Abstract
J Clin Hypertens (Greenwich).
The objective of this study was to evaluate the prevalence and effect of the metabolic syndrome (MetS) on patients with acute coronary syndrome (ACS) in six Middle Eastern countries using the new definition of MetS. Analysis of the Gulf Registry of Acute Coronary Events (Gulf RACE), which included 8716 consecutive patients hospitalized with ACS, was conducted and patients were divided into two groups: patients with and patients without the MetS. Overall, 46% of patients had MetS. Patients with MetS were more likely to be female and less likely to be smokers. In‐hospital mortality and cardiogenic shock were comparable between the two groups, although MetS patients were more likely to have congestive heart failure and recurrent ischemia. In ST‐elevation myocardial infarction, MetS was also associated with increased risk of recurrent myocardial infarction and stroke. Using the recent MetS definition, MetS is highly prevalent among Middle Eastern patients presenting with ACS. MetS is associated with higher‐risk profile characteristics and increased risk for development of heart failure and recurrent myocardial ischemia without an increase in hospital mortality. J Clin Hypertens (Greenwich). 2010;12:890–899.
Worldwide, the metabolic syndrome (MetS) is a major health problem associated with increased morbidity and mortality. The syndrome was first described in Western societies, but it is now emerging in developing countries. MetS represents a constellation of cardiovascular risk factors, including elevated blood pressure (BP), hyperglycemia, elevated triglyceride levels, central obesity, and decreased high‐density lipoprotein cholesterol. 1 The prevalence of MetS among patients with acute coronary syndrome (ACS) is limited by small registries and post hoc analyses of randomized trials that use three different guideline definitions for MetS (Third Report of the Adult Treatment Panel National Cholesterol Education Program [NCEP‐ATP III] definition; National Heart, Lung and Blood Institute/American Heart Association [NHLBI/AHA] definition; and International Diabetes Federation [IDF] definition), making consensus about their findings difficult. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 More recently, a joint statement of the IDF Task Force on Epidemiology and Prevention, NHLBI, AHA, the World Heart Federation, the International Atherosclerosis Society, and the International Association for the Study of Obesity unified the diagnostic criteria of MetS. 16 In this study we evaluate the prevalence of MetS and patient outcomes based on the new definition among a large cohort of patients presenting with ACS in six Middle Eastern countries.
Methods
The Gulf Registry of Acute Coronary Events (Gulf RACE) prospectively enrolled 8716 patients (of which 6071 patients had data on weight and abdominal waist circumference) with ACS from 65 centers in six adjacent Middle Eastern gulf countries (Bahrain, Kuwait, Qatar, Oman, United Arab Emirates, and Yemen) during a six‐month period. The diagnosis of ACS included unstable angina (UA) and non–ST‐segment myocardial infarction (NSTEMI), collectively referred to here as non–ST‐segment elevation ACS (NSTEACS) or ST‐segment elevation myocardial infarction (STEMI). The study received ethical approval from the institutional ethical bodies in all participating countries. There were no exclusion criteria. Full details of the methods have been previously published. 17 , 18 Diagnosis of the different types of ACS and definitions of data variables were based on the American College of Cardiology clinical data standards. 19
Patients were evaluated for the presence of MetS based on the most recent definition that was outlined in a joint statement of the IDF Task Force on Epidemiology and Prevention, NHLBI, AHA, World Heart Federation, International Atherosclerosis Society, and International Association for the Study of Obesity. Patients were classified as having MetS when at least three of the following were present: (1) abdominal obesity (waist circumference in Middle Eastern and Mediterranean populations: men ≥94 cm and women ≥80 cm); (2) high triglyceride level (≥150 mg/dL [≥1.7 mmol/L]); (3) low high‐density lipoprotein cholesterol level (<40 mg/dL [<1.04 mmol/L] for men and <50 mg/dL [<1.30 mmol/L] for women); (4) high BP (systolic ≥130 mm Hg or diastolic ≥85 mm Hg or taking antihypertensive medication); and (5) high fasting plasma glucose concentration (≥100 mg/dL [≥6.10 mmol/L]) or a clinical history of diabetes mellitus. 16
Statistical Analysis
Baseline and clinical characteristics of patients are presented as median and 25th and 75th percentiles for continuous variables, whereas frequency distribution and percentages are presented for categoric variables. Independent t tests were used for comparing continuous variables, and chi‐square tests for categoric variables have been used for comparing MetS vs non‐MetS cases. A univariate logistic regression was performed for all important variables, and important variables were considered multivariate logistic regression analysis to examine the importance of MetS in heart failure (HF) patients. Chi‐square test was performed. A P≤.05 was considered statistically significant. All data analyses were carried out using the Statistical Package for Social Sciences version 14 (SPSS Inc, Chicago, IL).
