Abstract
J Clin Hypertens (Greenwich). 2010;12:181–186. ©2010 Wiley Periodicals, Inc.
The authors assessed the validity of a hand‐carried cardiac ultrasound device operated by an internal medicine resident for left ventricular geometric abnormalities (LVGAs) in mild hypertensive patients. LVGAs were diagnosed when at least one of the following was present: left ventricular mass index exceeding 125 g/m2 and 110 g/m2 for men and women, respectively; intraventricular septum thickness ≥10 mm; posterior wall thickness ≥10 mm; and left ventricular end‐diastolic diameter ≥5.3 mm. For validation, a cardiologist performed standard echocardiography in all patients. A total of 85 patients completed both echocardiographic studies. LVGAs were diagnosed in 19 (22.4%) cases, 18 of which were confirmed by standard echocardiography. Standard echocardiography did not detect any case of LVGA among the hand‐carried cardiac ultrasonography LVGA‐negative patients. The sensitivity and specificity of the resident’s examination were 100% and 98.78%, respectively. Agreement between the two studies was 99% (κ 0.97, 95% confidence interval). Hand‐carried cardiac ultrasonography may be used as a screening tool for LVGA in hypertensive patients.
Left ventricular geometric changes are indications of hypertensive subclinical target organ damage, which is an intermediate stage in cardiovascular morbidity. 1 Subclinical target organ damage should be detected as soon as possible in order to attenuate the progression of vascular disease to major cardiovascular and cerebrovascular insult. 2 , 3 Left ventricular geometric changes as a consequence of increased afterload usually presents with an elevated wall and septal thickness, with or without an increase in cavity size. 4 , 5 Isolated septal thickening in hypertensive patients is shown to be associated with poorer control and longer duration of hypertension. 6 In addition, left ventricular wall thickness in hypertensive patients has been demonstrated to correlate with diastolic dysfunction. 7
Early detection of left ventricular geometric abnormalities (LVGAs) such as isolated septal and wall thickening has an influence on decision making in treatment and follow‐up of hypertensive patients. Echocardiography can provide important information about these early changes; however, due to personal and cost considerations it is currently not recommended as a routine test in the assessment of all hypertensive patients. 1 , 8
In recent years, a battery‐operated hand‐carried ultrasonography (HCU) device has been successfully introduced into clinical practice. The use of HCU demonstrated unsuspected findings such as systolic dysfunction and left atrial enlargement that resulted in management change. 9 It was found to be a useful tool in assessing the presence of heart failure, valvular regurgitation, and pericardial effusion. 10 , 11 Its use has been successfully applied in emergency as well as outpatient settings. 12 , 13 Recently, a large‐scale research study demonstrated its efficacy in detection of left ventricular hypertrophy (LVH) in hypertensive African American patients in a rural setting. 14 Its main advantage besides small dimensions and higher portability is its ability to be operated by personnel briefly trained in detection of a limited number of abnormal parameters. 13 , 15 , 16
The aim of the present study was to evaluate the diagnostic accuracy of an HCU device operated by a briefly trained medical resident, in detection of left ventricular geometry changes in a population of mild hypertensive patients referred to an outpatient clinic. The accuracy of HCU diagnosis was compared with a full featured standard echocardiography (SE) examination conducted by an expert echocardiographic cardiologist blinded to the HCU examination results.
Methods
Study Population
The study population included grade I hypertensive patients visiting the hypertensive clinic of the Soroka University Medical Center. Patients eligible for the study were those older than 18 years, with pretreatment blood pressure grade I essential hypertension according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) classification (systolic blood pressure ≤159 mm Hg, diastolic blood pressure ≤99mm Hg), who were treated with ≤2 antihypertensive drugs. During a 6‐month period (from July 2004 to December 2004), all eligible patients visiting the hypertensive clinic were enrolled. Patients with secondary hypertension, known LVH, or existing cardiovascular disease (such as ischemic heart disease, renal insufficiency, or cerebrovascular damage) were excluded from the study.
