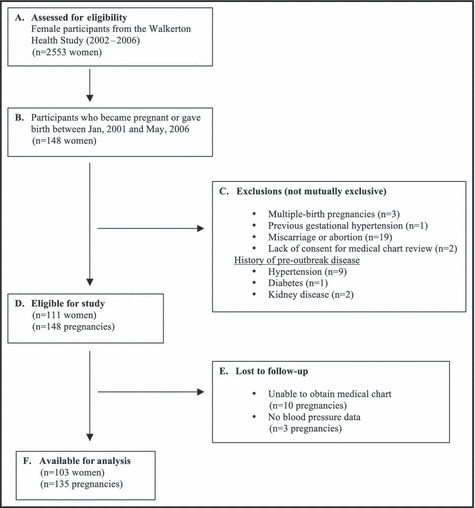
Flow chart of study participants.
Table I describes the characteristics of the pregnant women of which 48 (36%) were asymptomatic and 87 (64%) were symptomatic at the time of the outbreak. Median gravida (2), number of prenatal visits (10), and average age of women (28 years) were similar between exposure groups; however, the symptomatic group was more likely to have a family history of hypertension as compared to the asymptomatic group (35% vs 40%).
Table I.
Characteristics of Pregnancies Within 5 Years of an Outbreak of Acute Gastroenteritis From Bacteria‐Contaminated Drinking Water (N=135)
| Maternal Characteristics | Symptoms of Gastroenteritis During Outbreak in May, 2000 | |
|---|---|---|
| None (n=48) | Moderate to Severe (n=87) | |
| Age at first prenatal visit (y), mean (SD) | 28.1 (6.0) | 28.2 (5.3) |
| Family history of hypertension, % | 35.4 | 40.23 |
| Diabetes (prepregnancy or gestational), % | 4.2 | 5.8 |
| Smoking, % | 27.1 | 25.3 |
| Prepregnancya | ||
| Height (cm), mean (SD) | 163.3 (4.7) | 164.3 (5.6) |
| Weight (kg), mean (SD) | 71.1 (16.6) | 75.1 (18.8) |
| BMI (kg/m2), mean (SD) | 26.8 (6.5) | 27.8 (6.7) |
| Obese BMI (≥30 kg/m2), % | 27.1 | 29.9 |
| Systolic blood pressure (mm Hg), mean (SD) | 108.3 (8.5) | 113.1 (9.2) |
| Diastolic blood pressure (mm Hg), mean (SD) | 64.7 (7.2) | 67.4 (5.8) |
| Mean arterial pressure (mm Hg) | 79.3 (6.4) | 82.6 (5.8) |
| Albumin to creatinine ratio (mg/mmol), mean (SD) | 1.2 (2.8) | 1.1 (2.7) |
| Estimated glomerular filtration rate (mL/min/1.73 m2), mean (SD) | 97.9 (16.9) | 103.0 (22.4) |
| Obstetrical history | ||
| Gravida, median | 2 | 2 |
| Previous premature birth, % | 4.2 | 6.9 |
| Previous low birth weight, % | 4.2 | 4.6 |
| Prenatal care | ||
| Gestational age at first prenatal visit (weeks), mean (SD) | 13.0 (6.6) | 12.6 (5.8) |
| Gestational age at last prenatal visit (weeks), mean (SD) | 38.1 (1.7) | 38.1 (2.5) |
| Total number of prenatal visits, mean (SD) | 9.5 (2.7) | 9.8 (2.9) |
| Distribution by year of birth, % | ||
| 2001 | 25.0 | 19.5 |
| 2002 | 20.8 | 13.8 |
| 2003 | 16.7 | 21.8 |
| 2004 | 10.4 | 20.7 |
| 2005 | 18.8 | 11.5 |
| 2006 | 8.3 | 12.6 |
Abbreviations: BMI, body mass index; SD, standard deviation. aData on prepregnancy blood pressure and renal function were only available for women who became pregnant after study enrollment, which began in 2002 (n=74; 44% of those with no symptoms and 61% of those with moderate to severe symptoms of gastroenteritis).
Pregnancy‐Related Hypertension
PRH was detected in 28 (20.7%) pregnancies, which included 9 (6.7%) cases of chronic hypertension and 19 (14.1%) cases of gestational hypertension. Figure 2 shows the crude rates of hypertension outcomes compared between symptomatic and asymptomatic groups. The rates of PRH, chronic, and gestational hypertension were higher among symptomatic compared to asymptomatic groups; however no differences were statistically significant.
Figure 2.