Results
Study Population Characteristics
Of the 6701 patients studied, 3108 patients (46%) had MetS. Patients with MetS were more likely to present with NSTEACS, NSTEMI (37% vs 28%), or UA (33% vs 25%) than STEMI (30% vs 47%) (all P=.001).
Presenting Symptoms and Baseline Clinical Characteristics
Overall, MetS patients with ACS were three years older than those without MetS, more likely to be female, and less likely to be smokers. Patients with MetS were more likely to have a history of previous coronary artery disease and prior coronary revascularization and were more likely to be taking aspirin therapy. Patients with MetS were also more likely to have renal impairment at presentation. The admission heart rate and systolic and diastolic BPs were significantly higher in MetS patients. MetS patients were more likely to have Killip class >1 on admission. While MetS patients were more likely to present with atypical presentations, they were more likely to present earlier than patients without MetS. MetS patients had higher first blood sugar level at admission, serum creatinine, and triglyceride levels when compared when patients without MetS. Subset analysis of patients with STEMI, NSTEMI, and UA demonstrated similar clinical characteristics and risk factor findings regardless of the admission diagnosis (Table I).
Table I.
Clinical Characteristics, Management, and Hospital Outcomes in Patients With and Without the Metabolic Syndrome Presenting With Acute Coronary Syndrome
| No Metabolic Syndrome | Metabolic Syndrome | P Value | |
|---|---|---|---|
| No. % | 3593 (54) | 3108 (46) | |
| Age, mean, y | 55±13 | 58±11 | <.001 |
| Female, % | 15 | 35 | <.001 |
| Diabetes mellitus, % | 18 | 67 | <.001 |
| Hypertension, % | 28 | 76 | <.001 |
| Dyslipidemia, % | 20 | 46 | <.001 |
| Smoking, % | 43 | 32 | <.001 |
| Prior aspirin use, % | 32 | 53 | <.001 |
| Family history of CAD, % | 13 | 14 | .43 |
| Prior CAD, % | 38 | 55 | <.001 |
| Prior revascularization, % | 11 | 21 | <.001 |
| Renal impairment, % | 15 | 21 | <.001 |
| At presentation | |||
| Heart rate, beats per min | 84±22 | 87±32 | <.001 |
| Systolic BP, mm Hg | 135±30 | 145±31 | <.001 |
| Diastolic BP, mm Hg | 83±19 | 85±18 | <.001 |
| Body mass index, mean | 26±4 | 29±6 | <.001 |
| Killip class >1% | 20 | 25 | <.001 |
| GRACE risk score | |||
| Low, % | 45 | 38 | .001 |
| High, % | 27 | 33 | |
| Laboratory results | |||
| Total cholesterol | 5±2 | 5±3 | .33 |
| Serum triglyceride | 1.5±1 | 2.3±4 | .001 |
| First blood sugar | 9.4±8 | 12.5±12 | .001 |
| First serum creatinine | 103±78 | 114±110 | .001 |
Abbreviations: CAD, coronary artery disease; BP, blood pressure.
A subset analysis of the interaction between age at presentation with ACS and sex was performed (Figure 1) The prevalence of MetS among the different age groups was consistently higher among women when compared with men and was >70% among women in the age group of 50 to 79 years.
Figure 1.
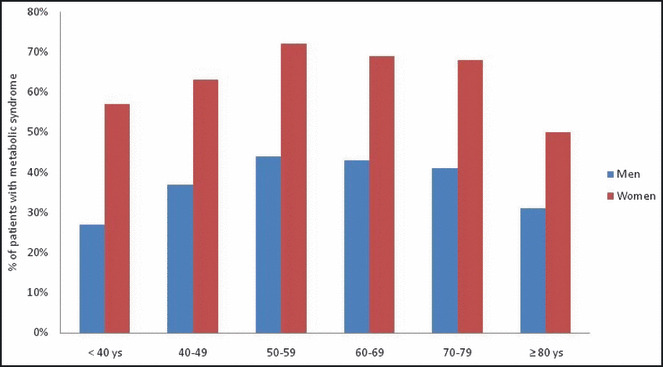
Prevalence of metabolic syndrome stratified according to age and gender.