Demographic data, disease history, laboratory test results, and medication information were obtained from the medical records of the patients. Study protocol was approved by the local ethical committee of the Soroka University Medical Center. Prior to recruitment, all patients signed an informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Echocardiography
All patients underwent focused HCU study performed by an internal medicine resident. The resident went through a 2‐hour session of cardiac ultrasound interpretation conducted by an expert echocardiographic cardiologist, and another 6 hours of training with a certified echocardiographic technician. To validate the diagnostic accuracy of the tests performed by the primary physician, an expert echocardiographic cardiologist, unaware of the first HCU study, performed SE in all patients. Performance of both echocardiographic examinations was a predefined inclusion criterion.
The OptiGo HCU device (Philips Medical Systems, Andover, MA) was used. It is equipped with a 2.5‐MHz phased array broadband transducer and operates on a rechargeable lithium ion battery or alternating current. Three‐dimensional imaging, color flow Doppler imaging, and 3 calipers for linear measurements are also integrated with the system. Images were documented on a CompactFlash card.
Echocardiographic Measurements and LVGA Definition
Left ventricular mass was calculated using the Devereux‐modified American Society of Echocardiography equation: 0.80 {1.04 [(IVST+PWT+LVEDD)3−LVEDD3]}+0.6. Where IVST was intraventricular septal thickness, PWT was posterior wall thickness, and LVEDD was left ventricular end‐diastolic diameter. Left ventricular mass index (LVMI) was calculated by dividing left ventricular mass by body surface area using the DuBois formula. Increased LVMI was considered to be present when LVMI exceeded 125 g/m2 for men and 110 g/m2 for women. 14 LVGAs were considered to be present when at least one of the following was present: LVMI >125 g/m2 for men and 110 g/m2 for women, increased intraventricular septum thickness (≥10 mm), increased posterior wall thickness (≥10 mm), or increased LVEDD (≥5.3 mm). 4
Statistical Analysis
Bivariate hypotheses involving continuous variables were tested with a t test for independent groups with normal distribution and Mann–Whitney test for not normal distribution. Normality of the study data was tested with a 1‐sample Kolmogorov‐Smirnov test to indicate the appropriateness of parametric testing. For tests of whether the distribution of categoric variables differed across study groups, chi‐square test was used. Fisher exact test was applied when appropriate. The correlation between continuous variables was evaluated by Pearson method.
The agreement rate between definition of left ventricular geometric changes by the internal medicine resident and cardiology specialist was assessed in a one‐by‐one manner with κ statistic method. Boundaries of 95% confidence interval (CIs) for sensitivity, specificity, and positive and negative predicting values were calculated according to the efficient score method. 17 The comparison between echocardiographic assessment by internal medicine resident and cardiologist (LVMI, posterior wall and septum thickness) was presented as regression lines and Bland‐Altman plots. Continuous variables were expressed as mean ± standard deviation (SD), and categoric variables were expressed as percentages. All reported P values are 2‐sided and P<.05 was considered significant. Statistical analyses were performed with SPSS software (version 12.0.1; SPSS Inc, Chicago, IL).
Results
During the 6‐month study period, 530 new patients were referred to the hypertension clinic of the Soroka University Medical Center. Eighty‐eight patients answered the inclusion criteria, had grade I hypertension, and were treated with ≤2 antihypertensive medications. Three patients refused to participate, thus 85 patients were included in the study.
Of the 85 patients enrolled in the study, 19 were found to have abnormal cardiac geometry by HCU. The abnormalities were demonstrated as either abnormal septal or posterior wall thickening (12.4± 0.7 mm and 10.8±1 mm, respectively). LVEDD and LVMI were not found to be abnormally enlarged (43.4±3.7 mm and 95.5±14.3 gm2/m2, respectively). The projected prevalence of LVGA in the study population was 22.4%, with a 95% CI of 13.6% to 31.4%.
Table I outlines the demographic and clinical characteristics of the study population divided into two groups according to the presence or absence of LVGA as measured by the internal medicine resident using an HCU device. Patients with LVGA were older and predominantly men. No difference was found in hypertension duration between LVGA‐positive and ‐negative patients (10.8±8.8 vs 12.0±10.5 years, respectively; P=.64). Overall, women were older than men, with a mean age of 62.1±11.2 vs 53.4± 15.9 years, respectively (P=.004). Age of hypertension diagnosis was lower in men than in woman (43.3±14.5 vs 49.4±11.6 years, respectively; P=.04). More women were treated with 2 antihypertensive drugs for blood pressure control compared with men (75.9% vs 58.1%, respectively; P=.08). Patients with a family history of hypertension tended to be younger at the time of hypertension diagnosis (45.9±11.9 vs 51.3±15.7 years, respectively; P=.10), and had longer duration of hypertension (13.0±10.7 vs 7.7±6.9 years, respectively; P=.04).