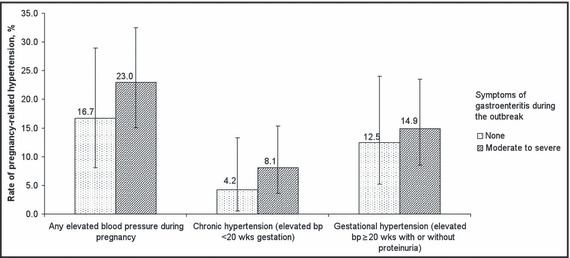
Rates of pregnancy‐related hypertension within 5 years of an outbreak of acute gastroenteritis from bacteria‐contaminated drinking water. Error bars represent 95% confidence intervals. bp indicates blood pressure. 29
The RRs for chronic and gestational hypertension are presented in Table II. After adjusting for age, obesity, smoking, and family history of hypertension, the RRs for chronic and gestational hypertension were 1.5 (95% CI, 0.3–7.7) and 1.0 (95% CI, 0.4–2.5) respectively. The adjusted RR for PRH was 1.1 (95% CI, 0.5–2.3). A body mass index ≥30 kg/m2 was strongly associated with chronic hypertension (adjusted RR=9.3; 95% CI, 2.1–41.8) and smoking was strongly associated with gestational hypertension (RR=3.8; 95% CI, 1.5–9.5).
Table II.
RR for Chronic and Gestational Hypertension Among Pregnant Women Within 5 Years of Drinking Water Contaminated With Escherichia coli 0157:H7 (N=135)
| Chronic Hypertension | Gestational Hypertension a | |||
|---|---|---|---|---|
| Unadjusted b RR (95% CI) | Adjusted b,c RR (95% CI) | Unadjusted b RR (95% CI) | Adjusted b,c RR (95% CI) | |
| Moderate to severe symptoms of gastroenteritis | 2.0 (0.4–9.1) | 1.5 (0.3–7.7) | 1.1 (0.4–2.7) | 1.0 (0.4–2.5) |
| Obese (BMI ≥30 kg/m2) | 9.3 (2.1–41.8) | 1.7 (0.8–3.9) | ||
| Smoking | 2.4 (0.7–7.6) | 3.8 (1.5–9.5) | ||
Abbreviations: BMI, body mass index; CI, confidence interval; RR, relative risk. aGestational hypertension with or without proteinuria. bComparison group: women who were asymptomatic during the outbreak. cFull models were reduced using Backward Elimination (P=.15). Adjusted models included age in 1‐year increments and family history of hypertension.
Mean Arterial Pressure
Over the course of pregnancy, MAP was consistently higher among symptomatic vs asymptomatic women, with the greatest difference exhibited prior to 20 weeks gestation (Figure 3). After adjusting for age, obesity, previous premature birth, and family history of hypertension, the difference in MAP before 20 weeks gestation between exposure groups became marginally significant, and was 2.7 mm Hg higher in symptomatic vs asymptomatic participants (95% CI, 0.05–5.4).
Figure 3.
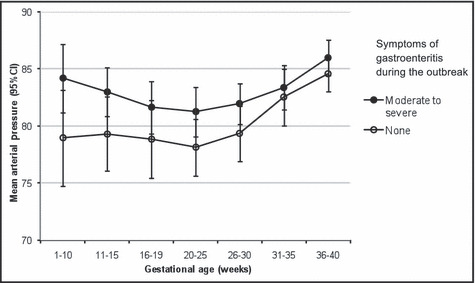
Mean arterial pressure compared between exposure groups over the course of pregnancy (5 years after an outbreak of acute gastroenteritis from bacteria‐contaminated drinking water). Error bars represent 95% confidence intervals (CI).
When gastroenteritis was categorized into 3 categories of increasing severity, there was a nonsignificant dose‐response association between acute gastroenteritis and chronic hypertension. Compared to asymptomatic women, the RR for chronic hypertension among those who experienced moderate symptoms of gastroenteritis was 1.7 (95% CI, 0.3–8.6), compared to 2.6 (95% CI, 0.5–15.3) among those reporting severe symptoms of gastroenteritis at the time of the outbreak. Similarly, a nonsignificant dose‐response relationship was observed between symptoms of gastroenteritis and MAP measured prior to 20 weeks gestation. Compared to asymptomatic women, MAP (<20 weeks gestation) was 1.9 mm Hg (95% CI, −1.5 to 5.2) higher among women with moderate symptoms, and 2.9 mm Hg (95% CI, −1.8to 7.8) higher among those reporting severe symptoms at the time of the outbreak. No dose‐response relationship was observed between gastroenteritis severity and gestational hypertension.