Treatment
In‐Hospital Overall, aspirin, clopidogrel, and β‐blockers use was high and glycoprotein IIb/IIIa inhibitor use was low and comparable between the two groups. Patients with MetS were more likely to receive low molecular weight heparin than unfractionated heparin; they were also more likely to receive angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and statins. Thrombolytic therapy was the primary reperfusion strategy in our registry, and no differences were observed in its use between the two groups (Table II).
Table II.
Variability in Presentation, Final Diagnosis, Hospital Treatment, and Outcome
| No Metabolic Syndrome | Metabolic Syndrome | P Value | |
|---|---|---|---|
| Presentation >12 h, % | 33 | 28 | .007 |
| Atypical presentation, % | 18 | 23 | <.001 |
| Final diagnosis | |||
| STEMI, % | 47 | 30 | .001 |
| Non‐STEMI, % | 28 | 37 | |
| Unstable angina, % | 25 | 33 | |
| Coronary angiogram, % | 18 | 19.5 | .09 |
| In‐hospital therapy | |||
| Thrombolytic therapy, %a | 57 | 59 | .35 |
| Primary PCIa | 5 | 3 | .006 |
| Aspirin, % | 97 | 98 | .62 |
| Clopidogrel, % | 54 | 53 | .95 |
| Unfractionated heparin, % | 52 | 42 | .001 |
| Low molecular weight heparin, % | 42 | 53 | <.001 |
| Glycoprotein IIb/IIIa inhibitors, % | 10 | 11 | .07 |
| Angiotensin‐converting enzyme inhibitor, % | 64 | 66 | .03 |
| Angiotensin receptor blocker, % | 3 | 8 | .001 |
| β‐blockers, % | 64 | 66 | .09 |
| Therapy at discharge | |||
| Aspirin, % | 93 | 95 | .005 |
| Clopidogril, % | 48 | 51 | .004 |
| Angiotensin receptor blocker, % | 4 | 10 | .001 |
| Angiotensin‐converting enzyme inhibitor, % | 70 | 69 | .99 |
| β‐Blockers, % | 75 | 76 | .07 |
| Statins, % | 79 | 83 | .001 |
| Hospital outcomes | |||
| Recurrent ischemia, % | 8 | 10 | .01 |
| Reinfarction,% | 2 | 2.4 | .37 |
| Heart failure, % | 15 | 18 | .002 |
| Cardiogenic shock, % | 5.5 | 5 | .20 |
| Major bleeding, % | 0.8 | 0.7 | .55 |
| Stroke, % | 0.6 | 0.9 | .56 |
| Mortality, % | 3 | 2.4 | .22 |
| Hospital stay, d | 5.5±4 | 5.7±5 | .19 |
Abbreviations: PCI, percutaneous coronary intervention; ST‐elevation myocardial infarction. aOf patients eligible for reperfusion.
At Discharge Patients with MetS were more likely to be prescribed aspirin, clopidogrel, ACE inhibitors, ARBs, and statins at discharge. There was no difference in β‐blocker prescription at discharge between the two groups.
In‐Hospital Outcome
In all ACS patients, there were no differences in mortality, cardiogenic shock, major bleeding, or recurrent myocardial infarction (MI) between the two groups, although MetS patients were more likely to develop HF and recurrent myocardial ischemia. In NSTEMI, MetS patients had more HF and recurrent myocardial ischemia than non‐MetS patients, with no differences in the incidence of other complications between the groups. In UA, MetS patients had comparable complication rates to non‐MetS patients. In the STEMI group, there were no differences between the two groups regarding mortality, cardiogenic shock, or major bleeding complications, although MetS patients had a significantly higher incidence of recurrent MI, stroke, and HF (Table II).
Multivariate Predictors of HF
Multivariate regression demonstrated age, female sex, diabetes mellitus (odds ratio [OR], 1.6; 95% confidence interval [CI], 1.31–1.88; P<.001), and hypertension (OR, 1.4; 95% CI, 1.16–1.69; P<.001) to be independent predictors for HF complications. MetS was not an independent predictor of HF (OR, 0.082; P=.08) (Figure 2).
Figure 2.