Table I.
Study Population Characteristics According to Left Ventricular Geometric Abnormalities (LVGAs)
| All Patients (N=81) | Patients With LVGAs (n=19) | Patients Without LVGAs (n=66) | P Value | |
|---|---|---|---|---|
| Age, y | 58.9±13.7 | 65.1±11.0 | 57.2±14.0 | .03 |
| Male sex, % | 31 (36.5) | 11 (57.9) | 20 (30.2) | .03 |
| Age of hypertension diagnosis, y | 47.2±13.0 | 54.3±11.9 | 45.2±12.7 | .01 |
| Years of hypertension | 11.8±10.1 | 10.8±8.9 | 12.0±10.5 | .64 |
| Systolic blood pressure, mm Hg | 132.2±13.4 | 133.4±14.9 | 131.9±13.0 | .66 |
| Diastolic blood pressure, mm Hg | 75.3±11.4 | 75.5±15.1 | 75.2±10.3 | .93 |
| Body mass index, kg/m2 | 30.9±5.7 | 30.5±5.6 | 31.0±5.8 | .74 |
| Dyslipidemia, % | 38 (44.7) | 7 (37.0) | 31 (47.0) | .43 |
| Smoker, % | 10 (11.8) | 4 (21.0) | 6 (9.1) | .22 |
| Family history of hypertension, % | 65 (76.5) | 12 (63.0) | 53 (80.3) | .12 |
| Patients treated with 2 drugs | 59 (69.4) | 15 (79.0) | 44 (66.7) | .31 |
| Medication | ||||
| Calcium channel blockers | 38 (44.7) | 8 (42.1) | 30 (45.5) | .80 |
| Diuretics | 28 (32.9) | 9 (47.4) | 19 (28.8) | .13 |
| Angiotensin‐converting enzyme inhibitors | 37 (43.5) | 9 (47.4) | 28 (42.4) | .70 |
| β‐Blockers | 29 (34.1) | 4 (21.1) | 25 (37.9) | .17 |
| α‐Blockers | 4 (4.7) | 2 (10.5) | 2 (3.0) | .22 |
| Angiotensin receptors blockers | 7 (8.2) | 2 (10.5) | 5 (7.6) | .65 |
Table II compares echocardiographic measurements between the two examiners. As depicted, all other parameters measured by the two devices were comparable, except for a mild difference in PWT measurement that did not approach statistical significance.
Table II.
The Different Echocardiographic Characteristics as Measured by the Cardiologist and Internal Medicine Resident
| Parameter | Absence of LVGA | Presence of LVGA | ||||
|---|---|---|---|---|---|---|
| Internal Medicine Resident | Cardiologist | P Value | Internal Medicine Resident | Cardiologist | P Value | |
| IVS, mm | 9.5±0.9 | 9.5±1.3 | .89 | 12.4±0.7 | 12.6±1.1 | .14 |
| PWT, mm | 9.1±1.0 | 8.9±1.3 | .09 | 10.8±1.0 | 10.1±1.2 | .02 |
| LVEDD, mm | 42.3±4.3 | 42.4±4.7 | .81 | 43.4±3.7 | 42.7±4.6 | .35 |
| LVMI, gm2/m2 | 70.4±13.9 | 69.8±15.4 | .62 | 95.5±14.3 | 91.0±16.9 | .20 |
Abbreviations: IVS, intraventricular septum; LVEDD, left ventricular end‐diastolic diameter; LVGA, left ventricular geometric abnormality; LVMI, left ventricular mass index; PWT, posterior wall thickness.
SE study found LVGA in 18 of the 19 patients diagnosed by the HCU device (sensitivity of 1.00 and specificity of 0.99; Table III). The agreement between the two examinations (by the internal medicine resident using an HCU device and expert echocardiographic cardiologist performing SE) was 94.7%, with a κ statistic of 0.97 (95% CI, 0.9–1.0). All patients diagnosed as negative for LVGA by HCU were also found to be LVGA negative by SE.
Table III.