Discussion
Results of our previous study of WHS participants identified a significantly increased risk of hypertension in adults who reported moderate to severe gastroenteritis after exposure to drinking water contaminated with E. coli O157:H7. 1 We hypothesized that exposure to E. coli O157:H7 may impose a subclinical renal injury that is not immediately apparent by either a rise in creatinine nor an increase in BP. The increased demand of glomerular filtration during pregnancy may unmask the occult injury and present as an increase in BP in the first 20 weeks of pregnancy. We did observe a marginally significant trend toward higher MAP in the first 20 weeks of gestation among women within 5 years of exposure to bacterially‐contaminated drinking water. No increased risk for gestational hypertension was detected.
Although nonsignificant, the results of our study concur with our initial hypothesis suggesting that any effect of exposure to bacteria‐contaminated drinking water on BP occurs prior to or early in pregnancy. This is supported by the gradient effect, when acute gastroenteritis was defined as 3 categories of increasing severity, a dose‐response increased risk for chronic hypertension was observed, which was not apparent for gestational hypertension. Also, the difference in MAP between exposure groups was greatest prior to 20 weeks gestation after which time the difference narrowed considerably. Women with gastrointestinal symptoms at the time of the outbreak had more risk factors for PRH. This suggests that exposure may have a greater effect in a compromised host; however, it was not possible to test this hypothesis within this study. It is important to note that any increased risk for chronic hypertension from exposure to bacterial‐contaminated water is small, and far outweighed by the risks from smoking and unhealthy weight.
Unlike chronic hypertension, gestational hypertension is a condition unique to pregnancy and in the case of preeclampsia, is more related to the function of the placenta than the kidney. 23 The increase in BP that occurs in preeclampsia represents an indicator of an underlying multisystem syndrome, and may be a compensatory mechanism to increase vascular exchange between the mother and a poorly perfused placenta. 24 , 25 , 26 Poor placental perfusion is caused by defective trophoblastic implantation, which results from an immunologic maladaptation between maternal tissue and fetal (paternal) allograph. 23 , 24 , 27 Thus, it is not surprising that no increase in gestational hypertension occurred.
The incidence of pregnancy‐related hypertension in this study (20.7%) was higher than that reported in the literature. 13 , 23 Although this may represent a real difference, an overestimate is possible given that our definition of hypertension was based on a single elevated BP rather than >1 elevation within 4 to 6 hours. 28 In addition, 2 participants with gestational hypertension that did not present for prenatal care until after 20 weeks gestation may have had undiagnosed chronic hypertension. The incidence of pregnancy‐related hypertension varies widely in the literature due to differences in definition, population composition, demographic and obstetric characteristics, and actual disease incidence. 23 The incidence of chronic hypertension is estimated to range from 3% to 5% in developed countries, 13 , 26 but may be as high as 22% among women older than 30 years of age. 13 The incidence of gestational hypertension (with or without proteinuria) ranges from 5% to 9%. 23
Our study has several limitations. First, the small sample size in this study makes it difficult to draw firm conclusions regarding the risk for chronic hypertension from exposure to E. coli O157:H7 in this population. Small sample sizes are unavoidable when evaluating rare outcomes in selected populations. However, the unique circumstances of this outbreak in which an entire community was exposed to bacteria‐contaminated drinking water permits hypotheses related to E. coli O157:H7 exposure to be tested within a single, large population, which is normally not possible given the wide geographical spread of E. coli O157:H7 outbreaks. 8 Given the small number of pregnancies in the WHS cohort, and rareness of the outcome, several more years of data collection would be necessary to ensure a sufficiently large sample; however, any conclusions reached from such an analysis would be limited by the longer time span between exposure and outcome. The small sample meant we were also underpowered to evaluate interactions between exposure and other risk factors such as smoking or body mass index. Finally, since all participants reported drinking the water during the outbreak it is possible that the asymptomatic group was exposed, but did not experience acute gastroenteritis. Such misclassification would attenuate any associations between exposure and outcome.
Conclusion
Our results suggest the need for close monitoring of BP among pregnant women after exposure to E. coli O157:H7. Consistent with our hypothesis of an increased risk for hypertension among women exposed to E. coli O157:H7, there was a slight elevation in BP during early pregnancy among women who experienced gastroenteritis during the water‐contamination outbreak. However, this BP elevation is transient, is unlikely to be materially different than prepregnancy blood pressure, and confers no additional risk for gestational hypertension.
Financial Support: This research was funded by the Ontario Ministry of Health and Long‐Term Care and the Lawson Health Research Institute‐Internal Research Fund.