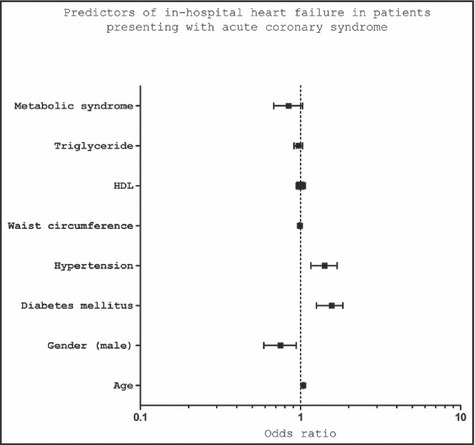
Predictors of in‐hospital heart failure in patients presenting with acute coronary syndrome. HDL indicates high‐density lipoprotein.
Discussion
The present study is the largest registry to date that reports the prevalence of MetS among patients with ACS and is the first that applies the new criteria of MetS that was recently jointly approved by multiple international associations. MetS was highly prevalent among Middle Eastern patients presenting with ACS (46%). ACS patients with MetS were more likely to present with atypical presentations and had higher Killip class. Although MetS in ACS patients was not associated with increased in‐hospital mortality, it was associated with increased risk of congestive HF. This increased risk was mainly attributed to diabetes mellitus and hypertension, as MetS was not an independent predictor of HF. In STEMI, patients with MetS had double the risk of developing stroke and recurrent MI.
Prevalence and Characteristics of MetS Among Patients With ACS
Few studies reported the prevalence of MetS, as defined by the NCEP‐ATP III, NHLBI/AHA, and/or IDF criteria in patients with ACS, 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 (Table III), which were mostly registry data conducted in North America, Europe, Japan, and Australia and included a small number of patients (107–633 patients) with an MetS prevalence ranging from 20.8% to 79.1%. Our study confirms this high prevalence of MetS (46%) using the new MetS definition in a larger unselected population (6701 patients). Patients with MetS were consistently younger (age range, 67.8 to 70 years) when compared with non‐MetS patients in almost all studies except two (Yasar 10 and Dohi 12 and colleagues). The current study extends these observations in an overall younger population with a mean age of 56 years (55 years with MetS vs 58 years without MetS). Moreover, it extends the observations of Zeller and colleagues 3 of higher female and lower smoking prevalence among MetS patients. Our study, based on an unselected large population of ACS patients in the Middle East reports the highest prevalence of diabetes mellitus (40%) when compared with previous reports, which ranged from 19% (in Australia) 8 to 34% (in Japan). 9 In a post hoc analysis of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI‐Prevenzione) trial, which included 10,384 patients who had recent MI (within 3 months), Levantesi and colleagues 13 reported a 29.3% and 21% prevalence of MetS and diabetes mellitus, respectively. To further expand on the current observations, additional large‐scale multi‐ethnic studies using the new definition of MetS are urgently needed.
Table III.
Reported Studies of the MetS and ACS
| Author, Year (Origin) | Study Design | No. of Patients | Previous DM, % | Previous MetS Definition, % | Follow‐Up | Outcome |
|---|---|---|---|---|---|---|
| Boulon, 2006 (France) 2 | Registry | 480 | 21.6 | ATP III: 20.8; IDF: 27.7 | 19 mo | Increase in total mortality in the MetS group (5.2% vs 1.4%, P<.01) due in part to an increase in noncardiovascular death. No increase in minor or major cardiovascular events. |
| Zeller, 2005 (France) 3 | Registry | 633 | 26 | ATP III: 46 | In‐hospital | Independent predictor of heart failure (OR, 2.13; 95% CI, 1.28–3.57; P=.003) but not death. |
| Clavijo, 2006 (United States) 4 | Registry | 405a | b | ATP III: 41b | In‐hospital | Associated with larger infarct size determined by cardiac enzymes (P<.001), more complications (21.1% vs 9.2%, P=.003), and a marked increase of acute renal failure (7.9% vs 0.8%, P=.007). |
| Celik, 2006 (Turkey) 5 | Registry | 283a | b | ATP III: 25 | In‐hospital | Independent predictor of poor myocardial perfusion grade post‐primary PCI (OR, 2.54; 95% CI, 1.35–4.75; P=.003). |
| Chung, 2007 (United States)6 | Registry | 223c | 13 | ATP III: 47 | Not provided | MetS highly prevalent in young (≤45 y) with MI. |