Agreement Between Internal Medicine Resident and Cardiology Specialist Assessment of Left Ventricular Geometric Abnormality (LVGA)
| Cardiology Specialist | Internal Medicine Resident | ||
|---|---|---|---|
| Presence of LVGA | Absence of LVGA | Total | |
| Presence of LVGA | 18 (21.2%) | 0 (0%) | 18 |
| Absence of LVGA | 1 (1.2%) | 66 (77.6%) | 67 |
| Total | 19 | 66 | 85 |
Sensitivity=1.00 (95% confidence interval [CI], 0.78–1.00). Specificity=0.99 (95% CI, 0.91–1.00). Positive predictive value=0.95 (95% CI, 0.72–1.00). Negative predictive value=1.00 (95% CI, 0.93–1.00). κ coefficient=0.97 (95% CI, 0.9–1.0).
The Figure presents Bland‐Altman plots for the comparison of measurements performed by the internal medicine resident and cardiologist. The Bland‐Altman plot is a statistical method to compare two measurement techniques. In this graphical method, the differences between the two techniques (ie, internal medicine resident estimation vs cardiologist estimation) are plotted against the averages of the two techniques. Horizontal lines are drawn at the mean difference and at the mean difference ± 1.96 times the SD of the differences. Mean difference (internal medicine resident – cardiologist) for LVMI, PWT, and IVST was −1.7 g/m2, with the boundaries (1.96×SD) of ±22.5 g/m2, 0.3 with the boundaries (1.96×SD) of ±2.2 mm, and −0.1 with the boundaries (1.96×SD) of ±1.7 mm, respectively.
Figure.
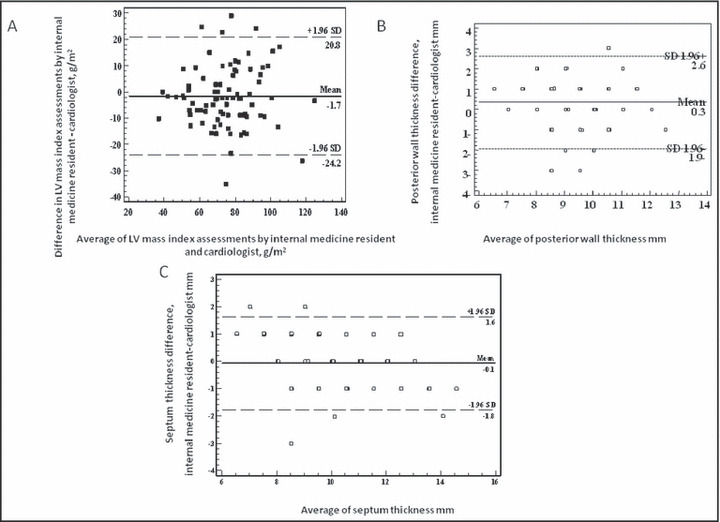
Bland‐Altman plot for comparison between two assessments. (A) Comparison between LV mass index assessment by cardiologist and internal medicine resident. (B) Comparison between posterior wall thickness assessment by cardiologist and internal medicine resident. (C) Comparison between septum thickness assessment by cardiologist and internal medicine resident.
Discussion
The main goal of our study was to address the question of whether primary physicians briefly trained in cardiac ultrasound operation could efficiently detect LVGA in patients with mild hypertension using an HCU device. Using HCU we found that 19 of 85 patients (22.4%) in our study group had cardiac geometric abnormalities. The abnormalities included increased septal and posterior wall thickness (12.4±0.7 and 10.8±1.0, respectively). Mean LVMI, although above normal values (95.5±14.3), did not met criteria for LVH diagnosis. Of 19 patients diagnosed by the medical resident, 18 patients with the abnormality were verified by a cardiologist with echocardiographic expertise blinded to the resident’s examination (sensitivity –1.0, specificity –0.99).