References
- 1. Garg AX, Moist L, Matsell D, et al. Risk of hypertension and reduced kidney function after acute gastroenteritis from bacteria‐contaminated drinking water. CMAJ. 2005;173:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hrudey S. Drinking‐water risk management principles for a total quality management framework. J Toxicol Environ Health A. 2004;67:1555–1566. [DOI] [PubMed] [Google Scholar]
- 3. Salvadori M, Sontrop JM, Garg AX, et al. Factors that led to the Walkerton tragedy. Kidney Int. 2009;175:S33–S34. [DOI] [PubMed] [Google Scholar]
- 4. Mead PS, Slutsker L, Dietz V, et al. Food‐related illness and death in the United States. J Environ Health. 2000;62:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frenzen PD, Drake A, Angulo FJ. Economic cost of illness due to Escherichia coli O157 infections in the United States. J Food Prot. 2005;68:2623–2630. [DOI] [PubMed] [Google Scholar]
- 6. Powell M, Ebel E, Schlosser W. Considering uncertainty in comparing the burden of illness due to foodborne microbial pathogens. Int J Food Microbiol. 2001;69:209–215. [DOI] [PubMed] [Google Scholar]
- 7. Garg AX, Suri RS, Barrowman N, et al. Long‐term renal prognosis of diarrhea‐associated hemolytic uremic syndrome: a systematic review, meta‐analysis, and meta‐regression. JAMA. 2003;290:1360–1370. [DOI] [PubMed] [Google Scholar]
- 8. Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157: H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. [DOI] [PubMed] [Google Scholar]
- 9. Hughes AK, Ergonul Z, Stricklett PK, et al. Molecular basis for high renal cell sensitivity to the cytotoxic effects of shigatoxin‐1: upregulation of globotriaosylceramide expression. J Am Soc Nephrol. 2002;13:2239–2245. [DOI] [PubMed] [Google Scholar]
- 10. Van Setten PA, Van HV, Van den Heuvel LP, et al. Verocytotoxin inhibits mitogenesis and protein synthesis in purified human glomerular mesangial cells without affecting cell viability: evidence for two distinct mechanisms. J Am Soc Nephrol. 1997;8:1877–1888. [DOI] [PubMed] [Google Scholar]
- 11. Hughes AK, Stricklett PK, Kohan DE. Cytotoxic effect of Shiga toxin‐1 on human proximal tubule cells. Kidney Int. 1998;54:426–437. [DOI] [PubMed] [Google Scholar]
- 12. Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22:203–212. [DOI] [PubMed] [Google Scholar]
- 13. Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369–377. [DOI] [PubMed] [Google Scholar]
- 14. Garg AX, Macnab J, Clark W, et al. Long‐term health sequelae following E. coli and campylobacter contamination of municipal water. Population sampling and assessing non‐participation biases. Can J Public Health. 2005;96:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chobanian AV, Bakris GL, Black HR, et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 16. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 17. Cnossen JS, Vollebregt KC, Vrieze N, et al. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre‐eclampsia: systematic review and meta‐analysis. BMJ. 2008;336:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walsh CA, Baxi LV. Mean arterial pressure and prediction of pre‐eclampsia. BMJ. 2008;336:1079–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Safar ME. Pulse pressure in essential hypertension: clinical and therapeutical implications. J Hypertens. 1989;7:769–776. [DOI] [PubMed] [Google Scholar]
- 20. Benetos A, Laurent S, Asmar RG, et al. Large artery stiffness in hypertension. J Hypertens Suppl. 1997;15:S89–S97. [DOI] [PubMed] [Google Scholar]
- 21. Garg AX, Marshall J, Salvadori M, et al. A gradient of acute gastroenteritis was characterized, to assess risk of long‐term health sequelae after drinking bacterial‐contaminated water. J Clin Epidemiol. 2006;59:421–428. [DOI] [PubMed] [Google Scholar]
- 22. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 23. Zhang J, Zeisler J, Hatch MC, et al. Epidemiology of pregnancy‐induced hypertension. Epidemiol Rev. 1997;19:218–232. [DOI] [PubMed] [Google Scholar]
- 24. Robillard PY, Dekker GA, Hulsey TC. Evolutionary adaptations to pre‐eclampsia/eclampsia in humans: low fecundability rate, loss of oestrus, prohibitions of incest and systematic polyandry. Am J Reprod Immunol. 2002;47:104–111. [DOI] [PubMed] [Google Scholar]
- 25. Roberts JM, Balk JL, Bodnar LM, et al. Nutrient involvement in preeclampsia. J Nutr. 2003;133:1684S–1692S. [DOI] [PubMed] [Google Scholar]
- 26. Roberts JM, Pearson G, Cutler J, et al. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41:437–445. [DOI] [PubMed] [Google Scholar]
- 27. Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99:2152–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Broughton PF, Roberts JM. Hypertension in pregnancy. J Hum Hypertens. 2000;14:705–724. [DOI] [PubMed] [Google Scholar]
- 29. Moist L, Sontrop JM, Garg A, et al. Risk of pregnancy‐related hypertension within five years of exposure to bacteria‐contaminated drinking water. Kidney Int. 2009;75(suppl 112):S47–S49. [DOI] [PubMed] [Google Scholar]