| Milionis, 2007 (Greece) 7 | Registry | 136c | 2.2 | ATP III: 40.4 | In‐hospital | MetS strongly associated with ACS in young persons, independent of other risk factors. Smoking and a positive family history of premature CAD in young persons with MetS had an incremental effect on the odds of having ACS (OR, 7.12; 95% CI, 2.42–20.96; P<.001). |
| Prasad, 2009 (Australia) 8 | Registry | 107a | 19 | IDF: 54 ATP III: 49 | In‐hospital | Same extent of coronary atherosclerosis and coronary flow post‐PCI. No differences in systolic function but greater diastolic dysfunction with MetS (P<.05). |
| Tokeno, 2008 (Japan) 9 | Registry | 461a | 34 | ATP III: 37 | 17.6 mo | Independent predictor of heart failure (OR, 2.60; 95% CI, 1.01–6.66; P=.04) and repeat revascularization (OR, 2.1; 95% CI, 1.27–3.47; P<.01). |
| Yasar, 2009 (Turkey) 10 | Registry | 116 | 27 | ATP III: 55.2 | In‐hospital | MetS patients have lower rates of TIMI grade 3 flow (OR, 3.545; 95% CI, 1.064–11.808; P=.03) and higher corrected TIMI frame counts after thrombolytic therapy for AMI. |
| Koutsovasilis, 2009 (Greece) 11 | Registry | 211 | 30.3 | ATP III: 72.5 NHLBI/AHA: 81.2 IDF: 79.1 | Compared with control | Only IDF‐defined MetS was significantly associated with ACS (OR, 2.23; 95% CI, 1.3–3.82; P=.003). Of the MetS components, only waist circumference was independently associated with ACS (OR, 1.045; 95% CI, 1.014–1.078; P=.005). |
| Dohi, 2009 (Japan) 12 | Registry | 384 (with complete revascularization) | ATP III: 42.5 | 10.4±3.4 y | Independent predictor of long‐term all‐cause (OR, 1.62; 95% CI, 1.01–2.59; P=.046) and cardiovascular death (OR, 2.40; 95% CI, 1.16–4.94; P=.018). | |
| Levantesi, 2005 (Italy)d 13 | RCT‐post hoc (GISSI‐Prevenzione) | 10,384 | 20.6 | ATP III: 29.3 | 3.5 y | Increased probability of death (OR, 1.29; 95% CI, 1.1–1.51; P=.002) and cardiovascular events (OR, 1.23; 95% CI, 1.06–1.42; P=.005) at follow‐up; however, hospitalization for heart failure at follow‐up was not increased. |
| Germain, 2004 (Europe‐United States)e 14 | RCT‐post hoc (4S and AFCAPS/TexCAPS) | 5179 (not all with prior ACS) | b | ATP III: 20.6–46 | 5–5.4 y | 4S: Independent predictor of major adverse cardiac events (OR, 1.5; 95% CI, 1.2–1.8). AFCAPS/TexCAPS: Independent predictor of major adverse cardiac events (OR, 1.4; 95% CI, 1.04–1.9). |
| Schwatrz, 2005 (United States) 15 | RCT‐Post hoc (MIRACL) | 3083 | 23 | ATP III: 38 | 16 wk | Independent predictor of major adverse cardiac events at 16‐week follow‐up (OR, 1.49; 95% CI, 1.24–1.79; P<.0001). Independent predictor of all‐cause mortality (OR, 1.49; 95% CI, 1.04–2.14; P=.029). |
| Current study (6 Middle Eastern countries) | Registry | 6701 | 40 | New unified: 46 | In‐hospital | Increased risk of heart failure and recurrent myocardial ischemia but not death. STEMI patients have double the risk of recurrent myocardial infarction and stroke. |
Abbreviations: ACS, acute coronary syndrome; ATP III, National Cholesterol Education Program Adult Treatment Panel III; CI, confidence interval; DM, diabetes mellitus; IDF, International Diabetes Federation; MIRACL, the Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering trial; OR, odds ratio; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; STEMI, ST‐elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction. aAcute myocardial infarction (AMI) patients only. bNondiabetic with metabolic syndrome (MetS). cThe study included only patients younger than 45 years. dPost hoc analysis of randomized trial (the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico [GISSI‐Prevenzione] Trial), which included patients who had a recent MI (within 3 months). ePost hoc analysis of two randomized trials (the Scandinavian Simvastatin Survival Study [4S] and the Air Force/Texas Coronary Atherosclerosis Prevention Study [AFCAPS/TexCAPS]).