The diagnosis of LVGA in hypertensive patients marks a turning point in the progression of disease. Various cardiac geometric abnormalities such as cardiac concentric remodeling and interventricular septal hypertrophy are independent risk factors for the development of cerebrovascular damage, 18 elevation of atrial natriuretic peptides (a marker for left ventricular dysfunction), 19 , 20 diastolic dysfunction, and dysrhythmias. 21 The detection of LVGA in patients with mild hypertension has an important impact on further medical therapy and follow‐up. An appropriate blood pressure reduction regimen can improve diastolic dysfunction and reduce relative PWT. 22 , 23
Notwithstanding the fact that echocardiography is an excellent tool with high sensitivity and specificity in diagnosing LVGA, systematic echocardiography was not found to be a cost‐effective tool in assessment of mild hypertensive patients and its use as screening tool is not supported by current recommendations. 1 , 24 On the other hand, electrocardiography has low sensitivity in detecting structural cardiac abnormalities. 1 The advantage of portable echocardiographic devices has the potential to revolutionize the field of LVGA screening. Its precision in the diagnosis of a variety of cardiac pathologies has been proved in several studies. 25 , 26 , 27 In previous studies comparing LVH rate of detection between HCU and SE, the operators of the HCU population consisted of expert cardiologists or cardiology residents with an average of 6 months of echocardiographic training. 27 In addition, HCU was proven to be a cost‐effective tool by reducing the SE needed to be performed. 28 , 29 In our study, a medical resident correctly identified LVGA after focused cardiac ultrasound training. The diagnosis of LVGA was established in 19 of 85 patients (22.4%) with mild hypertension participating in the study. LVGA was found at higher rates in men and older patients, demographic features known in the literature to be associated with this condition. 24
Limitations
In our study there were two limitations: (1) the CI for sensitivity, specificity, and positive and negative predictive estimates was wide due to small sample size. Studies with a larger number of patients are needed to confirm these results. (2) The measurements by HCU were performed by one resident, and so intraobserver variability was not examined.
Conclusions
These findings have important implications to the early diagnosis and treatment of LVGA in the hypertensive population. Since the management of hypertensive patients is conducted mainly by primary physicians, the HCU device might become a powerful bedside tool for early detection and management of patients at higher risk for cardiovascular complications. Finally, taking into consideration its higher portability and lower cost of operation, HCU should be considered as a tool for mass screening for LVGA in hypertensive populations.
Acknowledgments
Acknowledgment: Drs Perez‐Avraham and Kobal contributed equally to the study.
References
- 1. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 2. Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy by losartan versus atenolol: the Losartan Intervention for Endpoint reduction in Hypertension (LIFE) Study. Circulation. 2003;108:684–690. [DOI] [PubMed] [Google Scholar]
- 3. Muiesan ML, Salvetti M, Rizzoni D, et al. Association of change in left ventricular mass with prognosis during long‐term antihypertensive treatment. J Hypertens. 1995; 13:1091–1095. [DOI] [PubMed] [Google Scholar]
- 4. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 5. Nen‐Chung Chang T‐CWaZ‐YL. Patterns of isolated septal hypertrophy and their clinical correlations in essential hypertension. Int J Angiol. 1996;5:175–180. [Google Scholar]
- 6. Verdecchia P, Porcellati C, Zampi I, et al. Asymmetric left ventricular remodeling due to isolated septal thickening in patients with systemic hypertension and normal left ventricular masses. Am J Cardiol. 1994;73:247–252. [DOI] [PubMed] [Google Scholar]
- 7. Muller‐Brunotte R, Kahan T, Malmqvist K, et al. Blood pressure and left ventricular geometric pattern determine diastolic function in hypertensive myocardial hypertrophy. J Hum Hypertens. 2003;17:841–849. [DOI] [PubMed] [Google Scholar]
- 8. Lemogoum D, Seedat YK, Mabadeje AF, et al. Recommendations for prevention, diagnosis and management of hypertension and cardiovascular risk factors in sub‐Saharan Africa. J Hypertens. 2003;21:1993–2000. [DOI] [PubMed] [Google Scholar]