MetS and HF
One of the main findings of the current study is higher Killip class (Killip class >1, overall 25% vs 20% in non‐MetS patients) and higher incidence of HF complications among patients with MetS (overall ACS group, 18% vs 15%). The increased HF risk mainly occurs in patients with MI only, STEMI (20% vs 14%), and NSTEMI (18% vs 14%). The incidence of HF among MetS patients with UA was not increased (15% vs 15.2%, P=.09). However, DM and hypertension (not MetS) were independent predictors of HF. Several investigators reported the prevalence of HF complications among MetS patients with ACS. The Observatoire des Infarctus de Cote‐d’Or Survey 3 reported higher Killip class (Killip class >1, 26% vs 16%) and HF complications among MetS patients. In that survey (n=633), the overall reperfusion therapy rate was low and was even lower among MetS patients (49% vs 38%). The investigators hypothesized that this increased risk may be related to fasting hyperglycemia, and this hypothesis was supported by earlier studies reporting increased risk of pump failure in patients with MI and hyperglycemia, even after adjustment for age. 20 , 21 , 22 Another explanation however, may be lower reperfusion therapy in the MetS group. More recently, in a case‐control study of 461 patients with MI, Takeno and colleagues 9 also reported increased risk of HF and revascularization rates among MetS patients despite high reperfusion therapy rates >70% and lack of significant differences in left ventricular ejection fraction when compared with non‐MetS patients. They attributed increased risk of these two complications (HF and revascularization) to the presumed development of new coronary lesions or restenosis during the follow‐up period (19 months). On the other hand, in a 36‐month follow‐up study of patients with recent MI by Laventsei and colleagues, 13 patients with diabetes mellitus and not MetS had increased risk of HF when compared with controls. Clavijo and colleagues 4 reported increased infarct size in 405 patients with MetS and acute MI. They determined the infarct size by peak creatine kinase–MB (CK‐MB) levels. However, Zeller 3 and Yasar 10 and colleagues did not observe higher peak CK and CK‐MB levels or lower left ventricular ejection fraction in patients with MetS when compared with those without MetS. In conclusion, MetS patients presenting with MI (not UA) are at increased risk for HF, which appears to not be related to worse systolic function and appears to be related to the presence of diabetes and hypertension. Further studies specifically designed to elucidate the pathogenesis of this association are needed.
MetS and Prognosis
Several investigators (using either IDF or ATP III or both definitions of MS) reported in‐hospital and follow‐up mortality rates among MetS patients. In a 17.6‐month follow‐up of 461 patients, Takeno and colleagues 9 reported MetS (using the ATP III definition) no independent predictor of cardiovascular death (relative risk, 0.96; 95% CI, 0.3–3.30). Boulon and colleagues 2 reported an increase in total mortality in MetS patients at 19‐month follow‐up (using the IDF definition, among 480 ACS patients); however, this increase was in part due to an increase in noncardiovascular deaths. On the other hand, Zeller and colleagues 3 reported an increased in‐hospital mortality rate among MetS patients (10.7% vs 3.8%; P=.01). However, after adjustment for variables, MetS was no longer an independent predictor of mortality. On the other hand, in the Myocardial Ischemia Reduction With Aggressive Cholesterol Lowering (MIRACLE) trial, 15 patients with ACS and MetS had increased all‐cause mortality and an increased rate of reaching the primary end point (death, nonfatal MI, cardiac arrest, or recurrent unstable myocardial ischemia) during a 16‐week follow‐up. More recently, Dohi and colleagues 12 reported an increased risk of cardiovascular mortality among MetS patients in a 10‐year follow‐up of 383 Japanese patients.
The current study, which involved a much larger cohort of patients of different ethnicities than those previously reported, supports lack of increased hospital mortality risk among MetS patients. On the other hand, diabetes mellitus was an independent predictor of increased mortality. However, patients with MetS were at increased risk for recurrent myocardial ischemia in the overall ACS group (P=.01). Furthermore, it reports for the first time that STEMI patients with MetS have double the risk of stroke and recurrent MI. Increased risk of recurrent myocardial ischemia, MI, and stroke may be attributed to prothrombotic state associated with MetS. 23 , 24 , 25 Increased levels of plasminogen activator inhibitor‐1 (PAI‐1) are reported in patients with MetS. PAI‐1 inhibits spontaneous and pharmacologically induced fibrinolysis. In patients with MetS, fibrinolytic dysfunction together with increased platelet activation may lead to a prothrombotic state and impaired response to thrombolytic agents. Arenillas and colleagues 24 investigated the impact of MetS on the response to systemic thrombolysis in patients with acute middle cerebral artery ischemic stroke and reported that MetS was associated with a poor response to thrombolysis. They suggested that insulin resistance might exert independent deleterious actions that contribute to limit the efficacy of thrombolytic therapy. More recently, Mente and colleagues, 26 using the IDF and ATP III criteria among patients in the INTERHEART study, reported increased risk of MI among patients with MetS; however, this risk does not appear to be greater than the risk conferred by its component factors. It appears that the behavior of MetS as a cardiovascular risk factor is similar to that of hypertension and different from diabetes mellitus. MetS increases the risk of developing MI and, subsequently, HF and recurrent myocardial ischemia complications without increased in‐hospital mortality. Further studies, are required to clarify the prognostic impact of MetS among MI patients.
Study Limitations
Our data were collected from an observational study, which is a limitation. The fundamental limitations of observational studies cannot be eliminated because of the nonrandomized nature and unmeasured confounding factors. However, well‐designed observational studies provide valid results and do not systematically overestimate the results compared with the results of randomized controlled trials. Long‐term follow‐up is needed in both groups to consolidate our findings.
Conclusions
MetS is highly prevalent among patients presenting with ACS in six Middle Eastern countries. MetS is associated with increased risk for development of HF and recurrent myocardial ischemia without an increase in hospital mortality. STEMI patients have double the risk of recurrent MI and stroke. To further expand on our understanding of the prevalence and outcome of MetS in patients with ACS, further large‐scale studies in several ethnicities using the new definition of MetS are urgently needed.
Acknowledgments
Acknowledgements and disclosures: We thank the staff in all the participating centers for their invaluable cooperation. Gulf RACE is a Gulf Heart Association project and was financially supported by Sanofi Aventis, Paris, France, and Qatar Telecommunications Company, Doha, Qatar. The sponsors had no role in the study design, data collection, or data analysis. The sponsors had no role in the writing of the report and submission of the manuscript. The authors have no conflict of interest to disclose.
References
- 1. Zimmet P, Magliano D, Matsuzawa Y. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. [DOI] [PubMed] [Google Scholar]
- 2. Zeller M, Steg PG, Ravisy J, et al. Prevalence and impact of metabolic syndrome on hospital outcomes in acute myocardial infarction. Arch Intern Med. 2005;165:1192–1198. [DOI] [PubMed] [Google Scholar]
- 3. Boulon C, Lafitte M, Richeboeuf V, et al. Prevalence of metabolic syndrome after acute coronary syndrome and its prognostic significance. Am J Cardiol. 2006;89:1429–1434. [DOI] [PubMed] [Google Scholar]
- 4. Clavijo LC, Pinto TL, Kuchulakanti RT, et al. Metabolic syndrome in patients with acute myocardial infarction is associated with increased infarct size and in‐hospital complications. Cardiovasc Revasc Med. 2006;7:7–11. [DOI] [PubMed] [Google Scholar]
- 5. Celik T, Turhan H, Kursaklioglu H, et al. Impact of metabolic syndrome on myocardial perfusion grade after primary percutaneous coronary intervention in patients with acute ST elevation myocardial infarction. Coron Artery Dis. 2006;17:339–343. [DOI] [PubMed] [Google Scholar]