- 9. Kimura BJ, Shaw DJ, Agan DL, et al. Value of a cardiovascular limited ultrasound examination using a hand‐carried ultrasound device on clinical management in an outpatient medical clinic. Am J Cardiol. 2007;100:321–325. [DOI] [PubMed] [Google Scholar]
- 10. Piccoli M, Trambaiolo P, Salustri A, et al. Bedside diagnosis and follow‐up of patients with pleural effusion by a hand‐carried ultrasound device early after cardiac surgery. Chest. 2005;128:3413–3420. [DOI] [PubMed] [Google Scholar]
- 11. Tsutsui JM, Maciel RR, Costa JM, et al. Hand‐carried ultrasound performed at bedside in cardiology inpatient setting ‐ a comparative study with comprehensive echocardiography. Cardiovasc Ultrasound. 2004;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vourvouri EC, Poldermans D, Deckers JW, et al. Evaluation of a hand carried cardiac ultrasound device in an outpatient cardiology clinic. Heart. 2005;91:171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kobal SL, Trento L, Baharami S, et al. Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination. Am J Cardiol. 2005; 96:1002–1006. [DOI] [PubMed] [Google Scholar]
- 14. Kobal SL, Czer LS, Czer PC, et al. Making an impossible mission possible. Chest. 2004;125:293–296. [DOI] [PubMed] [Google Scholar]
- 15. Brennan JM, Blair JE, Goonewardena S, et al. A comparison by medicine residents of physical examination versus hand‐carried ultrasound for estimation of right atrial pressure. Am J Cardiol. 2007;99:1614–1616. [DOI] [PubMed] [Google Scholar]
- 16. Croft LB, Duvall WL, Goldman ME. A pilot study of the clinical impact of hand‐carried cardiac ultrasound in the medical clinic. Echocardiography. 2006;23:439–446. [DOI] [PubMed] [Google Scholar]
- 17. Newcombe RG. Two‐sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. [DOI] [PubMed] [Google Scholar]
- 18. Kohara K, Zhao B, Jiang Y, et al. Relation of left ventricular hypertrophy and geometry to asymptomatic cerebrovascular damage in essential hypertension. Am J Cardiol. 1999;83:367–370. [DOI] [PubMed] [Google Scholar]
- 19. Muscholl MW, Schunkert H, Muders F, et al. Neurohormonal activity and left ventricular geometry in patients with essential arterial hypertension. Am Heart J. 1998; 135:58–66. [DOI] [PubMed] [Google Scholar]
- 20. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B‐type natriuretic peptide in comparison with those of A‐type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. [DOI] [PubMed] [Google Scholar]
- 21. Nunez BD, Lavie CJ, Messerli FH, et al. Comparison of diastolic left ventricular filling and cardiac dysrhythmias in hypertensive patients with and without isolated septal hypertrophy. Am J Cardiol. 1994;74:585–589. [DOI] [PubMed] [Google Scholar]
- 22. Wachtell K, Dahlof B, Rokkedal J, et al. Change of left ventricular geometric pattern after 1 year of antihypertensive treatment: the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study. Am Heart J. 2002;144:1057–1064. [DOI] [PubMed] [Google Scholar]
- 23. Schulman DS, Flores AR, Tugoen J, et al. Antihypertensive treatment in hypertensive patients with normal left ventricular mass is associated with left ventricular remodeling and improved diastolic function. Am J Cardiol. 1996;78:56–60. [DOI] [PubMed] [Google Scholar]
- 24. Cuspidi C, Meani S, Valerio C, et al. Left ventricular hypertrophy and cardiovascular risk stratification: impact and cost‐effectiveness of echocardiography in recently diagnosed essential hypertensives. J Hypertens. 2006;24: 1671–1677. [DOI] [PubMed] [Google Scholar]
- 25. Martin LD, Howell EE, Ziegelstein RC, et al. Hand‐carried ultrasound performed by hospitalists: does it improve the cardiac physical examination? Am J Med. 2009; 122:35–41. [DOI] [PubMed] [Google Scholar]
- 26. Rugolotto M, Chang CP, Hu B, et al. Clinical use of cardiac ultrasound performed with a hand‐carried device in patients admitted for acute cardiac care. Am J Cardiol. 2002;90:1040–1042. [DOI] [PubMed] [Google Scholar]
- 27. Vourvouri EC, Poldermans D, Schinkel AF, et al. Left ventricular hypertrophy screening using a hand‐held ultrasound device. Eur Heart J. 2002;23:1516–1521. [DOI] [PubMed] [Google Scholar]
- 28. Trambaiolo P, Papetti F, Posteraro A, et al. A hand‐carried cardiac ultrasound device in the outpatient cardiology clinic reduces the need for standard echocardiography. Heart. 2007;93:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greaves K, Jeetley P, Hickman M, et al. The use of hand‐carried ultrasound in the hospital setting – a cost‐effective analysis. J Am Soc Echocardiogr. 2005;18:620–625. [DOI] [PubMed] [Google Scholar]