- 6. Chung EH, Curran PJ, Sivasankaran S, et al. Prevalence of metabolic syndrome in patients ≤45 years of age with acute myocardial infarction having percutaneous coronary intervention. Am J Cardiol. 2007;100:1052–1055. [DOI] [PubMed] [Google Scholar]
- 7. Milionis HJ, Kalantzi KJ, Papathanasiou AJ, et al. Metabolic syndrome and risk of acute coronary syndromes in patients younger than 45 years of age. Coronary Artery Dis. 2007;18:247–252. [DOI] [PubMed] [Google Scholar]
- 8. Prasad S, Fahratsh F, Malaipan Y, et al. Obesity and the metabolic syndrome in patients with acute myocardial infarction. Int J Cardiol. 2009 Apr 6. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9. Takeno M, Yasuda S, Otsuka Y, et al. Impact of metabolic syndrome on long‐term survival of patients with acute myocardial infarction. Circ J. 2008;72:415–419. [DOI] [PubMed] [Google Scholar]
- 10. Yasar AS, Bilen E, Bilge M, et al. Impact of metabolic syndrome on coronary patency after thrombolytic therapy for acute myocardial infarction. Coron Artery Dis. 2009;20:387–391. [DOI] [PubMed] [Google Scholar]
- 11. Koutsovasilis A, Protopsaltis J, Triposkiadis F, et al. Comparative performance of three metabolic syndrome definitions in the prediction of acute coronary syndrome. Intern Med. 2009;48:179–187. [DOI] [PubMed] [Google Scholar]
- 12. Dohi T, Miyauchi K, Kasai T, et al. Impact of metabolic syndrome on 10‐year clinical outcomes among patients with acute coronary syndrome. Circ J. 2009;73:1454–1458. [DOI] [PubMed] [Google Scholar]
- 13. Levantesi G, Macchia A, Marfisi RM, et al. Metabolic syndrome and risk of cardiovascular events after myocardial infarction. J Am Coll Cardiol. 2005;46:277–283. [DOI] [PubMed] [Google Scholar]
- 14. Girman CJ, Rhodes T, Mercuri M, et al. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Am J Cardiol. 2004;93:136–141. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz GG, Szarek M, Olsson AG, et al. Relation of characteristics of metabolic syndrome to short‐term prognosis and effects of intensive statin therapy after acute coronary syndrome. Diabetes Care. 2005;28:1508–1513. [DOI] [PubMed] [Google Scholar]
- 16. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 17. El‐Menyar A, Zuabid M, Rashed W, et al. Comparison of men and women with acute coronary syndrome in six Middle Eastern countries. Am J Cardiol. 2009;104(8):1018–1022. [DOI] [PubMed] [Google Scholar]
- 18. Alsheikh‐Ali AA, Al‐Mallah MZ, Al Mahmeed W, et al. Heart failure in patients hospitalized with acute coronary syndrome: observations from the Gulf Registry of acute coronary syndrome. Eur J Heart Fail. 2009;11(12):1135–1142. [DOI] [PubMed] [Google Scholar]
- 19. Al Suwaidi J, Reddan DN, Williams K, et al. Prognostic implications of abnormalities in renal function in patients with cute coronary syndrome. Circulation. 2002;106:974–980. [DOI] [PubMed] [Google Scholar]
- 20. Bellodi G, Manicardi V, Malavasi V, et al. Hyperglycemia and prognosis after myocardial infarction in patients without diabetes mellitus. Am J Cardiol. 1989;64:885–888. [DOI] [PubMed] [Google Scholar]
- 21. O’Sullivan JJ, Conroy RM, Robinson K, et al. In‐hospital prognosis of patients with fasting hyperglycemia after first myocardial infarction. Diabetes Care. 1991;14:758–760. [DOI] [PubMed] [Google Scholar]
- 22. Leor J, Goldbourt U, Reicher‐Reiss H, et al. Cardiogenic shock complicating acute myocardial infarction in patients without heart failure on admission. Am J Med. 1993;226:297–301. [DOI] [PubMed] [Google Scholar]
- 23. Serebrany VL, Malinin A, Ong S, et al. Patients with metabolic syndrome exhibit higher platelet activity than those with conventional risk factors for vascular disease. J Thromb Thrombolysis. 2008;25:207–213. [DOI] [PubMed] [Google Scholar]
- 24. Arenillas JF, Ispierto L, Millan M, et al. Metabolic syndrome and resistance to IV thrombolysis in middle cerebral artery ischemic stroke. Neurology. 2008;71:190–195. [DOI] [PubMed] [Google Scholar]
- 25. Anand SS, Yi Q, Gerstein H, et al. Relationship of metbolic syndrome and fibrinolytic dysfunction to cardiovascular disease. Circulation. 2003;108:420–425. [DOI] [PubMed] [Google Scholar]
- 26. Mente A, Yusuf S, Islam S, et al. Metabolic syndrome and risk of acute myocardial infarction: a case‐control study of 26,903 subjects from 52 countries. J Am Coll Cardiol. 2010;55(21):2390–2398. [DOI] [PubMed] [Google Scholar]


